Abstract
Aims
Adverse cardiovascular events in humans occur with a day/night pattern, presumably related to a daily pattern of behaviours or endogenous circadian rhythms in cardiovascular variables. Healthy humans possess a scale-invariant/fractal structure in heartbeat fluctuations that exhibits an endogenous circadian rhythm and changes towards the structure observed in cardiovascular disease at the circadian phase corresponding to the time of the broad peak of adverse cardiovascular events (at about 10 AM). To explore the relationship between the rest/activity cycle, endogenous circadian rhythmicity, and cardiac vulnerability, we tested whether the fractal structure of heart rate exhibits a similar circadian rhythm in a mammalian species that is nocturnally active (Wistar rats) compared with diurnally active humans, and how this fractal structure changes after lesioning the circadian pacemaker (suprachiasmatic nucleus, SCN) in rats.
Methods and results
Analysis of heart rate data collected over 10 days in eight intact and six SCN-lesioned Wistar rats during constant darkness revealed that: (i) as with humans, rats exhibit an endogenous circadian rhythm in the scaling exponent characterizing the hourly fractal structure of heart rate (P = 0.0005) with larger exponents during the biological day (inactive phase for rats; active phase for humans); (ii) SCN lesioning abolished the rhythm in the fractal structure of heart rate and systematically increased the scaling exponent (P = 0.01).
Conclusion
Rats possess a circadian rhythm of fractal structure of heart rate with a similar temporal pattern as previously observed in humans despite opposite rest/activity cycles between the two species. The SCN imparts this endogenous rhythm. Moreover, lesioning the SCN in rats results in a larger scaling exponent, as occurs with cardiovascular disease in humans.
Keywords: Heart rate control, Cardiac vulnerability, Circadian rhythms, Scale-invariant/fractal structure, Behaviour, Rest/activity cycle, Suprachiasmatic nucleus
1. Introduction
Healthy heartbeat fluctuations possess a scale-invariant/fractal structure characterized by long-range power-law correlations over a range of time scales.1–3 These scale-invariant correlations persist during different behaviours and in varied environments, but change with autonomic blockade4–6 and under pathological conditions such as congestive heart failure.7–9 In addition, these scale-invariant correlations provide one of the most sensitive heart rate variability (HRV) biomarkers for predicting survival rates in patients following stroke10 and acute myocardial infarction.11 These findings suggest that the scale-invariant correlations are intrinsic patterns of cardiac dynamics with relevance to cardiovascular health and survival.
There is considerable epidemiological evidence of a day/night pattern in the timing of stroke, myocardial infarction, ventricular arrhythmias, and sudden cardiac death with a broad peak at about 10 AM.12 However, it is not known whether this pattern is caused by the day/night patterns of behaviours or endogenous circadian rhythms in relevant physiological variables.12 In mammals, endogenous circadian rhythms are generated by the circadian pacemaker located in the suprachiasmatic nucleus (SCN) of the hypothalamus.13,14 We recently discovered that the scale-invariant/fractal structure in human heartbeat fluctuations exhibits an endogenous circadian rhythm, independent of scheduled behaviours such as the sleep/wake cycles15 and independent from overall activity level while awake.16 In healthy subjects, the statistical index that quantifies the fractal structure was highest at the specific circadian phase that corresponds to the time of day when adverse cardiovascular events are most frequent,15,16 moving this index closer to that observed in cardiovascular disease.7–9 These findings lead us to hypothesize that the SCN, which generates circadian rhythms in many physiological functions including the cardiovascular system,17–21 may play a role in the timing of adverse cardiovascular events.15,16 In this study, we explored endogenous circadian rhythmicity in the fractal structure of heart rate and its relationship with the rest/activity cycle by testing: (i) whether or not there exists a circadian rhythm in the fractal structure of heart rate in a mammalian species that is nocturnally active; (ii) whether the phase relationship between the rest/activity cycle and the circadian rhythm of fractal structure of heart rate is altered in nocturnally active mammals relative to that in humans; and (iii) how the degree and circadian rhythmicity of the fractal structure of heart rate change after lesioning the circadian pacemaker.
2. Methods
2.1. Protocol
Recordings were made in nocturnally active Wistar rats during constant dark conditions (DD protocol) as described previously.22 Each rat lived individually in a cage with a dimension of 39 × 38 × 38 cm3. Two groups of rats were used: eight control rats, and six rats following successful SCN lesion. Preceding the DD protocol, all rats underwent 10 light/dark cycles (12 h light, 100 lux; 12 h dark, 0.1 lux; LD protocol). The LD protocol ensured that the circadian pacemaker and rest/activity cycles were synchronized to the light–dark cycles (i.e. 12 h inactive phase and 12 h active phase) prior to the DD cycle. The investigation was approved by the Animal Care Committee of the Royal Netherlands Academy of Arts and Sciences and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.2. Suprachiasmatic nucleus lesion
Standardized surgical techniques were used to ablate the SCN as described previously.22 Briefly, 30 rats were anaesthetized (Hypnorm; 0.8 mL/kg i.m.), mounted in a stereotactic instrument, and electrode tips (0.2 mm diameter) were placed bilaterally in the SCN and heated to 80°C for 1 min. To avoid inclusion of rats with incomplete lesions, the day/night rhythms in drinking water were investigated at least 1 month after surgery, and vasoactive intestinal polypeptide (VIP) and vasopressin (VP) within the SCN in perfusion-fixed brains were quantified.23,24 Rats with rhythms in drinking behaviour, or with VIP or VP present, were excluded from analysis. Seven of the 30 rats met both behavioural and anatomical verification of complete SCN lesion. These SCN-lesioned (SCNx) rats were recorded throughout the same LD and DD protocols as the control rats. During DD, there were technical difficulties in the data collection of one SCNx rat, so data from only six SCNx rats are presented.
2.3. Data acquisition
This paper presents a re-analysis of previously published data.22 For the collection of heart rate data, a transmitter was implanted in each rat under anaesthesia (0.8 mL/kg i.m. Hypnorm and 0.4 mL/kg s.c. Dormicum) and secured to the inner muscle wall of the abdomen, one rostral and one caudal to the heart, as described previously.22 After surgery, animals recovered for at least 2 weeks and then heart rate data were transmitted from the freely moving rat to a telemetry antenna underneath the cage. Every 4 min, heart rate was sampled at 500 Hz for 10 s and the average was stored. Core body temperature was also measured every 4 min. Long-range correlations (across 4–24 h) in locomotor data25 and in heart rate data26 from the same study have been published previously.
2.4. Data analysis
2.4.1. Scale-invariant/fractal structure in heart rate fluctuations
To quantify the scale-invariant/fractal structure in heart rate fluctuations, we used detrended fluctuation analysis (DFA) on hourly segments of heart rate data. DFA is designed to quantify correlations in signals that may be masked by underlying non-stationarities or trends.27,28 This method quantifies the detrended fluctuation function of heart rate at different time scales, n (see Supplementary material online for detailed procedures). A power-law functional form indicates self-similarity (scale-invariance). This power-law functional form is defined by a straight line on a log–log plot of F(n) vs. time scale (bin width), yielding F(n) ∼ nα. The parameter α, called the scaling exponent, quantifies the correlation properties in the signal: if α = 0.5, there is no correlation in the fluctuations (random noise); if α < 0.5, the signal is anticorrelated, where large heart rate values are more likely to be followed by small heart rate values; if α > 0.5, there are positive correlations, where large heart rate values are more likely to be followed by large heart rate values (and vice versa for low heart rate values). For each rat and for each circadian bin (see 2.4.2 Circadian phase), we estimated the scaling exponent α over the same range of time scales, 4–40 min (Figure 2A). The variation of the scaling exponent α at different bins reveals the effect caused by circadian pacemaker.
Figure 2.
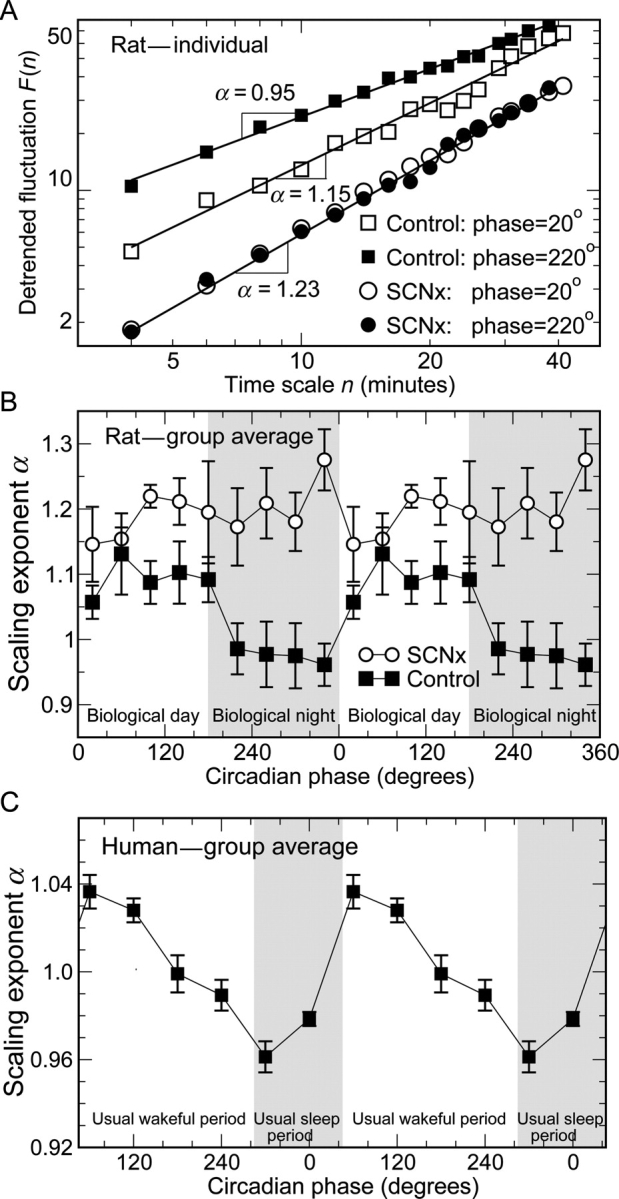
Circadian rhythms in the correlation property of heart rate dynamics. (A) Detrended fluctuation analysis results for a control rat at two different circadian phases [biological day (open squares) and biological night (filled squares)] and for an suprachiasmatic nucleus-lesioned rat at the same extrapolated circadian phases (open and closed circles). (B) Significant circadian rhythms are observed in the group average of the scaling exponent α in control rats (black squares). α is larger during the biological day (inactive phases 0°–180°) than during the biological night (active phases 180°–360°). There was no significant circadian rhythm in α of suprachiasmatic nucleus-lesioned rats (circles), yet α is significantly larger in suprachiasmatic nucleus-lesioned rats than in controls across all circadian phases. The grey region indicates circadian phases corresponding to the biological night for rats. (C) Similar circadian rhythms in the group average of the scaling exponent α were also observed in healthy humans (adapted from Hu et al.15). As in control rats, core body temperature of each human subject was used to determine the individual circadian period. In human data, circadian phase 0° was assigned to the time of the fitted minimum core body temperature (corresponding to about 5 AM),15 whereas phase 0° was assigned to the start of the DD cycle in control rats (corresponding to the onset of the light period in the preceding LD protocol; see Methods). The grey region indicates circadian phases corresponding to the usual sleep period of human subjects. The results of α in (B) and (C) are double-plotted to better visualize circadian rhythmicity.
To quantify the scale-invariant patterns in cardiac dynamics, most other studies have used continuous beat-to-beat intervals,4,27,29 but beat-to-beat data were unavailable in the current study. Instead, we analysed heart rate data of rats that were collected every 4 min on the basis of 10 s sampling (see 2.3 Data acquisition). Such difference in sampling frequency and variable (i.e. heart rate vs. inter-beat interval) can systematically affect the scaling exponent obtained by the DFA. To account for the sampling effect and enable the comparison of the current and previous studies, all scaling exponents presented in the current study were adjusted by adding 0.15 (see Supplementary material online for details).
2.4.2. Circadian phase
Core body temperature was used to determine the individual circadian periods of control rats during the DD protocol, with the start of the DD cycle (corresponding to the onset of the light period in the preceding LD protocol) being assigned circadian phase 0°. To appropriately align data from SCNx rats for comparison with control rats, the start of the DD cycle was again assigned circadian phase 0°, and the average circadian period of the control rats in the DD protocol, i.e. 24.1 h,22 was used for assignment of extrapolated circadian phase bins.
2.4.3. Statistical analysis
For data analysis, the circadian cycle (360°) was divided into nine non-overlapped circadian phase bins (equivalent to 40° or ∼2.67 h per bin). To determine whether or not there exists an endogenous circadian variation in the heart rate-scaling exponent, data were normalized (deviation from the mean), and subjected to one-way ANOVA, separately for control and SCNx groups. To test for any interaction effects upon the scaling exponent between group and circadian phase (biological day vs. biological night), a two-way ANOVA was performed. Unpaired t-tests were used for the comparisons of mean heart rate and scaling exponent between control and SCNx groups.
3. Results
3.1. Endogenous circadian rhythm of cardiac dynamics in rats
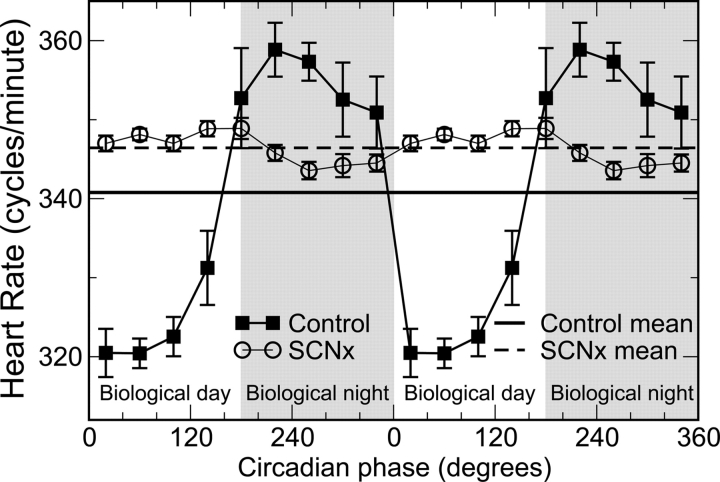
During DD, there was a significant circadian rhythm in heart rate in control rats (Figure 1; black squares). Mean heart rate was 341 b.p.m. (SEM = 4 b.p.m.), and the average peak-to-trough circadian fluctuation was ∼40 b.p.m. Heart rate was lower during the biological day (inactive phase in these animals, corresponding to the light periods in the preceding LD protocol). Similarly, during DD, there was a significant circadian rhythm in the scaling exponent of heart rate fluctuations in control rats (Figure 2B; black squares). The average adjusted scaling exponent α for control rats across all circadian phases was 1.03 ± 0.04 (SEM). Notably, there was a substantially larger α exponent at circadian phases 0°–180°, corresponding to the inactive biological day in these nocturnal animals when compared with the biological night (Δα ≈ 0.15; P = 0.0005). The α exponent is also larger during the biological day in diurnally active humans (Figure 2C).15,16 Taken together, these results suggest that the circadian rhythm in α is independent of activity level because the relationships between α and activity are reversed between these species.
Figure 1.
Circadian rhythms in the group average of mean heart rate during constant dark conditions. In control rats, heart rate exhibits a significant circadian variation (black square: P < 0.0001) with lower values at 0°–180° [corresponding to biological day (inactive phase)] and higher values at 180°–360° [grey region; corresponding to biological night (active phase)]. No circadian rhythms occur in the heart rate of suprachiasmatic nucleus-lesioned rats (open circle: P > 0.45). The mean heart rate across all circadian phases is slightly higher in suprachiasmatic nucleus-lesioned rats (dashed line) than in controls (black line), but the difference is not significant (P > 0.47). Data are double-plotted to better visualize circadian rhythmicity.
3.2. Suprachiasmatic nucleus lesion abolished circadian rhythms in heart rate and cardiac dynamics
The circadian rhythms of core temperature, activity, and heart rate were abolished by SCN lesion,22 with the heart rate data shown in Figure 1 (circles). The group average heart rate was not significantly different between the two groups (SCNx 346 ± 7 b.p.m., mean ± SEM; control rats 341 ± 4 b.p.m.; P = 0.47). Similarly, the circadian rhythm in the scaling exponent α was abolished by SCN lesion (Figure 2B, circles), indicating that the SCN generates a circadian rhythm in scale-invariant cardiac dynamics. Overall, the scaling exponent α was larger in SCNx rats than in control rats (SCNx α = 1.20 ± 0.04, mean ± SEM; control 1.03 ± 0.04; P = 0.01). The group difference in α was most pronounced during the biological night (P = 0.003, Figure 2B). ANOVA confirmed the phase dependence of the α difference between the control and SCNx rats (P = 0.01).
4. Discussion
This study yielded four main findings. (i) As with humans, there exists a circadian rhythm in the fractal structure of heart rate fluctuations in rats. This suggests that there may be a common scale-invariant heart rate control mechanism in mammals. (ii) As with humans, the scaling exponent characterizing the fractal structure of heart rate in rats is larger during the biological day compared with the biological night, i.e. the period when it is normally dark (although the rats and humans were studied in constant darkness or dim light conditions). Since the average neuronal activity of the central circadian pacemaker is normally in synchrony with the environmental day/night (or light/dark) cycle in both nocturnal and diurnal species, peaking during the biological day (or light phase when under L/D conditions),21,30–34 these findings suggest that the phase relationship between the endogenous neuronal activity cycle of the SCN and the circadian cycle of the fractal structure of heart rate is similar between the two species. In contrast, the phase relationship between the rest/activity cycle and the circadian cycle of the fractal structure of heart rate is clearly different between these species. This suggests that the circadian variation in the fractal structure in heart rate fluctuations is not mediated via changes in the mean level of activity or heart rate. (iii) Lesioning the SCN in rats completely abolished the circadian rhythm in the fractal structure of heart rate. This demonstrates that in mammals, the SCN is critical for the circadian rhythm in scale-invariant heart rate control. (iv) The scaling exponent α is systematically larger when the SCN is ablated. This suggests that in control rats, the SCN normally decreases α throughout all circadian phases with a stronger influence during the biological night when compared with during the biological day (as discussed in Section 4.3).
4.1. Potential mechanisms underlying changes in scale invariance of heart rate fluctuations across the circadian cycle
The scale-invariant/fractal heart rate pattern reveals a complex temporal organization in heart rate fluctuations and provides complementary dynamical information of heart rate control that is independent of traditional HRV parameters used for the assessment of mean heart rate and sympatho-vagal activity.10,35–39 This is exemplified by the observations that vagal tone decreases in humans and increases in rats at circadian phases corresponding to the biological day, and vice versa during the biological night,20,21 but the scaling exponent characterizing heart rate scale invariance has similar circadian rhythm profiles in humans and rats, with larger exponents during the biological day (Figure 3). Mathematical models of physical systems reveal that such scale-invariant patterns can be explained by interactions between multiple control components that affect the overall system at different time scales.40–42 Thus, scale invariance in heartbeat fluctuations could emanate from a variety of interacting influences on heart rate including sympathetic and parasympathetic nervous system activity, circulating catecholamines and cortisol, changes in temperature, and intrinsic properties of the heart itself.43 The SCN generates and coordinates circadian rhythms in many physiological and behavioural functions.44,45 Thus, the SCN could exert its influences on scale-invariant heart rate control via direct projections influencing autonomic and humoral activation of the heart,19,21,46–48 or via indirect pathways whereby circadian-related changes in activity or temperature could affect heart rate fluctuations.
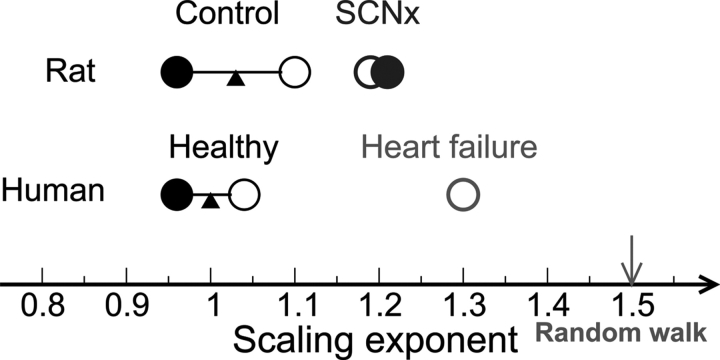
Figure 3.
Illustration of influences of the suprachiasmatic nucleus on scale-invariant correlations of heart rate dynamics. The scale-invariant correlations in healthy humans and control rats, as characterized by a scaling exponent α, are similar and exhibit endogenous circadian rhythms, with larger α at circadian phases corresponding to the biological day (open circles) and smaller α at circadian phases corresponding to the biological night (solid circles). Following suprachiasmatic nucleus lesion, the circadian rhythm in the scale-invariant correlations in rats was abolished, as indicated by the similar α values at all circadian phases. In addition, suprachiasmatic nucleus lesion causes a significant increase in α value, indicating that the correlations change towards the direction of a random walk—an integration of white noise that is associated with a simple process without any underlying feedback control and characterized by α = 1.5 (arrow). The scale-invariant cardiac correlations in cardiac diseases such as congestive heart failure are characterized by higher values of the scaling exponent α ≈ 1.3 during daytime.7
In our previous human studies, the timing of the circadian peak in the scaling exponent characterizing the fractal structure of heart rate is not in alignment with the circadian peak in mean heart rate and motor activity levels,15 and the circadian rhythm of α persists when circadian rhythms of motor activity were experimentally abolished using a constant routine protocol.16 These findings clearly indicate that the circadian rhythm of the HRV fractal structure in humans is independent of circadian influence on activity.
4.2. Limitations
In humans, heart beat fractal patterns at very small time scales (<10 beats) and at larger time scales (from 11 to ∼10 000 heartbeats) differ and provide complementary information about cardiac control.7,49 However, studies of heart rate in rats have not reported any significant difference in the fractal patterns at different time scale ranges.29,50 The scaling exponent characterizing the patterns in rats is identical to that in humans at time scales >11 beats. In this study, we note that since only average heart rates were stored every 4 min, we cannot study fractal structure in heart rate at time scales close to a few heartbeats.
In this study, we focused on the modulation on cardiac scale invariance at difference circadian phases. In order to examine changes across 24 h, the bin width must be sufficiently small to ensure capturing the peaks and troughs. This approach does not allow us to study scale invariance at large time scales (i.e. changes at different circadian phases can be smoothed out when considering a larger time scale). Evidence that the SCN is involved in cardiac scale-invariant control across a larger time scale (from ∼4 to 24 h) has been previously published.26
Currently, we could not identify the underlying pathways responsible for the SCN influence on the scale-invariant heart rate control. The SCN influences the autonomic nervous system,18,20,46,51,52 and sympathetic and parasympathetic blockade or autonomic dysfunction can alter scale-invariant cardiac dynamics.4–6 In the current study, we could not assess the involvement of the branches of autonomic nervous system to the circadian modulation of the fractal structure of heart rate because beat-to-beat recordings were not available for the estimation of standard HRV indices.
4.3. Significance of changes in scale invariance of heart rate fluctuations across the circadian cycle
Scale-invariant regulation of many physiological variables appears to provide greater adaptability and biological advantage compared with simple homeostatic control.53 The scale-invariant structure of heart rate fluctuations changes under pathological conditions7–9 and provides one of the most sensitive HRV biomarkers for predicting survival rates in patients after stroke10 and acute myocardial infarction.11 Thus scale-invariant correlations are intrinsic patterns of heart rate dynamics, with relevance to cardiovascular health and survival.
Almost identical scale-invariant HRV patterns are observed in human, dog, mouse, rat, swine, and sheep,1–3,29,54–57 suggesting that there may exist a common scale-invariant cardiac control mechanism in mammals. In humans, epidemiological studies reveal a 24 h daily pattern in adverse cardiac events with a broad peak at ∼9–11 AM,58–60 presumably caused by the day/night patterns of behaviours or internal influences from the circadian pacemaker.12 We previously found in healthy humans an increase in the scaling exponent α at the circadian phases corresponding to the time window of highest cardiac risk (9–11 AM).15 In humans, cardiovascular diseases are associated with a larger α value compared with healthy controls, thus we speculated that the time of peak cardiac risk may be partly caused by an endogenous circadian-mediated increase in α.15 In the present study, we found that nocturnally active rats possess an endogenous circadian rhythm in scale-invariant cardiac control, and the phase relationship between circadian changes in α and the normal day/night cycle is similar between humans and rats.21,30–34 Using an animal model of cardiovascular disease, it would be worthwhile to determine whether such animals exhibit an increased exponent α as in diseased humans, and whether or not these animals exhibit an endogenous circadian rhythm in adverse cardiovascular events. Furthermore, although we speculate that the endogenous circadian influence on the scale-invariant cardiac control may contribute to an underlying day/night pattern of cardiac risk, it is likely that behavioural factors such as motor activity levels also provide a major contribution to cardiac vulnerability.12 Future studies could test the relative contribution of dynamics vs. mean levels of heart rate and other physiological markers to cardiac risk in nocturnal mammals.
SCN lesion systematically increased the scaling exponent α, suggesting an altered scale-invariant heart rate control in the SCNx rats that resembles humans under pathological conditions (Figure 3). This finding raises the intriguing hypothesis that the SCN, as a master clock promoting adaptation of body functions to daily rest and activity, may have a protective role in heart rate control. This is consistent with the observation that a decreased activity of the SCN is associated with a history of hypertension, which is a major risk for cardiovascular diseases.61 Such a hypothesized protective role of the SCN in heart rate control could also provide an explanation for previous observations that disturbing the circadian system is associated with increased risk of cardiovascular diseases62–64 and increased morbidity and mortality.65,66 Further studies will be required in order to test this hypothesis.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
The research is supported by grants from NIH/NHLBI K24 HL076446 and R01 HL076409 to S.A.S. and R21 AT002713 to F.A.J.L.S.
References
- 1.Kobayashi M, Musha T. 1f fluctuations of heartbeat period. IEEE Trans Biomed Eng. 1982;29:456–457. doi: 10.1109/TBME.1982.324972. [DOI] [PubMed] [Google Scholar]
- 2.Peng CK, Mietus J, Hausdorff JM, Havlin S, Stanley HE, Goldberger AL. Long-range anticorrelations and non-Gaussian behavior of the heartbeat. Phys Rev Lett. 1993;70:1343–1346. doi: 10.1103/PhysRevLett.70.1343. [DOI] [PubMed] [Google Scholar]
- 3.Ivanov PC, Bunde A, Amaral LA, Havlin S, Fritsch-Yelle J, Baevsky RM, et al. Sleep-wake differences in scaling behavior of the human heartbeat: analysis of terrestrial and long-term space flight data. Europhys Lett. 1999;48:594–600. doi: 10.1209/epl/i1999-00525-0. [DOI] [PubMed] [Google Scholar]
- 4.Penttila J, Helminen A, Jartti T, Kuusela T, Huikuri HV, Tulppo MP, et al. Effect of cardiac vagal outflow on complexity and fractal correlation properties of heart rate dynamics. Auton Autacoid Pharmacol. 2003;23:173–179. doi: 10.1046/j.1474-8673.2003.00293.x. [DOI] [PubMed] [Google Scholar]
- 5.Merati G, Di Rienzo M, Parati G, Veicsteinas A, Castiglioni P. Assessment of the autonomic control of heart rate variability in healthy and spinal-cord injured subjects: contribution of different complexity-based estimators. IEEE Trans Biomed Eng. 2006;53:43–52. doi: 10.1109/TBME.2005.859786. [DOI] [PubMed] [Google Scholar]
- 6.Aoyagi N, Struzik ZR, Kiyono K, Yamamoto Y. Autonomic imbalance induced breakdown of long-range dependence in healthy heart rate. Methods Inf Med. 2007;46:174–178. [PubMed] [Google Scholar]
- 7.Peng CK, Havlin S, Hausdorff JM, Mietus JE, Stanley HE, Goldberger AL. Fractal mechanisms and heart rate dynamics. Long-range correlations and their breakdown with disease. J Electrocardiol. 1995;28(suppl.):59–65. doi: 10.1016/s0022-0736(95)80017-4. [DOI] [PubMed] [Google Scholar]
- 8.Ivanov PC, Amaral LA, Goldberger AL, Havlin S, Rosenblum MG, Struzik ZR, et al. Multifractality in human heartbeat dynamics. Nature. 1999;399:461–465. doi: 10.1038/20924. [DOI] [PubMed] [Google Scholar]
- 9.Goldberger AL, Amaral L, Hausdorff JM, Ivanov PCh. Fractal dynamics in physiology: alterations with disease and aging. Proc Natl Acad Sci USA. 2002;99:2466–2472. doi: 10.1073/pnas.012579499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makikallio AM, Makikallio TH, Korpelainen JT, Sotaniemi KA, Huikuri HV, Myllyla VV. Heart rate dynamics predict poststroke mortality. Neurology. 2004;62:1822–1826. doi: 10.1212/01.wnl.0000125190.10967.d5. [DOI] [PubMed] [Google Scholar]
- 11.Bigger JT, Jr, Steinman RC, Rolnitzky LM, Fleiss JL, Albrecht P, Cohen RJ. Power law behavior of RR-interval variability in healthy middle-aged persons, patients with recent acute myocardial infarction, and patients with heart transplants. Circulation. 1996;93:2142–2151. doi: 10.1161/01.cir.93.12.2142. [DOI] [PubMed] [Google Scholar]
- 12.Shea SA, Hilton MF, Muller JE. Clinical Hypertension and Vascular Disease: Blood Pressure Monitoring in Cardiovascular Medicine and Therapeutics. 2nd ed. Totowa, NJ: Humana Press Inc; 2007. Day/night pattern of myocardial infarction and sudden cardiac death. Interacting roles of the endogenous circadian system and behavioral triggers; pp. 253–291. [Google Scholar]
- 13.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 15.Hu K, Ivanov PC, Hilton MF, Chen Z, Ayers RT, Stanley HE, et al. Endogenous circadian rhythm in an index of cardiac vulnerability independent of changes in behavior. Proc Natl Acad Sci USA. 2004;101:18223–18227. doi: 10.1073/pnas.0408243101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivanov PC, Hu K, Hilton MF, Shea SA, Stanley HE. Endogenous circadian rhythm in human motor activity uncoupled from circadian influences on cardiac dynamics. Proc Natl Acad Sci USA. 2007;104:20702–20707. doi: 10.1073/pnas.0709957104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saleh MA, Winget CM. Effect of suprachiasmatic lesions on diurnal heart rate rhythm in the rat. Physiol Behav. 1977;19:561–564. doi: 10.1016/0031-9384(77)90234-7. [DOI] [PubMed] [Google Scholar]
- 18.Warren WS, Champney TH, Cassone VM. The suprachiasmatic nucleus controls the circadian rhythm of heart rate via the sympathetic nervous system. Physiol Behav. 1994;55:1091–1099. doi: 10.1016/0031-9384(94)90392-1. [DOI] [PubMed] [Google Scholar]
- 19.Scheer FA, Ter Horst GJ, Van der Vliet J, Buijs RM. Physiological and anatomic evidence for regulation of the heart by suprachiasmatic nucleus in rats. Am J Physiol Heart Circ Physiol. 2001;280:H1391–H1399. doi: 10.1152/ajpheart.2001.280.3.H1391. [DOI] [PubMed] [Google Scholar]
- 20.Hilton MF, Umali MU, Czeisler CA, Wyatt JK, Shea SA. Endogenous circadian control of the human autonomic nervous system. Comput Cardiol. 2000;27:197–200. [PubMed] [Google Scholar]
- 21.Scheer FA, Kalsbeek A, Buijs RM. Cardiovascular control by the suprachiasmatic nucleus: neural and neuroendocrine mechanisms in human and rat. Biol Chem. 2003;384:697–709. doi: 10.1515/BC.2003.078. [DOI] [PubMed] [Google Scholar]
- 22.Scheer FA, Pirovano C, Van Someren EJ, Buijs RM. Environmental light and suprachiasmatic nucleus interact in the regulation of body temperature. Neuroscience. 2005;132:465–477. doi: 10.1016/j.neuroscience.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Buijs RM, Kalsbeek A, Van der Woude TP, Van Heerikhuize JJ, Shinn S. Suprachiasmatic nucleus lesion increases corticosterone secretion. Am J Physiol. 1993;264:R1186–R1192. doi: 10.1152/ajpregu.1993.264.6.R1186. [DOI] [PubMed] [Google Scholar]
- 24.Kalsbeek A, Buijs RM, Van Heerikhuize JJ, Arts M, Van der Woude TP. Vasopressin-containing neurons of the suprachiasmatic nuclei inhibit corticosterone release. Brain Res. 1992;580:62–67. doi: 10.1016/0006-8993(92)90927-2. [DOI] [PubMed] [Google Scholar]
- 25.Hu K, Scheer FA, Ivanov PC, Buijs RM, Shea SA. The suprachiasmatic nucleus functions beyond circadian rhythm generation. Neuroscience. 2007;149:508–517. doi: 10.1016/j.neuroscience.2007.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu K, Scheer FA, Buijs RM, Shea SA. The endogenous circadian pacemaker imparts a scale-invariant pattern of heart rate fluctuations across time scales spanning minutes to 24 h. J Biol Rhythms. 2008;23:265–273. doi: 10.1177/0748730408316166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng CK, Buldyrev SV, Havlin S, Simons M, Stanley HE, Goldberger AL. Mosaic organization of DNA nucleotides. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1994;49:1685–1689. doi: 10.1103/physreve.49.1685. [DOI] [PubMed] [Google Scholar]
- 28.Hu K, Ivanov PC, Chen Z, Carpena P, Stanley HE. Effect of trends on detrended fluctuation analysis. Phys Rev E Stat Nonlin Soft Matter Phys. 2001;64:011114. doi: 10.1103/PhysRevE.64.011114. [DOI] [PubMed] [Google Scholar]
- 29.Beckers F, Verheyden B, Ramaekers D, Swynghedauw B, Aubert AE. Effects of autonomic blockade on non-linear cardiovascular variability indices in rats. Clin Exp Pharmacol Physiol. 2006;33:431–439. doi: 10.1111/j.1440-1681.2006.04384.x. [DOI] [PubMed] [Google Scholar]
- 30.Shibata S, Oomura Y, Kita H, Hattori K. Circadian rhythmic changes of neuronal activity in the suprachiasmatic nucleus of the rat hypothalamic slice. Brain Res. 1982;247:154–158. doi: 10.1016/0006-8993(82)91041-1. [DOI] [PubMed] [Google Scholar]
- 31.Groos G, Hendriks J. Circadian rhythms in electrical discharge of rat suprachiasmatic neurones recorded in vitro. Neurosci Lett. 1982;34:283–288. doi: 10.1016/0304-3940(82)90189-6. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz WJ, Reppert SM, Eagan SM, Moore-Ede MC. In vivo metabolic activity of the suprachiasmatic nuclei: a comparative study. Brain Res. 1983;274:184–187. doi: 10.1016/0006-8993(83)90538-3. [DOI] [PubMed] [Google Scholar]
- 33.Sato T, Kawamura H. Circadian rhythms in multiple unit activity inside and outside the suprachiasmatic nucleus in the diurnal chipmunk (Eutamias sibiricus) Neurosci Res. 1984;1:45–52. doi: 10.1016/0168-0102(84)90029-4. [DOI] [PubMed] [Google Scholar]
- 34.Meijer JH, Schaap J, Watanabe K, Albus H. Multiunit activity recordings in the suprachiasmatic nuclei: in vivo versus in vitro models. Brain Res. 1997;753:322–327. doi: 10.1016/s0006-8993(97)00150-9. [DOI] [PubMed] [Google Scholar]
- 35.Ho KKL, Moody GB, Peng CK, Mietus JE, Larson MG, Levy D, et al. Predicting survival in heart failure case and control subjects by use of fully automated methods for deriving nonlinear and conventional indices of heart rate dynamics. Circulation. 1997;96:842–848. doi: 10.1161/01.cir.96.3.842. [DOI] [PubMed] [Google Scholar]
- 36.Makikallio TH, Koistinin J, Jordeans L, Tulppo MP, Wood N, Golosarsky B, et al. Heart rate dynamics before spontaneous onset of ventricular fibrillation in patients with healed myocardial infarcts. Am J Cardiol. 1999;83:880–884. doi: 10.1016/s0002-9149(98)01068-6. [DOI] [PubMed] [Google Scholar]
- 37.Huikuri HV, Makikallio TH, Peng CK, Goldberger AL, Hintze U, Moller M. Fractal correlation properties of R-R interval dynamics and mortality in patients with depressed left ventricular function after an acute myocardial infarction. Circulation. 2000;101:47–53. doi: 10.1161/01.cir.101.1.47. [DOI] [PubMed] [Google Scholar]
- 38.Penzel T, Kantelhardt JW, Grote L, Peter JH, Bunde A. Comparison of detrended fluctuation analysis and spectral analysis for heart rate variability in sleep and sleep apnea. IEEE Trans Biomed Eng. 2003;50:1143–1151. doi: 10.1109/TBME.2003.817636. [DOI] [PubMed] [Google Scholar]
- 39.Stein PK, Domitrovich PP, Huikuri HV, Kleiger RE. Traditional and nonlinear heart rate variability are each independently associated with mortality after myocardial infarction. J Cardiovasc Electrophysiol. 2005;16:13–20. doi: 10.1046/j.1540-8167.2005.04358.x. [DOI] [PubMed] [Google Scholar]
- 40.Mandelbrot B. The Fractal Geometry of Nature. New York: W.H. Freeman and Company; 1982. [Google Scholar]
- 41.Bak P, Tang C, Wiesenfeld K. Self-organized criticality: an explanation of the 1/f noise. Phys Rev Lett. 1987;59:381–384. doi: 10.1103/PhysRevLett.59.381. [DOI] [PubMed] [Google Scholar]
- 42.Basu S, Foufoula-Georgiou E, Porte-Agel F. Synthetic turbulence, fractal interpolation, and large-eddy simulation. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;70:026310. doi: 10.1103/PhysRevE.70.026310. [DOI] [PubMed] [Google Scholar]
- 43.Yoneyama M, Kawahara K. Coupled oscillator systems of cultured cardiac myocytes: fluctuation and scaling properties. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;70:021904. doi: 10.1103/PhysRevE.70.021904. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz WJ. Suprachiasmatic nucleus. Curr Biol. 2002;12:R644. doi: 10.1016/s0960-9822(02)01155-7. [DOI] [PubMed] [Google Scholar]
- 45.Herzog ED. Neurons and networks in daily rhythms. Nat Neurosci. 2007;8:790–802. doi: 10.1038/nrn2215. [DOI] [PubMed] [Google Scholar]
- 46.Buijs RM, Scheer FA, Kreier F, Yi C, Bos N, Goncharuk VD, et al. Organization of circadian functions: interaction with the body. Prog Brain Res. 2006;153:341–360. doi: 10.1016/S0079-6123(06)53020-1. [DOI] [PubMed] [Google Scholar]
- 47.Kalsbeek A, Perreau-Lenz S, Buijs RM. A network of (autonomic) clock outputs. Chronobiol Int. 2006;23:521–535. doi: 10.1080/07420520600651073. [DOI] [PubMed] [Google Scholar]
- 48.Reilly DF, Westgate EJ, FitzGerald GA. Peripheral circadian clocks in the vasculature. Arterioscler Thromb Vasc Biol. 2007;27:1694–1705. doi: 10.1161/ATVBAHA.107.144923. [DOI] [PubMed] [Google Scholar]
- 49.Karasik R, Sapir N, Ashkenazy Y, Ivanov PC, Dvir I, Lavie P, et al. Correlation differences in heartbeat fluctuations during rest and exercise. Phys Rev E Stat Nonlin Soft Matter Phys. 2002;66:062902. doi: 10.1103/PhysRevE.66.062902. [DOI] [PubMed] [Google Scholar]
- 50.Perlstein I, Sapir N, Backon J, Sapoznikov D, Karasik R, Havlin S, et al. Scaling vs. nonscaling methods of assessing autonomic tone in streptozotocin-induced diabetic rats. Am J Physiol Heart Circ Physiol. 2002;283:H1142–H1149. doi: 10.1152/ajpheart.00519.2001. [DOI] [PubMed] [Google Scholar]
- 51.Furlan R, Crivellaro W, Dell’Orto S, Gentile E, Piazza S, Pagani MR, et al. Circadian changes in vascular sympathetic activity in ambulant subjects. J Hypertens Suppl. 1989;7:S30–S31. doi: 10.1097/00004872-198900076-00012. [DOI] [PubMed] [Google Scholar]
- 52.Scheer FA, Cajochen C, Turek FW, Czeisler CA. Melatonin in the regulation of sleep and circadian rhythms. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia: W B Saunders; 2005. pp. 395–404. [Google Scholar]
- 53.Goldberger AL, Giles F. Filley lecture. Complex systems. Proc Am Thorac Soc. 2006;3:467–471. doi: 10.1513/pats.200603-028MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Minors SL, O’Grady MR. Heart rate variability in the dog: is it too variable? Can J Vet Res. 1997;61:134–144. [PMC free article] [PubMed] [Google Scholar]
- 55.Callaway CW, Sherman LD, Scheatzle MD, Menegazzi JJ. Scaling structure of electrocardiographic waveform during prolonged ventricular fibrillation in swine. Pacing Clin Electrophysiol. 2000;23:180–191. doi: 10.1111/j.1540-8159.2000.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 56.Koshino T, Kimura Y, Kameyama Y, Takahashi T, Yasui T, Chisaka H, et al. Fractal and periodic heart rate dynamics in fetal sheep: comparison of conventional and new measures based on fractal analysis. Am J Physiol Heart Circ Physiol. 2003;284:H1858–H1864. doi: 10.1152/ajpheart.00268.2002. [DOI] [PubMed] [Google Scholar]
- 57.Meyer M, Stiedl O. Fractal rigidity by enhanced sympatho-vagal antagonism in heartbeat interval dynamics elicited by central application of corticotropin-releasing factor in mice. J Math Biol. 2006;52:830–874. doi: 10.1007/s00285-006-0375-5. [DOI] [PubMed] [Google Scholar]
- 58.Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 59.Goldberg RJ, Brady P, Muller JE, Chen Z, de Groot M, Zonnefeld P, et al. Time of onset of symptoms of acute myocardial infarction. Am J Cardiol. 1990;66:140–144. doi: 10.1016/0002-9149(90)90577-n. [DOI] [PubMed] [Google Scholar]
- 60.Willich SN, Goldberg RJ, Maclure M, Perriello L, Muller JE. Increased onset of sudden cardiac death in the first three hours after awakening. Am J Cardiol. 1992;70:65–68. doi: 10.1016/0002-9149(92)91391-g. [DOI] [PubMed] [Google Scholar]
- 61.Goncharuk VD, van Heerikhuize J, Dai JP, Swaab DF, Buijs RM. Neuropeptide changes in the suprachiasmatic nucleus in primary hypertension indicate functional impairment of the biological clock. J Comp Neurol. 2001;431:320–330. doi: 10.1002/1096-9861(20010312)431:3<320::aid-cne1073>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 62.Knutsson A, Akerstedt T, Jonsson BG, Orth-Gomer K. Increased risk of ischaemic heart disease in shift workers. Lancet. 1986;2:89–92. doi: 10.1016/s0140-6736(86)91619-3. [DOI] [PubMed] [Google Scholar]
- 63.Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Speizer FE, et al. Prospective study of shift work and risk of coronary heart disease in women. Circulation. 1995;92:3178–3182. doi: 10.1161/01.cir.92.11.3178. [DOI] [PubMed] [Google Scholar]
- 64.Martino TA, Tata N, Belsham DD, Chalmers J, Straume M, Lee P, et al. Disturbed diurnal rhythm alters gene expression and exacerbates cardiovascular disease with rescue by resynchronization. Hypertension. 2007;49:1104–1113. doi: 10.1161/HYPERTENSIONAHA.106.083568. [DOI] [PubMed] [Google Scholar]
- 65.Penev PD, Kolker DE, Zee PC, Turek FW. Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. Am J Physiol. 1998;275:H2334–H2337. doi: 10.1152/ajpheart.1998.275.6.H2334. [DOI] [PubMed] [Google Scholar]
- 66.Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Curr Biol. 2006;16:R914–R916. doi: 10.1016/j.cub.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.