Abstract
18 F-fluorodeoxyglucose positron emission tomography (FDG-PET) is a useful technique to characterize the solitary pulmonary nodule, diagnose primary lung cancer, carry out mediastinal and extrathoracic staging, plan radiotherapy, therapeutic response assessment and detect recurrence. PET may help to determine the ideal site for tissue diagnosis as well as predict prognosis. Combined PET and computed tomography (PET / CT) has the best of both worlds of metabolic and anatomic imaging and may provide optimal disease assessment.
Keywords: Lung cancer, positron emission tomography (PET), PET/computed tomography, tumor staging, therapy response
Imaging plays a vital role in the diagnosis, staging, therapeutic assessment and follow-up of patients with lung cancer. In the past decade, FDG-PET has become a major adjunct to structural imaging techniques because it adds a biological dimension by the study of glucose metabolism in tissues. The development of integrated positron emission tomography and computed tomography (PET/CT) scanning has made it possible to acquire both morphological and functional information of the entire body in a single examination.[1] This article provides a brief history of PET and PET / CT imaging, a review of the current PET literature pertaining to lung cancer and gives specific recommendations for its use.
PET and PET/CT
Whole-body PET scanners provide the unique ability to quantitate metabolic processes in vivo. Tumors exhibit an accelerated glycolysis allowing 18F-fluorodeoxyglucose (FDG, an analog of glucose) to be trapped in tissues with an elevated metabolic rate compared with normal tissues.[2,3] Although PET images show functional information, they provide limited anatomical data, which in regions such as the head and neck, mediastinum and the pelvic cavity, is a significant drawback. The exact localization of lesions may also be difficult in some cases on the basis of PET images alone.[3–5] In the last 2 years, PET imaging in oncology has been migrating from the use of dedicated PET scanners to the use of PET / CT tomographs.[4]
Researchers realized early on that it would be ideal if the morphological information from CT and the functional information from PET were combined.[6,7] Software registration techniques and providing fusion images for the separate modalities provided more accurate localization than that provided by visual coregistration. However, erroneous registration resulted in several problems. Especially in the fusion of body images, deformable organs caused various errors. Time differences in imaging created other problems. For example, the contours of the abdomen are dependent on the pallet design; also, gastrointestinal organs move over time. This made accurate registration extremely difficult.
PET/CT was designed to provide the solution to these shortcomings.[7] In a PET/CT scanner, the PET and CT tomographs are housed in a single gantry with a single patient bed and workstation. PET/CT scanners can also be used either as a dedicated PET scanner or as a dedicated CT scanner. Upon reconstruction, both the PET images and the CT images are displayed side by side and overlaid (fused).[5] Use of the CT scan reduces the total PET acquisition time as well as enables improved accuracy and precision attenuation correction of the emission images. In addition, the CT scan gives a more precise localization and interpretation of the hypermetabolic lesions, thanks to the availability of anatomical landmarks.[3,5]
PET/CT solves the problems caused by body contour deformability and time difference, but does not routinely correct for breathing artifacts or inadvertent positioning changes between the two scans. PET/CT has been shown to reduce the false-positive interpretation of physiological uptake such as brown fat, muscle and colon uptake.[7–11] Multiple clinical trials revealed the superiority of a PET/CT image over either modality alone.[7] Clinical FDG studies are generally analyzed using qualitative (visual) and semi-quantitative data. A standardized uptake value (SUV) is a semi-quantitative index of glucose utilization that is obtained by normalizing the accumulation in the abnormal lesion to the injected dose and patient body weight. So SUV is calculated using the following formula:
SUV = mean lesion activity/[injected dose/body weight(g)]. Perhaps calculation of SUVmax using the pixel with greatest amount of radioactivity in the image of lesion instead of mean activity of several pixels of the lesion would be more appropriate.[12] Please explain what a pixel is here. Also this statement is confusing-do you mean using the most active pixel for calculation of SUV or just using the most active pixel to determine glucose utilization? If so, it would be better to state this.
Pulmonary Nodule
The crucial objective in the evaluation of the solitary pulmonary nodule (SPN) is the ability to noninvasively differentiate benign from malignant lesions in the most cost-effective manner before definitive therapy while minimizing patient morbidity and mortality.[13] FDG-PET has proven to be an accurate, noninvasive method for the management of (please substitute jargon for terms found in literature) patients with SPN.[14] The reported sensitivity and specificity have ranged from 89-100% and 77-100% respectively, for the detection of malignant pulmonary nodules.[13]
In addition to the importance of visual analysis of the PET images,[15,16] semi-quantitative analysis using a threshold SUV of > 2.5 for the diagnosis of malignancy in a pulmonary nodule has often been suggested, despite the fact that quite a few lesions with SUV < 2.5 are malignant.[15,17,18] False-positive results are seen in active granulomatous disease (tuberculoma and histoplasmosis) and certain other inflammatory processes due to increased glycolytic activity within the active macrophages. False-negative results may be seen in tissues with low metabolic activity such as bronchoalveolar carcinoma (BAC), pulmonary carcinoids or when the lesion is < 5-7 mm in diameter.[14]
The negative predictive power of PET is sufficiently high to obviate the necessity of a biopsy.[19] If FDG-PET is negative for lesions ≥ 7 mm in diameter, then the process is most likely benign and may be followed with serial surveillance. If the lesion is < 7 mm in diameter, then malignancy cannot be excluded with a negative PET.[14] When FDG-PET is positive, then diagnostic and definitive treatment may be instituted.[14] As seen from previous studies, most lesions that were found positive by PET were either malignant or required specific active management determined from subsequent histopathological analysis.[19] Another challenging field for PET is its additional value in lung cancer screening studies.
A sensitivity of 90% was seen in one of the first studies in this setting[20] requiring additional PET for nodules ≥ 7 mm. In a more recent study,[21] PET was also added to the screening protocol for nodules ≥ 10 mm or growing nodules ≥ 7 mm. The conclusion was that selected use of PET is useful in screening trials because it may minimize unnecessary invasive procedures for benign lesions. It was correctly concluded that PET can be of help in screening detected nodules but that its role there is more limited than in screening the general population presenting with pulmonary nodules.[22]
Lung Cancer Staging
Because survival is inversely correlated with the stage of the lung cancer, a meticulous staging procedure is required to determine the required treatment and prognosis.[23] CT is frequently unable to discriminate between malignant enlarged mediastinal lymph nodes and those that are enlarged due to benign reactive hyperplasia.[24] In addition, conventional imaging limited to the thorax and upper abdomen is unable to detect more distant metastatic disease which can occur in 9–11% of all patients with non-small cell lung cancer (NSCLC).[24,25] However, FDG-PET has been shown to have greater sensitivity for the detection of metabolically active malignant disease and can lead to changes in initial staging and treatment plans for lung cancer when used in combination with conventional work-up.[26,27] One retrospective study[28] on 198 patients confirmed that the use of PET has an important impact on stage designation and clinical decision-making. PET upstaged 16.2% and downstaged 6.1% of the patients.
Primary Tumor
CT is excellent for determining the location and anatomic size of the primary mass and its relationship to surrounding structures.[24] Compared to CT, “isolated” PET offers little additional information in the tumor (T) characterization of lung cancer due to its lack of spatial resolution and the invisibility of all but the grossest anatomical landmarks.[29] However, one exception is its usefulness in distinguishing between tumor and postobstructive atelectasis.[30] In addition, PET can be beneficial in evaluating the cause of pleural effusions. Gupta et al[31] quote an accuracy rate of 91% for PET in a study of 35 patients with lung cancer and suspected malignant pleural effusion.
However, FDG-PET imaging is of potential use in assessing the metabolic activity of the primary lung cancer, which reflects cell turnover rate and may indicate the biologic aggressiveness of the cancer.[24,32–34] Several studies have shown that the SUV has prognostic value independent of the conventional clinical tumor, node, metastasis (TNM) staging.[35,36] For example, Higashi et al[37] demonstrated that a primary tumor with SUV > 5 was associated with a significant increase in postoperative relapse in early stage lung cancer. Thus, PET imaging in initial T staging by predicting the likelihood of tumor recurrence after treatment, may help in selecting which patients are likely to respond to induction therapy before surgery and which patients should receive adjuvant chemotherapy / radiotherapy.[24,37]
Integrated PET / CT has been shown to be more useful than dedicated PET imaging in determining the T stage of the primary tumor and in assessing the presence of mediastinal or chest wall invasion[38] [Figure 1]. Halpern et al[40] demonstrated an accuracy rate of 97% with PET / CT compared with 67% with PET only. This superiority was attributed entirely to the CT component of the examination. By comparison, another report described accuracy rates for T staging with PET / CT and CT to be 88 and 58%, respectively.[38] The reasons for this surprising finding were not fully explored, but it is worth reiterating that PET can have a role in T staging by distinguishing between tumor and distal atelectasis.[29] It is important to remember that the CT component of PET / CT is acquired without IV contrast in mid-inspiration rather than full inspiration. Therefore, a diagnostic contrast-enhanced CT scan of the chest performed as part of the PET / CT study or independently as a separate scan is still recommended.[24]
Figure 1.
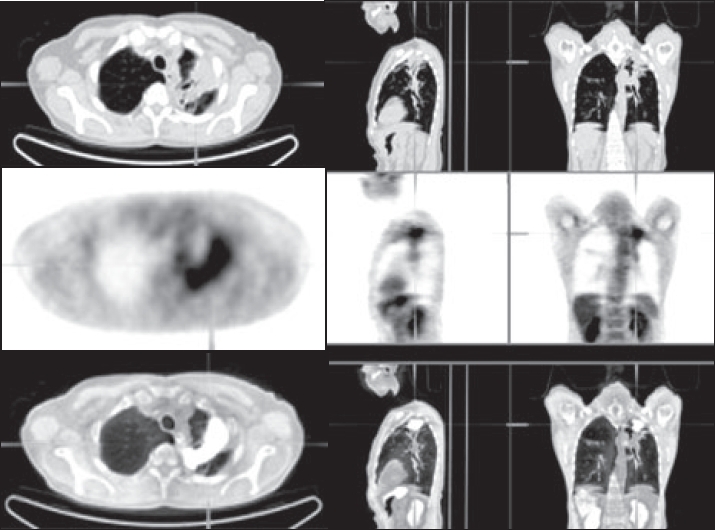
Non-small cell lung cancer. PET/CT images show invading the visceral pleura without chest wall invasion while on the CT alone it would be more difficult to determine the chest wall invasion (T2 N0 M0) (Reprinted with permission of reference).[39]
Nodal Involvement
Mediastinal lymph node staging can be divided into imaging and sampling. Analysis of the pooled receiver operating characteristic (ROC) curves indicated that PET was significantly more accurate than CT or MRI in identifying nodal metastasis[41] with a reported accuracy of 81–96%.[24,41] A meta-analysis of different imaging methods for determining nodal stage reported a sensitivity of 79% and a specificity of 91% for the detection of nodal metastases by PET (vs 60 and 77% respectively, for CT).[41] Overall, there is 20% improvement in accuracy of PET over CT imaging for mediastinal staging of NSCLC.[14] PET / CT has an even higher diagnostic accuracy than either CT or PET alone[38] with a reported sensitivity of 89% and specificity of 94% and an overall diagnostic accuracy of 93%.[24] A very well-designed prospective study[42] compared CT and PET in the diagnosis of mediastinal lymph node metastases in 132 consecutive patients with potentially resectable NSCLC. Negative predicted probability (NPP) was very high at 98%. The conclusion was that both imaging methods are complementary [Figure 2] and that their common strength is their powerful negative predictive value (NPV). Thus, integrated PET / CT may be more helpful than either of these techniques used alone. False-positive PET results in the mediastinum, which affect selection of treatment, have been reported in the range 13–17% and are mainly due to inflammatory lymph nodes (secondary to pneumonia, postobstructive pneumonitis or chronic granulomatous infection) which may lead to mistaken upstaging of the primary tumor.[24,43,44] Conversely, several reviews have found a false-negative rate as high as 8% for the detection of mediastinal metastases by PET imaging.[41,45,46]
Figure 2.
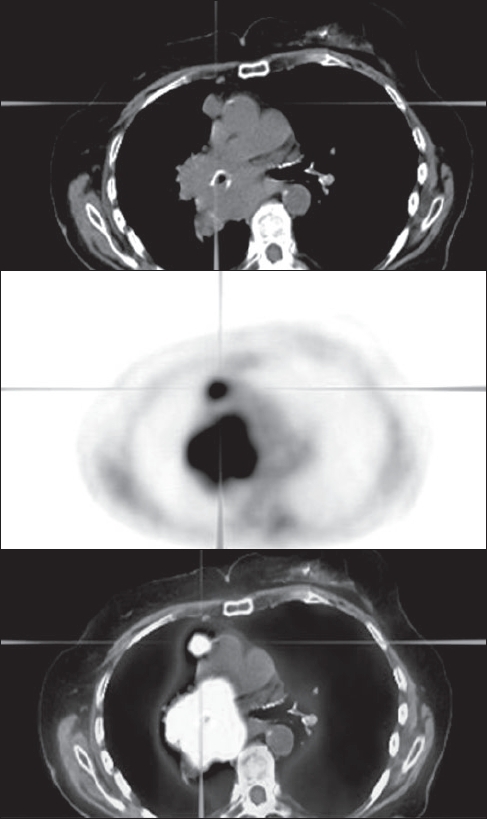
Non-small cell lung cancer. PET/CT images show a mass involving the right hilum with extension into mediastinum without extension to the contralateral mediastinum but a separable focus in the right superior mediastinum (stage IIIA) (Reprinted with permission of reference).[39]
The exact role of PET in the diagnostic algorithm of Node (N) staging has been the subject of much debate. However, staging of the mediastinum should not rely solely on PET and mediastinoscopy has still been advocated. It is suggested by some authors that one of the main values of PET is based on its high NPV for nodal disease, (estimated at > 90% in several studies).[24,25] This finding implies that eligible patients with negative mediastinal nodes on PET examinations may proceed directly to thoracotomy without the need for mediastinoscopy. False-negatives can occur in this group of patients with tumor subsequently being identified upon thoracotomy. These patients are, however, referred to by some as having “minimal N2 disease”, which confers a better prognosis.[29] De Langen and co-workers[47] have made recommendations based on the fact that the prevalence of nodal disease increases with size as seen in CT. They concluded that patients with nodes measuring < 15 mm on CT and a negative PET examination do not require mediastinoscopy, whereas those patients with negative PET but large lymph nodes on CT should nevertheless undergo invasive staging. It is suggested to avoid mediastinoscopy in patients with T1 tumors and negative PET scans.[24] The lower positive predictive value (PPV) makes cytologic or histologic confirmation necessary in case of a positive mediastinum on PET.
In patients with locally advanced but potentially operable tumors based on conventional clinical staging (stages II-IIIA), PET can detect nodal metastases that are inaccessible by cervical mediastinoscopy and that may be missed by conventional staging methods. It can change the work-up of the patient by indicating the need for a different approach to invasive lymph node sampling.[48]
Distant Metastasis
One of the advantages of FDG-PET vs CT is that the whole body can be imaged. Therefore, in addition to staging the mediastinum, PET has shown promise for identifying distant metastases. There are reports of the ability of PET to detect clinically unsuspected distant metastases in 10-29% of patients.[49,50]
Bury et al. found PET to have an accuracy of 96% and bone scanning, 66%, in the evaluation of osseous involvement in patients with NSCLC. Although these tests were very similar in high sensitivity for bone metastasis, PET had a much higher specificity for disease than bone scan.[51] Although adrenal adenomas are readily characterized on unenhanced or enhanced CT, indeterminate adrenal nodules are common and require further evaluation by magnetic resonance imaging (MRI), biopsy or PET scanning. On PET or PET / CT imaging, the finding of FDG uptake within the adrenal gland being greater than that of the liver is a highly sensitive and specific sign of adrenal metastatic disease, with an overall diagnostic accuracy of > 92%.[52]
In the brain and genitourinary system, PET is less accurate in identifying malignancy. The high metabolic activity of the brain and the concentration and excretion of FDG in the genitourinary system make it difficult to differentiate metastatic disease from normal activity. As the brain is a common site for metastatic lung cancer, CT or MRI has been recommended.[14]
Unfortunately, it is frequently difficult to establish the diagnosis of a malignant pleural effusion because cytology of fluid obtained at thoracentesis is only positive for malignancy in 66% of patients and more invasive tests such as pleural biopsy or thoracoscopy may be required for confirmation.[24] PET has promising diagnostic accuracy for the diagnosis of pleural metastases with reported sensitivities of 92-100%, specificities of 67-71%, NPVs of 100% and PPVs of 63-79%.[53,54] Interpretation of the PET findings should take into account the results of pleural fluid analysis and the patient's recent medical history because of false positive uptake of FDG by the pleura secondary to pleural infection or inflammation after talc pleurodesis. However, a negative PET result can be useful by confirming the absence of pleural metastatic disease, particularly when the results of thoracentesis are also negative.[24]
Although PET imaging has a higher overall detection rate for metastatic disease than conventional workup, it is recommended that PET complement rather than replace conventional imaging modalities.[24]
Treatment, Prognosis and Follow-up
Whole-body PET has potential value in treatment planning because it allows physicians to simultaneously assess for regional and metastatic disease. Therefore, PET imaging may result in the alteration of clinical staging and significantly alter management. A multivariate analysis in patients with NSCLC who were treated with either radical radiotherapy or surgery, found that the use of a cutoff of 5 for the SUV of FDG-PET in the primary tumor was the strongest prognostic factor for overall survival.[37] The use of PET clinical staging resulted in a different stage from that determined by standard methods in 62 of 102 patients (60%)-it was lowered in 20 and raised in 42.[25] In the study by Lewis et al., the PET findings resulted in patient management changes in 41% of the cases.[55]
Wolfgang et al[56] in a prospective study demonstrate that in patients with advanced NSCLC, effective chemotherapy causes a rapid reduction in the utilization of glucose by the tumor. After one cycle of platinum-based chemotherapy (21 days), a metabolic response in PET imaging was significantly correlated with the most positive response to this chemotherapy regimen. In patients without a metabolic response, the response rate was only 4%, whereas it was 71% in patients with a metabolic response. For patients with a metabolic response, the 1-year survival rate was 44%, whereas it was only 10% in patients with no metabolic response. A recent prospective study was conducted in 60 patients with stage III NSCLC who underwent neoadjuvant chemoradiotherapy before surgical resection. In these patients, a restaging PET scan 2 weeks after induction therapy was able to predict the pathological response in the primary tumor. This was later confirmed upon subsequent surgery, with a sensitivity of 86% and a specificity of 81%.[57] Another prospective study was conducted in 57 patients with locally advanced NSCLC. In these patients also who underwent restaging PET imaging after only 1 cycle of platinum-based chemotherapy, PET was able to predict the pathological response in the tumor. A fall in SUVmax of ≥ 20% in the primary tumor was an independent predictor of long-term survival in these patients.[58] These findings indicate that PET imaging after the initiation of chemotherapy / radiotherapy can assess the response of the primary tumor to treatment by detecting a reduction in the metabolic activity of the primary mass, which is a favorable prognostic indicator of survival.[24] Nevertheless, FDG-PET may provide a unique means to change the therapy regimen based on PET imaging findings in the early course of therapy.
Several studies reported that FDG-PET has a sensitivity of 98–100% and a specificity of 62–92% for the detection of recurrent malignancy after definitive treatment with surgery, chemotherapy or radiotherapy.[24,59,60] Normalization of FDG uptake after treatment appears to be a sensitive indicator of favorable response and good prognosis.[14] Hebert et al demonstrated that patients with negative PET scans were alive 2 years after treatment and 50% of patients with residual hypermetabolism on PET had died.[61] Sensitivity, specificity and accuracy of PET are high in differentiating recurrent malignancy from benign posttreatment changes in patients studied after therapy. Detection of recurrent disease using radiological changes is made difficult by the often extensive anatomic abnormalities that exist after definitive treatment, such as parenchymal scarring, distortion of normal bronchovascular architecture, pleural thickening and effusions and mediastinal fibrosis.[24] A PET evaluation has been shown to be more[30] useful than conventional imaging for diagnosing tumor recurrence.[12,60,62] Specificity of PET for malignant disease is lower than at initial staging because of the often coexisting inflammation secondary to radiotherapy or chemotherapy, which can appear FDG-avid on PET scans.[24] Diagnostic difficulties over the presence or absence of recurrent cancer most frequently arise after radiotherapy, in particular after three-dimensional (3D) conformal radiotherapy, which causes low-grade inflammation in the treated lung[24,63] [Figure 3]. On PET scans, areas of radiation pneumonitis characteristically appear as diffuse areas of mild to moderate FDG uptake that conform to the region of irradiated lung.[64] However, tumor recurrence is suspected where a focal site of more intense metabolic activity is seen. It is advisable to wait for a period of 3–6 months after the end of treatment before performing surveillance PET scans. Findings which arouse suspicion for tumor recurrence on a posttreatment PET scan should be confirmed by histological or cytological evidence to avoid diagnostic errors.[24]
Figure 3.
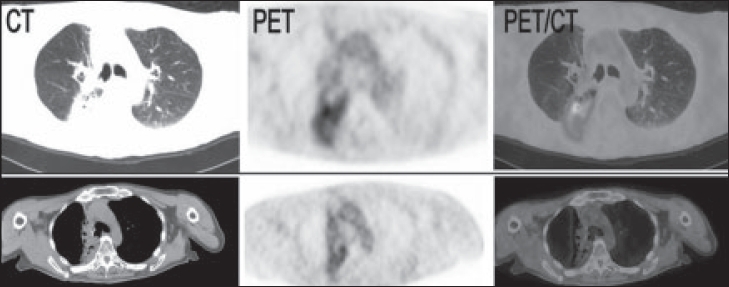
Top images: PET/CT abnormalities secondary to radiation pneumonitis. Bottom images: Resolving radiation changes 5 months later (Reprinted with permission of reference).[39]
Radiotherapy Planning
It is the radiotherapy oncologist's goal to optimize the beneficial effects of radiation therapy while at the same time, limiting the dose to normal surrounding tissue. FDG-PET can also assist in radiation therapy planning by focusing radiation ports to precise areas of tumor activity, preventing irradiation of uninvolved areas and omission of regions of active tumor from radiation ports.[12] Accurate identification of nodal metastases is crucial for planning curative radiotherapy, particularly as routine elective nodal irradiation is no longer recommend in NSCLC.[65] Different meta-analyses have shown FDG-PET to be superior to conventional mediastinal staging using CT scans and esophageal ultrasound.[41,44] A prospective clinical trial using this approach reported isolated nodal failures in only 1 of 44 patients.[66] Vander Wal et al studied whether fusion PET/CT-based radiotherapy planning could improve the therapeutic ratio in 21 patients with clinical N2-N3 NSCLC. Compared with 3D CT-based planning the gross tumor volume of the nodes decreased from 13.7 to 9.9 cm3 on PET/CT planning (P = 0.011). The delivered dose could be increased from 56.0 Gy with CT to 71 Gy with PET / CT planning (P = 0.038), leading to better tumor control probability for a similar toxicity risk for lung, esophagus and spinal cord.[67] Due to false positive results, PET findings that can have a major impact on treatment policy should ideally be confirmed by histology.[68]
In several studies, incorporation of PET / CT imaging into treatment planning resulted in an alteration of the initial radiotherapy plan in over 50% of patients with NSCLC, compared with the use of CT alone. This was possible because of the better differentiation of the metabolically active tumor from adjacent atelectasis and by increased sensitivity for nodal metastatic disease.[24]
One group used the term ‘anatomic biologic contour’ to express the potential advantages of using PET/CT fusion radiotherapy planning. Compared with contouring treatment volumes based on CT alone, PET / CT resulted in clinically significant (> 25%) treatment volume changes in 10/19 patients and better concordance in treatment planning with different observers.[69]
Several complexities, both in fused imaging and in new radiation therapy techniques, (such as 3D conformal radiation therapy) have meant that there is a greater need for interaction between radiologists, radiation oncologists and radiation therapy physicists when planning radiotherapy for NSCLC. Radiologists should always be available for consultation when radiation treatment plans are being formulated. However, data regarding radiotherapy planning and PET/CT are still sparse and several issues remain to be solved before PET / CT is routinely used for radiotherapy planning.
Cost-effectiveness
The general consensus is that PET can reduce needless thoracotomy rates.[29] These benefits can be quantified by calculating the incremental cost-effectiveness ratio (ICER), which may be measured in monetary terms per life year saved (LYS) or per quality adjusted life years (QALY). Gambhir et al used a decision analysis model to compare the cost-effectiveness of four strategies for the diagnosis and management of solitary pulmonary nodules. CT-plus-PET was the most cost-effective strategy when an intermediate pretest likelihood of 12–69% was present. In addition, a CT-plus-PET strategy over CT alone yielded cost savings of $91–2200 per patient.[70] Scott et al findings supported the use of thoracic PET as an adjunct to thoracic CT for preoperative staging. Furthermore, several different CT-plus-PET strategies resulted in a greater life expectancy than the CT-only strategy.[71] A recent French study used a decision tree analysis model to compare various strategies including CT only, PET for patients with negative CT and PET plus CT.[72] They concluded that employing a combination of PET and CT was the most cost-effective resulting in an ICER of -576 euros / LYS (i.e., a cost saving per life year saved). Analyses from other countries have reported similar cost savings by using PET and selective mediastinoscopy.[29,73] With PET / CT, it can be anticipated that examination costs may rise due to more expensive machinery, though this may be offset in part by improvements in staging accuracy and examination times.
Conclusion
FDG-PET has been approved by the Health Care Finance Administration for Medicare reimbursement for diagnosing, staging and restaging lung cancer. FDG-PET and PET / CT provide a noninvasive and cost-effective strategic approach to patient selection for interventional and therapeutic procedures without contributing to increased morbidity. FDG-PET is the recommended test for evaluation of the solitary pulmonary nodule which can be as small as 7 mm, mediastinal and extrathoracic staging excluding the brain, evaluation of therapy response and restaging following treatment.[14] PET / CT has the best of both worlds of metabolic and anatomic imaging and may likely be the first choice in lung cancer imaging of the future.[14] Currently available data on PET / CT suggests that its superiority to alone PET lies principally in better T staging, but it also provides tangible benefits for N and M staging. Also PET / CT is useful in prediction of prognosis, follow-up of patients, radiotherapy planning, facilitating image-guided biopsy for definitive diagnosis as well as differentiating viable tumor from adjacent or necrotic tissue and tumor recurrence from residual scar. The clinical applications of PET / CT are still evolving and future research will determine the precise role that metabolic imaging has to play in the management of patients with lung cancer.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Vansteenkiste JF, Stroobants SG. The role of positron emission tomography with 18F-fluoro-2-deoxy-D-glucose in respiratory oncology. Eur Respir J. 2001;17:802–20. doi: 10.1183/09031936.01.17408020. [DOI] [PubMed] [Google Scholar]
- 2.Gambhir SS, Czernin J, Schwimmer J, Silverman DH, Coleman RE, Phelps ME. A tabulated summary of the FDG PET literature. J Nucl Med. 2001;42:1S–93S. [PubMed] [Google Scholar]
- 3.Fanti S, Franchi R, Battista G, Monetti N, Canini R. PET and PET-CT. State of the art and future prospects. Radiol Med (Torino. 2005;110:1–15. [PubMed] [Google Scholar]
- 4.Czernin J, Schelbert H. PET/CT imaging: Facts, opinions, hopes and questions. J Nucl Med. 2004;45:1S. [Google Scholar]
- 5.Sureshbabu W, Mawlawi O. PET/CT imaging artifacts. J Nucl Med Technol. 2005;33:156–61. [PubMed] [Google Scholar]
- 6.Barra V, Boire JV. A general framework for the fusion of anatomical and functional medical images. Neuroimage. 2001;13:410–24. doi: 10.1006/nimg.2000.0707. [DOI] [PubMed] [Google Scholar]
- 7.Tsukamoto E, Ochi S. PET/CT today: System and its impact on cancer diagnosis. Ann Nucl Med. 2006;20:255–67. doi: 10.1007/BF02984642. [DOI] [PubMed] [Google Scholar]
- 8.Rosenbaum SJ, Lind T, Antoch G, Bockisch A. False-positive FDG PET uptake-the Role of PET/CT. Eur Radiol. 2006;16:1054–65. doi: 10.1007/s00330-005-0088-y. [DOI] [PubMed] [Google Scholar]
- 9.Kostakoglu L, Hardoff R, Mirtcheva R, Goldsmith SJ. PETCT fusion imaging in differentiating physiologic from pathologic FDG uptake. Radiographics. 2004;24:1411–31. doi: 10.1148/rg.245035725. [DOI] [PubMed] [Google Scholar]
- 10.Minotti AJ, Shah L, Keller K. Positron emission tomography/computed tomography fusion imaging in brown adipose tissue. Clin Nucl Med. 2004;29:5–11. doi: 10.1097/01.rlu.0000102761.90104.c6. [DOI] [PubMed] [Google Scholar]
- 11.Yeung HW, Grewal RK, Gonen M, Schoder H, Larson SM. Patterns of (18)F-FDG uptake in adipose tissue and muscle: A potential source of false-positives for PET. J Nucl Med. 2003;44:1789–96. [PubMed] [Google Scholar]
- 12.Low VJ, Delbeke D, Coleman RE. Applications of PET in oncologic imaging. In: Sandler MP, Coleman RE, Patton JA, Wackers FJ, Gottschalk A, editors. Diagnostic nuclear medicine. 4th ed. Philadelphia: Lippincott Williams and Wilkins; 2003. pp. 987–1014. [Google Scholar]
- 13.Sarinas PS, Chitkara RK, Buadu EO, Gould MK, Kuschner WG, Segall GM. Usefulness of positron emission tomography imaging in the management of lung cancer. Curr Opin Pulm Med. 1999;5:201–7. doi: 10.1097/00063198-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Sarinas PS, Chitkara RK. PET and SPECT in the management of lung cancer. Curr Opin Pulm Med. 2002;8:257–64. doi: 10.1097/00063198-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Nomori H, Watanabe K, Ohtsuka T, Naruke T, Suemasuk K, Uno k. Visual and semiquantitative analyses for F-18 fluorodeoxyglucose PET scanning in pulmonary nodules 1 cm to 3 cm in size. Ann Thorac Surg. 2005;79:984–9. doi: 10.1016/j.athoracsur.2004.07.072. [DOI] [PubMed] [Google Scholar]
- 16.Herder GJ, Van Tinteren H, Golding RP, Kostense PJ, Comans EF, Smit EF, et al. Clinical prediction model to characterize pulmonary nodules: Validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest. 2005;128:2490–6. doi: 10.1378/chest.128.4.2490. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto Y, Tsujikawa T, Kondo C, Maki M, Momose M, Nagai A, et al. Accuracy of PET for diagnosis of solid pulmonary lesions with 18F-FDG uptake below the standardized uptake value of 2.5. J Nucl Med. 2006;47:426–31. [PubMed] [Google Scholar]
- 18.Bryant AS, Cerfolio RJ. The maximum standardized uptake values on integrated FDG-PET/CT is useful in differentiating benign from malignant pulmonary nodules. Ann Thorac Surg. 2006;82:1016–20. doi: 10.1016/j.athoracsur.2006.03.095. [DOI] [PubMed] [Google Scholar]
- 19.Pitman AG, Hicks RJ, Kalff V, Binns DS, Ware RE, McKenzie AF, et al. Positron emission tomography in pulmonary masses where tissue diagnosis is unhelpful or not possible. Med J Aust. 2001;175:303–7. doi: 10.5694/j.1326-5377.2001.tb143587.x. [DOI] [PubMed] [Google Scholar]
- 20.Pastorino U, Bellomi M, Landoni C, De Fiori E, Arnaldi P, Picchio M, et al. Early lung-cancer detection with spiral CT and positron emission tomography in heavy smokers: 2-year results. Lancet. 2003;362:593–7. doi: 10.1016/S0140-6736(03)14188-8. [DOI] [PubMed] [Google Scholar]
- 21.Bastarrika G, Garcia-Velloso MJ, Lozano MD, Montes U, Torre W, Spiteri N, et al. Early lung cancer detection using spiral computed tomography and positron emission tomography. Am J Respir Crit Care Med. 2005;171:1378–83. doi: 10.1164/rccm.200411-1479OC. [DOI] [PubMed] [Google Scholar]
- 22.Vansteenkiste J, Dooms C. Positron emission tomography in non-small cell lung cancer. Curr Opin Oncol. 2007;19:78–83. doi: 10.1097/CCO.0b013e328013cd00. [DOI] [PubMed] [Google Scholar]
- 23.Mountain CF. The international system for staging lung cancer. Semin Surg Oncol. 2000;18:106–15. doi: 10.1002/(sici)1098-2388(200003)18:2<106::aid-ssu4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 24.Bruzzi JF, Munden RF. PET/CT imaging of lung cancer. J Thorac Imaging. 2006;21:123–36. doi: 10.1097/00005382-200605000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Pieterman RM, van Putten JW, Meuzelaar JJ, Mooyaart EL, Vaalburg W, Koeter GH, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med. 2000;343:254–61. doi: 10.1056/NEJM200007273430404. [DOI] [PubMed] [Google Scholar]
- 26.Herder GJ, Kramer H, Hoekstra OS, Smit EF, Pruim J, van Tinteren H, et al. Traditional versus up-front [18F] fluorodeoxyglucose-positron emission tomography staging of non-small-cell lung cancer: A Dutch cooperative randomized study. J Clin Oncol. 2006;24:1800–6. doi: 10.1200/JCO.2005.02.4695. [DOI] [PubMed] [Google Scholar]
- 27.Lardinois D, Weder W, Roudas M, von schulthess GK, Tutic M, Moch H, et al. Etiology of solitary extrapulmonary positron emission tomography and computed tomography findings in patients with lung cancer. J Clin Oncol. 2005;23:6846–53. doi: 10.1200/JCO.2005.10.116. [DOI] [PubMed] [Google Scholar]
- 28.Sachs S, Bilfinger TV. The impact of positron emission tomography on clinical decision making in a university-based multidisciplinary lung cancer practice. Chest. 2005;128:698–703. doi: 10.1378/chest.128.2.698. [DOI] [PubMed] [Google Scholar]
- 29.Devaraj A, Cook GJ, Hansell DM. PET/CT in non-small cell lung cancer staging-promises and problems. Clin Radiol. 2007;62:97–108. doi: 10.1016/j.crad.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Lavrenov K, Partridge M, Cook G, Brada M. Positron emission tomography for target volume definition in the treatment of non-small cell lung cancer. Radiother Oncol. 2005;77:1–4. doi: 10.1016/j.radonc.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Gupta NC, Rogers JS, Graeber GM, Graeber GM, Gregory JL, Waheed U, et al. Clinical role of F-18 fluorodeoxyglucose positron emission tomography imaging in patients with lung cancer and suspected malignant pleural effusion. Chest. 2002;122:1918–24. doi: 10.1378/chest.122.6.1918. [DOI] [PubMed] [Google Scholar]
- 32.Vesselle H, Schmidt RA, Pugsley JM, Li M, Kohlmyer SG, Vallires E, et al. Lung cancer proliferation correlates with [F-18]fluorodeoxyglucose uptake by positron emission tomography. Clin Cancer Res. 2000;6:3837–44. [PubMed] [Google Scholar]
- 33.Higashi K, Ito K, Hiramatsu Y, Ishikawa T, Sakuma T, Mastunari I, et al. 18F-FDG uptake by primary tumor as a predictor of intratumoral lymphatic vessel invasion and lymph node involvement in non-small cell lung cancer: analysis of a multicenter study. J Nucl Med. 2005;46:267–73. [PubMed] [Google Scholar]
- 34.Nomori H, Watanabe K, Ohtsuka T, Naruke T, Suemasa K, Kobayashi T, et al. Fluorine 18-tagged fluorodeoxyglucose positron emission tomographic scanning to predict lymph node metastasis, invasiveness or both, in clinical T1 N0 M0 lung adenocarcinoma. J Thorac Cardiovasc Surg. 2004;128:396–401. doi: 10.1016/j.jtcvs.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 35.Jeong HJ, Min JJ, Park JM, Chung JK, Kim BT, Jeong JM, et al. Determination of the prognostic value of [(18)F]fluorodeoxyglucose uptake by using positron emission tomography in patients with non-small cell lung cancer. Nucl Med Commun. 2002;23:865–70. doi: 10.1097/00006231-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Dunagan D, Chin R, Jr, McCain T, Case L, Harkness B, Oaks T, et al. Staging by positron emission tomography predicts survival in patients with non-small cell lung cancer. Chest. 2001;119:333–9. doi: 10.1378/chest.119.2.333. [DOI] [PubMed] [Google Scholar]
- 37.Higashi K, Ueda Y, Arisaka Y, Sakuma T, Nambu Y, Oguchi M, et al. 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med. 2002;43:39–45. [PubMed] [Google Scholar]
- 38.Lardinois D, Weder W, Hany TF, Kamel EM, Korom S, Seifert B, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500–7. doi: 10.1056/NEJMoa022136. [DOI] [PubMed] [Google Scholar]
- 39.Rajadhyaksha CD, Bader DA, Barbaras L, Parker JA. 18FDG-PETCT in lung cancer. An interactive web based image atlas. Joint Program Nucl Med. Available from: http://wwwjpnmorg/ [Google Scholar]
- 40.Halpern BS, Schiepers C, Weber WA, Crawford TL, Fueger BJ, Phelps ME, et al. Presurgical staging of non-small cell lung cancer: Positron emission tomography, integrated positron emission tomography/CT and software image fusion. Chest. 2005;128:2289–97. doi: 10.1378/chest.128.4.2289. [DOI] [PubMed] [Google Scholar]
- 41.Dwamena BA, Sonnad SS, Angobaldo JO, Wahl RL. Metastases from non-small cell lung cancer: Mediastinal staging in the 1990s-metaanalytic comparison of PET and CT. Radiology. 1999;213:530–6. doi: 10.1148/radiology.213.2.r99nv46530. [DOI] [PubMed] [Google Scholar]
- 42.Pozo-Rodriguez F, Martin de Nicolas JL, Sanchez-Nistal MA, Maldonado A, Garcia de Barajas S, Calero-Garcia R, et al. Accuracy of helical computed tomography and [18F]fluorodeoxyglucose positron emission tomography for identifying lymph node mediastinal metastases in potentially resectable non-small-cell lung cancer. J Clin Oncol. 2005;23:8348–56. doi: 10.1200/JCO.2004.00.6361. [DOI] [PubMed] [Google Scholar]
- 43.Gupta NC, Tamim WJ, Graeber GG, Bishop HA, Hobbs GR. Mediastinal lymph node sampling following positron emission tomography with fluorodeoxyglucose imaging in lung cancer staging. Chest. 2001;120:521–7. doi: 10.1378/chest.120.2.521. [DOI] [PubMed] [Google Scholar]
- 44.Gould MK, Kuschner WG, Rydzak CE, Maclean CC, Demas AN, Shigemitsu H, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: A meta-analysis. Ann Intern Med. 2003;139:879–92. doi: 10.7326/0003-4819-139-11-200311180-00013. [DOI] [PubMed] [Google Scholar]
- 45.Silvestri GA, Tanoue LT, Margolis ML, Barker J, Detterbeck F. American College of Chest Physicians. The noninvasive staging of non-small cell lung cancer: The guidelines. Chest. 2003;123:147S–56S. doi: 10.1378/chest.123.1_suppl.147s. [DOI] [PubMed] [Google Scholar]
- 46.Dietlein M, Weber K, Gandjour A, Moka D, Theissen P, Lauterback KW, et al. Cost-effectiveness of FDG-PET for the management of potentially operable non-small cell lung cancer: Priority for a PET-based strategy after nodal-negative CT results. Eur J Nucl Med. 2000;27:1598–609. doi: 10.1007/s002590000376. [DOI] [PubMed] [Google Scholar]
- 47.de Langen AJ, Raijmakers P, Riphagen I, Paul MA, Hoekstra OS. The size of mediastinal lymph nodes and its relation with metastatic involvement: A meta-analysis. Eur J Cardiothorac Surg. 2006;29:26–9. doi: 10.1016/j.ejcts.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Hoekstra CJ, Stroobants SG, Hoekstra OS, Vansteenkiste J, Biesma B, Schramel FJ, et al. The value of [18F]fluoro-2-deoxy-D-glucose positron emission tomography in the selection of patients with stage IIIA-N2 non-small cell lung cancer for combined modality treatment. Lung Cancer. 2003;39:151–7. doi: 10.1016/s0169-5002(02)00446-4. [DOI] [PubMed] [Google Scholar]
- 49.MacManus MP, Hicks RJ, Matthews JP, Hogg A, McKenzie AF, Wirth A, et al. High rate of detection of unsuspected distant metastases by pet in apparent stage III non-small-cell lung cancer: Implications for radical radiation therapy. Int J Radiat Oncol Biol Phys. 2001;50:287–93. doi: 10.1016/s0360-3016(01)01477-8. [DOI] [PubMed] [Google Scholar]
- 50.Eschmann SM, Friedel G, Paulsen F, Budach W, Harer-Mouline C, Dohmen BM, et al. FDG PET for staging of advanced non-small cell lung cancer prior to neoadjuvant radio-chemotherapy. Eur J Nucl Med Mol Imaging. 2002;29:804–8. doi: 10.1007/s00259-002-0801-x. [DOI] [PubMed] [Google Scholar]
- 51.Bury T, Barreto A, Daenen F, Barthelemy N, Ghaye B, Rigo P. Fluorine-18 deoxyglucose positron emission tomography for the detection of bone metastases in patients with non-small cell lung cancer. Eur J Nucl Med. 1998;25:1244–7. doi: 10.1007/s002590050291. [DOI] [PubMed] [Google Scholar]
- 52.Kumar R, Xiu Y, Yu JQ, Takalkar A, El-Haddad G, Potenta S, et al. 18F-FDG PET in evaluation of adrenal lesions in patients with lung cancer. J Nucl Med. 2004;45:2058–62. [PubMed] [Google Scholar]
- 53.Erasmus JJ, McAdams HP, Rossi SE, Goodman PC, Coleman RE, Patz EF. FDG PET of pleural effusions in patients with non-small cell lung cancer. AJR Am J Roentgenol. 2000;175:245–9. doi: 10.2214/ajr.175.1.1750245. [DOI] [PubMed] [Google Scholar]
- 54.Schaffler GJ, Wolf G, Schoellnast H, Groell R, Maier A, Smolle-Juttner FM, et al. Non-small cell lung cancer: Evaluation of pleural abnormalities on CT scans with 18F FDG PET. Radiology. 2004;231:858–65. doi: 10.1148/radiol.2313030785. [DOI] [PubMed] [Google Scholar]
- 55.Lewis P, Griffin S, Marsden P, Gee T, Nunan T, Malsey M, et al. Whole-body 18F-fluorodeoxyglucose positron emission tomography in preoperative evaluation of lung cancer. Lancet. 1994;344:1265–6. doi: 10.1016/s0140-6736(94)90753-6. [DOI] [PubMed] [Google Scholar]
- 56.Wolfgang A, Petersen V, Schmidt B, Tyndale-Hines L, Link T, Peschel C, et al. Positron emission tomography in non-small-cell lung cancer: Prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol. 2003;21:2651–7. doi: 10.1200/JCO.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 57.Choi NC, Fischman AJ, Niemierko A, Ryu JS, Lynch T, Wain J, et al. Dose-response relationship between probability of pathologic tumor control and glucose metabolic rate measured with FDG PET after preoperative chemoradiotherapy in locally advanced non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;54:1024–35. doi: 10.1016/s0360-3016(02)03038-9. [DOI] [PubMed] [Google Scholar]
- 58.Weber WA, Petersen V, Schmidt B, Tyndale-Hines L, Link T, Peschel C, et al. Positron emission tomography in non-small-cell lung cancer: Prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol. 2003;21:2651–7. doi: 10.1200/JCO.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 59.Ryu JS, Choi NC, Fischman AJ, Lynch TJ, Mathisen DJ. FDG-PET in staging and restaging non-small cell lung cancer after neoadjuvant chemoradiotherapy: correlation with histopathology. Lung Cancer. 2002;35:179–87. doi: 10.1016/s0169-5002(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 60.Hicks RJ, Kal V, MacManus MP, Ware RE, McKenzie AF, Matthews JP, et al. The utility of (18)F-FDG PET for suspected recurrent non-small cell lung cancer after potentially curative therapy: Impact on management and prognostic stratification. J Nucl Med. 2001;42:1605–13. [PubMed] [Google Scholar]
- 61.Hebert ME, Lowe VJ, Hoffman JM, Patz EF, Anscher MS. Positron emission tomography in the pretreatment evaluation and follow-up of non-small cell lung cancer patients treated with radiotherapy: preliminary findings. Am J Clin Oncol. 1996;19:416–21. doi: 10.1097/00000421-199608000-00020. [DOI] [PubMed] [Google Scholar]
- 62.Keidar Z, Haim N, Guralnik L, Wollner M, Bar-Shalom R, Ben-Nun A, et al. PET/CT using 18F-FDG in suspected lung cancer recurrence: Diagnostic value and impact on patient management. J Nucl Med. 2004;45:1640–6. [PubMed] [Google Scholar]
- 63.Koenig TR, Munden RF, Erasmus JJ, Sabloff BS, Gladish GW, Komaki R, et al. Radiation injury of the lung after three-dimensional conformal radiation therapy. AJR Am J Roentgenol. 2002;178:1383–8. doi: 10.2214/ajr.178.6.1781383. [DOI] [PubMed] [Google Scholar]
- 64.Hicks RJ, Mac Manus MP, Matthews JP, Hogg A, Binns D, Rischin D, et al. Early FDG-PET imaging after radical radiotherapy for non-small-cell lung cancer: Inflammatory changes in normal tissues correlate with tumor response and do not confound therapeutic response evaluation. Int J Radiat Oncol Biol Phys. 2004;60:412–8. doi: 10.1016/j.ijrobp.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 65.Senan S, De Ruysscher D, Giraud P, Mirimanoff R, Budach V. Radiotherapy Group of European Organization for Research and Treatment of Cancer. Literature-based recommendations for treatment planning and execution for high-precision radiotherapy in lung cancer. Radiother Oncol. 2004;71:139–46. doi: 10.1016/j.radonc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 66.De Ruysscher D, Wanders S, van Haren E, Hochstenbag M, Geeraedts W, Utama I, et al. Selective mediastinal node irradiation based on FDG-PET scan data in patients with non small cell lung cancer: A prospective clinical study. Int J Radiat Oncol Biol Phys. 2005;62:988–94. doi: 10.1016/j.ijrobp.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 67.van Der Wel A, Nijsten S, Hochstenbag M, Lamers R, Boersma L, Wanders R, et al. Increased therapeutic ratio by 18FDG-PET-CT planning in patients with clinical CT stage N2-N3 M0 non-small-cell lung cancer: A modeling study. Int J Radiat Oncol Biol Phys. 2005;61:649–55. doi: 10.1016/j.ijrobp.2004.06.205. [DOI] [PubMed] [Google Scholar]
- 68.Senan S, De Ruysscher D. Critical review of PET-CT for radiotherapy planning in lung cancer. Crit Rev Oncol Hematol. 2005;56:345–51. doi: 10.1016/j.critrevonc.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 69.Ashamalla H, Rafla S, Parikh K, Mokhtar B, Goswami G, Kambam S, et al. The contribution of integrated PET/ CT to the evolving definition of treatment volumes in radiation treatment planning in lung cancer. Int J Radiat Oncol Biol Phys. 2005;63:1016–23. doi: 10.1016/j.ijrobp.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 70.Gambhir SS, Shepherd JE, Shah BD, Hart E, Hoh CK, Valk PE, et al. Analytical decision model for the cost-effective management of solitary pulmonary nodules. J Clin Oncol. 1998;16:2113–25. doi: 10.1200/JCO.1998.16.6.2113. [DOI] [PubMed] [Google Scholar]
- 71.Scott WJ, Shepherd J, Gambhir SS. Cost-effectiveness of FDG-PET for staging non-small cell lung cancer: A decision analysis. Ann Thorac Surg. 1998;66:1876–85. doi: 10.1016/s0003-4975(98)01055-8. [DOI] [PubMed] [Google Scholar]
- 72.Alzahouri K, Lejeune C, Woronoff-Lemski MC, Arveux P, Gaillemin F. Cost-effectiveness analysis of strategies introducing FDG-PET into the mediastinal staging of non-small-cell lung cancer from the French healthcare system perspective. Clin Radiol. 2005;60:479–92. doi: 10.1016/j.crad.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 73.Yap KK, Yap KS, Byrne AJ, Berlangieri SU, Poon A, Mitchell P, et al. Positron emission tomography with selected mediastinoscopy compared to routine mediastinoscopy offers cost and clinical outcome benefits for pre-operative staging of non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2005;32:1033–40. doi: 10.1007/s00259-005-1821-0. [DOI] [PubMed] [Google Scholar]


