Abstract
Bone marrow stromal cells are multipotential cells that can be induced to differentiate into osteoblasts, chondrocytes, myocytes and adipocytes in different microenvironments. Recent studies revealed that bone marrow stromal cells could improve neurological deficits of various damages or diseases of the central nervous system such as Parkinson's disease, brain trauma, spinal cord injury and multiple sclerosis, and promote glia-axonal remodeling in animal brain subjected to an experimentally induced stroke. In the present study, bone marrow stromal cells were intracerebrally transplanted into the cerebrum following a transient middle cerebral artery occlusion. Our aim was to find out whether the bone marrow stromal cells could survive and express neural phenotypic proteins and, in addition, whether they could restore the behavioral and functional deficits of the cerebral ischemic rats. Our results demonstrated that transplanted bone marrow stromal cells survived and migrated to areas around the lesion site. Some of them exhibited marker proteins of astrocytes and oligodendrocytes. Bone marrow stromal cell implantation significantly reduced the transient middle cerebral artery occlusion-induced cortical loss and thinning of the white matter and enhanced cortical β-III-tubulin immunoreactivity. Rats implanted with bone marrow stromal cells showed significant improvement in their performance of elevated body swing test and forelimb footprint analysis and only transient recovery of the adhesive-removal test. Our data support bone marrow stromal cells as a valuable source of autologous or allogenic donor cells for transplantation to improve the outcome following cerebral ischemia.
Keywords: astrocyte, bone marrow stromal cells, cerebral stroke, differentiation, migration, oligodendrocyte
Introduction
A large number of reports have focused on the functional and phenotypical characteristics of bone marrow stromal cells (BMSCs) in view of their potential to differentiate and replace dying cells (Owen, 1988; Cao et al. 2007). Multiple lineages, including osteogenic, chondrogenic, tenogenic, hepatogenic, musclegenic and adipogenic, have been clonally derived from a single marrow stromal cell (MSC) (Owen & Friedenstein, 1988; Cheng et al. 1994; Pittenger et al. 1999; Kronenwett & Haas, 2006; Wlodarski et al. 2006;Chen et al. 2007a; Grassel & Ahmed, 2007). BMSCs also have the potential to differentiate into neuronal, glial and endodermal cell types in vitro and in vivo (Chopp et al. 2000; Munoz-Elias et al. 2004; Suzuki et al. 2004; Hermann et al. 2006; Lu et al. 2006; Cao et al. 2007). These cells are still capable of expanding while showing a stable phenotype, thus opening a way to the transplantation of nonhematopoietic stem cells for reconstituting a variety of damaged tissues. Recent reports demonstrate that BMSCs can express growth factors, such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), and neurotrophin-3 (NT-3) in vitro and in vivo (Garcia et al. 2004; Chen et al. 2005). These factors are known to promote or maintain neural survival and function following CNS damages. The potential of using BMSCs to restore nervous tissue after injuries has been explored extensively using the cerebral ischemia model. BMSCs were found to decrease the infarct size and improve the functional deficits associated with brain stroke (e.g. Chen et al. 2001a,b; Li et al. 2001; Iihoshi et al. 2004; Shen et al. 2006). Many of these studies introduced BMSCs through blood vessels or intraventricularly. Mechanisms associated with the functional improvement have been hypothesized to result from the ability of BMSCs to reduce apoptosis (Chen et al. 2003a), promote angiogenesis (Tanaka et al. 1995; Chen et al. 2003b), differentiate into other cells (Chen et al. 2000; Li et al. 2002; Zhao et al. 2002; Iihoshi et al. 2004; Mimura et al. 2005; Seyfried et al. 2006; Shen et al. 2006), promote endogenous cell proliferation (Chen et al. 2003a,b) and enhance glial–axonal remodeling (Li et al. 2006; Shen et al. 2006). In the present study, we used a rat transient middle cerebral artery occlusion (tMCAo) cerebral infarction model whereby BMSCs were introduced directly into the brain, to explore whether direct cerebral administration could improve the outcome. We carried out histological and behavioural analyses to find out whether and how intracerebral BMSCs transplantation might improve the outcome of cortical infarction. We employed three behavioral measures, the elevated body swing test, tape removal test and forelimb footprint analysis, to evaluate functional changes following infarction. These allowed us to estimate neurological dysfunctions including asymmetrical motor behavior and somatosensory deficit, which were found to correlate well with the regional infarction of the cerebral cortex induced in our experimental protocol (Ishibashi et al. 2003a,b; Vendrame et al. 2004).
Materials and methods
Animals
A total of 20 adult female Sprague-Dawley rats, aged between 8 and 10 weeks (250–300g) were used. Bone marrow was harvested from two adult female rats and the other 18 rats were divided into three groups: a sham control group (n = 2), an infarcted group (vehicle-treated, n = 8), and a transplanted group (n = 8). The rats were housed and cared for according to guidelines of the animal research committee of the National Chung-Hsing University. All efforts were taken to minimize animal suffering during and following surgery.
BMSC preparation
Culture of BMSCs was performed as described previously (Yu et al. 2006). The purification of BMSCs was confirmed by fibronectin and vimentin immunohistochemistry as these cells have been shown to express both proteins (Azizi et al. 1998; Hofstetter et al. 2002). BMSCs could be isolated from the whole marrow suspension by their adherent capability on plastic culture plates. The hematopoietic stem cells would be washed out by persisting culture media change. In brief, BMSCs were harvested from two adult female rats under anesthesia with 7% chloral hydrate (4.5mLkg−1; Merck, Darmstadt, Germany). BMSCs were collected from femurs with syringes containing heparin, followed by culturing with Dulbecco's modified Eagle's medium-Ham's F-12 (DMEM/F-12, Gibco Life Technologies, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS), 100UmL−1 penicillin and 100µg mL−1 streptomycin at 37°C in a 5% CO2 water-jacketed incubator. Non-adherent cells were removed 3days later by washing the flask with phosphate-buffered saline (PBS), and fresh medium was added. The medium was then changed every 3days. After the culture reached confluence, cells were trypsinized and re-plated at the density of 5000∼8000cm−2, which served as passage1 (P1). In preliminary studies, it was found that BMSCs at P8, P10, P12 and P14 displayed the same phenotype, and over 90% of them were immunopositive for vimentin and fibronectin, demonstrating their stability. On the basis of this, P10∼P12 BMSCs were used as the donor in the present study. Additional support for the stability of the relatively high passage cells we used came from our finding that the grafted BMSCs were well mixed and nonaggregative around the injection site in the brain sections. BMSCs at P10–P12 were labeled by incubation with 5-bromo-2′-deoxyuridine (BrdU, Sigma, St. Louis, MO, USA) in DMEM/F-12 for 5 days before transplantation.
Stroke surgery and BMSC implantation
Sixteen Sprague-Dawley rats were subjected to temporary focal cerebral ischemia. The method to induce ischemia was modified from that of Liao et al. (2004). Briefly, the two common carotid arteries (CCAs) and the right middle cerebral artery (MCA) exposed were clamped for 30min. Reflow of the right MCA and both CCAs was confirmed visually during surgery before closure of the wound. Two sham-operated animals served as control.
Sixteen stroke-induced rats were then randomly divided into two transplantation groups, receiving either 1×106 BMSCs in 20µL DMEM/F-12 medium or DMEM/F-12 medium (vehicle) alone (n = 8 each). The transplantation was performed immediately after stroke surgery. A 25-µL Hamilton syringe was filled with a 20-µL cell suspension and the injection was accomplished automatically at an infusion rate of 1µL min−1 using a programmable syringe pump (KDS-100, KD Scientific Inc.). The BMSCs were injected intracerebrally into the striatum of the infracted hemisphere at the following coordinates: 0.3mm posterior to the bregma, 3.00mm laterally, 4.0mm in depth. Animals were allowed to survive for 14 days following transplantation and prepared for the various examinations detailed below.
Histological and immunohistochemical procedures
At the end of the survival period, rats were anesthetized and perfused transcardially, first with normal saline, followed by a fixative containing 4% paraformaldehyde in 0.1 m phosphate buffer (PB), pH 7.3, at room temperature for 30min. After perfusion, the brains containing the areas of interest were removed and postfixed in the same fixative for 4h. Following this, tissues were cryoprotected in 30% sucrose in 0.1 m PB. Cryosections, 25µm thick, were prepared and collected in series in 0.1 m PB and all sections were divided into six groups. Sections from four groups were processed with antiserum for the glial fibrillary acidic protein (GFAP), galactocerebroside (GalC), microtubule-associated protein 2 (MAP2) and βIII tubulin (Tuj 1) to reveal astrocytes, oligodendrocytes, neurons and sprouting axons, respectively. The fifth group sections were treated with Griffonia simplicifolia isolectin B4 (GSA I-B4) conjugated with horseradish peroxidase (HRP) (25µgmL−1, Sigma) to reveal microglia. The last group sections were directly mounted on slides and processed for H&E staining to estimate the infarcted area. To study the differentiation, transplanted BMSCs were labeled with bromodeoxyuridine (BrdU, Sigma) before transplantation and double-stained with the following markers for identification of neurons and various glial cell types. To process for GFAP, GalC, MAP2, Tuj1 and BrdU, sections were treated in 10% normal serum in 0.1m PBS for 1h at room temperature. Following three rinses in 0.1m PBS, sections were incubated in solution containing the rabbit anti-GFAP (1:1000, Chemicon, Temecula, CA, USA), rabbit anti-GalC (1:50, Sigma), rabbit anti-MAP2 (1:1000, Chemicon), mouse anti-Tuj1 (1:1000, Chemicon), and mouse anti-BrdU (1:1000, Dako, Carpinteria, CA, USA) in 0.1m PBS containing 0.5% Triton X-100 for 18h at 4°C, respectively. For BrdU immunocytochemistry, before anti-BrdU serum treatment, sections were treated with 2N HCl for 30min and then three rinses in 0.1m borax solution, pH 8.5. They were then washed with three changes of 0.1m PBS and incubated in secondary antibody solution containing fluorescein isothiocyanate-tagged horse anti-mouse IgG (FITC, 1:100, Vector, Burlingame, CA, USA) or tetramethylrhodamine goat anti-rabbit IgG (TRITC, 1:100, Vector) and 0.5% Triton X-100 in 0.1m PBS at room temperature for 1h. All sections were washed and mounted in fluorescence mounting medium (Dako).
Functional test
Behavioral measurements including tape removal test, elevated body swing test, and footprint analysis were adopted to assess the extent of functional recovery. All tests were conducted by two individuals blinded to the treatment of the animals. The elevated body swing test (Borlongan et al. 1998) is a reflex test that does not require training. It examines the extent and direction of the swing of the body while the rat is held elevated by the tail. Normal rats swing to the right or left equally. Rats swung predominantly to the left after right tMCAo. The tape removal test (Bradbury et al. 2002; Sughrue et al. 2006) produced separate scores for sensory and motor behavior. Adhesive paper dots (each 50mm2) were used as tactile stimuli on the wrist of the left forepaw, and animals were observed in a cage (20×40×30cm). The time, to a maximum of 3min, for each rat to remove the tape from the forelimb (removal time) was recorded in three trials per day. Individual trials were separated by at least 5min. In the footprint analysis (de Medinaceli et al. 1982), rat forepaws were covered with ink to record walking patterns during continuous locomotion across a wooden runway (4×36 inches) and the stride length and width were measured. Data were collected from animals before surgery and 1, 4, 7 and 14 days after surgery and treatment. We averaged right and left forepaw scores as no difference was observed between them. Data was expressed as mean±SE. Statistical significance between groups was determined using two-tailed Student's t-test.
Tissue analysis
To evaluate the migrating abilities of the implanted BMSCs in the host tissue, the migrated BMSCs were divided into lesion and contralateral sides by their directional migration from the central axis of the injection site. The linear distances of the grafted BMSCs from the axis were measured from digital micrographs of each section with a PC-based image analysis program (Freeman Image-ProPlus, Media Cybernetics, Silver Spring, MD, USA) and compared. Infarction-induced cerebral tissue loss was estimated by measuring the area of the affected cortical area and the thickness of selected underlying white matter as expressed as a fraction of the measurements of the corresponding areas of the contralateral brain of each animal. These were measured with PC-based software (Image-ProPlus) from Nissl-stained coronal sections of the brain at the sectional plane –0.12mm∼–0.48mm in reference to the bregma.
Results
Characterization of BMSCs
BMSCs could be isolated from the whole marrow suspension by their adherent capability on plastic culture plates during the multiple passages of medium changes in the purification process. The cells were initially heterogeneous and turned into a homogeneous monolayer after passages. Almost all the BMSCs in our culture at P10 expressed the proteins vimentin (99.0±0.1%) and fibronectin (98.0± 1.6%), which are known to be expressed by these cells. The BMSCs treated with 3µgmL−1 BrdU-containing medium, a thymidine analogue, for 72h before transplantation exhibited 97.9±0.7% BrdU-immunopositivity (BrdU-IR).
BMSCs were transplanted intracerebrally into the striatum of the infarct side at the coordinates specified earlier. BrdU-IR BMSCs were found to migrate radially from the injected site (Fig. 1A) in the striatum of normal and infarct rats. To find out whether BMSCs migrated better in the stroke environment, we measured the distances of the grafted BMSCs from the central axis of the injection tract and found that the transplanted BMSCs moved equally in all directions in the normal rats but traveled a longer distance toward the lesion sites in the infarct brains (Fig. 1B).
Fig. 1.
Distribution of the engrafted BMSCs in the transplanted brain as revealed by BrdU-immunoreactivity. The section was counterstained with Hoechst (blue). BrdU-IR cells (red) survived around the injection site (arrow, A). The distance that they traveled from the center of the injection site into the infarct brain was significantly longer than that when they were introduced into the brain of normal rats. *P<0.05 between the infarct and normal rat; #P<0.01 between the infarct and the contralateral side (unpaired 2-tailed Student's t-test). Scale bar=50µm in A.
Differentiation of the injected BMSCs
To investigate the differentiation of BMSCs, we looked for the double-labeling of various cell markers with the BrdU-labeling of the cells 14 days following transplantation. Most of the 485 BrdU-labeled grafted BMSCs examined were negative for the neural marker (MAP2), and 9.3±1.7%, 2.0±1.0%, and 4.3±1.2% were immunopositive for the astrocyte marker GFAP), oligodendrocyte marker GalC, and microglia marker GSA I-B4, respectively (Fig. 2). In addition to a preferential migration toward the infarcted cortex, these cells were often found near white matter and close to blood vessels.
Fig. 2.
Photomicrographs showing the differentiation of the engrafted BrdU-labeled BMSCs. Immunohistochemical staining shows that some of the BrdU-positive cells (A,D) also expressed the astrocyte marker GFAP (B) and oligodendrocyte marker GalC (E). Photophraphs were taken from the border of the layer VI and the underlying white matter of the sensorimotor cortex of the BMSC-implanted rats. (C,F) Merged photographs of A, B, E and F, respectively. Arrows in C and F point to examples of BrdU-immunoreactive cells expressing marker for astrocyte and oligodendrocyte, respectively. Inset in E shows a high-power image of the colocalization of BrdU-IR cell with oligodendrocyte marker. Scale bar: A–C=25µm, D,E=50µm.
Effect of BMSC grafting on the cortical lesion induced by ischemia
Gross examination showed that infarction induced a marked loss of the sensorimotor cortex, including the hindlimb and forelimb representation areas and areas 1 and 2 of the parietal cortex of the affected hemisphere. To estimate the degree of tissue loss we measured the area of remaining cortical tissue and the thicknesses of the white matter at the midline of the corpus callosum and directly above the top of the lateral ventricle of the infarcted hemisphere (Fig. 3A,B) and expressed this as a fraction of the respective measurements of the corresponding contralateral hemisphere of the Nissl-stained coronal sections of the brain of the rats (Fig. 3C,D). Our tMCAo protocol caused significant loss of the affected cortex and reduction in the thickness of the underlying white matter (Fig. 3). BMSC transplantation significantly reduced the cerebral cortical tissue loss and specifically reduced the thinning of the white matter of the infarct hemisphere (Fig. 3C,D). Immunohistochemical examination of the cortical tissue showed that the neurite outgrowth marker βIII tubulin, (Tuj1) was evenly expressed along the white matter of the lesion and contralateral sides of the brain of the tMCAo-treated animals. Immunoreactivity was, however, more intense on the lesion side than on the contralateral side in tMCAo animals with BMSC transplantation (Fig. 4).
Fig. 3.
The amount of cortical tissue (grey matter) loss 14 days following tMCAo-induced cerebral ischemia. A and B were taken from Nissl-stained sections of the infarct brains 14 days after vehicle (DF12) and BMSC implantation, respectively. C) Histogram of the tissue loss of the infarct brain of the control (infarct only) and BMSC-engrafted animals (infarct+BMSCs). D) Histogram of the thickness of the corpus callosum at the midline of the brain (L1 in A) and the white matter above the lateral ventricle of the infarct side (L2 in A). *P<0.05, **P<0.01 between the marked and infarct rat (unpaired 2-tailed Student's t-test). Scale bar=1mm in B.
Fig. 4.
The expression of β-III-tubulin in the white matter underlying the sensorimotor cortex 14 days after tMCAo. Vehicle (DF12) treatment did not affect the intensity of the β-III-tubulin immunoreactivity of the infarct or the contralateral hemisphere of the brain (A,B). However, in rats subjected to tMCAo and treated with BMSC transplantation, the infarct side of the brain (D) stained more positively, with higher intensity and over a larger area, than that of the contralateral control side (C). D) Notice that there is an additional layer of staining under the bulk of the white matter. Arrows point to the dorsal border of white matter of the brain. Scale bar=50µm.
Functional test
In the elevated body swing test, infarct animals (vehicle-treated) started to show a strong and persistent tendency to turn their upper bodies to the side contralateral to their stroked hemisphere starting 1 day following tMCAo (Fig. 5A). This phenomenon was more dramatic at 4 days and persisted through the 2-week period examined following the induction of the cerebral ischemia (Fig. 5A). Sham-operated controls showed no such preference. The ischemia-induced unilateral body swinging preference was significantly reduced in animals receiving BMSC implantation starting at 4 days, and lasted for 2weeks, the longest survival that we have examined, following BMSC implantation when compared to the performance of the vehicle-treated animals (Fig. 5A). However, this improvement was still limited and failed to restore the behavior in these animals before tMCAo surgery (Fig. 5A).
Fig. 5.
Graphs of the result of the elevated body swing test (A) and adhesive-removal somatosensory test (B) of BMSC-implanted and vehicle-treated rats. A) Incidence of body swinging to the left as a fraction of the total number of body swings for a given number of tests. B) Time required to remove the tape, normalized to the maximal duration of observation, 3min, for each trial. Animals at 1 day after surgery usually failed to remove the tape within 3min after it was placed. Data were normalized to those of animals before surgery (A) and animals 1 day after surgery (B). Rats implanted with BMSCs showed significant improvement over the elevated body swing test starting at 4 days and in the adhesive-removal test only at 7 days after tMCAo. *P<0.05 between the marked and infarct-only rat; #P<0.05 between the marked and normal rat (unpaired 2-tailed Student's t-test) (n = 12).
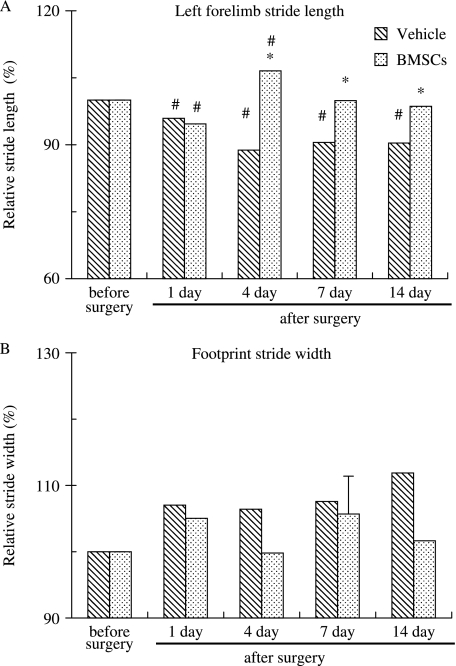
When animals were tested with the tape removal test, infarct animals took longer to remove the tape starting 1day following cerebral ischemia (P<0.05) as compared to their performance before tMCAo. Infarct rats often took longer than 3 min to remove the tape 1day after stroke but improved over time, 124±24, 71±30 and 43±13s on days, 4, 7 and 14, respectively, throughout the 2-week period examined following tMACo. Their performance at the end of the 2nd week following tMCAo was still significantly slower than their performance before tMCAo, which took 9±6.1s. BMSC-treated animals tested on the same days took 180, 118±27, 31±7 and 30±6s, respectively, to remove the tape. The plot in Fig. 5B showed that BMSC implantation failed to improve the performance of tMCAo animals for all the days tested, with the exception of 7 days, that we examined following tMCAo. Grafted animals performed slightly but significantly better than vehicle-treated animals 7 days following tMCAo. Analysis of the forelimb footprints showed that the stride length became significantly shorter following tMCAo (Fig. 6A). BMSC implantation significantly improved the stride length of rats starting at 4days and remain improved to the longest time that we have examined at 2weeks following tMCAo (Fig. 6A). Analysis of the relative stride width showed that there was a tendency to become wider following tMCAo; this improved in animals receiving BMSC implantation but was rather variable and failed to reach statistical significance (Fig. 6B).
Fig. 6.
Histograms showing the forelimb footprint measurements of vehicle-treated and BMSC-implanted rats. Data were normalized to the value of animals before surgery in both A and B. A) The stride length of the left forelimb of BMSC-implanted rats improved significantly starting 4 days after tMCAo, whereas those receiving vehicle implantation failed to improve. *P<0.05 between the marked and infarct-only rat; #P<0.05 between the marked and normal rat (n = 16).
Discussion
The present results have shown that BMSC implantation reduced the cortical damages caused by tMCAo and significantly improved the behavior deficits associated with tMCAo-induced ischemia. Behavioral improvement of the elevated body swing test and forelimb stride length, although still short of that of the normal levels, was achieved as early as 4days following BMSC implantation. Large and extended improvement in the elevated body swing test and the stride length during locomotion, but minor improvement only at one time point for the adhesive sensory stimulus-induced test, suggest that BMSC implantation preferentially enhanced motor functional recovery. This could result either from a preferential effect on the motor system or a lower threshold for motor recovery. The association between the amelioration of the tMCAo-induced sensorimotor cortical loss and the improvement of the tested behaviors is intriguing. Whether this is specifically related to the morphological or cortical volume recovery of motor-associated cortices and its associated mechanism remains to be explored.
In the present study we used a tMCAo scheme modified from Liao et al. (2004) to induce cortical infarction in rats. The ischemic infarction centered in the cerebral cortex and the areas affected were similar to those of the previous reports (Liao et al. 2004; Kao et al. 2006; Chen et al. 2007b). To obtain BMSCs for implantation, we took advantage of their adherent capability on plastic culture plates and purified them to a high density of a homogeneous population after 10 passages. BMSCs still retained pluripotency after this purification process (Lu et al. 2001, 2006; Jiang et al. 2002; Li et al. 2006). In our results, most of the grafted BMSCs were negative for the neural markers and only around 15% of them turned into astrocytes, oligodendrocytes and microglia 14 days after implantation in the brain. The lack of neuronal progenitor pretreatment, the short survival that we allowed before histological examination and perhaps some other unknown causes could have explained why the remaining population of the BMSCs did not differentiate into neurons (Brazelton et al. 2000; Chopp et al. 2000; Mezey et al. 2000; Lu et al. 2001, 2006; Mahmood et al. 2003).
Stems cells have been introduced intravenously (Mahmood et al. 2004), intraperitoneally (Gao et al. 2001), intraventricularly (Ohta et al. 2004), intracerebrally (Mahmood et al. 2004) or even direct to the lesion site (Hofstetter et al. 2002). To increase the number of grafted BMSCs effectively into the ischemic cortex, in the present study we chose intracerebral transplantation, although this method might entail the complication of introducing a needle puncture injury. Previous studies showed that grafted BMSCs might be attracted by damaged tissue and migrate to the injury site (Gao et al. 2001; Chen et al. 2003b; Ohta et al. 2004). The transplanted BMSCs in our study migrated radially from the injection site in the striatum of both normal and infarct rats; however, they migrated for significantly longer distances toward the lesion site of the infarct brain. Grafted BMSCs appeared to be able to migrate through the striatum and then the white matter above to reach the cortex damaged by tMCAo.
Regarding the measurement of the size of the infarct cortex, triphenyltetrazolium chloride (TTC) staining was often used to evaluate the infarct volume of the brain (Liao et al. 2004; Kao et al. 2006; Chen et al. 2007b), although this method failed to yield other morphological details. Comparative studies of TTC method and HE staining on the quantification of brain ischemic injury found that TTC measurements were slightly smaller than those of HE staining (Bederson et al. 1986; Isayama et al. 1991; Bednar et al. 1994). In the present study, we chose to examine sections following histochemical staining to evaluate the cortical damage area and adjacent sections for double immunohistochemical labeling to determine the distribution and differentiation of the grafted BMSCs in the same infarct cortical area. In addition, we also measured the thicknesses of the white matter at two locations, above the lateral ventricle and at the midline of the corpus callosum as additional indicators of the ischemic cortical damage. Measurements of the white matter thickness and the cortical infarct area yielded comparable results, although the thickness of corpus callosum at the midline was less but still significantly affected.
It has been well documented that ischemia induces progressive cerebral damages. BMSC implantation might be effective in slowing down or halting this process. As to how the engrafted BMSCs affect brain and promoted functional recovery after stroke, our results showed that a small percentage of BMSCs differentiated into glial cells, whereas none of them could be identified to express neuronal phenotypic proteins. Studies have shown that the introduction of BMSCs ameliorated at least some of the stroke-induced damages or behavioral deficits within 1–2weeks. Our results showed that significant behavioral improvement was already achieved in 4days if BMSCs were introduced intracerebrally. This argues against the notion that BMSCs ameliorated cortical damages by neuronal replacement. A more reasonable explanation for the benefit provided by the BMSCs is that these cells had activated endogenous restorative mechanisms of the brain. It is proposed that the functional benefit observed in the BMSC-grafted rats may be due to production of some trophic substances beneficial for the nervous tissue, including neurons and astrocytes (Bjorklund & Lindvall, 2000; Mahmood et al. 2002). BMSCs secrete cytokines such as colony-stimulating factor-1 (CSF-1), interleukins, stem cell factors (Eaves et al. 1991), nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), and hepatocyte growth factor (HGF) (Sensebe et al. 1997; Dormady et al. 2001; Chen et al. 2002). It has also been reported that BMSCs stimulate glial cells to produce neurotrophic factors like BDNF and NGF (Mahmood et al. 2002; Wang et al. 2002). These factors might promote the migration of grafted BMSCs, neuronal survival, differentiation, axonal regeneration and new vessel formation of the infarct cortex. Therefore, the reduction of the damages to the infarct cortex in the present study is more likely to be attributed to less cell apoptosis, the proliferation of glial cells, or angiogenesis in the ischemic boundary zone as well as axonal extension of the infarct area.
In summary, our study demonstrated that adult rat brain retains the capacity to respond to exogenous BMSCs. Introduction of BMSCs directly to the brain enhanced the endogenous restorative mechanisms of the infarct cortex. In our studies, allogeneic BMSCs did not induce a cellular or humoral immune response, suggesting that these cells would not be eliminated by the immune system. The advantage of using allogeneic BMSCs is that they can be given as an off-the-shelf product that has the benefits of early administration after stroke as well as cost effectiveness of delivery and characterization inherent in large-scale production of a universal product.
Acknowledgments
This work was supported by research grants from the National Science Council of Taiwan (NSC-95-2313-B-005-046 and NSC-95-2320-B-438-001) to J.-R.C. and T.-J.W.
References
- Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats–similarities to astrocyte grafts. Proc Natl Acad Sci USA. 1998;95:3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of. 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- Bednar MM, Fanburg JC, Anderson ML, Raymond SJ, Dooley RH, Gross CE. Comparison of triphenyltetrazolium dye with light microscopic evaluation in a rabbit model of acute cerebral ischaemia. Neurol Res. 1994;16:129–132. doi: 10.1080/01616412.1994.11740210. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000;3:537–544. doi: 10.1038/75705. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Hida H, Nishino H. Early assessment of motor dysfunctions aids in successful occlusion of the middle cerebral artery. Neuroreport. 1998;9:3615–3621. doi: 10.1097/00001756-199811160-00012. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- Cao Q, Ding P, Lu J, Dheen ST, Moochhala S, Ling EA. 2′,3′-Cyclic nucleotide 3′-phosphodiesterase cells derived from transplanted marrow stromal cells and host tissue contribute to perineurial compartment formation in injured rat spinal cord. J Neurosci Res. 2007;85:116–130. doi: 10.1002/jnr.21092. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Chopp M. Intracerebral transplantation of bone marrow with BDNF after MCAo in rat. Neuropharmacology. 2000;39:711–716. doi: 10.1016/s0028-3908(00)00006-x. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001a;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001b;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chen X, Li Y, Wang L, et al. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002;22:275–279. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Katakowski M, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003a;73:778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003b;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Chen Q, Long Y, Yuan X, et al. Protective effects of bone marrow stromal cell transplantation in injured rodent brain: synthesis of neurotrophic factors. J Neurosci Res. 2005;80:611–619. doi: 10.1002/jnr.20494. [DOI] [PubMed] [Google Scholar]
- Chen Y, Dong XJ, Zhang GR, Shao JZ, Xiang LX. In vitro differentiation of mouse bone marrow stromal stem cells into hepatocytes induced by conditioned culture medium of hepatocytes. J Cell Biochem. 2007a;102:52–63. doi: 10.1002/jcb.21275. [DOI] [PubMed] [Google Scholar]
- Chen C, Hu Q, Yan J, et al. Multiple effects of 2ME2 and D609 on the cortical expression of HIF-1alpha and apoptotic genes in a middle cerebral artery occlusion-induced focal ischemia rat model. J Neurochem. 2007b;102:1831–1841. doi: 10.1111/j.1471-4159.2007.04652.x. [DOI] [PubMed] [Google Scholar]
- Cheng SL, Yang JW, Rifas L, Zhang SF, Avioli LV. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994;134:277–286. doi: 10.1210/endo.134.1.8275945. [DOI] [PubMed] [Google Scholar]
- Chopp M, Zhang XH, Li Y, et al. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport. 2000;11:3001–3005. doi: 10.1097/00001756-200009110-00035. [DOI] [PubMed] [Google Scholar]
- Dormady SP, Bashayan O, Dougherty R, Zhang XM, Basch RS. Immortalized multipotential mesenchymal cells and the hematopoietic microenvironment. J Hematother Stem Cell Res. 2001;10:125–140. doi: 10.1089/152581601750098372. [DOI] [PubMed] [Google Scholar]
- Eaves CJ, Cashman JD, Kay RJ, et al. Mechanisms that regulate the cell cycle status of very primitive hematopoietic cells in long-term human marrow cultures. II. Analysis of positive and negative regulators produced by stromal cells within the adherent layer. Blood. 1991;78:110–117. [PubMed] [Google Scholar]
- Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- Garcia R, Aguiar J, Alberti E, de la Cuetara K, Pavon N. Bone marrow stromal cells produce nerve growth factor and glial cell line-derived neurotrophic factors. Biochem Biophys Res Commun. 2004;316:753–754. doi: 10.1016/j.bbrc.2004.02.111. [DOI] [PubMed] [Google Scholar]
- Grassel S, Ahmed N. Use of bone marrow mesenchymal stem cells for ex vivo cartilage regeneration. Orthopade. 2007;36:227–235. doi: 10.1007/s00132-007-1058-7. [DOI] [PubMed] [Google Scholar]
- Hermann A, Liebau S, Gastl R, et al. Comparative analysis of neuroectodermal differentiation capacity of human bone marrow stromal cells using various conversion protocols. J Neurosci Res. 2006;83:1502–1514. doi: 10.1002/jnr.20840. [DOI] [PubMed] [Google Scholar]
- Hofstetter CP, Schwarz EJ, Hess D, et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iihoshi S, Honmou O, Houkin K, Hashi K, Kocsis JD. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res. 2004;1007:1–9. doi: 10.1016/j.brainres.2003.09.084. [DOI] [PubMed] [Google Scholar]
- Isayama K, Pitts LH, Nishimura MC. Evaluation of 2,3,5-triphenyltetrazolium chloride staining to delineate rat brain infarcts. Stroke. 1991;22:1394–1398. doi: 10.1161/01.str.22.11.1394. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Kuroiwa T, Endo S, Okeda R, Mizusawa H. Neurological dysfunctions versus regional infarction volume after focal ischemia in Mongolian gerbils. Stroke. 2003a;34:1501–1506. doi: 10.1161/01.STR.0000074034.32371.13. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Kuroiwa T, Endo S, Okeda R, Mizusawa H. A comparison of long-term neurological symptoms after two different focal ischemic models in Mongolian gerbils. Acta Neurochir Suppl. 2003b;86:159–162. doi: 10.1007/978-3-7091-0651-8_33. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Kao TK, Ou YC, Kuo JS, et al. Neuroprotection by tetramethylpyrazine against ischemic brain injury in rats. Neurochem Int. 2006;48:166–176. doi: 10.1016/j.neuint.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Kronenwett R, Haas R. Differentiation potential of stem cells from bone marrow. Med Klin (Munich) 2006;101(Suppl 1):182–185. [PubMed] [Google Scholar]
- Li Y, Chen J, Wang L, Lu M, Chopp M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001;56:1666–1672. doi: 10.1212/wnl.56.12.1666. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Chen XG, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- Li Y, McIntosh K, Chen J, et al. Allogeneic bone marrow stromal cells promote glial-axonal remodeling without immunologic sensitization after stroke in rats. Exp Neurol. 2006;198:313–325. doi: 10.1016/j.expneurol.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Liao SL, Kao TK, Chen WY, et al. Tetramethylpyrazine reduces ischemic brain injury in rats. Neurosci Lett. 2004;372:40–45. doi: 10.1016/j.neulet.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Lu D, Mahmood A, Wang L, Li Y, Lu M, Chopp M. Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport. 2001;12:559–563. doi: 10.1097/00001756-200103050-00025. [DOI] [PubMed] [Google Scholar]
- Lu J, Moochhala S, Moore XL, et al. Adult bone marrow cells differentiate into neural phenotypes and improve functional recovery in rats following traumatic brain injury. Neurosci Lett. 2006;398:12–17. doi: 10.1016/j.neulet.2005.12.053. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Wang L, Chopp M. Intracerebral transplantation of marrow stromal cells cultured with neurotrophic factors promotes functional recovery in adult rats subjected to traumatic brain injury. J Neurotrauma. 2002;19:1609–1617. doi: 10.1089/089771502762300265. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697–702. doi: 10.1227/01.neu.0000079333.61863.aa. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery. 2004;55:1185–1193. doi: 10.1227/01.neu.0000141042.14476.3c. [DOI] [PubMed] [Google Scholar]
- de Medinaceli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol. 1982;77:634–643. doi: 10.1016/0014-4886(82)90234-5. [DOI] [PubMed] [Google Scholar]
- Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- Mimura T, Dezawa M, Kanno H, Yamamoto I. Behavioral and histological evaluation of a focal cerebral infarction rat model transplanted with neurons induced from bone marrow stromal cells. J Neuropathol Exp Neurol. 2005;64:1108–1117. doi: 10.1097/01.jnen.0000190068.03009.b5. [DOI] [PubMed] [Google Scholar]
- Munoz-Elias G, Marcus AJ, Coyne TM, Woodbury D, Black IB. Adult bone marrow stromal cells in the embryonic brain: engraftment, migration, differentiation, and long-term survival. J Neurosci. 2004;24:4585–4595. doi: 10.1523/JNEUROSCI.5060-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M, Suzuki Y, Noda T, et al. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp Neurol. 2004;187:266–278. doi: 10.1016/j.expneurol.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Owen M. Marrow stromal stem cells. J Cell Sci Suppl. 1988;10:63–76. doi: 10.1242/jcs.1988.supplement_10.5. [DOI] [PubMed] [Google Scholar]
- Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. CIBA Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Sensebe L, Deschaseaux M, Li J, Herve P, Charbord P. The broad spectrum of cytokine gene expression by myoid cells from the human marrow microenvironment. Stem Cells. 1997;15:133–143. doi: 10.1002/stem.150133. [DOI] [PubMed] [Google Scholar]
- Seyfried D, Ding J, Han Y, Li Y, Chen J, Chopp M. Effects of intravenous administration of human bone marrow stromal cells after intracerebral hemorrhage in rats. J Neurosurg. 2006;104:313–318. doi: 10.3171/jns.2006.104.2.313. [DOI] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Zhang J, Vanguri P, Borneman J, Chopp M. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 2006;137:393–399. doi: 10.1016/j.neuroscience.2005.08.092. [DOI] [PubMed] [Google Scholar]
- Sughrue ME, Mocco J, Komotar RJ, et al. An improved test of neurological dysfunction following transient focal cerebral ischemia in rats. J Neurosci Methods. 2006;151:83–89. doi: 10.1016/j.jneumeth.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Taguchi T, Tanaka H, et al. Neurospheres induced from bone marrow stromal cells are multipotent for differentiation into neuron, astrocyte, and oligodendrocyte phenotypes. Biochem Biophys Res Commun. 2004;322:918–922. doi: 10.1016/j.bbrc.2004.07.201. [DOI] [PubMed] [Google Scholar]
- Tanaka R, Miyasaka Y, Yada K, Ohwada T, Kameya T. Basic fibroblast growth factor increases regional cerebral blood flow and reduces infarct size after experimental ischemia in a rat model. Stroke. 1995;26:2154–2158. doi: 10.1161/01.str.26.11.2154. discussion 2158–2159. [DOI] [PubMed] [Google Scholar]
- Vendrame M, Cassady J, Newcomb J, et al. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35:2390–2395. doi: 10.1161/01.STR.0000141681.06735.9b. [DOI] [PubMed] [Google Scholar]
- Wang ZH, Ji Y, Shan W, et al. Therapeutic effects of astrocytes expressing both tyrosine hydroxylase and brain-derived neurotrophic factor on a rat model of Parkinson's disease. Neuroscience. 2002;113:629–640. doi: 10.1016/s0306-4522(02)00204-x. [DOI] [PubMed] [Google Scholar]
- Wlodarski KH, Galus R, Wlodarski PK. Osteogenic potential of bone marrow stromal cells. Orthop Traumatol Rehabil. 2006;8:573–577. [PubMed] [Google Scholar]
- Yu S, Tanabe T, Dezawa M, Ishikawa H, Yoshimura N. Effects of bone marrow stromal cell injection in an experimental glaucoma model. Biochem Biophys Res Commun. 2006;344:1071–1079. doi: 10.1016/j.bbrc.2006.03.231. [DOI] [PubMed] [Google Scholar]
- Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. 2002;174:11–20. doi: 10.1006/exnr.2001.7853. [DOI] [PubMed] [Google Scholar]