SUMMARY
This communication explores the temporal link between the age-associated increase in body iron stores and the age-related incidence of Alzheimer’s disease (AD), the most prevalent cause of senile dementia. Body iron stores that increase with age could be pivotal to AD pathogenesis and progression. Increased stored iron is associated with common medical conditions such as diabetes and vascular disease that increase risk for development of AD. Increased stored iron could also promote oxidative stress/free radical damage in vulnerable neurons, a critical early change in AD. A ferrocentric model of AD described here forms the basis of a rational, easily testable experimental therapeutic approach for AD, which if successful, would be both widely applicable and inexpensive. Clinical studies have shown that calibrated phlebotomy is an effective way to reduce stored iron safely and predictably without causing anemia. We hypothesize that reducing stored iron by calibrated phlebotomy to avoid iron deficiency will improve cerebrovascular function, slow neurodegenerative change, and improve cognitive and behavioral functions in AD. The hypothesis is eminently testable as iron reduction therapy is useful for chronic diseases associated with iron excess such as nonalcoholic steatohepatitis (NASH), atherosclerosis, hereditary hemochromatosis and thalassemia. Testing this hypothesis could provide valuable insight into the causation of AD and suggest novel preventive and treatment strategies.
Introduction
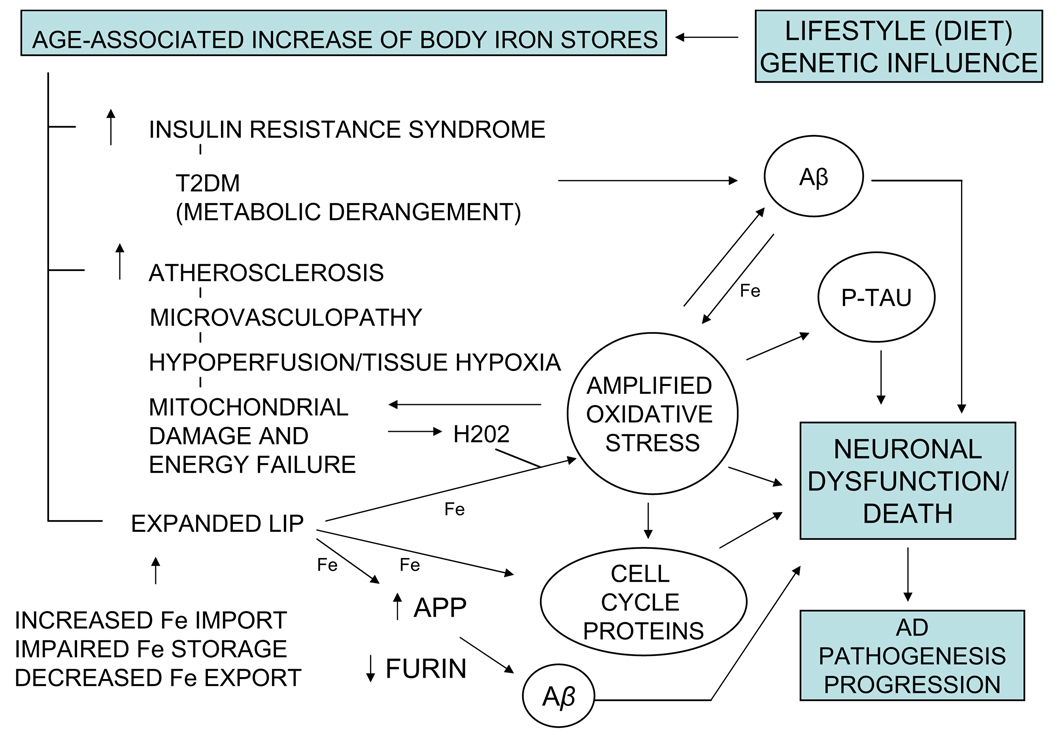
AD is a progressive and invariably fatal neurodegenerative disease. By recent estimates, the $100 billion annual cost of providing care for the 4.5 million Americans with AD is expected to rise as the American population ages and prevalence of AD-related risk factors such as adiposity, hyperinsulinemia, and type 2 diabetes mellitus (T2DM) continue to increase [1]. Sporadic, late onset AD comprising the great majority of AD cases, most likely results from the interactions of numerous genetic and environmental factors. Individuals diagnosed with AD survive an average of 5–10 years. During this period, they become increasingly and severely incapacitated. There is no cure for AD. Present pharmaceutical interventions are palliative and marginally effective. New therapies for AD should ideally minimize the impact of coexistent risk factors, and target the earliest and most basic pathophysiological changes in AD when intervention would likely provide the greatest benefit. Further, any intervention should be minimally toxic since long-term treatment will likely be necessary. Advances in brain imaging technologies offer much hope for early diagnosis of AD, but incomplete understanding of AD etiology and pathogenesis has hindered development of effective therapies. An explanatory model of AD etiology and pathogenesis should reconcile risk factors identified from epidemiological studies, the histopathological changes characteristic of AD, and age-related onset and the inexorable progression of the disease. A ferrocentric model of AD development (Fig. 1) aims to achieve this goal.
Fig. 1.
The pervasive and modifiable role of iron on the development of AD. The most widely accepted theories of AD causation implicate four significant changes: (1) Aβ accumulation, (2) tau hyperphosphorylation and development of neurofibrillary pathology, (3) oxidative stress and free radical damage in vulnerable neurons, and (4) expression of cell cycle proteins in post-mitotic neurons. Age-related increase in stored iron is at the apex of a pathophysiological cascade that could promote these critical changes by increasing the risk for diabetes and cardiovascular disease, two common medical conditions associated with increased risk for AD, and by expanding the pool of redox active iron (LIP) in brain cells. Insulin resistance syndrome and T2DM could increase Aβ accumulation because hyperinsulinemia modifies Aβ metabolism and glycation reactions alter Aβ aggregation [1,28]. Cardiovascular disease causing cerebral hypoperfusion and tissue hypoxia could be a cause of mitochondrial damage and dysfunction that results in increased generation of reactive oxygen species including hydrogen peroxide [45,50]. Increased redox active iron due to increased iron availability and impaired iron storage/detoxification could exacerbate Fenton reaction-mediated oxidative stress and free radical damage to essential cell components. Moreover, mitochondria are both a source of reactive oxygen species and a target of reactive oxygen-mediated oxidative stress, so a vicious circle could develop leading to increased mitochondrial damage and dysfunction. Iron-mediated oxidative stress, perhaps in association with cell signaling disturbances, could promote increased phosphorylation of microtubule-associated protein tau and expression of cell cycle proteins in post-mitotic neurons [91–94]. The figure also depicts a novel pathway by which iron could potentially regulate Aβ accumulation. Expression of furin, a subtilisin-like proprotein convertase that stimulates α-secretase activity, is decreased in AD brain, and brain from Tg2576 AD-transgenic mice [95]. Silvestri and Camaschella [96] proposed that iron-mediated decrease of furin protein and α-secretase activity in AD brain, and iron-mediated increase in APP expression [97], could favor the accumulation of Aβ. The figure does not presume to be an exhaustive review of how AD-related pathology develops, and indeed, does not depict other potentially important developments such as neuroinflammation. Our intent is to highlight potential linkage between increased body iron stores and some potentially important pathophysiological changes in AD that would possibly respond to iron reduction by calibrated phlebotomy.
Multiple studies associate disturbances in metal ion homeostasis with AD and suggest a pathogenic role [2]. Iron is the most abundant redox active metal in humans. Dietary iron is actively absorbed but minimally excreted [3], and there is mounting evidence that body iron stores present in free-living apparently healthy adults, rise with age reaching toxic levels [4–10]. Sullivan [6] raised two important questions in relation to iron and heart disease: first, are body iron stores excessive, even if they fall within clinically “normal” limits? Second, is stored iron safe? We believe these questions are relevant to AD whose risk increases with age [11].
Body iron stores increase with age and precede development of AD
Body iron stores increase with age in both men and women. Serum ferritin, which is a useful marker of body iron stores [12], increases sharply between the teenage years and age 40 in men [4,7]. A comparable increase in serum ferritin occurs in women after menopause, between 40–49 and 60–69 years of age. Sullivan recognized that the risk of heart disease in men and women paralleled the increases in body iron stores. He formulated the iron hypothesis of coronary artery disease, which suggests that pre-menopausal women are protected from atherosclerosis and atherosclerotic events because of low body iron stores until menopause after which body iron stores rise [4–6]. Iron is implicated in the pathogenesis of atherosclerosis, which begins relatively early in life and involves oxidation of low-density lipoprotein by incompletely understood mechanisms [9]. Significantly, the relationship between increased body iron stores and risk of heart disease is observed in men under 50 [13]. AD is a disease of aging; its incidence rises dramatically after 60 years of age [11]. However, changes in brain metabolism can precede disease-associated cognitive decline by decades [14]. Thus, it is likely that an age-associated increase in body iron stores will precede or overlap the early cerebral metabolic changes that presage AD in the majority of elderly individuals eventually diagnosed with this disease.
Cardiovascular disease and diabetes are associated with increased body iron stores and AD risk
AD shares common risk factors with vascular dementia including aging, atherosclerosis, stroke, and type 2 diabetes mellitus (T2DM) [15–18]. Vasculopathy, cerebral hypoperfusion, and tissue hypoxia biologically link these risk factors, and are implicated in the etiology of AD [19,20]. Moreover, these common risk factors for AD are associated with increased body iron stores. Thus, atherosclerosis is associated with increased risk for AD [18,21], and age-associated increase in stored iron is associated with atherosclerosis and vascular disease (discussed in [9]). Stroke increases risk for AD [18], and increased stored iron is associated with poor outcome in stroke [22], possibly due to iron-dependent hypoxia-reperfusion injury. T2DM increases risk for AD and for mild cognitive impairment (MCI), the transitional stage between normal cognition and AD [1,23], and increased stored iron is associated with risk for T2DM [24–27]. T2DM is a complex metabolic disease-associated with obesity, insulin resistance, hypo- and hyperglycemia, atherosclerosis and microvascular disease, and cognitive dysfunction [1]. Insulin resistance syndrome (IRS) (metabolic syndrome) could be an important link between T2DM and risk for AD [1,28]. IRS is a cluster of medical conditions that includes chronically elevated levels of insulin, reduced insulin activity, hyperlipidemia, and cardiovascular effects such as hypertension and atherosclerotic heart disease [29], and the incidence of IRS in both men and women is associated with increased body iron stores [30]. Genetic influences could also be an important link between increased stored iron and AD. Hereditary hemochromatosis (HH) is an autosomal recessive disorder resulting from mutation of the HFE gene. In HH, iron absorption from the gut is increased and iron overload in body tissues can lead to heart disease and diabetes and organ failure [31]. The most common HFE gene variants are C282Y and H63D; the H63D variant, in particular, has been associated with increased risk for AD in several studies [32].2
An important question is whether the association between age-related increase in stored iron and the development of diseases that may increase risk for AD is merely coincidental. In other words, is there evidence of molecular biological links between iron excess and abnormal lipid/insulin metabolism that could be important in development of AD? While a definitive answer is unavailable, experimental evidence suggests more than coincidence may be involved. Cholesterol, a key molecule in AD pathogenesis [33], may provide an example of an important interaction between iron and lipid metabolism. Hypercholesterolemia is a risk factor for both cardiovascular disease and AD [18]. The potential importance of excess iron in the development of atherosclerosis is suggested by inhibition of atherosclerotic lesion formation in cholesterol-fed rabbits by the iron chelator desferrioxamine [34]. In addition, experimental hypercholesterolemia in rabbits increased cerebral deposition of Aβ and iron, which preferentially accumulated around amyloid plaques [35]. Interestingly, DNA microarray analysis performed on primary human vascular smooth muscle cells from umbilical vein revealed that cholesterol synthesis genes were upregulated by iron supplementation [36]. While vascular smooth muscle cells are not neurons, this study is important because it suggests a mechanism by which increased brain iron could lead to increased neuronal cholesterol and in turn to increased Aβ [33]. Age-related iron increase could also contribute to abnormalities in glucose and insulin metabolism [37]. Iron-induced oxidative stress could inactivate insulin-degrading enzyme (IDE) [38] and contribute to increased levels of Aβ [39].
Are levels of stored iron excessive?
Studies demonstrating positive clinical benefits of phlebotomy for varied conditions, including those associated with risk for AD, suggest that the answer to this question is affirmative. Phlebotomy is an efficient means for reducing body iron stores. Frequent blood donation was associated with reduced risk for cardiovascular events [40,41]. The iron (Fe) and atherosclerosis study (FeAST) found evidence that iron reduction improved outcomes for patients with symptomatic but stable peripheral vascular disease if intervention began before age 60 [primary endpoint - all cause mortality; secondary endpoint - death plus nonfatal myocardial infarction and stroke] [9]. Flow-mediated dilation of the brachial artery, a noninvasive biomarker of vascular function, was enhanced in frequent blood donors (mean serum ferritin = 17 ng/ml), compared to infrequent blood donors (mean serum ferritin = 52 ng/ml) [42,43]. In addition, serum biomarkers of oxidative stress that are associated with development of atherosclerosis, were reduced [42]. The cause of improved vascular function in this study appeared to be increased responsiveness to endothelial-released nitric oxide, a regulator of flow-mediated dilation of the brachial artery. This observation could be particularly relevant for AD because chronic cerebral hypoperfusion and hypoxia, caused by perturbation of the balance between endothelial-derived vasoconstrictor and vasodilator substances, may be a significant factor in the etiology of AD [18–20,44,45]. Significantly, blood donation reduced insulin resistance in patients with hyperferritinemia and nonalcoholic fatty liver disease, the hepatic expression of insulin resistance syndrome [46] In addition, impaired vascular endothelial function in HH correlated with increased body iron stores (increased serum ferritin and transferrin saturation), and phlebotomy was reported to improve vascular function [47,48].
Is stored iron safe?
Sullivan [6] raised a second question in relation to iron and heart disease – Is stored iron safe? This question is important for AD because disturbances in iron homeostasis contribute to AD pathophysiology. Thus, oxidative stress/free radical damage is increased in brain in AD and mild cognitive impairment [49,50], and development of oxidative stress may be a critical early event in AD pathogenesis [51]. As was noted earlier, iron is the most abundant redox active metal in humans and iron could become more available in AD brain. Thus, blood-brain barrier dysfunction caused by AD-associated risk factors such as hypercholesterolemia could increase cerebrovascular permeability to circulating iron [52]. In addition, microhemorrhages are prominent in aging brain and implicated in AD progression [53,54]; heme oxygenase-mediated degradation of blood-derived heme releases iron that could be a source of oxidative stress and mitochondrial dysfunction in CNS [55]. Anomalies in iron regulatory proteins have been reported in AD brain [56,57]. Iron uptake in neurons could be increased in AD brain because a more stable IRE/IRP1 complex observed by Pinero and coworkers [57] is predicted to increase the stability of transferrin receptor mRNA. Intracellular iron storage in ferritin could be impaired in AD brain [57–60]. Consistent with the possibility that stored iron is unsafe, in vitro ferritin iron release assays suggest that elevated levels of molecules such as superoxide anion and nitric oxide in AD could release iron from ferritin in vivo [61] but the importance of this iron release mechanism in vivo is uncertain. If iron storage in ferritin is impaired in AD, ferroportin-mediated cellular iron efflux, which is briefly discussed below, could become an important mechanism for iron detoxification.
Ferroportin-1 (Fpn1, SLC11A3, IREG-1, MTP1) is a transmembrane iron exporting protein [3]. Dietary iron is absorbed through the apical surface of enterocytes that line the intestinal villi. Fpn1 mediates transport of ferrous iron through the basolateral membrane of the enterocyte. Fpn1 is widely distributed in the CNS where it was localized in most cell types, albeit with some inconsistency [62–65]. Its localization in neuronal perikarya, axons, dendrites and synaptic vesicles [63] is important because it suggests iron efflux is a potentially important mechanism for managing neuronal iron excess. In enterocytes, efficient cellular iron export by Fpn1 requires the copper-dependent ferroxidase, hephaestin, which oxidizes ferrous iron to ferric iron enabling iron binding by transferrin in interstitial fluid [3]. In the CNS, two ferroxidases, hephaestin and ceruloplasmin, may cooperate with Fpn1 in exporting iron to maintain optimal intracellular iron levels. The potential importance of neuronal iron export by Fpn1 is underscored by the observation that retinal iron overload and retinal degeneration occur in mice deficient in both ceruloplasmin and hephaestin, in spite of increased ferritin [66,67]. Ceruloplasmin levels in brain may be altered in AD [68,69] and it is noteworthy that increased redox active iron was present in basal ganglia obtained at autopsy from two individuals with aceruloplasminemia, an autosomal recessive disorder characterized by defective or absent ceruloplasmin function [70]. Additional studies will be needed to establish the significance of neuronal iron export by Fpn1 as an iron detoxifying mechanism in brain. The answer could be important for AD. Microtubule modification and dysfunctional axonal transport are early developments in AD [71,72]. Impaired distribution of Fpn1 and accessory proteins to appropriate membrane sites in cells could contribute to disturbances in iron homeostasis and oxidative stress.
Impaired iron management increases redox active iron in AD
Impaired physiological iron management could help to explain accumulation of hemosiderin and biogenic magnetite in AD brain [73], and redox active iron associated with senile plaques and neurofibrillary tangles, the pathological hallmarks of AD [74]. Possibly the most significant consequence of iron excess is expansion of the labile iron pool (LIP). The LIP is a small fraction of total cellular iron. It consists of Fe+2 and Fe+3 ions that are loosely bound to a variety of organic anions and macromolecules and in dynamic equilibrium with transport and stored forms of cellular iron [75,76]. Labile iron (so called free or redox active iron) is predominantly cytosolic but is also present in other subcellular compartments including the nucleus. Traditionally viewed as an “intracellular iron transit pool”, the LIP is a source of readily available iron to meet physiological needs. It is also a source of redox active iron capable of catalyzing formation of reactive oxygen species. The size of the LIP is subject to change depending on physiological conditions. Short-term low-level expansion of the LIP consistent with normal physiological functions may result in manageably increased production of reactive oxygen species. However, prolonged expansion of the LIP could be a cause of oxidative stress/ free radical damage, a critical early neuronal change in AD [51]. Does the LIP expand in AD? This is unknown and direct measurement of the LIP in vivo presents formidable technical problems. Nevertheless, indirect evidence supports this possibility. Redox active iron is increased in vulnerable neurons in AD brain and associates with nucleic acid [77,78]. This would explain conspicuous oxidative damage to DNA [79] and RNA [80]. The potential importance of iron-mediated oxidative stress/free radical damage in the development and progression of AD is underscored by efforts to develop iron chelators as therapeutic agents for AD [81,82]. In the sections to follow, we suggest that calibrated phlebotomy could also be a useful therapeutic option to reduce iron in AD.
A ferrocentric model of AD pathogenesis
We propose that an age-associated increase in body iron stores, which is potentially modifiable by diet and genetics, could be at the apex of a pathophysiological cascade that increases risk for AD and promotes disease progression (Fig. 1). A key idea is that increased body iron stores could increase risk for AD, even if they fall within the clinically “normal” range (see [12]). The model shown in Fig. 1 suggests how physiological iron excess could be a common cause for major pathological features of AD - oxidative stress and free radical damage to cell macromolecules, expression of cell cycle proteins in post-mitotic neurons, Aβ accumulation, and aberrant tau protein metabolism.
The model is consistent with important hypotheses regarding AD pathogenesis:
The “two-hit” hypothesis of AD causation that proposes development of oxidative stress/free radical damage and cell cycle abnormalities in vulnerable neurons, can act independently to initiate AD [51].
The two-hit hypothesis that development of cell cycle abnormalities in neurons and a second hit, perhaps inflammation or hypoxia, are critical for development of AD [83].
The amyloid cascade hypothesis that proposes accumulation of toxic forms of Aβ is the cause of neuronal dysfunction and cell loss in AD.
The tau hypothesis that proposes development of neurofibrillary tangles consisting primarily of hyperphosphorylated forms of microtubule-associated protein tau causes neuronal dysfunction and cell loss in AD.
The model also recognizes that Aβ accumulation and altered tau could possibly be a protective cellular response (albeit ineffective in the long-term) to cell stress in AD [84].
Implication for AD therapy
The ferrocentric model shown in Fig. 1 suggests a testable hypothesis on the causation and progression of AD: reducing body iron stores will improve vascular function, slow or possibly prevent neurodegenerative change, and improve cognitive and behavioral functions in individuals with AD. If begun early, iron reduction could prevent or delay disease onset in at-risk individuals. The hypothesis is readily testable. Phlebotomy and common sense measures such as limiting dietary intake of iron, is a safe and effective way to reduce body iron stores [8,9]. A single donation of 500 ml of whole blood removes 200–250 mg of iron and reduces serum ferritin levels by about 50 ng/ml in healthy men [42]. Graded phlebotomy as used in the FeAST study predictably reduced body iron stores to levels of serum ferritin approximating 25 ng/ml, which is typical of premenopausal women and conditioned athletes [7]. A simple formula is used to calculate the volume of blood to be removed to achieve this targeted nadir. Because blood donation 2–3 times per year should be sufficient to maintain targeted levels of serum ferritin, a high rate of compliance is expected. Iron reduction in peripheral tissues by phlebotomy is well documented, but how might phlebotomy reduce brain iron? Recent detection of Fpn1 in brain cells suggests cellular iron export is an important iron detoxifying mechanism [62–67]. Thus, iron exported from brain cells into extracellular fluid enters cerebrospinal fluid (CSF), which is continuous with the extracellular fluid, and returns to the blood stream when CSF is reabsorbed [87,88]. However, MRI measurement of brain iron stores (ferritin/hemosiderin) [85,86] may be desirable in initial studies because the rate of reduction of brain iron stores may lag the reduction of body iron stores. There is evidence for optimism with this experimental approach. Treatment with the iron chelator desferrioxamine (125 mg intramuscularly twice daily, 5 days per week for 24 months), slowed the rate of decline of daily living skills in AD patients [89]. In addition, intravenous infusion of desferrioxamine (500 mg twice a week for 10 months) reduced serum ferritin, decreased brain iron (by MRI), and improved clinical status of a patient with aceruloplasminemia [90]. Phlebotomy could be useful as an adjunct therapy. It could improve the efficacy of novel metal iron chelators, minimize potential toxicities associated with their use, and supplement other types of treatment such as those designed to reduce Aβ accumulation. Finally, testing the ferrocentric hypothesis could provide valuable mechanistic insight into competing theories of the etiology and pathophysiology of AD.
Acknowledgement
The authors would like to acknowledge Peter R. Sinclair PhD and Meghan L. Stone BS for valuable discussions on iron metabolism and help in the preparation of this manuscript.
Role of the funding source
Supported by the Office of Research and Development, Medical Research Service (BED) and the Cooperative Studies Program (LRZ), Department of Veterans Affairs, and by the National Institutes of Health and the Alzheimer’s Association (GP, MAS, XZ).
Abbreviations
- Aβ
amyloid-β
- AD
Alzheimer’s disease
- Fpn1
ferroportin
- HH
hereditary hemochromatosis
- IRE
iron responsive element
- IRP
iron regulatory protein
- LIP
labile iron pool
- T2DM
type 2 diabetes mellitus.
Footnotes
Inconsistent attempts to find linkage between Alzheimer’s disease and individual genes may reflect, as suggested by Connor and Lee’s review [32], numerous environmental and genetic factors affecting a complex disease.
Conflict of interest statement
The authors report no conflicts of interest.
Contributor Information
Barney E. Dwyer, Email: Barney.E.Dwyer@Dartmouth.edu.
Mark A. Smith, Email: mark.smith@case.edu.
References
- 1.Luchsinger JA. Adiposity, hyperinsulinemia, diabetes, and Alzheimer’s disease. An epidemiological perspective. Eur J Pharmacol. 2008;585:119–129. doi: 10.1016/j.ejphar.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bush AI. The metallobiology of Alzheimer’s disease. Trends Neurosci. 2003;26:207–214. doi: 10.1016/S0166-2236(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 3.Dunn LL, Rahmanto YS, Richardson DR. Iron uptake and metabolism in the new millennium. Trends Cell Biol. 2006;17:93–100. doi: 10.1016/j.tcb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan JL. Iron and the sex difference in heart disease rate. Lancet. 1981;1:1293–1294. doi: 10.1016/s0140-6736(81)92463-6. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan JL. Misconceptions in the debate on the iron hypothesis. J Nutr Biochem. 2001;12:33–37. doi: 10.1016/s0955-2863(00)00142-x. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan JL. Is stored iron safe? J Lab Clin Med. 2004;144:280–284. doi: 10.1016/j.lab.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Zacharski LR, Ornstein DL, Woloshin S, Schwartz LM. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am Heart J. 2000;140:98–104. doi: 10.1067/mhj.2000.106646. [DOI] [PubMed] [Google Scholar]
- 8.Zacharski LR, Chow BK, Lavori PW, et al. The iron (Fe) and atherosclerosis study (FeAST): a pilot study of reduction of body iron stores in atherosclerotic peripheral vascular disease. Am Heart J. 2000;139:337–345. doi: 10.1067/mhj.2000.102909. [DOI] [PubMed] [Google Scholar]
- 9.Zacharski LR, Chow BK, Howes PS, et al. Reduction of iron stores and cardiovascular outcomes in patients with peripheral artery disease. A randomized controlled trial. JAMA. 2007;297:603–610. doi: 10.1001/jama.297.6.603. [DOI] [PubMed] [Google Scholar]
- 10.Brewer GJ. Iron and copper toxicity in diseases of aging, particularly atherosclerosis and Alzheimer’s disease. Exp Biol Med. 2007;232:323–335. [PubMed] [Google Scholar]
- 11.McDowell I. Alzheimer’s disease: insights from epidemiology. Aging Clin Exp Res. 2001;13:143–162. doi: 10.1007/BF03351474. [DOI] [PubMed] [Google Scholar]
- 12.Zacharski LR, Gerhard GS. Atherosclerosis: a manifestation of chronic iron toxicity? Vasc Med. 2003;8:153–155. doi: 10.1191/1358863x03vm492ed. [DOI] [PubMed] [Google Scholar]
- 13.Haidari M, Javadi E, Sanati A, Hajilooi M, Ghanbili J. Association of increased ferritin with premature coronary stenosis in men. Clin Chem. 2001;47:1666–1672. [PubMed] [Google Scholar]
- 14.Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de la Torre JC. Alzheimer disease as a vascular disorder. Nosological evidence. Stroke. 2002;33:1152–1162. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- 16.de la Torre JC. Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184–190. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- 17.de la Torre JC. Is Alzheimer’s disease preceded by neurodegeneration or cerebral hypoperfusion. Ann Neurol. 2005;57:783–784. doi: 10.1002/ana.20516. [DOI] [PubMed] [Google Scholar]
- 18.de la Torre JC. Alzheimer’s disease: how does it start? J Alzheimers Dis. 2002;4:497–512. doi: 10.3233/jad-2002-4606. [DOI] [PubMed] [Google Scholar]
- 19.Kalback W, Esh C, Castano EM, et al. Atherosclerosis, vascular amyloidosis and brain hypoperfusion in the pathogenesis of sporadic Alzheimer’s disease. Neurol Res. 2004;26:525–539. doi: 10.1179/016164104225017668. [DOI] [PubMed] [Google Scholar]
- 20.Ruitenberg A, den Hejier T, Bakker SLM, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam study. Ann Neurol. 2005;57:789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 21.Hofman A, Breteler MM, Bots ML, et al. Atherosclerosis, apolipoprotein E and prevalence of dementia and Alzheimer’s disease in the Rotterdam study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- 22.Millan M, Sobrino T, Castellanos M, et al. Increased body iron stores are associated with poor outcome after thrombolytic treatment in acute stroke. Stroke. 2007;38:90–95. doi: 10.1161/01.STR.0000251798.25803.e0. [DOI] [PubMed] [Google Scholar]
- 23.Luchsinger JA, Reitz C, Patel B, Tang M-X, Manly JJ, Mayeaux R. Relation of diabetes to mild cognitive impairment. Arch Neurol. 2007;64:570–575. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 24.Jiang R, Manson JE, Meigs JB, Ma J, Rifai N, Hu FB. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA. 2004;291:711–717. doi: 10.1001/jama.291.6.711. [DOI] [PubMed] [Google Scholar]
- 25.Forouhi NG, Harding AH, Allison M, et al. Elevated serum ferritin levels predict new-onset type 2 diabetes: results from the EPIC-Norfolk prospective study. Diabetologia. 2007;50:949–956. doi: 10.1007/s00125-007-0604-5. [DOI] [PubMed] [Google Scholar]
- 26.Jehn ML, Guallar E, Clark JM, et al. A prospective study of plasma ferritin level and incident diabetes. The atherosclerosis risk in communities (ARIC) study. Am J Epidemiol. 2007;165:1047–1054. doi: 10.1093/aje/kwk093. [DOI] [PubMed] [Google Scholar]
- 27.Swaminathan S, Fonseca VA, Alam MG, Shah SV. The role of iron in diabetes and its complications. Diabetes Care. 2007;30:1926–1933. doi: 10.2337/dc06-2625. [DOI] [PubMed] [Google Scholar]
- 28.Craft S. Insulin resistance syndrome and Alzheimer disease: pathophysiologic mechanisms and therapeutic implications. Alzheimer Dis Assoc Disord. 2006;20:298–301. doi: 10.1097/01.wad.0000213866.86934.7e. [DOI] [PubMed] [Google Scholar]
- 29.Rao G. Insulin resistance syndrome. Am Fam Physician. 2001;63:1165–1166. [PubMed] [Google Scholar]
- 30.Vari IS, Balkau B, Kettaneh A, et al. Ferritin and transferrin are associated with metabolic syndrome abnormalities and their change over time in a general population. Data from an epidemiological study on insulin resistance syndrome (DESIR) Diabetes Care. 2007;30:1795–1801. doi: 10.2337/dc06-2312. [DOI] [PubMed] [Google Scholar]
- 31.Batts KP. Iron overload syndromes and the liver. Mod Pathol. 2007;20:S31–S39. doi: 10.1038/modpathol.3800715. [DOI] [PubMed] [Google Scholar]
- 32.Connor JR, Lee SY. HFE mutations and Alzheimer’s disease. J Alzheimers Dis. 2006;10:267–276. doi: 10.3233/jad-2006-102-311. [DOI] [PubMed] [Google Scholar]
- 33.Puglielli L, Tanzi RE, Kovacs DM. Alzheimer’s disease: the cholesterol connection. Nat Neurosci. 2003;6:345–351. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- 34.Minquin R, Rajendran R, Pan N, et al. The iron chelator desferrioxamine inhibits atherosclerotic lesion development and decreases lesion iron concentrations in the cholesterol-fed rabbit. Free Rad Biol Med. 2005;38:1206–1211. doi: 10.1016/j.freeradbiomed.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Ghribi O, Golovko MY, Larsen B, Schrag M, Murphy EJ. Deposition of iron and β-amyloid plaques is associated with cortical cellular damage in rabbits fed longterm cholesterol-enriched diets. J Neurochem. 2006;99:438–449. doi: 10.1111/j.1471-4159.2006.04079.x. [DOI] [PubMed] [Google Scholar]
- 36.Mueller PP, May T, Perz A, Hauser H, Peuster M. Control of smooth muscle cell proliferation by ferrous iron. Biomaterials. 2006;27:2193–2200. doi: 10.1016/j.biomaterials.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Real JM, Lopez-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes. 2002;51:2348–2354. doi: 10.2337/diabetes.51.8.2348. [DOI] [PubMed] [Google Scholar]
- 38.Shinall H, Song ES, Hersh LB. Susceptibility of amyloid beta peptide degrading enzymes to oxidative damage: a potential Alzheimer’s disease spiral. Biochemistry. 2005;44:15345–15350. doi: 10.1021/bi050650l. [DOI] [PubMed] [Google Scholar]
- 39.Farris W, Mansourian S, Chang Y, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci USA. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salonen JT, Tuomainen T-P, Salonen R, Lakka TA, Nyyssonen K. Donation of blood is associated with reduced risk of myocardial infarction. The Kuopio ischaemic heart disease risk factor study. Am J Epidemiol. 1998;148:445–451. doi: 10.1093/oxfordjournals.aje.a009669. [DOI] [PubMed] [Google Scholar]
- 41.Meyers DG, Jensen KC, Menitove JE. A historical cohort study of the effect of lowering body iron through blood donation on incident cardiac events. Transfusion. 2002;42:1135–1139. doi: 10.1046/j.1537-2995.2002.00186.x. [DOI] [PubMed] [Google Scholar]
- 42.Zheng H, Cable R, Spencer B, Votto N, Katz SD. Iron stores and vascular function in voluntary blood donors. Arterioscler Thromb Vasc Biol. 2005;25:1577–1583. doi: 10.1161/01.ATV.0000174126.28201.61. [DOI] [PubMed] [Google Scholar]
- 43.Sullivan JL. Stored iron and vascular reactivity. Arterioscler Thromb Vasc Biol. 2005;25:1532–1535. doi: 10.1161/01.ATV.0000174124.20147.22. [DOI] [PubMed] [Google Scholar]
- 44.Aliev G, Smith MA, Obrenovich ME, de la Torre JC, Perry G. Role of vascular hypoperfusion-induced oxidative stress and mitochondrial failure in the pathogenesis of Alzheimer disease. Neurotox Res. 2003;5:491–504. doi: 10.1007/BF03033159. [DOI] [PubMed] [Google Scholar]
- 45.Zhu X, Smith MA, Honda K, et al. Vascular oxidative stress in Alzheimer disease. J Neurol Sci. 2007;257:240–246. doi: 10.1016/j.jns.2007.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valenti L, Fracanzani AL, Dongiovanni P, et al. Iron depletion by phlebotomy improves insulin resistance in patients with nonalcoholic fatty liver disease and hyperferritinemia: evidence from a case-control study. Am J Gastroenterol. 2007;102:1251–1258. doi: 10.1111/j.1572-0241.2007.01192.x. [DOI] [PubMed] [Google Scholar]
- 47.Failla M, Giannattasio C, Piperno A, et al. Radial artery wall alterations in genetic hemochromatosis before and after iron depletion. Hepatology. 2000;32:569–573. doi: 10.1053/jhep.2000.16265. [DOI] [PubMed] [Google Scholar]
- 48.Gaenzer H, Marschang P, Sturm W, et al. Association between increased iron stores and impaired endothelial function in patients with hereditary hemochromatosis. J Am Coll Cardiol. 2002;40:2189–2194. doi: 10.1016/s0735-1097(02)02611-6. [DOI] [PubMed] [Google Scholar]
- 49.Lovell MA, Markesbery WR. Oxidative damage in mild cognitive impairment and early Alzheimer’s disease. J Neurosci Res. 2007;85:3036–3040. doi: 10.1002/jnr.21346. [DOI] [PubMed] [Google Scholar]
- 50.Zhu X, Su B, Wang X, Smith MA, Perry G. Causes of oxidative stress in Alzheimer disease. Cell Mol Life Sci. 2007;64:2202–2210. doi: 10.1007/s00018-007-7218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu X, Lee H, Perry G, Smith MA. Alzheimer disease, the two-hit hypothesis: an update. Biochim Biophys Acta. 2006;1772:494–502. doi: 10.1016/j.bbadis.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 52.Ong W-Y, Halliwell B. Iron, atherosclerosis, and neurodegeneration. A key role for cholesterol in promoting iron-dependent oxidative damage. Ann NY Acad Sci. 2004;1012:51–64. doi: 10.1196/annals.1306.005. [DOI] [PubMed] [Google Scholar]
- 53.Cullen KM, Kocsi Z, Stone J. Pericapillary haem-rich deposits: evidence for microhaemorrhages in aging human cerebral cortex. JCBFM. 2005;25:1656–1667. doi: 10.1038/sj.jcbfm.9600155. [DOI] [PubMed] [Google Scholar]
- 54.Cullen KM, Kocsi Z, Stone J. Microvascular pathology in the aging human brain: evidence that senile plaques are sites of microhaemorrhages. Neurobiol Aging. 2006;27:1786–1796. doi: 10.1016/j.neurobiolaging.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 55.Schipper HM. Brain iron deposition and the free radical-mitochondrial theory of ageing. Ageing Res Rev. 2004;3:265–301. doi: 10.1016/j.arr.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Smith MA, Wehr K, Harris PL, Siedlak SL, Connor JR, Perry G. Abnormal localization of iron regulatory protein in Alzheimer’s disease. Brain Res. 1998;788:232–236. doi: 10.1016/s0006-8993(98)00002-x. [DOI] [PubMed] [Google Scholar]
- 57.Pinero DJ, Hu J, Connor JR. Alterations in the interaction between iron regulatory proteins and their iron responsive element in normal and Alzheimer’s diseased brains. Cell Mol Biol. 2000;46:761–776. [PubMed] [Google Scholar]
- 58.Grundke-Iqbal I, Fleming J, Tung YC, Lassman H, Iqbal K, Joshi JG. Ferritin is a component of the neuritic (senile) plaque in Alzheimer dementia. Acta Neuropathol. 1990;81:105–110. doi: 10.1007/BF00334497. [DOI] [PubMed] [Google Scholar]
- 59.Connor JR, Snyder BS, Arosio P, Foeffler DA, LeWitt P. A quantitative analysis of isoferritins in select regions of aged, Parkinsonian and Alzheimer’s diseased brains. J Neurochem. 1995;65:717–724. doi: 10.1046/j.1471-4159.1995.65020717.x. [DOI] [PubMed] [Google Scholar]
- 60.Zecca L, Youdim MBH, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 61.Reif DW. Ferritin as a source of iron for oxidative damage. Free Rad Biol Med. 1992;12:417–427. doi: 10.1016/0891-5849(92)90091-t. [DOI] [PubMed] [Google Scholar]
- 62.Burdo JR, Menzies SL, Simpson IA, et al. Distribution of divalent metal transporter 1 and metal transport in the normal and Belgrade rat. J Neurosci Res. 2001;66:1198–1207. doi: 10.1002/jnr.1256. [DOI] [PubMed] [Google Scholar]
- 63.Wu LJ, Leenders AGM, Cooperman S, et al. Expression of the iron transporter ferroportin in synaptic vesicles and the blood-brain barrier. Brain Res. 2004;1001:108–117. doi: 10.1016/j.brainres.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 64.Moos T, Nielsen TR. Ferroportin in the postnatal rat brain: implications for axonal transport and neuronal export of iron. Semin Pediatr Neurol. 2006;13:149–157. doi: 10.1016/j.spen.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 65.Rouault TA, Cooperman S. Brain iron metabolism. Semin Pediatr Neurol. 2006;13:142–148. doi: 10.1016/j.spen.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Hahn P, Dentchev T, Qian Y, Rouault T, Harris ZL, Dunaief JL. Immunolocalization and regulation of iron handling proteins ferritin and ferroportin in the retina. Mol Vis. 2004;10:598–607. [PubMed] [Google Scholar]
- 67.Hahn P, Qian Y, Dentchev T, et al. Disruption of ceruloplasmin and hephaestin in mice causes retinal iron overload and retinal degeneration with features of age-related macular degeneration. Proc Natl Acad Sci USA. 2004;101:13850–13855. doi: 10.1073/pnas.0405146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Castellani RJ, Smith MA, Nunomura A, Harris PLR, Perry G. Is increased redoxactive iron in Alzheimer disease a failure of the copper-binding protein ceruloplasmin? Free Rad Biol Med. 1999;26:1508–1512. doi: 10.1016/s0891-5849(99)00016-7. [DOI] [PubMed] [Google Scholar]
- 69.Loeffler DA, Sima AAF, LeWitt PA. Ceruloplasmin immunoreactivity in neurodegenerative disorders. Free Rad Res. 2001;35:111–118. doi: 10.1080/10715760100300651. [DOI] [PubMed] [Google Scholar]
- 70.Gonzalez-Cuyar LF, Perry G, Miyajima H, et al. Redox active iron accumulation in aceruloplasminemia. Neuropathol. 2008 doi: 10.1111/j.1440-1789.2008.00901.x. [DOI] [PubMed] [Google Scholar]
- 71.Stokin GB, Lillo C, Falzone TL, et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- 72.Zhu X, Moreira PI, Smith MA, Perry G. Alzheimer’s disease: an intracellular movement disorder? Trends Mol Med. 2005;11:391–393. doi: 10.1016/j.molmed.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 73.Castellani RJ, Moreira PI, Liu G, et al. Iron: the redox center of oxidative stress in Alzheimer disease. Neurochem Res. 2007;32:1640–1645. doi: 10.1007/s11064-007-9360-7. [DOI] [PubMed] [Google Scholar]
- 74.Sayre LM, Perry G, Harris PLR, Liu Y, Schubert KA, Smith MA. In situ oxidative catalysis by neurofibrillary tangles and senile plaques in Alzheimer’s disease: a central role for bound transition metals. J Neurochem. 2000;74:270–279. doi: 10.1046/j.1471-4159.2000.0740270.x. [DOI] [PubMed] [Google Scholar]
- 75.Petrat F, de Groot H, Sustmann R, Rauen U. The chelatable iron pool in living cells: a methodically defined quantity. Biol Chem. 2000;383:489–502. doi: 10.1515/BC.2002.051. [DOI] [PubMed] [Google Scholar]
- 76.Kruszewski M. Labile iron pool: the main determinant of cellular response to oxidative stress. Mutat Res. 2003;531:81–92. doi: 10.1016/j.mrfmmm.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 77.Smith MA, Harris PLR, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci USA. 1997;94:9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Honda K, Smith MA, Zhu X, et al. Ribosomal RNA in Alzheimer disease is oxidized by bound redox-active iron. J Biol Chem. 2005;280:20978–20986. doi: 10.1074/jbc.M500526200. [DOI] [PubMed] [Google Scholar]
- 79.Wang J, Xiong S, Xie C, Markesberry WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer’s disease. J Neurochem. 2005;93:953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- 80.Castellani RJ, Nunomura A, Rolston RK, et al. Sublethal RNA oxidation as a mechanism for neurodegenerative disease. Int J Mol Sci. 2008;9:789–806. doi: 10.3390/ijms9050789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Whitnall M, Richardson DR. Iron: a new target for pharmacological intervention in neurodegenerative diseases. Semin Pediatr Neurol. 2006;13:186–197. doi: 10.1016/j.spen.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 82.Amit T, Avramovich-Tirosh Y, Youdim MBH, Mandel S. Targeting multiple Alzheimer’s disease etiologies with multimodal neuroprotective and neurorestorative iron chelators. FASEB J. 2008;22:1296–1305. doi: 10.1096/fj.07-8627rev. [DOI] [PubMed] [Google Scholar]
- 83.Yang Y, Herrup K. Cell division in the CNS: protective response or lethal event in post-mitotic neurons. Biochim Biophys Acta. 2007;1772:457–466. doi: 10.1016/j.bbadis.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Castellani RJ, Lee H-G, Zhu X, Nunomura A, Perry G, Smith MA. Neuropathology of Alzheimer disease: pathognomonic but not pathogenic. Acta Neuropathol. 2006;111:503–509. doi: 10.1007/s00401-006-0071-y. [DOI] [PubMed] [Google Scholar]
- 85.Bartzokis G, Tishler TA, Shin I-S, Lu PH, Cummings JL. Brain ferritin iron as a risk factor for age at onset in neurodegenerative diseases. Ann NY Acad Sci. 2004;1012:224–236. doi: 10.1196/annals.1306.019. [DOI] [PubMed] [Google Scholar]
- 86.Bartzokis G, Tishler TA, Lu PH, et al. Brain ferritin iron may influence age- and gender-related risks of neurodegeneration. Neurobiol Aging. 2007;28:414–423. doi: 10.1016/j.neurobiolaging.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 87.Johanson CE, Duncan JA, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. doi: 10.1186/1743-8454-5-10. Available from < http://www.cerebrospinalfluidresearch.com/content/5/1/ 10>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bradbury MWB. Transport of iron in the blood-brain-cerebrospinal fluid system. J Neurochem. 1997;69:443–454. doi: 10.1046/j.1471-4159.1997.69020443.x. [DOI] [PubMed] [Google Scholar]
- 89.Crapper McLachlan DR, Dalton AJ, Kruck TP, et al. Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet. 1991;337:1304–1308. doi: 10.1016/0140-6736(91)92978-b. [DOI] [PubMed] [Google Scholar]
- 90.Miyajima H, Takahashi Y, Kamata T, Shimizu H, Sakai N, Gitlin JD. Use of desferrioxamine in the treatment of aceruloplasminemia. Ann Neurol. 1997;41:404–407. doi: 10.1002/ana.410410318. [DOI] [PubMed] [Google Scholar]
- 91.Zhu X, Rottkamp CA, Boux H, Takeda A, Perry G, Smith MA. Activation of p38 kinase links tau phosphorylation, oxidative stress, and cell cycle-related events in Alzheimer disease. J Neuropath Exp Neurol. 2000;59:880–888. doi: 10.1093/jnen/59.10.880. [DOI] [PubMed] [Google Scholar]
- 92.Egana JT, Zambrano C, Nunez MT, Gonzalez-Billault C, Maccioni RB. Iron-induced oxidative stress modify tau phosphorylation patterns in hippocampal cell cultures. Biometals. 2003;16:215–223. doi: 10.1023/a:1020727218493. [DOI] [PubMed] [Google Scholar]
- 93.Zhu X, Raina AK, Lee H, Casadesus G, Smith MA, Perry G. Oxidative stress signaling in Alzheimer’s disease. Brain Res. 2004;1000:32–39. doi: 10.1016/j.brainres.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 94.Kondziella D, Zetterberg H. Hyperphosphorylation of tau protein in superficial siderosis. J Neurol Sci. 2008 doi: 10.1016/j.jns.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 95.Hwang EM, Kim S-Y, Sohn J-H, et al. Furin is an endogenous regulator of α-secretase associated with APP processing. Biochem Biophys Res Commun. 2006;349:654–659. doi: 10.1016/j.bbrc.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 96.Silvestri L, Camaschella C. A potential pathogenetic role of iron in Alzheimer’s disease. J Cell Mol Med, Postprint. doi: 10.1111/j.1582-4934.2008.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rogers JT, Randall JD, Cahill CM, et al. An iron-responsive element type II in the 5’-untranslated region of the Alzheimer’s amyloid precursor protein transcript. J Biol Chem. 2002;277:45518–45528. doi: 10.1074/jbc.M207435200. [DOI] [PubMed] [Google Scholar]