Abstract
Electron microscopy has been used to investigate whether the transversely striated columns of the connecting piece in the neck region of guinea pig spermatozoa, undergo lengthening and shortening as a result of the forces generated during motility. Motile spermatozoa were subjected to near-instantaneous rapid freezing, followed by freeze-substitution fixation and epoxy embedment. Thin sections passing longitudinally through the striated columns revealed that the periodicity was indeed variable. The repeat period, taken to have an unstressed width of 60nm, could be found extended to 75nm in some specimens, and reduced to 54nm in others. The estimates of the coefficients of variation were 6.6% for the width of the ‘dense’ band and 33.5% for the ‘pale’ band. The ‘pale’ band in the extended state showed longitudinal striae. Such variations in length, which – it is suggested – are physiological, and passively induced, would have functional implications for the flagellum – for both bend initiation and bend growth. Also, hypothetically, any mechanism that could increase the degree of compliance in these columns, such as perhaps phosphorylation of the constituent proteins, could permit the flagellum to develop the exaggerated bend angles and asymmetries of the ‘hyperactivated’ state.
Keywords: spermatozoa, connecting piece, flagellar motility, hyperactivation, guinea pig
Introduction
In mammalian spermatozoa the ‘9+9+2’ flagellum is attached to the base of the sperm head by the connecting piece. The structure of this connection is well known. It has been reconstructed from serial sections for several species (Iwashita & Oura, 1980; Hamasaki et al. 1994; Vernon & Woolley, 2004). In its distal region, the connecting piece consists of nine columns, each of which is fused to one of the nine outer dense fibres. More proximally, these nine columns fuse together progressively until two large masses remain to contact the sperm head via the basal plate, an electron-dense lamina adjacent to the nuclear envelope. The morphology of the connecting piece is complicated by the presence of the proximal centriole (or remnants of it) embedded within its basal region. The entire structure, including the part that encases the proximal centriole, has periodic striations in electron micrographs (Randall & Friedlaender, 1950; Fawcett & Phillips, 1969).
In terms of function, it is thought that the forces developed by the axonemal dyneins are transmitted via the nine microtubule doublets, first to the attached outer dense fibres, and from them to the connecting piece (Lindemann, 1996). It is important that the connecting piece anchors together the outer dense fibres (Lindemann & Gibbons, 1975). In this way, it takes the role of the distal centriole, which anchors the doublets in simpler flagella. (It is therefore not surprising that the distal centriole degenerates during spermiogenesis in mammals (Fawcett & Phillips, 1969; Zamboni & Stefanini, 1971; Gordon, 1972).) In all flagella, a basal anchorage of some kind is essential for sustained, regular flagellar beating (Woolley & Bozkurt, 1995; Fujimura & Okuno, 2006).
Recently, however, it has been suggested that the connecting piece might be mechanically compliant, even while serving as an anchorage. Evidence was produced for Chinese hamster sperm that the striated columns undergo compressive/tensile strain during beating (Vernon & Woolley, 2002). Further work showed that, in chinchilla sperm, flagellar beating was accompanied by shear strain between the two fused masses of the basal connecting piece (Vernon & Woolley, 2004). These observations mean that the nine doublets of the axoneme can apparently undergo some sliding relative to each other at the flagellar base. Basal sliding, as this is called, has implications for bend initiation, bend development, and for the form of the wave in mammalian spermatozoa (Vernon & Woolley, 2004; Riedel-Kruse et al. 2007).
The present study is an attempt to demonstrate conclusively that the compressive/tensile type of strain does occur in the columns of the connecting piece. The plan was to search for dynamic variation in the periodicity of the columns. Near-instantaneous fixation was a necessity in preparing the cells for electron microscopy.
Materials and methods
The method was to slam-freeze suspensions of living spermatozoa, as hanging droplets, by allowing them to fall against a liquid helium-cooled copper block. Large numbers of sperm needed to be swimming close to the lower surface of the droplet, preferably with a planar beat. Beat frequency needed to be regular and fairly slow. These requirements were met by choosing epididymal spermatozoa from the guinea pig. In this species, the sperm heads are attached together in stacks, or rouleaux (Bremser, 1819). Because of this, sedimentation occurred rapidly. Slow, synchronized flagellar beating was obtained by raising the viscosity of the medium (Cody, 1925). The propulsive thrust tends to trap the spermatozoa very close to the surface of the fluid (as in other species – see Woolley, 2003). A further advantage of guinea pig spermatozoa is that the connecting piece is well developed (Fawcett, 1965).
Adult guinea pigs (ex-breeding colony) were killed by injecting pentobarbital sodium i.p. The cauda epididymidis was punctured and 10µL of its contents were layered over 1mL of a diluent consisting of Hanks BSS with 3mgmL−1 bovine serum albumin and 2% methyl cellulose (Sigma M0262) to give a nominal viscosity of 400cP at 20°C. After 2h the sperm rouleaux had dispersed throughout the fluid and all of them showed vigorous motility. From a videotape sequence, the mean beat frequency was 1.60Hz (range 1.22–2.08Hz; n = 10).
Droplets (∼5µL) of this sperm suspension were each placed on a supporting slice of fixed-and-washed lung tissue and slam-frozen using a Cryopress (Med-Vac Inc.) apparatus, which causes the specimen to fall on to a liquid helium-cooled copper block (Heuser, 1981). A 30-s delay before freezing was imposed to allow the rouleaux to accumulate at the lower surface of the hanging droplet. Frozen samples were stored under liquid N2 until they were transferred to a Reichert AFS apparatus and chemically fixed by freeze-substitution for 68h at –90°C in acetone containing 2% OsO4 and 1% tannic acid. After a slow (6–7h) return to room temperature, the specimens were embedded conventionally in Epon 812. Each block was trimmed to select the centre of the impact zone. Thin sections were made parallel to, and as close as possible to, the surface that had been rapidly frozen; a few specimens were sectioned in a plane perpendicular to that surface. The sections (∼60nm) were stained with uranyl acetate and lead citrate, mounted on single-hole grids and examined in a Philips CM 100 electron microscope.
The only rule in selecting the specimens to be photographed was that the periodicity of at least one column of the connecting piece should be well defined. All images of the connecting piece were photographed at an instrument magnification of 21000×. Measurements were made from highly enlarged prints with the aid of a 10× measuring loupe. Because the electron microscope was not repeatedly re-calibrated, the key comparative measurements have been made on the periodicities within one column of the connecting piece, between columns within a single micrograph and between micrographs made during the same session at the microscope.
Results
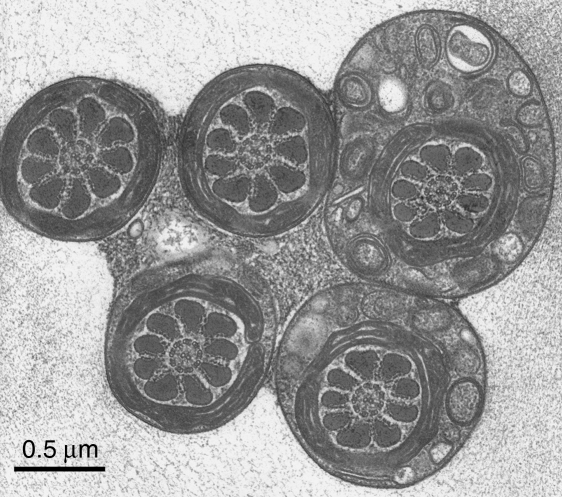
The rapid-freeze, freeze-substitution technique worked satisfactorily. The rouleaux formations were preserved in position throughout the process, probably owing to the cross-linking of the serum albumin that had been included in the medium (Fig. 1). The groups of synchronized flagella were co-aligned as expected. Within a few micrometres of the impact surface the structure of the cell membranes and the axonemal complex were very well preserved, showing the contrast typical for tannic acid (Fig. 2). In passing, it is worth stating that there was no sign of functional variation in the interdoublet spacings. An unexpected finding was that the sperm heads, and the flagella at the level of the midpiece, were linked together by extracellular material containing vesicles etc. (Figs 1 & 3).
Fig. 1.
A demonstration that the normal ‘rouleaux’ formation of the spermatozoa is preserved throughout the slam-freezing and freeze-substitution procedure. This rouleau comprises four sperm sectioned at the level of the acrosome. The heads are linked together by the well known membrane appositions (arrowed) – see Friend & Fawcett (1974). However, granular material, containing vesicles, is also present between the sperm heads (double arrows); this material is scarcely preserved at all in conventionally fixed cells (loc. cit.). Scale bar=2µm.
Fig. 2.
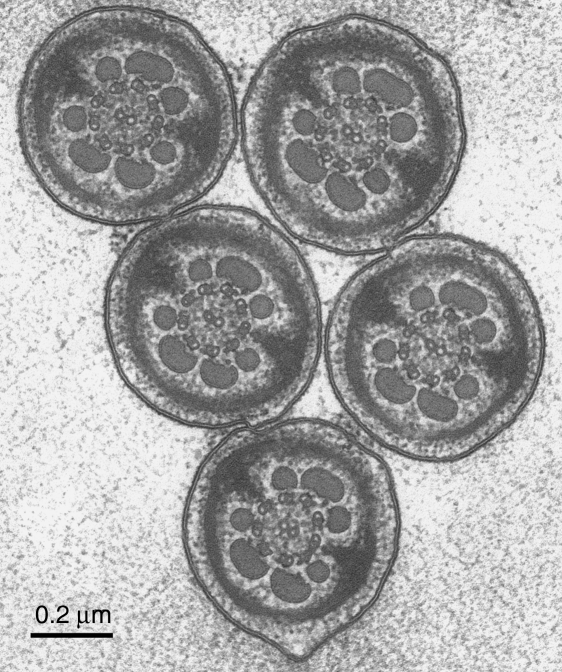
A group of sperm flagella sectioned at the level of the principal piece, from a thin section cut perpendicularly to the plane of rapid freezing. As expected from the nature of the head rouleau, groups of tails such as this are always similarly orientated. The axoneme itself has the standard arrangement, without any obvious dynamic variations in the interdoublet spacings. Scale bar=0.2µm.
Fig. 3.
A group of sperm flagella sectioned at the level of the midpiece, from a thin section cut perpendicularly to the freezing plane. At this level the flagella are stuck together by the granular material, which has been shown to lie also between the sperm heads (Fig. 1). This mechanical coupling must have a role in the flagellar synchronization – which is generally explained in the literature on cilia and flagella as being due to viscous coupling. Again, the structure of the midpiece and of the cytoplasmic droplet (top right) is well preserved and conventional. Scale bar=0.5µm.
It was possible to collect a set of micrographs showing longitudinal sections through the sperm necks. It was not possible, however, to see enough of the flagellum in the same section to be able to relate neck morphology to the phase of the flagellar beat cycle. This limitation frustrated the plan to show that changes in the connecting piece periodicities were in accordance with prediction and so could not be artefactual. Twenty-four micrographs were included in the study, showing details of 26 spermatozoa and 45 connecting piece columns.
In conventionally fixed material, the periodic pattern within each striated column is well known to consist of a dense (wider) band alternating with a pale (narrower) band; also, the dense band is traversed by a very narrow band of even greater density (e.g. Fawcett, 1965). This pattern was easily recognised after fixation by freeze-substitution but, unaccountably, the contrast was reversed in most specimens. Therefore we shall call them the ‘dense’ and the ‘pale’ bands. Fawcett & Phillips (1969) measured the periodicity to be 66.5nm in the Chinese hamster.
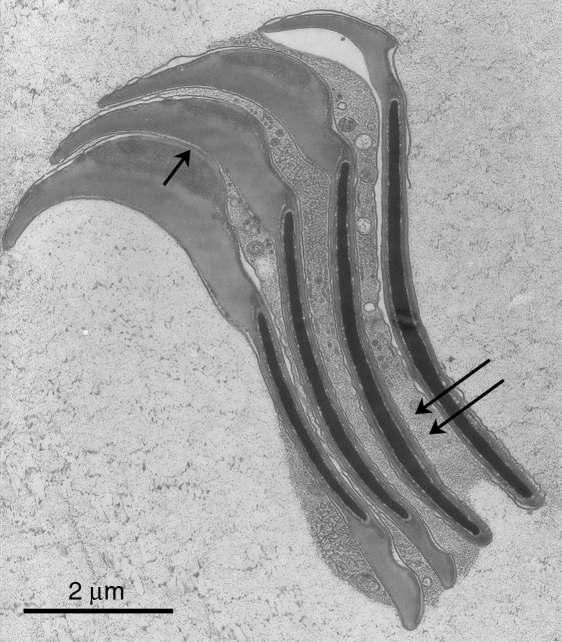
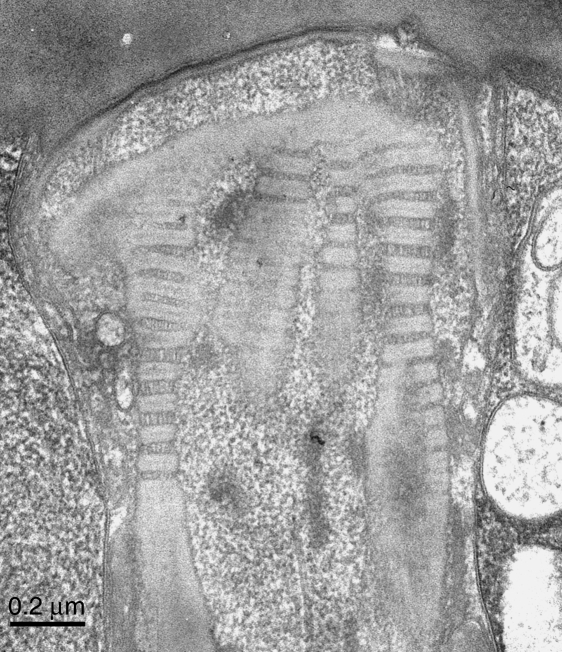
The mean periodicity measured in the present material was 62.3nm (global mean, n = 45 columns, average measurement from each column based on between 2 and 8 repeats, typically 4 repeats). However, the variability was appreciable. The SD of the global mean was 4.4nm and the range between column-averages was from 54.8nm to 75.3nm, suggesting that the columns are compressible and extensible. Figure 4 illustrates uniformly compressed columns; Figure 5 shows considerable extension of the periodicities. Comparing these two micrographs, it is evident that the extension has been due to a widening of the ‘pale’ band. This band is not only wider in much of Fig. 5; additionally, longitudinal striae are now resolved within it. The widths of all the ‘dense’ and ‘pale’ bands in Fig. 5 were measured. The mean width (±SD) of the ‘dense’ bands was 42.06±2.78nm (n = 40) and that of the ‘pale’ bands was 24.16±8.09nm (n = 38). The coefficients of variation were 6.6% and 33.5%, respectively.
Fig. 4.
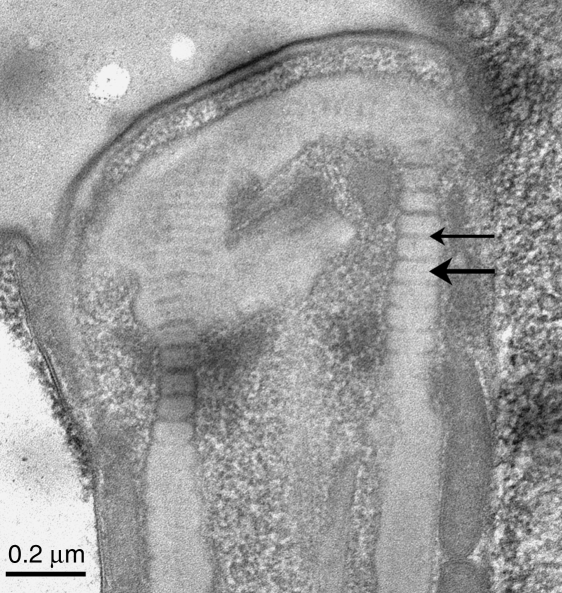
A longitudinal section through the connecting piece. This and all subsequent specimens are from sections cut parallel to the freezing plane. This example resembles the pattern seen after conventional fixation except that the contrast is reversed – for unknown reasons. Thus, the normally dense and wide bands of the striated columns appear pale (large arrow) and the normally pale and thin bands appear dense (small arrow). (For continuity, the bands are nevertheless referred to as ‘dense’ and ‘pale’, respectively.) The periodicities in this specimen were rather uniform. The mean repeat periods, as measured, were 60.6nm (left side) and 59.0nm (right side). Scale bar=0.2µm.
Fig. 5.
This micrograph was taken a few minutes before that in Fig. 4, that is, at the same instrumental settings. Here the periodicities within the columns are highly variable. It is evident that most of the variation is in the ‘pale’ bands, which in places are increased in width to equal the width of the ‘dense’ bands’. Where this is so, for example at the lower left, the ‘pale’ bands show longitudinal striae. The variations in period are analysed further in the text. Scale bar=0.2µm.
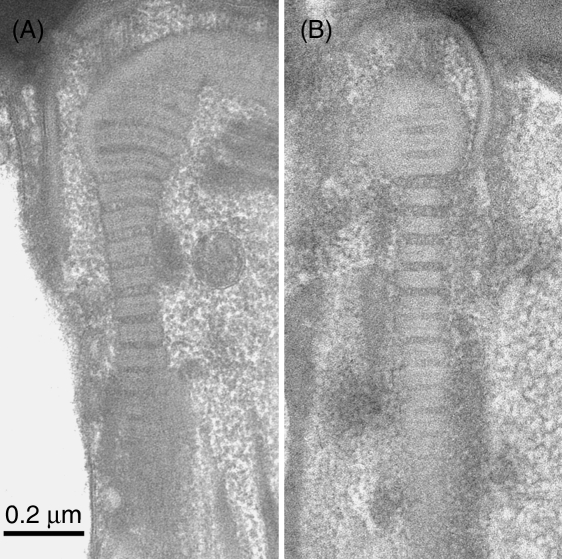
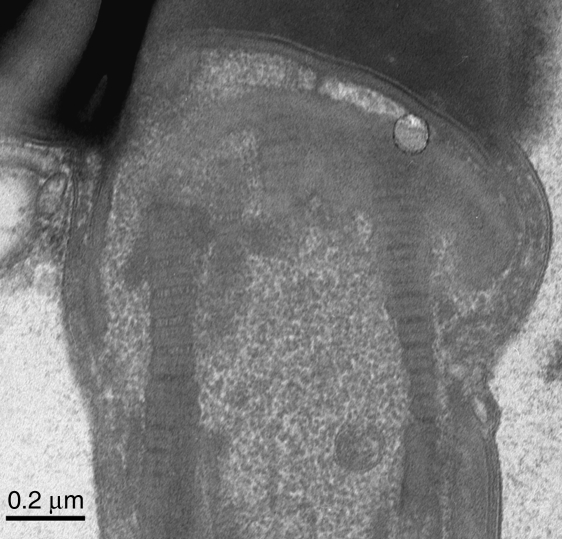
It was frequently noticed that the periodicity could vary within a single column of the connecting piece. The common pattern was that the region of minimal period occurred distally, where the column is spliced against the origin of the outer dense fibre (Fig. 6A,B). In a few examples it was apparent that the periodicities on the left and right edges of the connecting piece were different (Fig. 7). Surprisingly, however, it was more often found that columns of similar average periodicity occurred on each side of the sperm neck.
Fig. 6.
(A, B) These two examples show that the periodicity in the columns may be extended in the middle regions but is at a standard width in the distal region, where the column abuts the origin of the outer dense fibre. This suggests that there is no appreciable compliance in the substance of the latter. Scale bar=0.2µm.
Fig. 7.
This micrograph demonstrates within-sperm variation, with the periodicity of the right side column (mean width 64.4nm) less than that on the left side (74.1nm). Scale bar=0.2µm.
Discussion
In this work, the assumption can be made that virtually all the spermatozoa were alive at the moment of freezing and that they were physically stabilized in amorphous (or near-amorphous) ice relatively quickly, in a time equating to less than 1% of their beat cycle duration. This calculation is based on the measurements of Heuser et al. (1979), who showed that this apparatus provides a stabilization time of ∼2ms at a depth of 10µm. Because of this rapid stabilization, there is an expectation that physiological variations may have been captured.
Assuming that the variations seen are physiological, the implications can be explored. At the extremes of the variation, how much basal sliding would be occurring? Let us take the unstressed periodicity as being that of the columns where they are attached to the outer dense fibres, say 60nm (from Fig. 6). The length of the deformable part of the column may be taken as, say, 600nm, or 10 repeat periods. Now, if the columns on one side had their periodicity reduced to 55nm, while those on the other side were extended to 75nm, the total basal sliding, between the two sides, would amount to {10×[60–55]}+{10×[75–60]}, i.e. 200nm.
Is this amount of basal sliding likely to represent the maximum that occurs? Although there is little certainty, we may suppose that the phase of maximal basal sliding is when each new bend is held at its greatest angle, before it propagates. This is hard to measure accurately. Informally, it seems to be about 15% of the cycle duration. Probably, therefore, it has been captured in a sample of 24 micrographs.
How significant would 200nm of basal sliding be, in relation to bend growth? The angular size (in radians) of a bend underlain by 200nm of sliding is obtained by dividing the amount of sliding by the functional diameter of the axonemal complex (Satir, 1968; Woolley & Vernon, 2002). This diameter, in the proximal part of the guinea pig sperm, is approximately 500nm (e.g. from Fig. 3). (It is greater than the axonemal diameter, as the sliding units are the conjoined doublet-outer dense fibres.) Thus, 200nm of basal sliding would fully accommodate the sliding within a bend of 0.4 radians, or 23°.
From the videotapes, we estimated that the angular size of the reverse bends in guinea pig rouleaux is approximately 25°, whereas the principal bends reach 150°. Thus, basal sliding would accommodate the growth of the reverse bend. This is, in fact, an anticipated conclusion. In an earlier study of the asymmetrical bends of hamster sperm, it was inferred that the reverse bend must grow by basal sliding because no further sliding could be accommodated in the antecedent principal bend, nor could any sliding be transmitted through that bend (Vernon & Woolley, 2004).
This concordance with previous work gives credence to the physiological validity of our electron micrographs. It was unexpected, admittedly, that some specimens, sectioned in the plane-of-flattening of the head, showed extended columns on both sides of the connecting piece (e.g. Fig. 5). This finding may possibly be a hydrodynamic consequence of the fact that the plane of the head is inclined to the plane of the flagellar beat (the effect described by Woolley, 2003). We cannot rule out that forces generated during impact freezing may have played a part – although there were no signs of gross damage in the zone adjacent to the impact surface. The use of immobilized samples, as controls, was not practicable because we depended on the sperms’ own motility to bring them, in sufficient numbers, parallel to the impact surface, and close enough to it to be in the ‘zone of good freezing’.
Compliance in the columns of the connecting piece could be important physiologically for several reasons. (1) To permit reverse bend formation in highly asymmetrical waveforms, as explained above. (2) To maintain the oscillation, through the storage of energy elastically. The columns could behave as springs which, when unloaded, reverse the sliding direction of the outer dense fibres and of the doublets, possibly triggering activity in the dynein arms. This, however, is likely to be an auxiliary way of driving the oscillation. It cannot have relevance to cilia and to simple flagella that do not have connecting pieces. A more general theory of the oscillation has recently been proposed (Woolley, 2007). (3) To permit the greater bend growth and asymmetry that characterize hyperactivation in mammalian spermatozoa. Hyperactivation normally occurs at the site of fertilization (see review by Suarez & Ho, 2003). The signalling pathway undoubtedly involves Ca2+ (Ho & Suarez, 2001; Quill et al. 2003). It seems that calcium ions, together with cAMP, lead to protein kinase C activation. Hyperactivation is associated with tyrosine phosphorylation in several species (Chan et al. 1998; Mahony & Gwathmey, 1999; Si & Okuno, 1999; Harayama & Miyake, 2006). And, in boar spermatozoa, activated protein kinase C has been localized to the connecting piece (Harayama & Mikaye, 2006). Thus, as a speculation, it is possible that phosphorylation or dephosphorylation of the protein of the connecting piece might increase (or decrease) its compliance, and thereby induce (or terminate) the hyperactivated state.
Acknowledgments
D.M.W. gratefully acknowledges the receipt of a Leverhulme Emeritus Research Fellowship and the hospitality of Professor C.H. Orchard.
References
- Bremser J. Gametes and Spores. Baltimore: Johns Hopkins University Press; 1819. (drawings re-published by J Farley (1982)) p. 45. [Google Scholar]
- Chan PJ, Corselli JU, Patton WC, Jacobson JD, King A. Enhanced fertility after heat-induced hyperactivation. Fertil Steril. 1998;69:118–121. doi: 10.1016/s0015-0282(97)00440-8. [DOI] [PubMed] [Google Scholar]
- Cody BA. Observations and experiments upon spermatozoa of the guinea pig. J Urol. 1925;13:175–191. [Google Scholar]
- Fawcett DW. The anatomy of the mammalian spermatozoon with particular reference to the guinea pig. Z Zellforsch Mikrosk Anat. 1965;67:279–296. doi: 10.1007/BF00339376. [DOI] [PubMed] [Google Scholar]
- Fawcett DW, Phillips DM. The fine structure and development of the neck region of the mammalian spermatozoon. Anat Rec. 1969;165:153–184. doi: 10.1002/ar.1091650204. [DOI] [PubMed] [Google Scholar]
- Friend DS, Fawcett DW. Membrane differentiations in freeze-fractured mammalian sperm. J Cell Biol. 1974;63:641–664. doi: 10.1083/jcb.63.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura M, Okuno M. Requirement of the fixed end for spontaneous beating in flagella. J Exp Biol. 2006;209:1336–1343. doi: 10.1242/jeb.02131. [DOI] [PubMed] [Google Scholar]
- Gordon M. The distal centriole in guinea pig spermiogenesis. J Ultrastruct Res. 1972;39:364–388. doi: 10.1016/s0022-5320(72)90029-9. [DOI] [PubMed] [Google Scholar]
- Hamasaki M, Wakimoto M, Maehara T, Matsuo H. Three-dimensional structures in the neck region of the hamster spermatozoa in the caudal epididymis. Arch Histol Cytol. 1994;57:59–65. doi: 10.1679/aohc.57.59. [DOI] [PubMed] [Google Scholar]
- Harayama H, Mikaye M. A cyclic adenosine 3′,5′-monophosphate-dependent protein kinase C activation is involved in the hyperactivation of boar spermatozoa. Mol Reprod Devel. 2006;73:1169–1178. doi: 10.1002/mrd.20460. [DOI] [PubMed] [Google Scholar]
- Heuser J. Quick-freeze, deep-etch preparation of samples for 3-D electron microscopy. Trends Biochem Sci. 1981;6:64–68. [Google Scholar]
- Heuser JE, Reese TS, Jan Y, Jan l, Dennis MJ, Evans L. Synaptic vesicle exocytosis captured by quick-freezing and correlated with quantal transmitter release. J Cell Biol. 1979;81:275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho H-C, Suarez SS. An inositol 1,4,5-trisphosphate receptor-gated intracellular Ca2+store is involved in regulating sperm hyperactivated motility. Biol Reprod. 2001;65:1606–1615. doi: 10.1095/biolreprod65.5.1606. [DOI] [PubMed] [Google Scholar]
- Iwashita T, Oura C. A three-dimensional analysis of the capitellum and striated columns in the sperm neck region of the mouse. Okajimas Folia Anat Jpn. 1980;56:361–382. doi: 10.2535/ofaj1936.56.6_361. [DOI] [PubMed] [Google Scholar]
- Lindemann CB. Functional significance of the outer dense fibres of mammalian sperm examined by computer simulation with the geometric clutch model. Cell Motil Cytoskel. 1996;34:258–270. doi: 10.1002/(SICI)1097-0169(1996)34:4<258::AID-CM1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Lindemann CB, Gibbons IR. Adenosine triphosphate-induced motility and sliding of filaments in mammalian sperm extracted with Triton X-100. J Cell Biol. 1975;65:147–162. doi: 10.1083/jcb.65.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony MC, Gwathmey T. Protein tyrosine phosphorylation during hyperactivated motility of cynomolgus monkey (Macaca fascicularis) spermatozoa. Biol Reprod. 1999;60:1239–1243. doi: 10.1095/biolreprod60.5.1239. [DOI] [PubMed] [Google Scholar]
- Quill TA, Sugden SA, Rossi KL, Doolittle LK, Hammer RE, Garbers D. Hyperactivated sperm motility driven by CatSper2 is required for fertilization. Proc Natl Acad Sci USA. 2003;100:14869–14874. doi: 10.1073/pnas.2136654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall JT, Friedlaender MHG. The microstructure of ram spermatozoa. Exp Cell Res. 1950;1:1–32. [Google Scholar]
- Riedel-Kruse IH, Hilfinger A, Howard J, Jůlicher F. How molecular motors shape the flagellar beat. HFSP J. 2007;1:192–208. doi: 10.2976/1.2773861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P. Studies on cilia. III. Further studies on the cilium tip and a ‘sliding filament’ model of ciliary motility. J Cell Biol. 1968;39:77–94. doi: 10.1083/jcb.39.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Y, Okuno M. Role of tyrosine phosphorylation of flagellar proteins in hamster sperm hyperactivation. Biol Reprod. 1999;61:240–246. doi: 10.1095/biolreprod61.1.240. [DOI] [PubMed] [Google Scholar]
- Suarez SS, Ho H-C. Hyperactivated motility in sperm. Reprod Domest Anim. 2003;38:119–124. doi: 10.1046/j.1439-0531.2003.00397.x. [DOI] [PubMed] [Google Scholar]
- Vernon GG, Woolley DM. Microtubule displacements at the tips of living flagella. Cell Motil Cytoskel. 2002;52:151–160. doi: 10.1002/cm.10041. [DOI] [PubMed] [Google Scholar]
- Vernon GG, Woolley DM. Basal sliding and the mechanics of oscillation in a mammalian sperm flagellum. Biophys J. 2004;87:3934–3944. doi: 10.1529/biophysj.104.042648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley DM. Motility of spermatozoa at surfaces. Reproduction. 2003;126:259–270. doi: 10.1530/rep.0.1260259. [DOI] [PubMed] [Google Scholar]
- Woolley DM. A novel motility pattern in quail spermatozoa with implications for the mechanism of flagellar beating. Biol Cell. 2007;99:663–675. doi: 10.1042/BC20070050. [DOI] [PubMed] [Google Scholar]
- Woolley DM, Bozkurt HH. The distal sperm flagellum: its potential for motility after separation from the basal structures. J Exp Biol. 1995;198:1469–1481. doi: 10.1242/jeb.198.7.1469. [DOI] [PubMed] [Google Scholar]
- Woolley DM, Vernon GG. Functional state of the axonemal dyneins during flagellar bend propagation. Biophysics J. 2002;83:2162–2169. doi: 10.1016/S0006-3495(02)73976-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni L, Stefanini M. The fine structure of the neck of mammalian spermatozoa. Anat Rec. 1971;169:155–172. doi: 10.1002/ar.1091690203. [DOI] [PubMed] [Google Scholar]