Abstract
Aims
Nuclear factor-kappa B (NF-κB) is a potent inducer of pro-inflammatory cytokines (PIC) and oxidative stress in cardiovascular disease. In this study, we determined whether upregulation of NF-κB in the hypothalamic paraventricular nucleus (PVN) contributed to neurohumoral excitation either directly, or via interaction with the renin–angiotensin system (RAS), in heart failure (HF).
Methods and results
Rats were implanted with intracerebroventricular (ICV) cannulae and subjected to coronary artery ligation, or sham surgery (SHAM). Subsequently, animals were ICV treated with the angiotensin type 1 receptor (AT1-R) antagonist losartan (LOS, 20 µg/h), or SN50 (2 µg/h), which inhibits nuclear translocation of NF-κB, or tempol (TEMP, 80 µg/h), a membrane-permeable superoxide scavenger, or vehicle for 4 weeks. HF induced a significant increase in the expression of AT1-R, PIC, and NAD(P)H oxidase genes and NF-κB p50 in the PVN and in plasma norepinephrine (NE) levels when compared with SHAM rats. In contrast, ICV LOS, SN50, or TEMP attenuated PIC, NF-κB p50, AT1-R and NAD(P)H oxidase genes in the PVN compared with vehicle-treated HF rats. Treatment with LOS, SN50, or TEMP also reduced plasma levels of NE, angiotensin II, and PIC, and decreased left ventricular end diastolic pressure.
Conclusion
These findings indicate that NF-κB mediates the cross-talk between RAS and PIC in the PVN in HF, and that superoxide stimulates more NF-κB in the PVN and contributes to neurohumoral excitation.
Keywords: NF-κB, Heart failure, Brain, Angiotensin, Superoxide
1. Introduction
Neurohumoral mechanisms play important roles in the pathophysiology of congestive heart failure (HF). Among the neurohormones, those of the renin–angiotensin system (RAS) play an important role in regulating body fluid homoeostasis and sympathetic activity.1 An increase in the components of RAS is not only found in peripheral tissues but also in the brain cardiovascular centres of HF rats, including the paraventricular nucleus (PVN) of the hypothalamus.2 Previous findings indicate that blocking components of brain RAS attenuated sympathetic drive, decreased retention of sodium and water, and ameliorated cardiac remodelling in HF rats.3–5 These findings indicate a role of brain RAS in inducing neurohumoral excitation in HF.
In recent years, immune-mediated mechanisms have also been shown to play important roles in the pathophysiology of cardiovascular diseases. Circulating levels of pro-inflammatory cytokines (PIC) such as tumour necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6 are increased in HF patients. These levels are increased with the severity of HF and are of prognostic significance.6,7 Recent findings have shown that, along with myocardial production of TNF-α, cytokine levels are also upregulated in the PVN of the hypothalamus of HF rats.8,9 In addition, these brain PICs also contribute to neurohumoral excitation in HF.9
Excess amounts of PIC5 and RAS3 are both present in the brain cardiovascular regions and contribute to neurohumoral excitation in HF; however, it is not known whether exaggerated neurohumoral excitation in HF is due to an interaction between these two systems. More importantly, it is not known if there is a downstream signalling molecule that links these two systems to some of the adverse effects in HF. The nuclear factor-kappa B (NF-κB) family of eukaryotic transcription factors is present in CNS neurons and plays an important role in inflammatory responses.10 Activated NF-κB has been shown to be the major regulator facilitating the synthesis of several different injury-responsive cytokines in neurons. These include TNF-α, IL-6, and angiotensin type 1 receptors (AT1-R).11 In our previous study, we showed that along with PIC activation, NF-κB is upregulated in the PVN of HF rats.12 In the present study, we determined whether NF-κB is the link in the cross-talk between RAS and PIC in HF.
The PICs or angiotensin II (ANG II) may activate NF-κB.13 In addition, NAD(P)H oxidase derived reactive oxygen species (ROS) induced by PIC or ANG II appear to facilitate the activation of NF-κB.14–16 Augmented ROS in the PVN in HF were also shown to contribute to increased sympathetic outflow.17 In this study, we also investigated whether NF-κB-induced oxidant signalling in the PVN contributes to the neurohumoral excitation in HF.
2. Methods
2.1. Animals
Adult male Sprague–Dawley rats weighing 250–275 g were housed in temperature- (23 ± 2°C) and light-controlled animal quarters and were provided with rat chow ad libitum. These studies were performed in accordance with the American Physiological Society’s ‘Guiding Principles for Research Involving Animals and Human Beings’. All experimental procedures were approved by the Louisiana State University Institutional Animal Care and Use Committee and the Shanxi Medical University Institutional Animal Care and Use Committee.
2.2. General experimental protocol
Under anaesthesia, an intracerebroventricular (ICV) cannula was implanted in the right lateral cerebral ventricle and fixed to the cranium using small screws and dental cement as previously described.3,7,18 Stereotaxic coordinates were used for cannula placement. The skin incision was closed with sterile 3-0 silk sutures. One week after the implantation of the cannula, anaesthetized rats underwent surgery to induce HF by ligating the left anterior descending coronary artery, or the same surgery without ligating the vessel (SHAM), as previously described.3,7,9,18 Animals received benzathine penicillin (30 000 units, im) and buprenorphine (0.1 mg/kg, sc) immediately after surgery and 12 h later. Echocardiography was performed ∼24 h after recovery from surgery to assess left ventricular (LV) function. Immediately thereafter, an Alzet osmotic mini-pump (DURECT Corporation) was implanted subcutaneously in the back of the neck3,7,18 and attached to the right lateral cerebral ventricle cannula for chronic ICV infusion of the angiotensin type 1 receptor (AT1-R) antagonist losartan (LOS, 20 µg/h) (Sigma-Aldrich), or SN50 (2 µg/h; a synthetic peptide carrying the nuclear localization sequence of the NF-κB p50 subunit, which competes for the cellular mechanisms mediating nuclear translocation and prevents NF-κB binding to DNA without affecting the level of the inhibitory protein IκB) (Biomol), the superoxide dismutase mimetic tempol (TEMP, 80 µg/h), or vehicle (VEH, artificial cerebrospinal fluid, aCSF). Mini-pumps were filled with SN50, LOS, TEMP, or vehicle and placed in a 0.9% saline solution at room temperature 24 h in advance to ensure a constant pumping rate during the treatment. A second echocardiogram was performed near the end of the treatment protocol. After 4 weeks of treatment, animals were anaesthetized for haemodynamic measurements and then euthanized to collect tissue and plasma for further analysis.
2.3. Echocardiographic assessment of LV function
Echocardiography was performed under ketamine (25 mg/kg, ip) sedation to assess LV function as previously described.9,18 Ischaemic zone (IZ) was estimated by planimetry of the region of the LV endocardial silhouette which demonstrated akinesis or dyskinesis, and expressed as a percentage of the whole (%IZ). From these measurements, %IZ, LV ejection fraction (LVEF), and LV end-diastolic volume (LVEDV), all of which are indexes of severity of congestive HF, were reported.
2.4. Measurement of haemodynamic parameters
Under pentobarbital anaesthesia (50 mg/kg, ip), a micromanometer-tipped catheter was inserted via the right carotid artery and advanced to the aorta. Arterial pressure was measured and the catheter was then advanced into the left ventricle for measurement of LV end-diastolic pressure (LVEDP). Mean arterial pressure (MAP), heart rate (HR), and pulse pressure (PP) were derived from the arterial pressure tracing.
2.5. Collection of blood and tissue samples
Rats were decapitated while still under deep anaesthesia to collect trunk blood. Trunk blood was collected in chilled EDTA tubes. Plasma samples were separated and stored at −80°C until assayed for IL-1β, IL-6, and norepinephrine (NE) levels. The brain and heart were harvested, the heart ventricles were separated, and the right and left ventricles were weighed. The lungs were also harvested and weighed wet. Right ventricular (RV) and lung weights were expressed as a function of body weight (BW).
2.6. Biochemical assays
Plasma and tissue cytokine levels were measured using ELISA (Biosource International Inc.) techniques, as described before.7,19 Plasma NE was measured using a high-sensitivity ELISA kit (Rocky Mountain Diagnostics). Plasma angiotensin II was measured using an EIA kit (Cayman Chemical Company).
2.7. Immunohistochemistry and immunofluorescence
Rats were anaesthetized with pentobarbital (50 mg/kg, ip) and transcardially perfused with PBS and 4% paraformaldehyde. Samples were fixed in 4% paraformaldehyde overnight at 4°C, and then immersed in 30% sucrose for at least 2 days. Samples were embedded in OCT and sectioned into several 40 µm transverse sections about −1.80 mm from the bregma using a sliding microtome; sections were put in PBS for immunohistochemistry.9 Remaining samples were used for Cryostat sections (10 µm) which were put on slides, and stored at −80°C for future use for immunofluorescence.
2.7.1. Immunohistochemistry
A general avidin–biotin–peroxidase complex procedure was used.20 A double-staining protocol was used for Fra-like (Fra-LI) activity (Santa Cruz Biotechnology) plus IL-1β (Santa Cruz Biotechnology) staining in PVN. Expression of Fra-LI (fos family gene) activity was used as an indicator of chronic neuronal activation.9 For each animal, labelled neurons within the bilateral borders of the PVN were counted manually in two representative 40 µm transverse sections, and an average value was reported. NIH ImageJ software was used to confirm the manual cell counts in the PVN.
2.7.2. Immunofluorescent labelling
Immunofluorescent staining was performed as previously described.8,9 The primary antibodies for NF-κB p50, gp91phox, and TNF-α were obtained from Santa Cruz Biotechnology. Superoxide generation was determined by fluorescent-labelled dihydroethidium (DHE; Molecular Probes). DHE staining was performed as previously described.21 Fluorescent intensity of DHE in PVN was measured using laser confocal microscopy. NF-κB p50, gp91phox, and TNF-α positive staining cells were counted under confocal microscopy in four view fields (equal area) randomly selected from bilateral PVN. One sample consisted of the average of four view fields from a section. The observer was blinded to the type of samples examined.
2.8. Western blot
Protein extracted from punches of the PVN was used for measurement of gp91phox (Santa Cruz Biotechnology) and AT1-R (Abcam Inc.) expression by western blot.22 Protein loading was controlled by probing all western blots with anti-β-actin antibody (Santa Cruz Biotechnology) and normalizing gp91phox and AT1-R protein intensities to that of β-actin. Band densities were analysed using NIH ImageJ software.
2.9. Statistical analysis
All data are expressed as mean ± SEM. Data were analysed by two-way ANOVA. Multiple testing was corrected for by using Tukey’s test. The echocardiography data were analysed by ANOVA allowing for repeated measurement. A probability value of P < 0.05 was considered statistically significant.
3. Results
3.1. Echocardiographic characterization of the study groups
Echocardiography performed within 24 h of surgery revealed that rats with ischaemic injury after coronary ligation had a significantly lower LV ejection fraction (LVEF), a higher LVEDV, and a higher LVEDV to mass (LVEDV/mass) ratio than the sham-operated rats. The %IZ, LVEF, LVEDV, and LVEDV/mass ratio were well matched among HF rats assigned to ICV VEH vs. ICV LOS, SN50 or TEMP treatments. Echocardiography performed at 4 weeks revealed that LOS-, SN50-, or TEMP-treated HF rats had no significant differences in these parameters when compared with VEH-treated HF rats (Figure 1).
Figure 1.
Echocardiographic assessment of left ventricular function within 24 h (left panels) of coronary ligation to induce heart failure (HF) or sham operation (SHAM) and after 4 weeks of treatment (right panels) with drugs or vehicle (VEH). Compared with SHAM rats, HF rats had reduced left ventricular ejection fraction (LVEF), increased left ventricular end diastolic volume (LVEDV), and increased LVEDV/mass (LVEDV/M) ratio at baseline. Rats assigned to treatment with drugs or VEH were well-matched with regard to LV function. No drug treatment regimen had any significant effect on LVEF, LVEDV, and LVEDV/M. *P < 0.05 vs. control (SHAM + treated or SHAM + VEH). ‡ P < 0.05, 4 week vs. baseline value.
3.2. Haemodynamic and anatomical indicators of HF
LVEDP was lower (P < 0.05, n = 6) in LOS-, SN50-, or TEMP-treated HF rats (HF + ICV LOS: 12.33 ± 1.35 mmHg; HF + ICV SN50: 10.52 ± 1.28 mmHg; HF + ICV TEMP: 14.64 ± 1.37 mmHg) than in VEH-treated (22.43 ± 1.58 mmHg) HF rats, but was still higher than in VEH-treated SHAM rats (4.84 ± 1.51 mmHg). There were no significant differences in MAP, HR, or PP attributable to LOS, SN50 or TEMP treatment. Lung/body weight ratio (mg/g) was lower (P < 0.05, n = 6) in LOS- or SN50-treated HF rats (HF + ICV LOS: 7.3 ± 0.3; HF + ICV SN50: 7.5 ± 0.4) than in VEH-treated HF rats (11.1 ± 0.5), but heart/body weight ratio was not affected (Table 1).
Table 1.
Haemodynamic and anatomical measurements (n = 6)
| Measurements at 4 weeks | RV/BW (mg/g) | Lung/BW (mg/g) | HR (beats/min) | MAP (mmHg) | PP (mmHg) | LVEDP (mmHg) |
|---|---|---|---|---|---|---|
| HF + ICV LOS | 1.10 ± 0.09* | 7.3 ± 0.3*,** | 337 ± 6 | 97 ± 4 | 26 ± 2 | 12.33 ± 1.35*,** |
| HF + ICV SN50 | 1.15 ± 0.11* | 7.5 ± 0.4*,** | 331 ± 8 | 94 ± 3 | 29 ± 2 | 10.52 ± 1.28*,** |
| HF + ICV TEMP | 1.17 ± 0.10* | 9.2 ± 0.4* | 335 ± 7 | 92 ± 3 | 27 ± 2 | 14.64 ± 1.37*,** |
| HF + ICV VEH | 1.2 ± 0.12* | 11.1 ± 0.5* | 349 ± 7 | 91 ± 5 | 30 ± 2 | 22.43 ± 1.58* |
| SHAM + ICV LOS | 0.61 ± 0.06 | 4.3 ± 0.2 | 326 ± 9 | 101 ± 5 | 34 ± 3 | 5.37 ± 1.62 |
| SHAM + ICV SN50 | 0.65 ± 0.07 | 4.7 ± 0.4 | 321 ± 8 | 98 ± 4 | 37 ± 4 | 6.28 ± 1.39 |
| SHAM + ICV TEMP | 0.63 ± 0.07 | 4.9 ± 0.3 | 327 ± 7 | 100 ± 4 | 36 ± 4 | 5.74 ± 1.42 |
| SHAM + ICV VEH | 0.69 ± 0.06 | 5.2 ± 0.3 | 329 ± 9 | 105 ± 5 | 35 ± 3 | 4.84 ± 1.51 |
Values are mean ± SEM.
SHAM, sham-operated control; HF, heart failure; BW, body weight; RV, right ventricular; HR, heart rate; MAP, mean arterial pressure; PP, pulse pressure; LVEDP, left ventricular end-diastolic pressure.
*P < 0.05 vs. control (SHAM + treated or SHAM + VEH).
**P < 0.05 HF + treated vs. HF + VEH.
3.3. Humoral indicators of HF
Plasma levels of NE, IL-1β, IL-6, and ANG II were all higher in HF rats than SHAM rats. Treatment with ICV LOS, SN50, or TEMP decreased plasma levels of NE, IL-1β, and ANG II in HF rats, but all levels, except that of IL-6 in the SN50- or TEMP-treated HF rats, remained higher than those observed in SHAM rats (Table 2).
Table 2.
The hypothalamus levels of pro-inflammatory cytokines and plasma humoral indicators (n = 6)
| Group | Hypothalamus (pg/mg protein, n = 6) |
Plasma (pg/mL, n = 6) |
|||||
|---|---|---|---|---|---|---|---|
| TNF-α | IL-1β | IL-6 | ANG II | IL-1β | IL-6β | NE | |
| HF + ICV LOS | 5.3 ± 0.5*,** | 34.5 ± 3.7*,** | 42.3 ± 3.9*,** | 87.2 ± 8.4*,** | 85.2 ± 8.3*,** | 59.3 ± 6.1*,** | 207.3±17.4*,** |
| HF + ICV SN50 | 4.9 ± 0.4*,** | 31.2 ± 2.9*,** | 38.6 ± 3.7*,** | 95.7 ± 10.2*,** | 90.5 ± 8.9*,** | 98.5 ± 8.7* | 213.2±19.3*,** |
| HF + ICV TEMP | 5.2 ± 0.5*,** | 36.7 ± 3.5*,** | 45.2 ± 4.2*,** | 97.3 ± 8.9*,** | 94.7 ± 9.1*,** | 101.5 ± 9.5* | 210.1±17.2*,** |
| HF + ICV VEH | 7.3 ± 0.7* | 51.4 ± 4.9* | 63.7 ± 6.1* | 127.6 ± 11.8* | 130.2 ± 12.8* | 110.6 ± 10.4* | 274.3±24.2* |
| SHAM + ICV LOS | 3.1 ± 0.3 | 17.2 ± 1.4 | 19.3 ± 1.7 | 58.1 ± 5.4 | 54.8 ± 4.7 | 33.1 ± 2.8 | 153.5±13.2 |
| SHAM + ICV SN50 | 2.9 ± 0.3 | 15.7 ± 1.5 | 17.6 ± 1.8 | 55.2 ± 4.9 | 59.1 ± 5.2 | 37.6 ± 3.4 | 149.6±11.5 |
| SHAM + ICV TEMP | 3.3 ± 0.4 | 19.2 ± 1.7 | 20.1 ± 1.9 | 62.5 ± 5.6 | 57.8 ± 4.9 | 38.2 ± 3.7 | 151.7±14.8 |
| SHAM + ICV VEH | 3.5 ± 0.4 | 19.4 ± 2.1 | 21.5 ± 2.0 | 64.8 ± 5.8 | 63.5 ± 6.1 | 35.8 ± 3.6 | 159.6±15.3 |
*P < 0.05 vs. control (SHAM + treated or SHAM + VEH).
**P < 0.05 HF + treated vs. HF + VEH.
3.4. Hypothalamic expression of PIC
Hypothalamic tissue levels of the PICs TNF-α, IL-1β, and IL-6 by ELISA were higher in HF rats than in SHAM rats. HF rats treated with LOS, SN50, or TEMP had lower TNF-α and IL-1β in the hypothalamus than VEH-treated HF rats, but values in the treated HF rats remained higher than in VEH-treated SHAM rats (Table 2).
3.5. PIC and NF-κB in the PVN
Immunohistochemistry revealed fewer IL-1β- and Fra-LI-positive neurons (Figure 2) in the PVN of LOS-, SN50-, or TEMP-treated HF rats when compared with VEH-treated HF rats; however, the number of positive neurons in the treated HF rats was higher than that of SHAM rats. Immunofluorescence revealed that HF rats had more TNF-α (Figure 3) and NF-κB p50 (Figure 4) in the PVN region than SHAM rats. The levels of TNF-α and NF-κB p50 in LOS-, SN50-, or TEMP-treated HF rats were lower than those of VEH-treated HF rats.
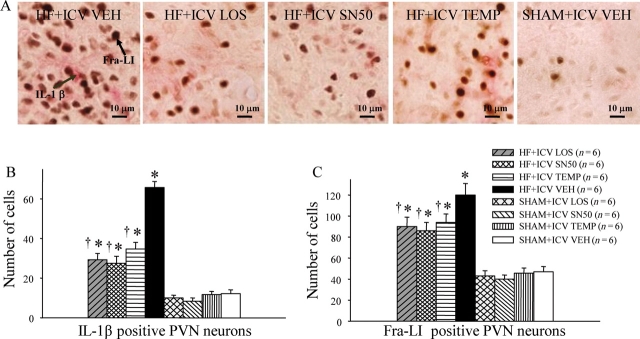
Figure 2.
(A) Immunohistochemistry studies revealed that VEH-treated HF rats had more IL-1β (pink dots) and Fra-LI (black dots), an indicator of chronic neuronal excitation, in a coronal section of the PVN region than ICV LOS-, SN50- or TEMP-treated HF rats. (B) The numbers of IL-1β positive neurons in PVN of LOS-, SN50-, or TEMP-treated HF rats were lower than VEH-treated HF rats, but still higher than SHAM rats. (C) The number of Fra-LI positive neurons in the PVN of LOS, SN50-, or TEMP-treated HF rats was lower than VEH-treated HF rats and higher than SHAM rats. *P < 0.05 vs. control (SHAM + treated or SHAM + VEH). †P < 0.05 HF + treated vs. HF + ICV VEH.
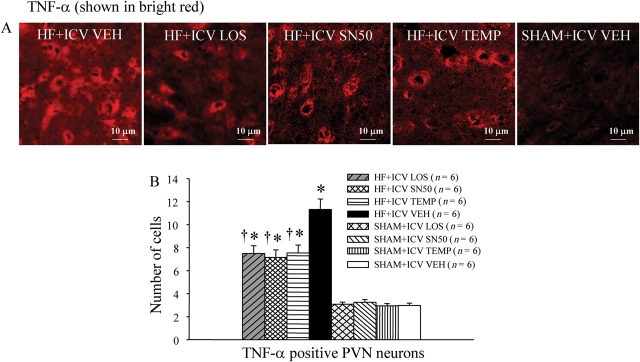
Figure 3.
(A) Immunofluorescence revealed VEH-treated HF rats had more TNF-α (bright red) in the PVN region than ICV LOS, SN50-, or TEMP-treated HF rats. (B) The numbers of TNF-α positive neurons in the PVN of LOS, SN50- or TEMP-treated HF rats were lower than those of VEH-treated HF rats, but still higher than those of SHAM rats. *P < 0.05 vs. control (SHAM + treated or SHAM + VEH). †P < 0.05 HF + treated vs. HF + VEH.
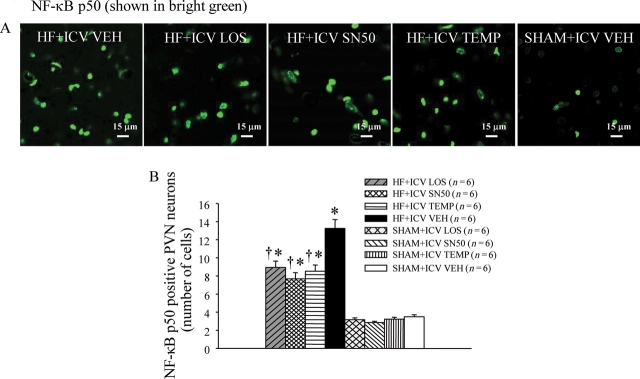
Figure 4.
(A) Immunofluorescence indicated that VEH-treated HF rats had more NF-κB p50 (bright green) in the PVN region than ICV LOS-, SN50-, or TEMP-treated HF rats. (B) Effects of treatment with LOS, SN50, or TEMP on the numbers of NF-κB p50 positive neurons in the PVN of HF and SHAM rats. *P < 0.05 vs. control (SHAM + treated or SHAM + VEH). †P < 0.05 HF + treated vs. HF + VEH.
3.6. Expression of angiotensin type 1 receptor (AT1-R) in the PVN
Western blot indicated that HF rats had increased AT1-R protein expression in the PVN when compared with SHAM rats. The levels of AT1-R in the PVN were lower in the LOS-, SN50-, or TEMP-treated HF rats when compared with VEH-treated HF rats, and higher than the levels of VEH-treated SHAM rats (Figure 5C).
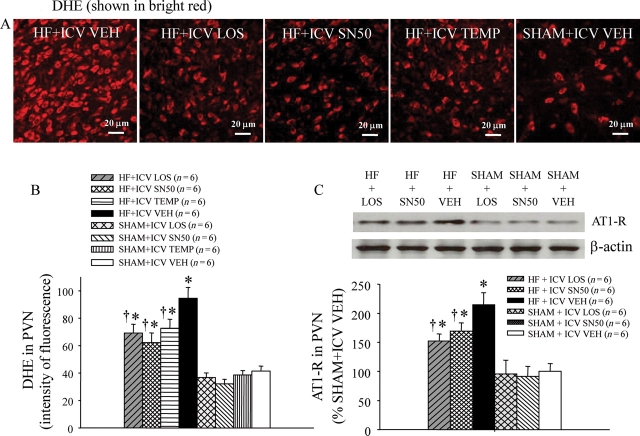
Figure 5.
(A) Immunofluorescence revealed increased superoxide in PVN neurons of VEH-treated HF rats compared with ICV LOS-, SN50-, or TEMP-treated HF rats, as determined by fluorescent-labelled dihydroethidium (DHE, bright red). (B) Effects of treatment with LOS, SN50, or TEMP on DHE staining in the PVN of HF and SHAM rats. (C) Western blot for AT1-R expression in the PVN of LOS-, SN50-, or TEMP-treated HF rats was lower than in VEH-treated HF rats. *P < 0.05 vs. control (SHAM + treated or SHAM + VEH). †P < 0.05 HF + treated vs. HF + VEH.
3.7. Superoxide and NAD(P)H oxidase subunits in the PVN
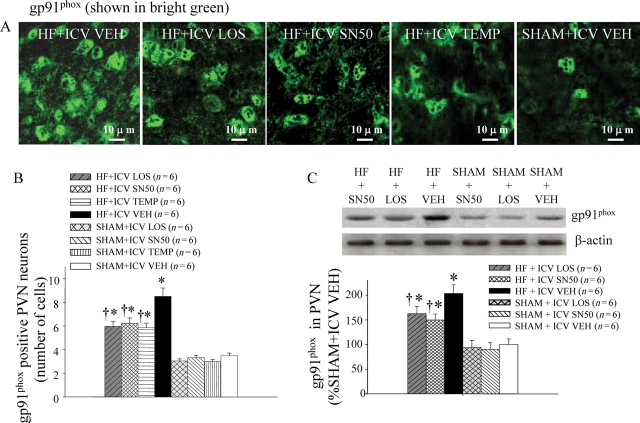
Immunofluorescence revealed increased superoxide in the PVN of VEH-treated HF rats, as determined by fluorescence-labelled dihydroethidium (DHE) (Figure 5A and B) and NAD(P)H oxidase subunit gp91phox (Figure 6). VEH-treated HF rats had more DHE fluorescent intensity and more gp91phox positive neurons in the PVN than did SHAM rats. In HF rats, treatment with ICV LOS, SN50, or TEMP decreased DHE fluorescent intensity and gp91phox positive neurons in the PVN of HF rats.
Figure 6.
(A) Immunofluorescence indicated NAD(P)H oxidase subunit expression in the PVN of VEH-treated HF rats was higher than ICV LOS-, SN50-, or TEMP-treated HF rats. (B) Effects of treatment with LOS, SN50, or TEMP on the numbers of gp91phox positive neurons in the PVN of HF and SHAM rats. (C) Western blot for NAD(P)H oxidase subunit gp91phox expression in the PVN was lower in LOS- or SN50-treated HF rats than in VEH-treated HF rats. *P < 0.05 vs. control (SHAM + treated or SHAM + VEH). †P < 0.05 HF + treated vs. HF + VEH.
4. Discussion
A number of studies have demonstrated that activation of the RAS in the PVN plays an important role in the exaggerated sympathoexcitation in HF.3,23,24 In this study, plasma NE (a marker of sympathetic activity) decreased in ICV LOS-treated HF rats compared with VEH-treated HF rats, suggesting that the excitation of brain RAS can promote sympathetic activity in HF. The angiotensin type 1 receptor (AT1-R) is the primary receptor inducing the action of RAS, and recent findings indicate that AT1-R is upregulated in brain cardiovascular centres, especially the PVN, in HF.24 Blockade of AT1-R2 or inhibition of ANG II production by administration of angiotensin-converting enzyme inhibitors3 can effectively lower sympathetic activity concomitant with abatement of water retention and cardiac ventricular remodelling. Although the activation of brain RAS has been shown to exaggerate HF, the mechanism is still not fully understood. The present study demonstrates that AT1-R upregulation contributes to the synthesis of PIC in the PVN region in HF, and that ICV administration of LOS reduces the levels of PIC in the PVN and in the plasma, suggesting that brain RAS influences the synthesis of PIC in the central nervous systems in HF.
A growing body of evidence suggests that immune-mediated mechanisms play an important role in the pathogenesis of HF. Plasma levels of cytokines such as TNF-α, IL-1β, and IL-6 are increased in HF patients and their levels increase with the severity of HF.7,25 Presently, these blood-borne PIC are considered as spillover from myocardial tissues in the early stages of HF. Subsequently, these cytokines stimulate their own production by both lymphoid and non-lymphoid tissues. Recent findings from our lab show that cardiac spinal afferent nerves may serve as a potential source of production of brain cytokines.19 Whatever the source of PIC, an overload of PIC in the brain can induce sympathoexcitation, downregulate cardiac function, and contribute to the pathophysiology of cardiovascular diseases.9
With the development of cell biology, NF-κB is now considered a major nuclear factor regulating the expression of PIC in signal transduction pathways. Functional NF-κB complexes are present in essentially all cell types in the nervous system. Activated NF-κB is the major regulator facilitating the synthesis of several different injury-responsive cytokines in neurons. These cytokines include TNF-α, IL-6, and AT1-R, a component of the RAS.11 Our previous study showed an elevated level of NF-κB p50 in the PVN neurons of HF rats.12 In this study, ICV treatment with SN50 (a selective inhibitor of NF-κB) reduced PIC, AT1-R, and NF-κB p50 in the PVN of HF rats, indicating that NF-κB is involved in regulating the production of PIC and AT1-R. In addition, ICV treatment with LOS also decreased PIC, AT1-R, and NF-κB p50 in the PVN of HF rats, suggesting that NF-κB might mediate the cross-talk between RAS and PIC in the PVN of HF rats. The present study also demonstrates that ICV treatment with SN50 decreased plasma PIC (TNF-α, IL-1β, and IL-6), ANG II, and NE (a marker of sympathetic activity) in HF. Since there is no direct administration of SN50 in peripheral tissues, we considered the reduction of plasma PIC and ANG II a direct result of decreases in both volume and pressure overload induced by sympathetic activity in the cardiovascular system; we further conclude that the reduction of plasma PIC and ANG II is likely to be an indirect consequence of brain NF-κB inhibition.
Although increased levels of PIC were found in the brains of HF rats and were considered as contributors to exaggerated sympathetic activity,19,26 the mechanism by which PIC exert this effect is still unclear. HF is characterized by augmented oxidative stress, which results in sympathoexcitation.17 NF-κB can be activated by NAD(P)H oxidase-dependent oxidative stress27,28 which serves as a significant source of intracellular reactive oxygen species (ROS) in many tissues.29 NAD(P)H oxidase, a complex enzyme consisting of two membrane-bound components (gp91phox and p22phox) and three cytosolic components (p67phox, p47phox, and p40phox),30 can be activated by PIC and ANG II in peripheral tissues. Interestingly, cytoplasmic and membrane-associated NAD(P)H oxidase proteins have been found throughout the neuraxis, indicating that NAD(P)H oxidase-dependent ROS are also generated within the nervous system. We have recently shown that cytokines induce an increase in ROS in the PVN of normal rats,31 and that cytokines produced in the PVN augmented the expression levels of gp91phox and its homologue, Nox1, thereby contributing to sympathoexcitation in HF rats. In the present study, we further demonstrate that treatment with LOS or SN50 downregulated the protein expression of the NAD(P)H oxidase subunit gp91phox and ROS (DHE staining) in the PVN of HF rats. This finding implicates NF-κB as a potential mediator in the upregulation of superoxide in the PVN of HF rats. At the same time, TEMP, a superoxide scavenger administered in this study, decreased NF-κB p50 in the PVN and plasma NE in HF rats, indicating that ROS activated by PIC or ANG II contribute to activation of NF-κB p50 in the PVN and facilitate the effects of NF-κB on neurohumoral excitation.
In summary, the present study indicates that treatment of HF rats with losartan prevents the increases of PIC, NF-κB, and superoxide in the PVN. The NF-κB inhibitor SN50 effectively blocks the upregulation of PIC, AT1-R, and superoxide in HF rats. Thus, our findings suggest that superoxide-induced NF-κB might be the link in the cross-talk between RAS and PIC in HF. This increased NF-κB in the PVN induces superoxide and contributes to neurohumoral excitation in HF. Thus, manipulations designed to inhibit superoxide may be effective adjuncts to the current treatment of neurohumoral excitation in HF.
Conflict of interest: none declared.
Funding
This work was supported by National Institutes of Health (NIH) Grant RO1-HL-080544-01 (PI: J. Francis) and National Natural Science Foundation of China (No. 30770867).
References
- 1.Dampney RA, Fontes MA, Hirooka Y, Horiuchi J, Potts PD, Tagawa T. Role of angiotensin II receptors in the regulation of vasomotor neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol. 2002;29:467–472. doi: 10.1046/j.1440-1681.2002.03658.x. [DOI] [PubMed] [Google Scholar]
- 2.Zhu GQ, Gao L, Li Y, Patel KP, Zucker IH, Wang W. AT1 receptor mRNA antisense normalizes enhanced cardiac sympathetic afferent reflex in rats with chronic heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1828–H1835. doi: 10.1152/ajpheart.01245.2003. [DOI] [PubMed] [Google Scholar]
- 3.Francis J, Wei SG, Weiss RM, Felder RB. Brain angiotensin-converting enzyme activity and autonomic regulation in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H2138–H2146. doi: 10.1152/ajpheart.00112.2004. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Huang BS, Ganten D, Leenen FH. Prevention of sympathetic and cardiac dysfunction after myocardial infarction in transgenic rats deficient in brain angiotensinogen. Circ Res. 2004;94:843–849. doi: 10.1161/01.res.0000120864.21172.5a. [DOI] [PubMed] [Google Scholar]
- 5.Zhang ZH, Wei SG, Francis J, Felder RB. Cardiovascular and renal sympathetic activation by blood-borne TNF in rat: the role of central prostaglandins. Am J Physiol Regul Integr Comp Physiol. 2003;284:R916–R927. doi: 10.1152/ajpregu.00406.2002. [DOI] [PubMed] [Google Scholar]
- 6.Dibbs Z, Kurrelmeyer K, Kalra D, Seta Y, Wang F, Bozkurt B, et al. Cytokines in heart failure: pathogenetic mechanisms and potential treatment. Proc Assoc Am Physicians. 1999;111:423–428. doi: 10.1111/paa.1999.111.5.423. [DOI] [PubMed] [Google Scholar]
- 7.Francis J, Weiss RM, Johnson AK, Felder RB. Central mineralocorticoid receptor blockade decreases plasma TNF-alpha after coronary artery ligation in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R328–R335. doi: 10.1152/ajpregu.00376.2002. [DOI] [PubMed] [Google Scholar]
- 8.Yu Y, Kang YM, Zhang ZH, Wei SG, Chu Y, Weiss RM, et al. Increased cyclooxygenase-2 expression in hypothalamic paraventricular nucleus in rats with heart failure: role of nuclear factor kappa B. Hypertension. 2007;49:511–518. doi: 10.1161/01.HYP.0000257356.20527.c5. [DOI] [PubMed] [Google Scholar]
- 9.Kang YM, Zhang ZH, Johnson RF, Yu Y, Beltz T, Johnson AK, et al. Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circ Res. 2006;99:758–766. doi: 10.1161/01.RES.0000244092.95152.86. [DOI] [PubMed] [Google Scholar]
- 10.La Rosa G, Cardali S, Genovese T, Conti A, Di Paola R, La Torre D, et al. Inhibition of the nuclear factor-kappaB activation with pyrrolidine dithiocarbamate attenuating inflammation and oxidative stress after experimental spinal cord trauma in rats. J Neurosurg Spine. 2004;1:311–321. doi: 10.3171/spi.2004.1.3.0311. [DOI] [PubMed] [Google Scholar]
- 11.Cowling RT, Gurantz D, Peng J, Dillmann WH, Greenberg BH. Transcription factor NF-kappa B is necessary for up-regulation of type 1 angiotensin II receptor mRNA in rat cardiac fibroblasts treated with tumor necrosis factor-alpha or interleukin-1 beta. J Biol Chem. 2002;277:5719–5724. doi: 10.1074/jbc.M107515200. [DOI] [PubMed] [Google Scholar]
- 12.Kang YM, Zhang ZH, Johnson RF, Weiss RM, Beltz TG, Johnson AK, et al. Oxidative stress mediates activation of NF-κB and upregulation of brain renin-angiotensin system (RAS) in ischemia-induced heart failure. Circulation. 2005;112:II-80. [Google Scholar]
- 13.Gregg D, Rauscher FM, Goldschmidt–Clermont PJ. Rac regulates cardiovascular superoxide through diverse molecular interactions: more than a binary GTP switch. Am J Physiol Cell Physiol. 2003;285:C723–C734. doi: 10.1152/ajpcell.00230.2003. [DOI] [PubMed] [Google Scholar]
- 14.Bonizzi G, Piette J, Schoonbroodt S, Greimers R, Havard L, Merville MP, et al. Reactive oxygen intermediate-dependent NF-kappaB activation by interleukin-1beta requires 5-lipoxygenase or NADPH oxidase activity. Mol Cell Biol. 1999;19:1950–1960. doi: 10.1128/mcb.19.3.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- 16.Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Gao L, Wang W, Liu D, Zucker IH. Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic heart failure. Circulation. 2007;115:3095–3102. doi: 10.1161/CIRCULATIONAHA.106.677989. [DOI] [PubMed] [Google Scholar]
- 18.Francis J, Weiss RM, Wei SG, Johnson AK, Beltz TG, Zimmerman K, et al. Central mineralocorticoid receptor blockade improves volume regulation and reduces sympathetic drive in heart failure. Am J Physiol Heart Circ Physiol. 2001;281:H2241–H2251. doi: 10.1152/ajpheart.2001.281.5.H2241. [DOI] [PubMed] [Google Scholar]
- 19.Francis J, Zhang ZH, Weiss RM, Felder RB. Neural regulation of the proinflammatory cytokine response to acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2004;287:H791–H797. doi: 10.1152/ajpheart.00099.2004. [DOI] [PubMed] [Google Scholar]
- 20.Huang J, Weiss ML. Characterization of the central cell groups regulating the kidney in the rat. Brain Res. 1999;845:77–91. doi: 10.1016/s0006-8993(99)01937-x. [DOI] [PubMed] [Google Scholar]
- 21.Miller FJ, Jr, Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. 1998;82:1298–1305. doi: 10.1161/01.res.82.12.1298. [DOI] [PubMed] [Google Scholar]
- 22.Zheng H, Li YF, Cornish KG, Zucker IH, Patel KP. Exercise training improves endogenous nitricoxide mechanisms within the paraventricular nuclei in rats with heart failure. Am J Physiol Heart Circ Physiol. 2005;288:H2332–H2341. doi: 10.1152/ajpheart.00473.2004. [DOI] [PubMed] [Google Scholar]
- 23.Tan J, Wang H, Leenen FH. Increases in brain and cardiac AT1 receptor and ACE densities after myocardial infarct in rats. Am J Physiol Heart Circ Physiol. 2004;286:H1665–H1671. doi: 10.1152/ajpheart.00858.2003. [DOI] [PubMed] [Google Scholar]
- 24.DiBona GF, Jones SY, Brooks VL. ANG II receptor blockade and arterial baroreflex regulation of renal nerve activity in cardiac failure. Am J Physiol Regul Integr Comp Physiol. 1995;269:R1189–R1196. doi: 10.1152/ajpregu.1995.269.5.R1189. [DOI] [PubMed] [Google Scholar]
- 25.Nozaki N, Yamaguchi S, Shirakabe M, Nakamura H, Tomoike H. Soluble tumor necrosis factor receptors are elevated in relation to severity of congestive heart failure. Jpn Circ J. 1997;61:657–664. doi: 10.1253/jcj.61.657. [DOI] [PubMed] [Google Scholar]
- 26.Francis J, Chu Y, Johnson AK, Weiss RM, Felder RB. Acute myocardial infarction induces hypothalamic cytokine synthesis. Am J Physiol Heart Circ Physiol. 2004;286:H2264–H2271. doi: 10.1152/ajpheart.01072.2003. [DOI] [PubMed] [Google Scholar]
- 27.Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996;10:709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- 28.Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 29.Li YL, Gao L, Zucker IH, Schultz HD. NADPH oxidase-derived superoxide anion mediates angiotensin II-enhanced carotid body chemoreceptor sensitivity in heart failure rabbits. Cardiovasc Res. 2007;75:546–554. doi: 10.1016/j.cardiores.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deleo FR, Quinn MT. Assembly of the phagocyte NADPH oxidase: molecular interaction of oxidase proteins. J Leukoc Biol. 1996;60:677–691. doi: 10.1002/jlb.60.6.677. [DOI] [PubMed] [Google Scholar]
- 31.Mariappan N, Soorappan RN, Haque M, Sriramula S, Francis J. TNF-alpha-induced mitochondrial oxidative stress and cardiac dysfunction: restoration by superoxide dismutase mimetic Tempol. Am J Physiol Heart Circ Physiol. 2007;293:H2726–H2737. doi: 10.1152/ajpheart.00376.2007. [DOI] [PubMed] [Google Scholar]