Abstract
The GABAB agonist baclofen has been shown to alter ethanol intake in both human and animal studies (Moore et al., 2007). GABAB receptors are located within the ventral tegmental area (VTA; Imperato and DiChiara, 1986) and therefore may be involved in modulating voluntary ethanol intake. The present study was designed to assess the effects of baclofen in a variation on a new mouse model of binge-like ethanol intake that takes advantage of the nocturnal nature of this species (Rhodes et al., 2005; 2007). Baclofen or saline microinjections directly into the anterior or posterior VTA were administered to male C57BL/6J mice. Immediately following microinjection, mice were presented with ethanol or water using the DID procedure (fluid administration for 2 h, 3 h into the dark cycle). Ethanol or water intake was recorded at 30, 60, 90, and 120 min, and retro-orbital sinus bloods were sampled upon termination of the 120 min ethanol access period. Baclofen reduced binge-like ethanol intake when microinjected into the anterior VTA, while posterior VTA microinjections did not alter ethanol intake. Baclofen had no effect on water intake when administered to either the anterior or posterior VTA. These results add to the growing literature suggesting that GABAB receptor systems are important in the modulation of binge-like ethanol intake and suggest that the GABAB receptor system may have different roles in the anterior versus posterior VTA.
Keywords: ethanol, intake, baclofen, GABAB, mouse
The gamma-aminobutryic acid type B (GABAB) receptor system has been implicated in the modulation of ethanol’s reinforcing properties; and GABAergic drugs targeting GABAB receptors have demonstrated efficacy in modulating ethanol administration and intake in rodents (Besheer et al. 2004; Anstrom et al. 2003; Colombo et al. 2000, 2003; Janak and Gill 2003; Liang et al. 2006; Walker and Koob 2007; Stromberg 2004; Smith et al. 1992, 1999; Petry 1997; Moore et al. 2007). Based on these rodent studies, the GABAB receptor system has emerged as a potential target for pharmacological intervention in drug abuse and dependence.
Recently, Moore et al., (2007) has shown baclofen (a GABAB agonist) to increase binge-like ethanol intake without influencing water intake in the Drinking in the Dark (DID) paradigm. In the Drinking in the Dark (DID) model (developed by Rhodes et al., 2005, 2007; adapted by Moore et al., 2007) C57BL/6J mice are presented with a 20% unsweetened ethanol solution for 2 h, 3 h into their dark cycle. Ethanol intake during DID has been reported to be upwards of 5 g/kg, producing pharmacologically relevant blood ethanol concentrations (BEC; Rhodes et al., 2005, 2007; Kamdar et al., 2007; Moore et al., 2007). The DID model provides voluntary access to ethanol, and yields high ethanol intake and corresponding BECs without food or water restriction.
The ventral tegmental area (VTA) is a likely neural substrate in the modulation of the binge-like ethanol intake characteristic of DID. The VTA is a part of the mesocorticolimbic pathway; it sends projections to both the nucleus accumbens and the prefrontal cortex, making it an important neural substrate of reward. Previously, Brodie and Appel (2000) demonstrated that mouse VTA dopamine neurons are directly excited by local application of ethanol. In addition, infusion of baclofen into the VTA of male rats caused a decrease in extracellular dopamine in the nucleus accumbens, suggesting that GABAB receptors are present on VTA dopaminergic cells (Westerink et al., 1996). Importantly, it has been suggested that the VTA may have regional heterogeneity; that is, the anterior and posterior VTA may be anatomically and functionally distinct. For example, Rodd-Henricks et al. (2000) demonstrated that rats will readily self administer ethanol into the posterior VTA, however this behavior is absent when rats are given the opportunity to administer ethanol into the anterior VTA. The GABAB receptor system may also have different roles in the anterior versus the posterior VTA. For example, intra-VTA microinjection of baclofen modulates ethanol’s locomotor stimulant properties in a region-specific manner. Microinjection of baclofen into the anterior VTA of FAST selectively bred mice attenuates ethanol-induced locomotor stimulation, and this behavior is potentiated by microinjection of baclofen into the posterior VTA (Boehm II et al., 2002).
In the current investigation the DID model was used to assess the effects of baclofen microinjections into either the anterior or posterior VTA on binge-like ethanol intake in mice. It was predicted that microinjections into the anterior VTA would reduce binge-like ethanol intake whereas microinjections into the posterior VTA would increase binge-like ethanol intake.
Materials and Methods
Animals
Male C57BL/6J inbred mice were used in the present work. Mice were purchased from the Jackson Laboratory and shipped to the animal facility at Binghamton University. Mice were individually housed in standard rat cages (24 × 47.5 × 20.5 cm), providing enough space between the cage floor and the cage top to prevent the animals from getting caught on the cage top after they were surgically implanted with bilateral guide cannulae. Mice were 70–100 days old at the start of the experiment. Lighting was maintained on a 12 h reverse light-dark cycle with lights off at 10 AM. The temperature of the colony room was maintained at 21 ± 1 degrees Celsius. Animals had free access to food at all times accept during stereotaxic surgery. Animals had free access to water at all times except during stereotaxic surgery and implementation of DID procedures when a small sipper tube containing either ethanol or tap water was instead made available (see below). All procedures were approved by the Binghamton University Institutional Animal Care and Use Committee and conformed to the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (The National Academies Press, 2003).
Drugs
Ethanol (200 proof) was obtained from Pharmco, Inc (Brookfield, CT). Ethanol solutions (20% v/v) were made with tap water. Baclofen was obtained from Sigma Aldrich (St. Louis, MO), and was dissolved in 0.9% saline at doses of 0.0, 0.01, or 0.02 µg, and delivered in a volume of 200 nl per side with a flow rate of 382 nl/min.
Drinking in the Dark: Pre-surgery Procedures
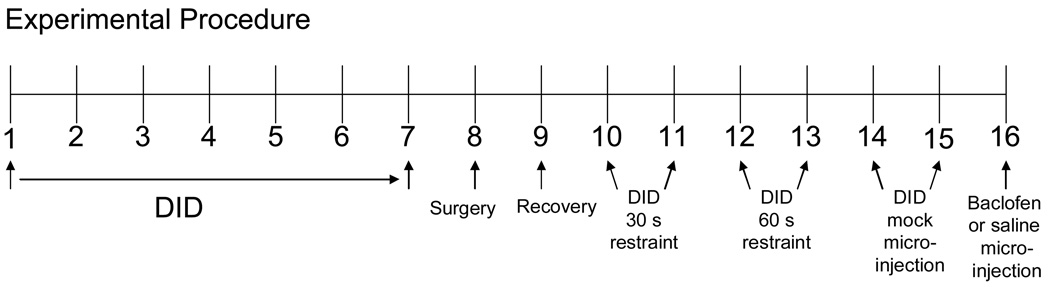
Our drinking in the dark procedure was similar to that of Moore et al. (2007). See Figure 1 for a layout of the 16-day DID microinjection procedure. During the dark phase of the light-dark cycle, mice were given a 2 h access to either an unsweetened 20% ethanol solution or water, 3 h into their dark cycle (1PM). Water bottles were removed and replaced with ethanol or water-filled 10 mL graduated cylinders attached to ball bearing sippers. Therefore, ethanol animals had 22 h access to water in standard water bottles and 2 h access to ethanol in ball-bearing sipper tubes, while water animals had 22 h access to water with the standard water bottles and 2 h access to water in ball-bearing sipper tubes. Ethanol or water sipper tube volumes were recorded both before and after the 2 h drinking period. In order to establish stable drinking, animals had fluid access using the DID model for 7 days prior to surgery.
Figure 1.
Drinking in the Dark microinjection procedures. Animals were given DID access to ethanol or water for 7 days before being surgically implanted with bilateral guide cannulae. DID access resumed on experimental day 10, and habituation to the microinjection procedure was gradually increased immediately prior to DID access over the next 6 days. Microinjections were administered on day 16.
Bilateral Cannulation of the VTA
Mice were anesthetized using a ketamine cocktail. Ketamine (1 mL of 100mg/ml solution) was mixed with xylazine (0.1 mL or 100mg/mL solution) and sterile saline (8.9 mL) and administered in a volume of 0.1mL/10 g mouse weight. The dorsal scalp was shaved, cleansed and a midline incision was made extending from bregma to lambda (about 3 mm wide). The exposed cranial surface was sterilized with 20% ethanol solution. The eyes were moistened with Major brand Lubrifresh sterile lubricant eye ointment (Livonia, MI). A Kopf stereotaxic alignment system (Tujunga, CA) was used for cannulations.
The distance between bregma and lambda was measured and subsequently divided by 4.21 mm, the published average distance between lambda and bregma for C57BL/6J mice (Franklin and Paxinos, 1997). This value was then multiplied by predetermined stereotaxic coordinates, resulting in stereotaxic coordinates adjusted for the size of the mouse brain. For the anterior VTA the predetermined coordinates were as follows: lateral 0.5 mm, caudal 3.16 mm, and ventral 2.0 mm, and for the posterior VTA: lateral 0.5 mm, caudal 3.64 mm, and ventral 2.0 mm. Two holes were drilled through the skull for simultaneous placement of guide cannulae. A third hole was drilled about 2 mm anterior to the guide cannulae holes; this hole was enlarged for placement of an anchor screw (1/8 inch; Small Parts, Miami Lakes, FL, USA) which provided an additional surface area for the skull cap to adhere. Following anchor screw placement, 2 guide cannulae (with stylets inserted) were simultaneously lowered into the brain (to a depth of 2 mm). Durelon carboxylate cement (Norristown, PA, USA) was applied to the exposed cranium, securing the guide cannulae in place and encasing the head of the anchor screw. Once the cement was dried, mice were removed from the stereotaxic apparatus and placed in heated rat cages for recovery from the effects of anesthesia. After approximately 4 h of recovery, animals were returned to their reverse light-dark cycle holding room and given free access to food and water.
Drinking in the Dark: Post-surgery Procedures
Following surgery animals were allowed to recover for 7 days (with daily stylet removal to prevent cannulae obstruction) before microinjection of baclofen. On the first day following surgery (experimental day 9) animals were left undisturbed. For the next four days (days 10–13), stylet removal and either 30 sec or 1 min animal restraint (to habituate the mice to the microinjection procedure; see Figure 1) occurred immediately prior to ethanol or water presentation. Fluid volumes were recorded both before and after the 2 h fluid access. On days 14–15, mice received mock microinjections (microinjectors extended 2 mm beyond guide cannulae; a distance that did not reach the VTA) and 1 min restraint immediately prior to fluid access. Fluid volumes were recorded every 30 min during the 2 h access period. On day 16 mice received an intra-VTA microinjection (microinjectors extended 3 mm beyond guide cannulae) of either baclofen or saline and were immediately given access to DID. Fluid volume was recorded every 30 min for the 2 h access period, and for animals receiving ethanol access, retro-orbital sinus blood samples were collected for determination of blood ethanol concentration (BEC).
Intra-VTA Microinjections
Guide cannulae, stylets and tubing to make microinjectors were obtained from Small Parts Inc. (Miami Lakes, FL). Guide cannulae were made of 25-gauge stainless steel tubing, pre-cut to 15.5 mm. Stainless steel wire (0.0095 inch) was used to make stylets, which were placed inside the guide cannulae to prevent obstruction. Microinjectors were made from two sections of stainless steel tubing: a 30-mm section of 32 gauge tubing was inserted into a 30-mm section of 25 gauge tubing so that 18 mm extended. Super glue was used to hold the sections together.
Two 0.5-m segments of PE-20 tubing were each attached to microinjectors and loaded with 0.9% saline or baclofen. The other ends of the tubing were fitted over two 10-µl Hamilton glass syringes (Hamilton Co., Reno, NV, USA) filled with distilled water. The syringes were fitted to a Cole-Parmer (74900-Series) dual infusion pump. For microinjection, the mouse was grasped by the scruff of the neck with the thumb and forefinger of the left hand while the microinjectors were slowly inserted and held in place with the thumb and forefinger of the right hand. The tips of the microinjectors extended 3 mm below the end of the guide cannulae to reach the anterior or posterior VTA. Saline or baclofen (200 nl per side) was microinjected at a rate of 382 nl/min. The microinjection took approximately 30 sec, and the microinjectors remained in place for an additional 30 sec following the end of the microinjection; this allowed for diffusion away from the microinjector tips. Each microinjector was removed slowly to prevent the fluid from being drawn back up through the guide cannulae.
Blood Ethanol Concentration
Retro-orbital blood samples were collected immediately after the ethanol access period was concluded on the day of baclofen or saline microinjections. Retro-orbital blood samples were only collected from mice given DID access to ethanol, and not those given similar access to water. Fifty µL microcapillary tubes were used to collect the sample. Samples were centrifuged and plasma was decanted and stored at −80°C until the time of BEC determination. Determination of BEC was achieved using an Analox Alcohol Analyzer (Analox Instruments, Lunenburg, MA).
Histological Verification of Cannulae Placements
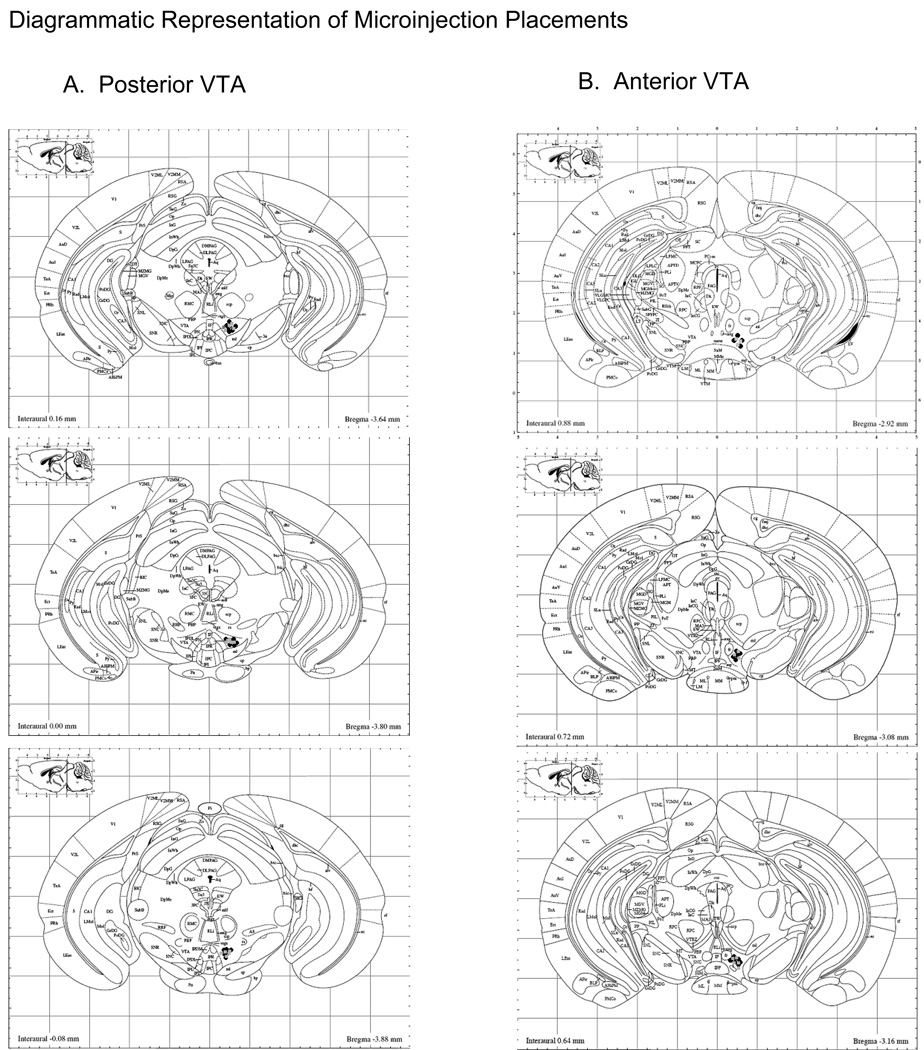
One day following intra-VTA microinjection, animals were sacrificed by cervical dislocation, brains were extracted and flash-frozen using ice-cold (−25°C) methylbutane. Forty micron brain slices were cut on a Microm HM505E cryostat (Walldorf, Germany), thaw-mounted on VWR microslides (25 × 75 × 1 mm; West Chester, PA), thionin stained (Sigma Aldrich, St. Louis, MO) and cover slipped using VWR microcover glass (24 × 60; West Chester, PA). Cannulae placement was inspected and verified by two different blind experimenters. A diagram schematic of anterior and posterior VTA microinjection placements can be seen in Fig 2.
Figure 2.
Cannulae placement. A. Location of microinjection tracts in the posterior VTA. B. Location of microinjection tracts in the anterior VTA. Black circles represent microinjection tract placements in animals given DID access to ethanol prior to drug delivery; grey squares represent microinjection tract placements in animals with DID access to water. Figures were adapted from Franklin & Paxinos (1997).
Statistical Analysis
Following histological verification of guide cannulae placement, 2–3 animals were removed from the experiment due to microinjection placement outside the target regions. Additionally, 1–2 animals per group were removed because microinjections were determined to be at the midline between anterior and posterior VTA (see Table 1).
Table 1.
| Region Targeted | Initial n | Final n | Animals removed due to improper placement |
Animals removed due to midline VTA placement |
|---|---|---|---|---|
| Experiment 1: Ethanol | ||||
| Anterior VTA Total | 29 | 18 | 6 | 5 |
| 0.0 µg baclofen | 10 | 6 | 2 | 2 |
| 0.1 µg baclofen | 9 | 6 | 1 | 2 |
| 0.2 µg baclofen | 10 | 6 | 3 | 1 |
| Posterior VTA Total | 27 | 19 | 4 | 4 |
| 0.0 µg baclofen | 8 | 6 | 1 | 1 |
| 0.1 µg baclofen | 9 | 6 | 1 | 2 |
| 0.2 µg baclofen | 10 | 7 | 2 | 1 |
| Experiment 2: Water | ||||
| Anterior VTA Total | 17 | 13 | 2 | 2 |
| 0.0 µg baclofen | 9 | 7 | 1 | 1 |
| 0.1 µg baclofen | 8 | 6 | 1 | 1 |
| Posterior VTA Total | 18 | 14 | 2 | 2 |
| 0.0 µg baclofen | 9 | 7 | 1 | 1 |
| 0.1 µg baclofen | 9 | 7 | 1 | 1 |
Note: A hit was difined as cannulation placement within either the anterior or posterior VTA. The hit rate for Experiment 1 was 66% and the hit rate for Experiment 2 was 77%.
Data in which guide cannulae placements were deemed accurate were analyzed using Statistica release 7 (StatSoft Inc.). Ethanol and water pre-surgery intake data were analyzed using a one-way repeated measures analysis of variance (ANOVA) with day as the within subjects factor. Ethanol and water post-surgery intake data were analyzed using a two-way mixed ANOVA, with day as the within subjects factor and VTA region (anterior or posterior) as the between subjects factor. The effects of baclofen microinjection on the pattern of ethanol and water intakes were analyzed with mixed three-way ANOVAs with time as the within subject factor and baclofen concentration and VTA region as the between subjects factors. Total 2h ethanol intakes, corresponding BECs, and 2h water intakes following baclofen microinjection were analyzed by two-way ANOVAs with VTA region and baclofen concentration as the between subject factors. Tukey post-hoc tests were carried out where appropriate. Results were considered significant at p<0.05.
Results
Ethanol Intake Before and After Cannulation
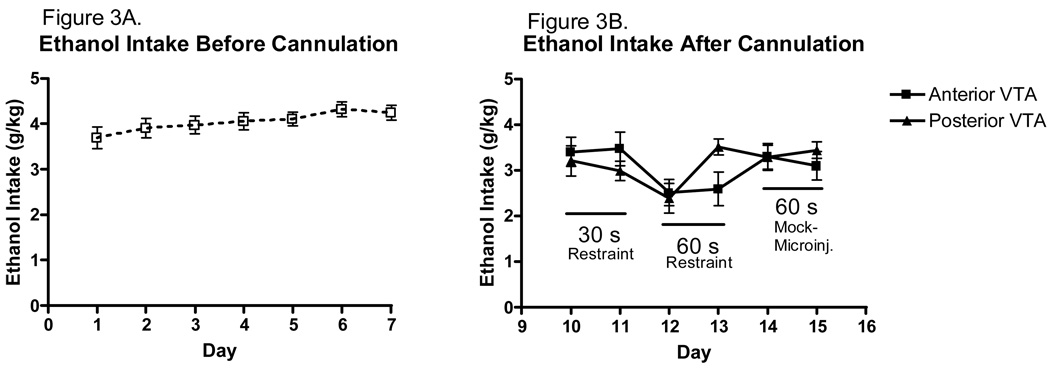
Mean ethanol intakes prior to surgery were fairly stable. Average ethanol intakes in g/kg can be seen in Figure 3. The interested reader is referred to Table 2 for ethanol intakes in ml/kg. The analysis of pre-surgery ethanol intake (days 1–7) revealed no significant main effect of day (Figure 3A). Following surgery, average daily ethanol intake was somewhat more variable, but approached 3.5g/kg by day 15 (Figure 3B). The analysis revealed a significant main effect of day [F(5,175)=3.7552, p<0.01], indicating that perhaps stress associated with the microinjection habituation procedures suppressed the ethanol intake but that the animals rapidly recovered. There was no main effect of VTA region, but a marginally significant day × VTA region interaction [F(5,175)=2.0849, p=0.069]. Tukey post hoc tests did not reveal any important differences. There were no differences in ethanol intake between animals with cannulae placements in the anterior or posterior VTA (prior to baclofen microinjections on Day 16).
Figure 3.
Daily pattern of ethanol intake in male C57BL/6J mice. A. The pattern of ethanol intake for male mice was relatively stable over days 1–7 of the procedure (n=37). B. The pattern of ethanol intake over days 9–14 was somewhat more variable (n=18–19).
Table 2.
| Ethanol Intake (mL/kg) | Water Intake (mL/kg) | |||
|---|---|---|---|---|
| Day | Mean | SEM | Mean | SEM |
| 1 | 18.47 | 1.18 | 31.41 | 2.58 |
| 2 | 19.53 | 1.05 | 30.04 | 2.47 |
| 3 | 19.86 | 0.97 | 37.60 | 2.77 |
| 4 | 20.28 | 0.92 | 32.65 | 3.39 |
| 5 | 20.53 | 0.74 | 35.75 | 1.96 |
| 6 | 21.61 | 0.82 | 34.21 | 2.52 |
| 7 | 21.22 | 0.83 | 37.70 | 2.30 |
| 10 | 17.10 | 1.02 | 33.19 | 1.83 |
| 11 | 16.10 | 1.04 | 31.37 | 1.96 |
| 12 | 12.56 | 1.05 | 22.14 | 1.96 |
| 13 | 15.86 | 0.97 | 23.06 | 1.78 |
| 14 | 16.44 | 0.97 | 15.20 | 1.72 |
| 15 | 16.38 | 0.88 | 15.12 | 1.30 |
| 16 | ||||
| Anterior VTA | ||||
| 0 μg baclofen | 21.63 | 1.84 | 15.49 | 3.53 |
| 0.1 µg baclofen | 5.02 | 0.83 | 13.27 | 3.26 |
| 0.2 µg baclofen | 7.16 | 1.90 | ||
| Posterior VTA | ||||
| 0 µg baclofen | 19.08 | 1.17 | 14.29 | 2.78 |
| 0.1 µg baclofen | 18.06 | 2.72 | 14.77 | 3.80 |
| 0.2 µg baclofen | 18.36 | 2.78 | ||
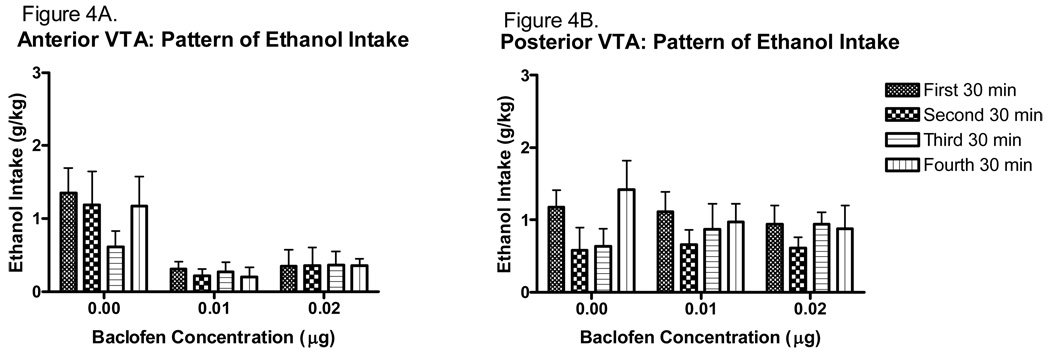
The effect of baclofen on the ethanol drinking pattern over the 2 h period is shown for the anterior VTA in Figure 4A and for the posterior VTA in Figure 4B. Baclofen appeared to reduce ethanol intake following both doses when administered into the anterior VTA, with no effect in the posterior VTA. The analysis did not detect a main effect of time or interaction of time and any other factor. However, a significant main effect of baclofen concentration [F(2,31)=11.239, p<0.001], and VTA region [F(1,31)=15.844, p<0.001], as well as a significant interaction of these factors [F(2,31)=7.729, p<0.01] was revealed. Tukey post hoc tests did not reveal any specific differences.
Figure 4.
Pattern of ethanol intake following intra-VTA microinjection of baclofen (30 min time bins). A. Ethanol intakes following anterior VTA baclofen microinjection at both concentrations are significantly reduced compared to saline microinjection at all time points (n=6). B. Ethanol intakes following posterior VTA baclofen microinjections do not differ from saline controls at any time point (n=6–7).
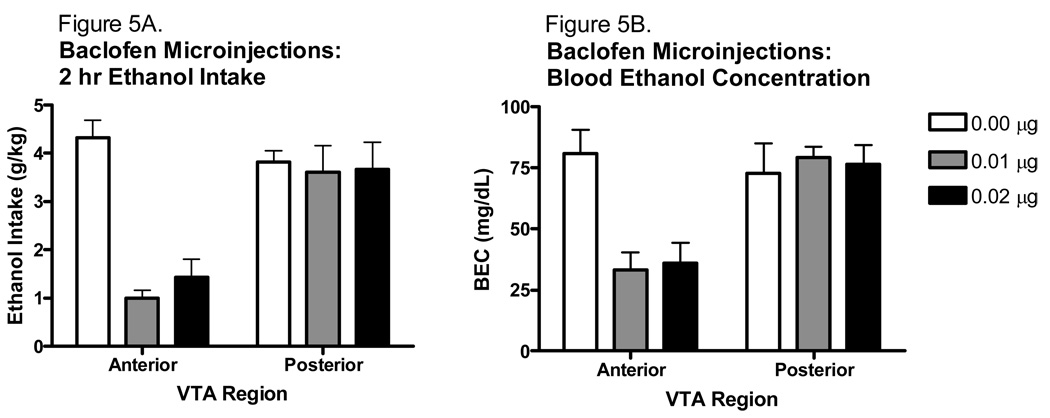
The effect of baclofen on 2 h binge-like ethanol intake and resulting blood ethanol concentrations are shown in Fig. 5. Analysis of the ethanol intakes revealed a significant main effect of baclofen concentration [F(2,31)=10.475, p<0.001], and VTA region [F(1,31)=18.287, p<0.001], as well as a significant interaction of these factors [F(2,31)=8.333, p<0.01]. Tukey post hoc analyses revealed that baclofen microinjections into the anterior VTA reduced ethanol intake at both concentrations tested (p values<0.001). However, neither concentrations altered total ethanol intake when microinjected into the posterior VTA. Due to an error, some of the blood samples were lost and therefore there are only 4–5 blood samples per group. Despite the small n, analysis of the blood ethanol concentrations following the ethanol access period were consistent with the ethanol intake findings, with a significant main effect of VTA region [F(1, 19)=12.420, p<0.01], baclofen concentration [F(2, 19)=3.541, p<0.05], and an interaction of these factors [F(2,19)=5.501, p<0.05]. Tukey post hoc tests revealed that BECs were significantly reduced following anterior VTA baclofen microinjection at both concentrations (p values<0.05); however, no differences in BEC were found following posterior VTA baclofen microinjection.
Figure 5.
Effect of baclofen microinjected into either the anterior or posterior VTA on 2 h binge-like ethanol intake and corresponding BECs. A. Baclofen reduced binge-like ethanol intake in the anterior VTA but had no effect in the posterior VTA (n=6–7). B. Blood ethanol concentrations followed the same pattern of ethanol intake following baclofen microinjections (n=4–5).
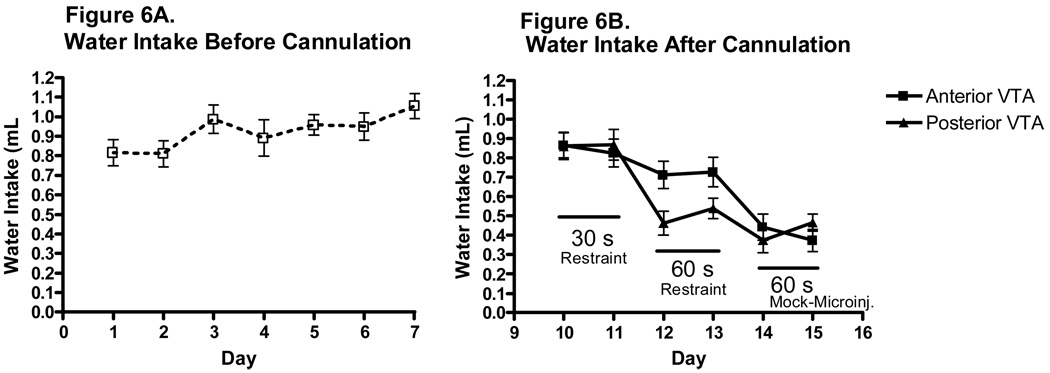
Water Intake Before and After Cannulation
Average water intake in ml can be seen in Figure 6, and average water intake in ml/kg can be seen in Table 2. The analysis of water intake prior to surgery revealed a significant main effect of day [F(5,125)=17.931, p<0.001]; Tukey post hoc tests revealed that 2 h water intake was significantly greater on day 7 than on day 1 (p<0.05). Following surgery, 2 h water intake was more varied, and by day 13 water intakes were reduced to 0.420±0.036 mL (Figure 5 B). The statistical analysis revealed a significant main effect of day [F(5,125)=17.931, p<0.001] as well as a marginal main effect of VTA region [F(1,25)=2.980, p=0.097] and a marginal day × VTA region interaction [F(5,125)=2.025, p=0.08]. Tukey post hoc tests indicated that water intake on day 12 and 13 was reduced compared to days 10 and 11 (p values<0.05); and day 14 and 15 water intakes were significantly reduced compared to days 10, 12 and 13 (p values<0.05). Importantly, there were no differences in water intake between animals targeted for anterior or posterior VTA before baclofen microinjections on Day 16.
Figure 6.
Daily pattern of water intake in male C57BL/6J mice. A. The pattern of water intake prior to surgery for male mice was relatively stable over days 1–7 of the procedure (n=27). B. The pattern of water intake over days 9–14 was more variable (n=13–14).
Effect of Intra-VTA Baclofen Microinjection on Water Intake
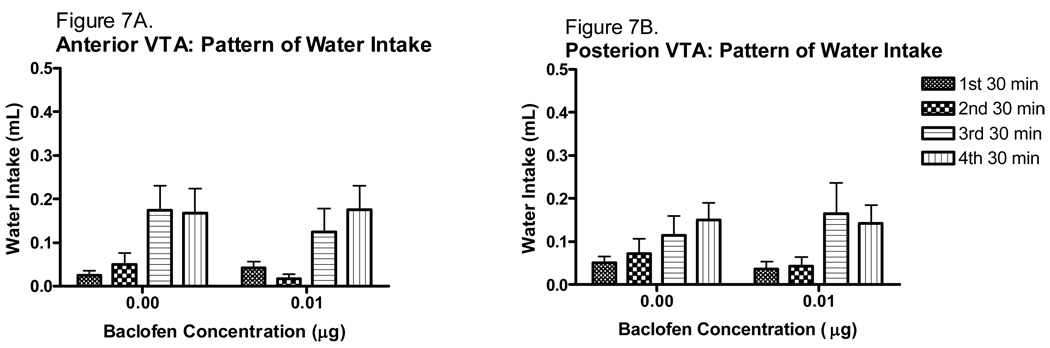
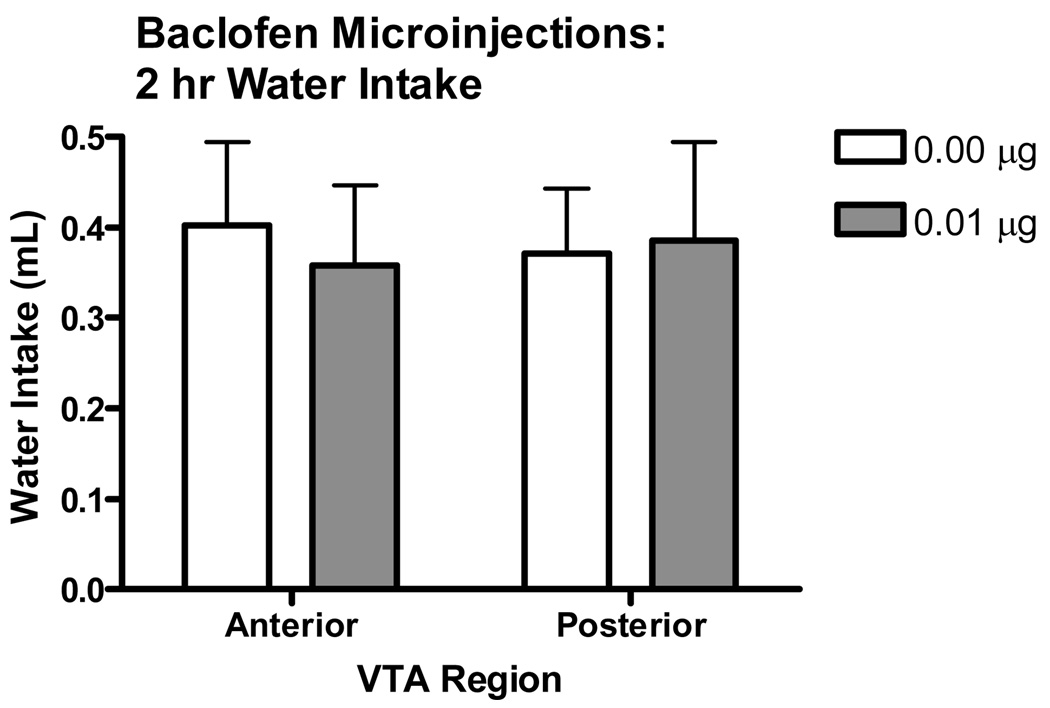
The effect of baclofen on the pattern of water intake is shown in Fig. 7. Because there was no difference between either the 0.01 or 0.02 µg baclofen concentrations on the modulation of binge-like ethanol intake, only the 0.01 µg concentration of baclofen was used to determine the effect of baclofen intra-VTA microinjection on water intake. It was of interest to find a baclofen concentration that altered ethanol intake but not water intake while limiting the number of animals used in this study. Therefore, the 0.1 µg baclofen concentration was chosen because it was just as effective as the higher concentration at reducing binge-like ethanol intake, while at the same time being less likely to produce a competing behavior that would inadvertently alter water intake. The statistical analysis revealed a significant main effect of time [F(3,69)=9.503, p<0.001], but no main effect of VTA region or baclofen treatment, and no significant interactions. Baclofen had no effect on total water intake (Figure 8). Analysis of the water intakes revealed no significant main effect of baclofen treatment or VTA region, and no significant interaction of these factors.
Figure 7.
Pattern of water intake following intra-VTA microinjection of baclofen (30 min time bins). A. Anterior intra-VTA baclofen microinjection did not alter water intake (n=6–7). B. Posterior intra-VTA baclofen microinjections also did not alter water intake (n=7).
Figure 8.
Effect of baclofen microinjected into either the anterior or posterior VTA on water intake. Baclofen had no effect on water intake in either the anterior or posterior VTA, suggesting that the effects of baclofen do not extend to general fluid intake (n=6–7).
Discussion
The goal of the current work was to investigate the role of anterior and posterior GABAB receptors in the modulation of voluntary ethanol intake. We chose the DID model to achieve this goal. The DID model was chosen in part because C57BL/6J mice will consume large amounts of ethanol over relatively short time periods, achieving pharmacologically relevant BECs (Moore et al. 2007; Rhodes et al. 2005, 2007; Kamdar et al. 2007). Additionally, the DID model was chosen because the ethanol access period is short and the experimenter knows precisely when to administer drug and monitor drinking; a critical issue in the examination of intra-cranial actions of neuromodulatory compounds on voluntary ethanol intake.
DID ethanol intakes prior to cannulation were consistent with the available literature demonstrating that the DID model consistently yields high ethanol consumption. After surgery and habituation to the microinjection procedure, ethanol intake was somewhat reduced, most likely due to the apparent stress associated with the microinjection procedure. However, by the end of the microinjection habituation period ethanol intakes appeared to recover, approaching 3.5 g/kg at conclusion of the 2 h ethanol access period. In fact, the BECs that were obtained from saline-injected control mice on the final day of ethanol access were similar in range as those reported by Moore et al. (2007) and sufficient to produce behavioral intoxication.
In contrast to post-surgery ethanol intake, water intake following surgery was reduced, and it continued to decrease as the apparent stress (30–60 s restraint prior to ethanol or water DID to habituate the mice to the microinjection procedure) associated with the microinjection habituation procedure increased. Considered along with the recovery of the post-surgery ethanol intakes, the continued reduction in water intakes may suggest that the animals were more motivated to consume the ethanol than the water. This would not be surprising as water was freely available to the animals for 24 h a day. Ethanol was only available for the 2 h period immediately following the habituation procedure. Thus, the mice may have maintained ethanol drinking levels despite the apparent habituation stress because the drug was inherently rewarding (and may have reduced DID water intake because water was not rewarding in the absence of a thirst component). Perhaps adding to this, the mice given daily access to ethanol may have developed some level of dependence to the drug. Indeed, prior to surgery such animals had already been allowed DID access to ethanol for 7 days. By the time these animals had to cope with the apparent restraint stress associated with habituation, they may have developed some level of dependence, perhaps further motivating them to consume the ethanol. The assessment of DID during a deliberate stress-inducing regimen will be necessary to determine to what extent dependence and/or reward is involved in maintaining ethanol intakes using this procedure. In any case, for the purposes of the current study, the level of ethanol (and water) intake using DID procedures appeared adequate for subsequent examination of intra-VTA microinjection of baclofen on such intake.
Additional evidence that the apparent restraint stress associated with our habituation procedure might have affected water (but not ethanol) intake can be seen in the pattern of fluid intake following microinjection. Compared to the saline-injected controls, anterior intra-VTA baclofen reduced ethanol intake at each of the 30-min intervals post-injection, whereas posterior intra-VTA baclofen produced no such effect. Further examination of the pattern of water intake following microinjection revealed that water intakes were low in the first two 30 min time bins following both baclofen and saline microinjection, but higher in the third and fourth 30 min time bins. This pattern of water intake may indicate that the stress associated with the microinjection procedure reduces water intake during the first hour of DID access, but that the animals begin to recover in the second hour of access. Indeed, ethanol intake was relatively consistent in each 30 min time bin following saline microinjection, perhaps again suggesting that the animals were more motivated to consume the ethanol in this procedure.
Anterior intra-VTA microinjection of baclofen reduced the total 2 h ethanol intake, and this effect was also observed in the corresponding blood ethanol concentrations. Posterior intra-VTA baclofen had no effect on binge-like ethanol intake. Neither anterior nor posterior intra-VTA microinjection of baclofen altered 2 h water intake, suggesting that although GABAB receptors in the anterior VTA modulate DID ethanol intake, they do not influence general fluid intake, at least of non-reinforcing fluids. This result might indicate that competing behaviors such as locomotor depression did not prevent us from assessing intra-VTA baclofen’s role on binge-like ethanol intake. However, it is possible that anterior intra-VTA baclofen combined with ethanol intake early on during the ethanol access period to produce a locomotor depressant response, limiting subsequent ethanol intake. We did not directly assess locomotor activity, but it is notable that visual observation during the course of the studies did not reveal any obvious effects of anterior intra-VTA baclofen on locomotor activity. Nevertheless, future work might examine this possibility by either directly examining anterior intra-VTA baclofen’s actions on locomotor activity in both the presence and absence of ethanol, or by indirectly assessing such actions by examining anterior intra-VTA baclofen’s actions on intake of some other reinforcer that is not known to produce locomotor depression on its own, such as sugar water.
We previously reported that systemic baclofen dose-dependently increased binge-like ethanol intake and had no effect on water intake using the DID procedure (Moore et al., 2007). If systemic baclofen indeed produced these effects via actions at the level of the VTA as we had speculated, then the current findings would seem to directly conflict with this previous work. There are several plausible explanations that may at least in part explain these differences. These explanations include heterogeneity of the VTA, differences in duration of ethanol access, and interactions between ethanol and stress.
Previous research has shown that the anterior and posterior VTA may be heterogeneous in function (Rodd-Henricks et al., 2000; Boehm II et al., 2002). Because Boehm II et al. (2002) reported that posterior intra-VTA baclofen potentiated ethanol-induced locomotor stimulation (and anterior intra-VTA baclofen attenuated it) we had expected to see an increase in DID after microinjection into the posterior region of the VTA, with a decrease in drinking after microinjection into the anterior region. Although baclofen administration into the anterior VTA did reduce binge-like ethanol intake, we did not see the increase in drinking we expected to see when baclofen was administered into the posterior VTA; instead we saw no effect. These results suggest that the GABAB receptor system in the anterior VTA may be more important in the modulation of binge-like ethanol intake than the GABAB receptor system in the posterior VTA. Moreover, the present data point to the importance of GABAB receptors in other brain structures in the modulation of DID ethanol intake; neither the anterior nor the posterior region of the VTA appears to account for the increase in drinking seen in Moore et al. (2007).
Another important consideration for interpretation of the present data is the duration of daily ethanol access. Although the previously published studies using DID procedures (Rhodes et al., 2005, 2007; Kamdar et al., 2007; Moore et al., 2007) were generally shorter in duration (2–5 days of DID ethanol exposure), the animals in the current study experienced 7 days of DID ethanol access prior to surgery, as well as an additional 7 days of DID ethanol access post-surgery. Thus, the chronic nature of the ethanol exposure in the current work may have altered the GABAB receptor system in the VTA, thereby altering the actions of intra-VTA baclofen. Unpublished data from our lab has found that 7 days of daily DID exposure to ethanol is sufficient to alter GABAB1 subtype mRNA expression in the nucleus accumbens using real-time PCR (unpublished observations). Additional work will be needed to determine whether long-term daily repeated binge-like ethanol intake as produced by DID also alters GABAB receptor expression in the anterior and/or posterior VTA.
Yet another factor that may have set the current data apart from Moore et al. (2007) was the apparent restraint stress associated microinjection. Prior to ethanol access post-surgery, animals were restrained for 30–60 s each day so that they might habituate to the microinjection procedure. Using a social isolation procedure, Knapp et al. (2007) recently demonstrated that baclofen alters the effects of stress in animals withdrawing from chronic ethanol diet. This may suggest that there could have been as of yet unidentified, but important interactions between baclofen, stress and ethanol intake/withdrawal that might have influenced the present results.
Finally, the current trend in the literature suggests that the VTA may be heterogeneous in structure and function (Rodd-Henricks et at., 2000; Boehm II et al., 2002), and the present data appear to support this conclusion. However, because baclofen had differential effects in the anterior versus the posterior VTA, the question remains: how do the subregions differ with respect to GABAB receptor systems? One possibility as previously proposed by Boehm et al. (2002) is that in the anterior and posterior regions of the VTA may differ with respect to GABAB receptor localization. In the anterior VTA, GABAB receptors may be located on dopamine cell bodies that project to the nucleus accumbens. Activation of these receptors by baclofen would directly inhibit ethanol’s stimulation of dopamine release in the nucleus accumbens. GABAB receptors in the posterior VTA may be located on GABA interneurons that synapse on dopamine cell bodies. In the posterior VTA baclofen would therefore inhibit the GABA interneurons, and therefore disinhibit the dopamine cell bodies; this series of events would be expected to result in an increase in dopamine release in the nucleus accumbens.
Imperato and Di Chiara (1986) noted that high doses of ethanol (administered via IP injection) inhibit dopamine release in the nucleus accumbens. The pattern of binge-like ethanol intake produced by DID may actually mimic such IP injections, resulting in a similar reduction in nucleus accumbens dopamine release. If this was the case, then at the same time high ethanol intake was attenuating dopamine release in the nucleus accumbens, anterior intra-VTA baclofen might be inhibiting dopamine release in the nucleus accumbens; such effects could sum to create lower than normal levels of dopamine in the nucleus accumbens, and perhaps a lower than normal rewarding effect of ethanol (and therefore lower ethanol intake). In contrast, baclofen administration into the posterior VTA (where GABAB receptors may be located on interneurons that synapse on dopamine cell bodies) would inhibit the GABA interneurons (disinhibiting the dopamine cell bodies) resulting in more dopamine in the nucleus accumbens. Binge-like ethanol intake would still result in overall reductions in nucleus accumbens dopamine release. Thus, these opposing actions would possibly result in no change in ethanol intake. Although certainly speculative, this is an interesting possibility, and more work will be needed to more fully understand the mechanisms surrounding the region-specific effects of intra-VTA baclofen on DID ethanol intake.
In conclusion, the current work demonstrates the usefulness of the DID model in examining the intra-cranial actions of neuromodulatory substances on ethanol intake in mice. Our data supports the growing literature indicating that the anterior and posterior VTA are two distinct subregions with differing roles relating to ethanol intake, particularly with respect to GABAB receptor systems. Moreover, it adds to the vast literature indicating that the GABAB receptor system is important in the modulation of ethanol intake, in particular binge-like ethanol intake. Future work will be necessary to determine the mechanism by which anterior intra-VTA baclofen reduces binge-like ethanol intake. Such work will be necessary to elucidate the complex interactions between baclofen, stress and ethanol intake/withdrawal in the modulation of binge-like ethanol intake, as well as determine whether the actions of anterior intra-VTA baclofen on binge-like ethanol intake generalize to other reinforcers. Such work will undoubtedly have important implications for studies aimed at further developing baclofen, and perhaps other drugs that target GABAB receptor systems, for the treatment of alcoholism in humans.
Acknowledgments
This work was supported in part by NIAAA grant AA015434.
References
- Anstrom KK, Cromwell HC, Markowski T, Woodward DJ. Effect of baclofen on alcohol and sucrose self-administration in rats. Alcohol Clin Exp Res. 2003;27:900–908. doi: 10.1097/01.ALC.0000071744.78580.78. [DOI] [PubMed] [Google Scholar]
- Besheer J, Lepoutre V, Hodge CW. GABAB receptor agonists reduce operant ethanol self-administration and enhance ethanol sedation in C57BL/6J mice. Psychopharmacology (Berl,) 2004;74:358–366. doi: 10.1007/s00213-003-1769-3. [DOI] [PubMed] [Google Scholar]
- Boehm SL, II, Piercy MM, Bergstrom HC, Phillips TJ. Ventral tegmental area region governs GABAB receptor modulation of ethanol-stimulated activity in mice. Neuroscience. 2002;115(1):185–200. doi: 10.1016/s0306-4522(02)00378-0. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Appel SB. Dopaminergic neurons in the ventral tegmental area of C57BL/6J and DBA/2J mice differ in sensitivity to ethanol excitation. Alcohol Clin Exp Res. 2000;24(7):1120–1124. [PubMed] [Google Scholar]
- Colombo G, Agabio R, Carai MAM, Lobina C, Pani M, Reali R, Addolorato G, Gessa GL. Ability of baclofen in reducing alcohol intake and withdrawal severity: I-preclinical evidence. Alcohol Clin Exp Res. 2000;24:58–66. [PubMed] [Google Scholar]
- Colombo G, Vacca G, Serra S, Brunetti G, Carai MAM, Gessa GL. Baclofen suppresses motivation to consume alcohol in rats. Psychopharmaclogy (Berl,) 2003;167:221–224. doi: 10.1007/s00213-003-1397-y. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego CA: Academic Press; 1997. [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- Janak PH, Gill TM. Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol. 2003;30:1–7. doi: 10.1016/s0741-8329(03)00068-5. [DOI] [PubMed] [Google Scholar]
- Kamdar MK, Miller SA, Syed YM, Bahyana R, Gupta T, Rhodes JS. Acute effects of Naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology (Berl) 2007;192:207–217. doi: 10.1007/s00213-007-0711-5. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Baclofen blocks expression and sensitization of anxiety-like behavior in an animal model of repeated stress and ethanol withdrawal. Alcohol Clin Exp Res. 2007;31(4):582–595. doi: 10.1111/j.1530-0277.2007.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JH, Chen F, Krstew E, Cowen MS, Carroll FY, Crawford D, Beart PM, Lawrence AJ. The GABAB receptor allosteric modulator CGP7930, like baclofen, reduces operant self-administration of ethanol in alcohol-preferring rats. Neuropharmacology. 2006;50:632–639. doi: 10.1016/j.neuropharm.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, Boehm SL., II GABAergic modulation of binge-like ethanol intake in C57BL/6J mice. Pharmacol, Biochem, Behav. 2007;88:105–113. doi: 10.1016/j.pbb.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council of the National Academies. Guidelines for the care and use of mammals in neuroscience and behavioral research. The National Academies Press; 2003. [PubMed] [Google Scholar]
- Petry NM. Benzodiazepine-GABA modulation of concurrent ethanol and sucrose reinforcement in the rat. Exp Clin Psychopharmacol. 1997;5(3):183–194. [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu C-H, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology. 2000;149:217–224. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- Smith BR, Robidoux J, Amit Z. GABAergic involvement in the acquisition of voluntary ethanol intake in laboratory rats. Alcohol Alcohol. 1992;27:227–231. [PubMed] [Google Scholar]
- Smith BR, Boyle AEL, Amit Z. The effects of the GABAB agonist baclofen on the temporal and structural characteristics of ethanol intake. Alcohol. 1999;17:231–240. doi: 10.1016/s0741-8329(98)00053-6. [DOI] [PubMed] [Google Scholar]
- Stromberg MF. The effect of baclofen alone and in combination with naltrexone on ethanol consumption in the rat. Pharmacol Biochem Behav. 2004;78:743–750. doi: 10.1016/j.pbb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The c-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31(1):11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerink BH, Kwint HF, deVries JB. The pharmacology of mesolimbic dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and nucleus accumbens of the rat brain. J Neurosci. 1996;16(8):2605–2611. doi: 10.1523/JNEUROSCI.16-08-02605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]