Abstract
In spite of recent advancements in the treatment of pulmonary hypertension (PH), successful control is yet to be accomplished. Abundant presence of ACE2 in the lungs, and its impressive effect in the prevention of acute lung injury, led us to test the hypothesis that pulmonary overexpression of this enzyme could produce beneficial outcomes against PH. Monocrotaline (MCT) treatment of mice for eight weeks resulted in significant increases in right ventricular systolic pressure (RVSP), right ventricle/left ventricle + septal (RV/LV+S) weight ratio and muscularization of pulmonary vessels. Administration of a lentiviral vector containing ACE2 (lenti-ACE2) seven days prior to MCT treatment prevented the increases in RVSP (Control: 25 ± 1 mmHg; MCT: 44 ± 5 mmHg; MCT+ACE2: 26 ± 1 mmHg, n=6, p<0.05) and RV/LV +S weight ratio (Control: 0.25 ± 0.01 mg/mg; MCT: 0.31 ± 0.01 mg/mg; MCT+ACE2: 0.26 ± 0.01mg/mg, n=8, p<0.05). A significant attenuation in muscularization of pulmonary vessels induced by MCT was also observed in animals overexpressing ACE2. These beneficial effects were associated with an increase in the AT2 receptor/AT1 receptor mRNA ratio. Also, PH-induced increases in pro-inflammatory cytokines were significantly attenuated by lenti-ACE2 treatment. Furthermore, ACE2 gene transfer in mice following six weeks of MCT treatment resulted in significant reversal of RVSP. These observations demonstrate that ACE2 overexpression prevents and reverses RVSP and associated pathophysiology in MCT-induced PH by a mechanism involving a shift from the vasoconstrictive, proliferative and fibrotic axis to the vasoprotective axis of the renin-angiotensin system and inhibition of pro-inflammatory cytokines.
Keywords: Cardiovascular diseases, Gene therapy, Hypertension, pulmonary, Lung, Remodeling
Introduction
Pulmonary hypertension (PH) is a refractory disease characterized by a progressive increase in pulmonary artery pressure and resistance. The remodeling in pulmonary arterioles results in PH, increased pulmonary vascular resistance, right ventricular (RV) hypertrophy, and right heart failure. Whereas the pathogenesis of PH is poorly understood, it has been proposed that endothelial dysfunction or damage could be involved.1, 2
Previous studies have implicated the involvement of the renin-angiotensin system (RAS) in the pathogenesis of PH. Evidence for this conclusion include the following: (i) lungs are the primary site for angiotensin-converting enzyme (ACE) expression3 and are responsible for the generation of high concentrations of circulating and pulmonary angiotensin (Ang) II4; (ii) other components of the RAS including renin, angiotensinogen, and both subtypes of Ang II receptors are expressed in the lungs4–10; (iii) increase in ACE in pulmonary vasculature has been associated with PH in both animal models and in patients11,12 and; (iv) ACE inhibitors have been shown to attenuate PH in animal models.11,13,14 In spite of this, ACE inhibitors (ACEi) and angiotensin receptor blockers (ARBs) have not proven to be very effective in the management of pulmonary diseases.15,16 This may be primarily due to the fact that these drugs lower basal systemic blood pressure. This would be counter-productive in PH patients since most of them already exhibit lower blood pressure. In addition, the limited/lack of success of these orally-administered therapeutic agents may be related to the differential tissue distribution and drug-specific pharmacodymanics that could limit their therapeutic concentrations in lung tissue.17,18,19 These observations, taken together with the well established hypertrophic actions and emerging role of pro-inflammatory signaling by Ang II and AT1 receptors,2,20,21 suggest that the involvement of the RAS in PH should be re-examined. This view takes on an added relevance since the discovery of ACE2, as a new member of the RAS.
ACE2, a homolog of ACE, shares approximately 42% structural identity with the catalytic domain of ACE and cleaves a single residue from Ang I to generate Ang-(1–9). More importantly, ACE2 degrades Ang II into Ang-(1–7) with high efficiency.22,23 Thus, ACE2 is an important player in the vasoprotective axis [ACE2-Ang-(1–7)-Mas] of the RAS and is critical in balancing the activity of the vasoconstrictive, proliferative and fibrotic axis (ACE-Ang II-AT1 receptor) of the RAS.24 ACE2 is highly expressed in the lungs25,26 and recent evidence suggest its pivotal role in pulmonary physiology and pathophysiology. This evidence include the following: (i) ACE2 knock-out mice develop pulmonary congestion and increased incidence of congestive heart failure27 (ii) ACE2 expression is down-regulated in both human and experimental lung fibrosis28 (iii) alterations in the expression of ACE2 in primary pulmonary hypertensive patients show a direct correlation between AT2 receptors and Ang-(1–7)-forming activity29 (iv) ACE2 has been shown to protect lungs from acute respiratory distress syndrome and acute lung injury, which involves increases in two vasoprotective members of the RAS: Ang-(1–7) and AT2 receptors25,26 and; (v) administration of recombinant ACE2 attenuates lung failure in ACE2 knock-out mice.30 Collectively, these observations led us to hypothesize that ACE2 overexpression would produce beneficial effects on PH. Thus, we employed gene transfer techniques utilizing lentiviral vector to obtain long-term expression of ACE2 in the lungs of mice.
Methods
Production of lentiviral-mediated overexpression of ACE2 viral Particles, determination of transduction efficiency of lung by lentivirus, cardiac hypertrophy, immunohistochemical analysis, RNA Isolation and Real-time PCR are described in the supplemental information (available online at http://hyper.ahajournals.org).
Animals
Five to six week old male C57/BL6 mice were utilized and housed in a temperature-controlled room (25 ± 1°C). Animals were maintained on a 12:12 hour light: dark cycle with free access to water and food. All procedures involving experimental animals were approved by the Institutional Animal Care and Use Committee (IACUC) at University of Florida and complied with NIH guidelines.
Experimental Design
PH was induced by weekly subcutaneous injections of 600 mg/kg monocrotaline (MCT) (Sigma-Aldrich) for 8 weeks. Control mice received saline (20 µl/g, s.c., 8 weeks). Two protocols were used, one to assess prevention of PH with ACE2 overexpression and another one to determine the ability of ACE2 gene transfer on the reversal of PH. In the prevention protocol, 7 days after lenti-GFP or lenti-ACE2 treatment, mice were treated with MCT or saline for 8 weeks, while in the reversal protocol, mice were started on MCT or saline for 6 weeks prior to GFP or ACE2 gene transfer and then continued on MCT or saline for an additional 2 weeks. A time course experiment demonstrated that six weeks of MCT treatment yielded near maximal histological changes in lung tissue that were not different than those observed at an eight week time point (data not shown). Animals in the control and MCT-treated group were the same for both the protocols. Gene delivery of lenti-GFP (control) and lenti-ACE2 (3×106 TU in 30 µl of PBS) was accomplished by injection of the virus into the trachea of anesthetized mice as described above.
Blood Pressure measurements
Seven to ten days after the last injection of MCT, systemic blood pressure was measured in conscious mice by the tail-cuff method (n=6–8 in each group) as previously described31. Right ventricular systolic pressure (RVSP) was used as an indicator for pulmonary blood pressure. For this measurement mice were anesthetized with a mixture of ketamine (100 mg/kg, s.c.) and xylazine (15 mg/kg, s.c.) and were placed in a supine position, breathing room air. A catheter was inserted into the right descending jugular vein and forwarded to the RV. The data was recorded after stabilization of the tracing using a liquid pressure transducer, which was interfaced to a PowerLab (AD Instruments) unit. The waveform was used to confirm the positioning of the catheter in the RV. Data were analyzed by using the Chart program that was supplied with the PowerLab system. After RVSP measurement, mice were euthanized and the hearts and lungs were harvested.
Statistical analysis
Data are presented as means ± SEM. Statistical differences were evaluated by either Student’s t-test, one-way ANOVA or two-way ANOVA wherever applicable, followed by the Newman-Keuls post-hoc test. p values <0.05 were considered statistically significant.
Results
In Vivo Gene Delivery Into the lungs
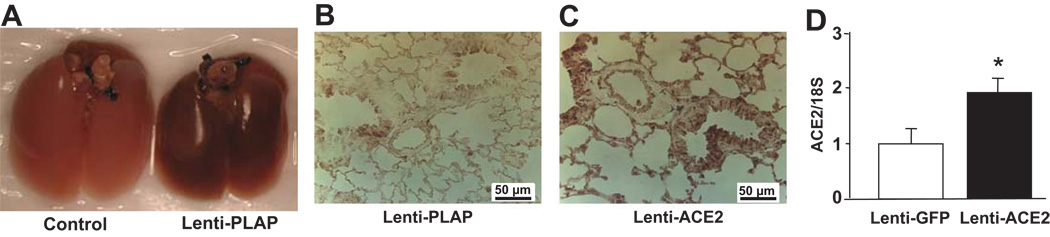
A robust, widespread and random transduction of pulmonary tissue was observed as early as 7 days following intratracheal gene transfer with lentiviral vector containing placental alkaline phosphatase (PLAP - Fig. 1a). The expression persisted for the duration of experimental protocols (8–10 weeks). No visible transduction was detected in kidneys and heart (data not shown). Intratracheal administration of lenti-ACE2 resulted in an indiscriminate expression ACE2 in the pulmonary tissue (Fig. 1b and c). Immunohistochemistry revealed that ACE2 immunoreactivity was observed in the bronchiolar epithelial and alveolar cells (Fig. 1c). Lentiviral-mediated ACE2 gene transfer into the lungs resulted in approximately 2 fold increase in ACE2 mRNA levels compared with control animals (Fig. 1d). Furthermore, Ang-(1–7) immunoreactivity was observed in the arterial epithelial and alveolar cells of the control lungs (Supplemental Figure 1- a and c) which was significantly increased (~30%) by lenti-ACE2 injection into the trachea (Supplemental Figure 1- b and d).
Figure 1.
a: Transduction of lungs with lenti-PLAP
Mice were injected with 3×106 TU of lenti-PLAP intratracheally as described in the Methods section. Seven days following viral administration, lungs were removed, inflated, fixed and subjected to PLAP staining. The dark staining in the right pair of lungs showed in Figure 1A indicates the expression of PLAP, demonstrating the effectiveness of the transduction of lungs with lenti-PLAP.
Figure 1b and c: Transduction of the lung with lenti-ACE2
Mice were injected with 3×106 TU of lenti-PLAP (c) or lenti-ACE2 (d). Seven days following transduction, lungs were inflated, fixed and subjected to ACE2 immunoreactivity as described in the Methods section. ACE2 immunoreactivity was significantly higher in lenti-ACE2-treated lungs compared to control lenti-PLAP-treated lungs.
Figure 1d: ACE2 mRNA in lungs treated with lenti-ACE2
Seven days following lenti-ACE2 or lenti-GFP gene transfer, as described before, total mRNA was isolated and subjected to real-time PCR to quantify ACE2 mRNA levels. Data are represented as mean ± SEM (n=4). * p<0.05 vs. Lenti-GFP.
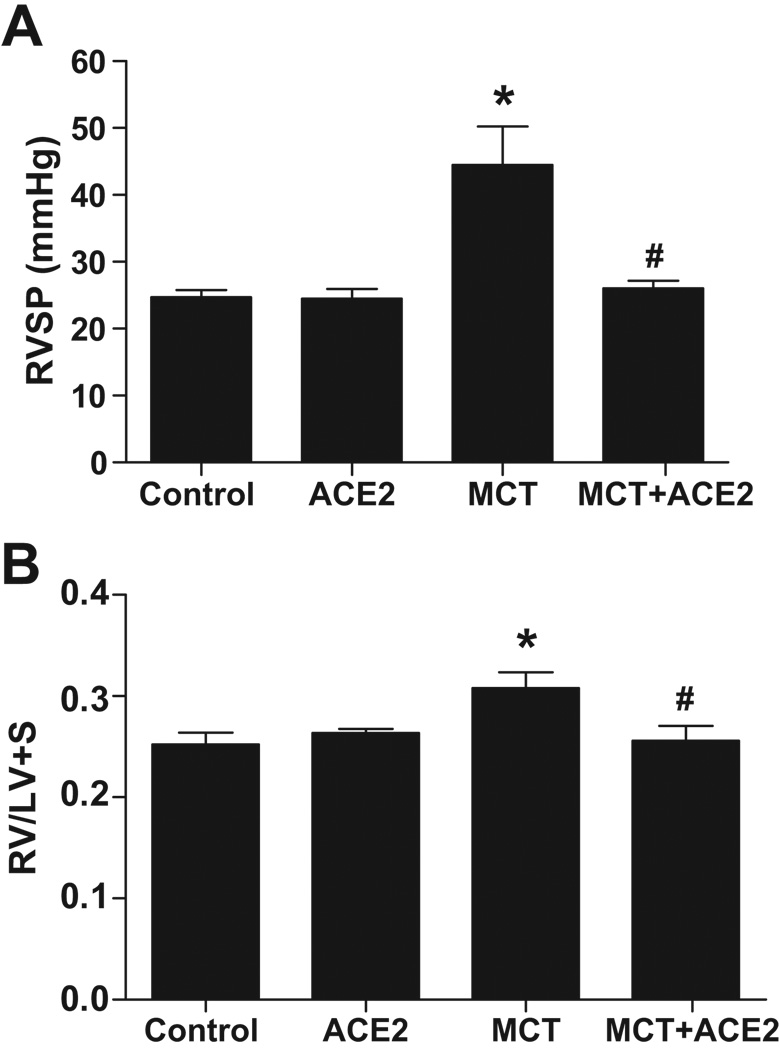
Prevention of PH and associated cardiac and pulmonary damages by ACE2
Two-way ANOVA revealed significant interaction between the pulmonary pressure lowering effect of lenti-ACE2 and monocrotaline administration. Weekly injection of MCT for 8 weeks resulted in an increase in RVSP (44 ± 5 mmHg, MCT vs 25 ± 1 mmHg, control, p<0.05, Fig. 2a), which was prevented with ACE2 gene transfer (26 ± 1 mmHg, Fig. 2a). Also, the RV/LV+S ratio was significantly increased in MCT-treated mice compared with control group (0.31 ± 0.01 mg/mg, MCT vs 0.25 ± 0.01 mg/mg, control, p<0.05, Fig. 2b). This increase was also prevented with ACE2 gene transfer treatment (0.26 ± 0.01 mg/mg, Fig. 2b). Lenti-ACE2 administration significantly reduced pulmonary hypertension only in the MCT treated group and not in the control group. No significant differences in systemic blood pressure were observed among any of the groups (systolic blood pressure: Control, 127 ± 3 mmHg; ACE2: 113 ± 4 mmHg; MCT: 119 ± 3 mmHg; MCT+ACE2: 123 ± 4 mmHg).
Figure 2. Effects of lenti-ACE2 on the prevention of MCT-induced PH.
Mice were injected with 3×106 TU of lenti-ACE2. Animals were subjected to weekly saline (control) or MCT treatment seven days following gene transfer for 8 weeks. Effects on (a) RVSP and (b) RV hypertrophy: RVSP and RV hypertrophy were measured as described in the Methods. Data are represented as mean ± SEM. * p<0.05 vs. control group, # p<0.05 vs MCT group (n=6–8).
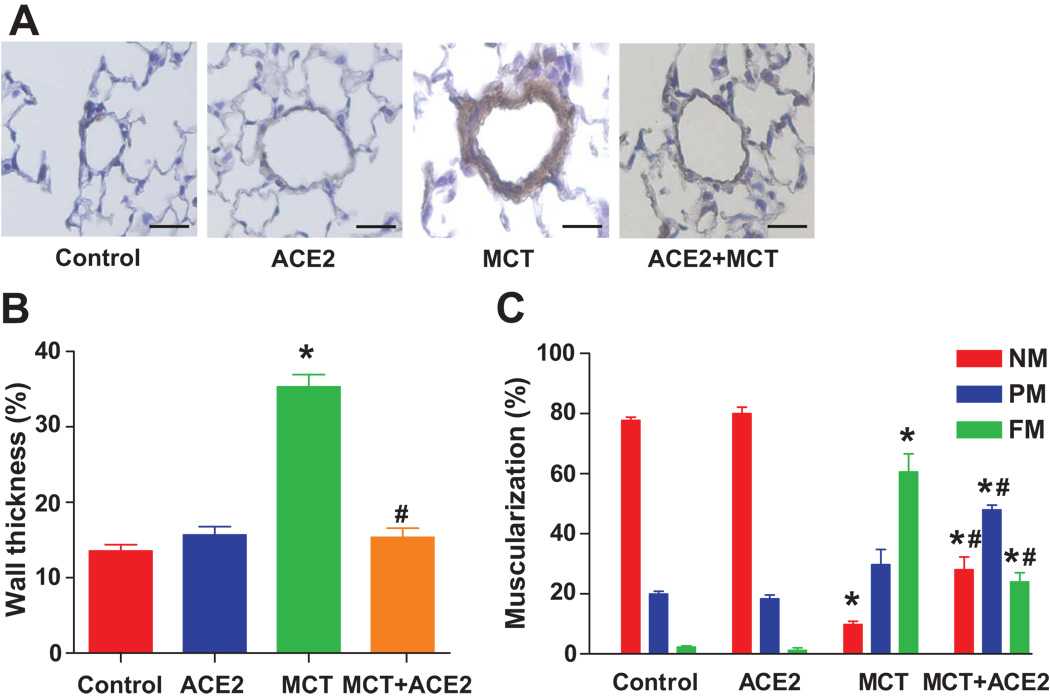
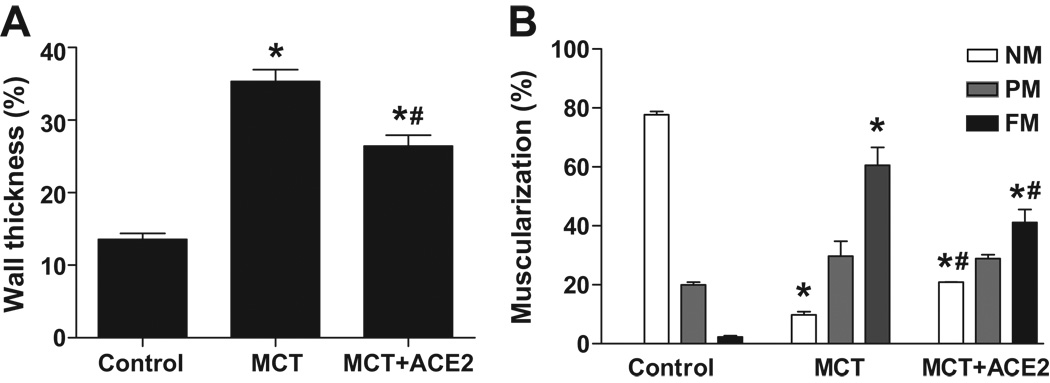
Immunostaining with an antibody directed to α-SMA showed that the medial walls of pulmonary arterioles were markedly thickened by MCT treatment for 8 weeks. This effect was attenuated by ACE2 gene transfer (Fig. 3a and 3b). In normal lungs, 77% of the arterioles were nonmuscularized and 2% were fully muscularized. In contrast, MCT-treated animals showed a substantially greater proportion of small vessels with full muscularization (60%) and lower proportion with nonmuscularization (10%). ACE2 gene transfer significantly reduced the percentage of small vessels exhibiting muscularization (24%, MCT+ACE2 vs 60%, MCT, p<0.05, Fig. 3c) and increased the percentage of nonmuscularized vessels.
Figure 3. Effects of lenti-ACE2 on preventing vessel wall thickness and vessel muscularization induced by MCT.
(a) Representative microphotographs of pulmonary vessels (scale bar=50 µm).
(b) Quantification of wall thickness as described in the Methods section. Data are expressed as mean ± SEM, * p<0.05 vs. control group, # p<0.05 vs MCT group (n=4–5).
(c) Quantification of vessel muscularization: degree of muscularization of vessels was carried out as described in the Methods section. Data are expressed as mean ± SEM, * p<0.05 vs. control group, # p<0.05 vs MCT group (n=4–5). NM: nonmuscularized vessels; PM: partially muscularized vessels; FM: fully muscularized vessels.
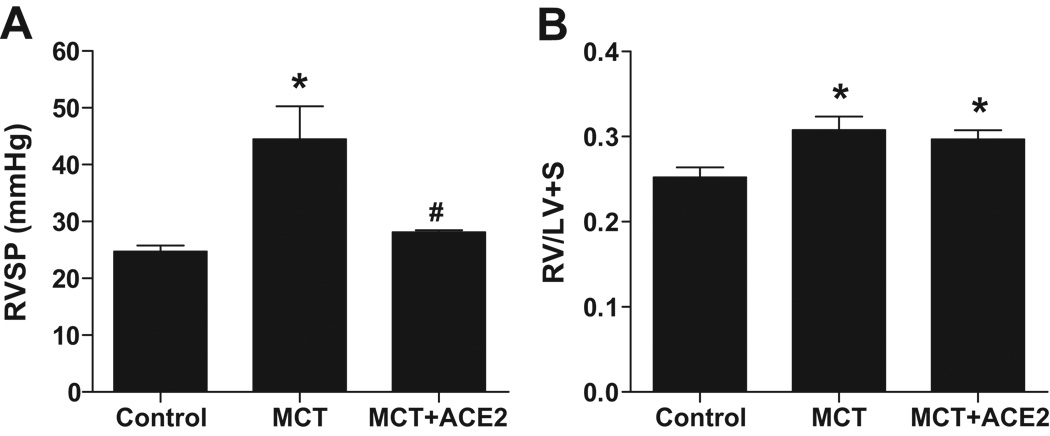
Reversal of PH and associated cardiac and pulmonary damages by ACE2
ACE2 gene transfer in mice following 6 weeks of pretreatment with MCT also resulted in attenuation of RVSP (28 ± 0.3 mmHg, p<0.05, Fig. 4a) compared with MCT-treated mice. However, the RV/LV+S ratio was similar in both groups (0.29 ± 0.01 mg/mg, MCT+ACE2 vs 0.31 ± 0.01 mg/mg, MCT, Fig. 4b). Immunohistochemical studies revealed that the increase in the wall thickness observed in small pulmonary vessels after MCT treatment was reversed by ~30% in mice overexpressing ACE2 (Fig. 5a). In addition, the percentage of fully muscularized intra-acinar vessels in lungs from MCT-treated mice increased, while the percentage of nonmuscularized vessels decreased compared with control mice. ACE2 gene transfer reversed the effect of MCT on muscularized vessels demonstrating a ~35% decrease (Fig. 5b). No significant difference was observed in the systolic blood pressure of MCT mice treated with lenti-ACE2.
Figure 4. Effects of Lenti-ACE2 on reversal of MCT-induced increases in (a) RVSP and (b) RV hypertrophy.
Experimental protocol is described in the Methods section. Data are represented as mean ± SEM, * p<0.05 vs. control group, # p<0.05 vs MCT group (n=6–8). Injection of lenti-ACE2 alone did not induce any significant change in RVSP and RV hypertrophy.
Figure 5. Effects of lenti-ACE2 on reversing vessel wall thickness and muscularization in MCT-treated mice.
Six weeks following MCT treatment, animals were subjected to lenti-ACE2 gene transfer. This was followed by an additional 2 weeks of MCT treatment.
(a) Quantification of wall thickness of vessels from control, MCT, and ACE2+MCT groups. Data are expressed as mean ± SEM, * p<0.05 vs. control group, # p<0.05 vs MCT group (n=4–5).
(b) Quantification of vessel muscularization. Injection of lenti-ACE2 alone did not induce any significant changes in vessel wall thickness and muscularization. Data are represented as mean ± SEM, * p<0.05 vs. control group, # p<0.05 vs MCT group (n=4–5). NM: nonmuscularized vessels; PM: partially muscularized vessels; FM: fully muscularized vessels.
Possible Mechanism of ACE2 action
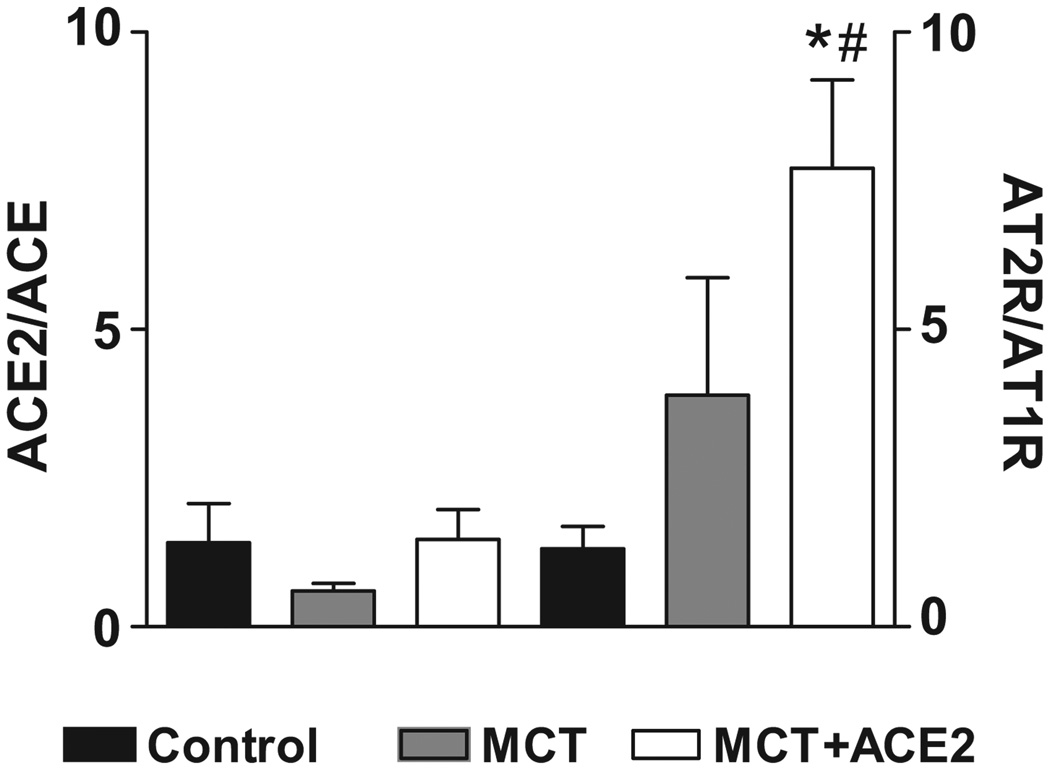
mRNA levels of certain members of the RAS and pro-inflammatory cytokines genes were measured to determine the possible mechanism of ACE2 gene transfer. MCT treatment showed no significant effects on the AT1 receptor, ACE, and ACE2 mRNA levels, while renin and AT2 receptor mRNA levels increased by 54% and 100%, respectively, albeit the increase in AT2R did not reach significance (Table 1). However, an interesting pattern emerged when the ratios between the mRNAs of the vasoprotective axis of the RAS (ACE2 and AT2 receptor) were compared with the vasoconstrictive, proliferative axis (ACE and AT1 receptor) in the lenti-ACE2-treated group in our prevention protocol. MCT treatment decreased the mRNA ratio of ACE2/ACE, while lenti-ACE2 treatment of MCT-treated mice restored this to control levels (Fig. 6). In addition, AT2 receptor/AT1 receptor levels increased ~4 fold in the MCT-treated animal and ~8-fold by lenti-ACE2 treatment of MCT-treated mice (Fig. 6). In addition, Ang II immunoreactivity was significantly increased in MCT-treated mice and this was significantly reduced in MCT-treated mice by lenti-ACE2 (Supplemental Figure S2).
Table 1.
mRNA levels of the members of the RAS genes following MCT treatment.
| Treatment | Renin | ACE | AT1R | ACE2 | AT2 |
|---|---|---|---|---|---|
| Control | 1.1 ± 0.2 | 1.3 ± 0.4 | 1.0 ± 0.06 | 1.0 ± 0.05 | 1.4 ± 0.4 |
| (n=4) | (n=5) | (n=5) | (n=5) | (n=5) | |
| MCT | 1.7 ± 0.1 * | 1.7 ± 0.4 | 0.7 ± 0.05 | 0.9 ± 0.03 | 2.8 ± 1.6 |
| (n=4) | (n=4) | (n=4) | (n=4) | (n=4) |
The data are expressed as mean ± SEM.
p<0.05 vs. controls (Student’s t-test).
Figure 6. Effects of lenti-ACE2 on ratios of ACE2/ACE, AT2 receptor/AT1 receptor mRNAs in the lungs of MCT-treated mice.
(a) Mice were injected with lenti-ACE2 and treated with MCT as described in the prevention protocol of legend to Figure 3. Total RNA was isolated and subjected to real-time RT-PCR as described in the Methods section to quantify mRNA levels of ACE, ACE2, AT1-and AT2 receptors. Data are presented as ratios and are the mean of three experiments. * p<0.05 vs. control group, # p<0.05 vs MCT group.
(b) Sections of lungs from control, MCT and MCT+ lenti-ACE2 mice from reversal protocol were fixed and incubated with anti-Ang II antibody. This was followed by incubation with FITC-labeled second antibody as described in the Methods.
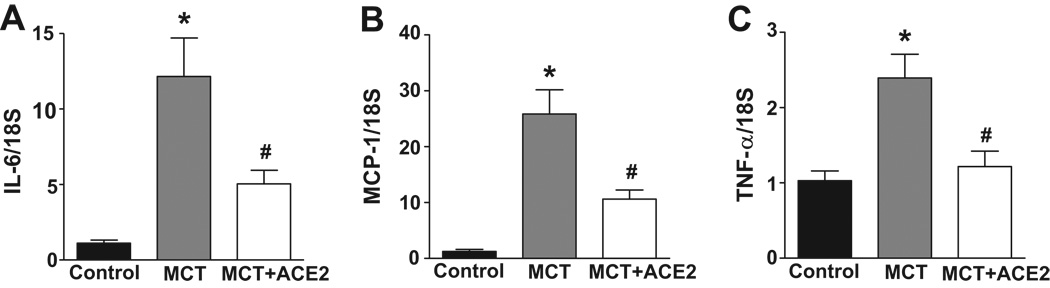
MCT treatment also resulted in ~12-fold, ~25-fold, and 2.5-fold increases in the inflammatory cytokines, IL-6, MCP-1, and TNF-α mRNA levels, respectively (Fig. 7). Treatment with lenti-ACE2 in the prevention protocol caused 65% decrease in MCP-1, 60% decrease in IL-6, and 90% decrease in TNF-α mRNA levels when compared with their levels in MCT-treated mice. Immunohistochemical data supported the mRNA changes. Supplemental Figures 3 and 4 show that MCT treatment resulted in significant increases in the intensities of MCP-1 and TNF-immunoreactivity in the lungs. This was significantly reduced in lenti-ACE2 lungs of MCT-treated mice. These observations suggest that the shifting of the RAS to vasoprotective axis is associated with attenuation of the increase in pro-inflammatory cytokines. This may be linked to the beneficial effects of lenti-ACE2 on PH.
Figure 7. Effects of lenti-ACE2 on mRNA levels of IL-6, MCP-1, and TNF-α.
Total RNA samples from the experiment in the legend to Figure 7a were analyzed for IL-6, MCP-1, and TNF-α as described in the Methods. Data are presented as relative expression using control as one. Data are mean ± SEM, *p<0.05 vs control group, # p<0.05 vs MCT group. ACE2 overexpression prevents an increased expression of these pro-inflammatory cytokines in MCT-treated mice.
Discussion
In this study we provide evidence that: (i) lentiviral vector is extremely efficient in transducing a wide variety of cells in pulmonary tissue on a long-term basis, (ii) overexpression of ACE2 results in almost complete attenuation of PH induced by MCT, and (iii) this strategy is also successful in a significant reversal of PH-induced lung damage. Thus, these observations provide supportive evidence that ACE2 overexpression or endogenous pulmonary ACE2 activation may have important implications in the development of a novel therapeutic strategy in the treatment and possibly reversal of PH and its associated complications.
Lenti-ACE2 treatment, prior to induction of PH, resulted in almost complete prevention of increases in RVSP, RV hypertrophy, and attenuation of thickening of pulmonary vessels. This was associated with a significant inhibition of muscularization of arterioles. Despite the increase in RVSP and RV hypertrophy, we did not observe significant differences in right ventricular end-diastolic pressure (RVEDP) or dP/dt in the right ventricle among the groups. It is conceivable the animals were still in an adaptive phase at this time and the pathophysioloical aspects would be manifested at a later time point. Hessel et al.32, using a rat model of MCT, also noted normal RV function with regard to dP/dt values in animals treated with a high and low dose of MCT for 4 weeks despite the increase in RSVP and RV hypertrophy.
In addition, lenti-ACE2 treatment at six weeks following MCT administration caused partial reversal of PH-linked pathophysiologies. The reason for a partial reversal may be related to the time course and levels of transgenic ACE2, since ACE2 overexpression was performed for only 2 weeks in animals that already exhibited long standing PH in the reversal study. However, the possibility that the complete reversal could not be accomplishable once the PH is well established cannot be ruled out at the present time.
Targeting of ACE2 in the lungs appears to be a better strategy than the use of systemic administration of AT1 receptor antagonists and ACE inhibitors, which have been found to have limited or no success in prevention of PH15–17. The precise mechanism of this success will await further investigation. However, it is tempting to suggest that ACE2 shifts the balance of the vasoconstrictive, proliferative, and fibrotic axis of the RAS (ACE-Ang II-AT1 receptor) toward the vasoprotective axis [ACE2-Ang-(1–7) and AT2 receptor].24,33,34 This contention is supported by our observation that ACE2 gene transfer increases Ang-(1–7) staining, decreases Ang II immunoreactivity and increases the ratio of AT2 receptor/AT1 receptor in the MCT treated mice. Further support for our hypothesis is our recent finding that an ACE2 activator inhibits fibrosis and has similar alterations in cytokines in a rat MCT-model of pulmonary hypertension35. Likewise, we have recently shown that overexpression of ACE2 or Ang-(1–7) provided protective pulmonary and cardiac effects in a bleomycin induced model of pulmonary fibrosis.36 Others have also suggested a similar shift in the vasoprotective axis by ACE2 for acute lung injury.25, 26 It is pertinent to state that the apparent increase in the AT2R expression observed in our study is consistent with the finding of an increase in AT2R expression observed in other cardiovascular diseases.33 Zisman et al 29 has previously demonstrated a direct correlation between AT2R expression and Ang-(1–7) forming activity (ACE2) in failing human heart ventricles from patients with primary pulmonary hypertension. In addition, AT2R have been shown to suppress myocardial hypertrophy37 and fibroblast proliferation.33 Thus, the beneficial effect of ACE2 overexpression may, in part, be due to an increase in the ratio of AT2 receptor/AT1 receptor. Furthermore, ACE2 overexpression has been shown to exert a negative influence on AT1 receptors.38
Previous studies have shown that induction of PH is associated with increased production of pro-inflammatory cytokines.2,39 Our data showing increases in MCP-1, IL-6, and TNF-α confirms this. Furthermore, lenti-ACE2 treatment prevents increases in these pro-inflammatory cytokines. This suggests that the attenuation of pro-inflammatory cytokines in combination with the shift towards the vasoprotective axis may be responsible for the overall beneficial effects of ACE2 gene transfer in PH. It remains to be determined if changes in the RAS are responsible for the changes in pro-inflammatory cytokines or if they are independently altered in PH. However, we favor the former situation since RAS is a potent regulator of pro-inflammatory cytokines.40 A limitation of the study is that the mRNA data is not confirmed by protein measurement. Nevertheless, evaluation of both types of gene product will be desirable in future work.
An interesting aspect of this study is that pulmonary overexpression of ACE2 did not influence systemic blood pressure (BP). This observation is supported by our previous study in which cardiac overexpression of ACE2, which causes significant attenuation of hypertension-induced cardiac hypertrophy, has little effect on high BP.41 This may turn out to be an important benefit if this strategy could be translated for therapeutics. Patients suffering from severe PH already express lower systemic blood pressure as a result of right ventricular overload and treatment with ACE inhibitors, AT1 receptor blockers or other currently available therapy would exacerbate systemic hypotension.42,43 However, it appears that pulmonary ACE2 overexpression circumvents influences on systemic hemodynamics and only influences pulmonary pathophysiology.
Finally, the most significant aspect of our observation is that it provides evidence that ACE2 overexpression/activation is an innovative strategy against PH. However, further evidence confirming the safety issues associated with lentiviral vector and validation with other animal models will be needed before the translation of this observation into preclinical strategy. Nonetheless, our data provide evidence that pulmonary ACE2 represents a novel target for therapeutic intervention aiming at the prevention and restoration of lung vascular remodeling and subsequent right heart hypertrophy.
Perspectives
Current therapeutic strategies for the control and treatment of PH are primarily based on pharmacological agents with limited efficacy. In spite of their relative success in PH treatment, frequently these pharmacological agents are associated with serious side effects. Clearly, there `is an urgent need to develop new strategies (for example: new drug targets, novel therapeutic molecule delivery methods, cell-based therapies) for successfully control this disease. The discovery of ACE2, with its potential to shift the adverse effects of RAS hyperactivity toward beneficial outcomes in the cardiovascular system, holds this promise. Our study is timely in that, since it presents evidence that overexpression of ACE2 prevents and reverses PH. It provides conceptual in vivo support for ACE2 as a viable target for future development of pharmacological and genetic upregulating strategies for the treatment of this disease.
Supplementary Material
Acknowledgments
We thank Mr. Dae Song Jang for preparing the lung sections for pathological examination, and Dr. Robson AS Santos for kindly providing the Ang-(1–7) antibody.
Funding Sources:
This work was supported by the National Institutes of Health Grants HL-56921 and HL-64024.
Footnotes
Disclosures:
None
References
- 1.Coggins MP, Bloch KD. Nitric oxide in the pulmonary vasculature. Arterioscler Thromb Vasc Biol. 2007;27:1877–1885. doi: 10.1161/ATVBAHA.107.142943. [DOI] [PubMed] [Google Scholar]
- 2.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 3.Studdy PR, Lapworth R, Bird R. Angiotensin-converting enzyme and its clinical significance-a review. J Clin Pathol. 1983;36:938–947. doi: 10.1136/jcp.36.8.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall RP. The pulmonary renin-angiotensin system. Curr Pharm Des. 2003;9:715–722. doi: 10.2174/1381612033455431. [DOI] [PubMed] [Google Scholar]
- 5.Dezsö B, Nielsen AH, Poulsen K. Identification of renin in resident alveolar macrophages and monocytes: HPLC and immunohistochemical study. J Cell Sci. 1988;91:155–159. doi: 10.1242/jcs.91.1.155. [DOI] [PubMed] [Google Scholar]
- 6.Ohkubo H, Nakayama K, Tanaka T, Nakanishi S. Tissue distribution of rat angiotensinogen mRNA and structural analysis of its heterogeneity. J Biol Chem. 1986;261:319–323. [PubMed] [Google Scholar]
- 7.Chassagne C, Eddahibi S, Adamy C, Rideau D, Marotte F, Dubois-Randé JL, Adnot S, Samuel JL, Teiger E. Modulation of angiotensin II receptor expression during development and regression of hypoxic pulmonary hypertension. Am J Respir Cell Mol Biol. 2000;22:323–332. doi: 10.1165/ajrcmb.22.3.3701. [DOI] [PubMed] [Google Scholar]
- 8.Bullock GR, Steyaert I, Bilbe G, Carey RM, Kips J, De Paepe B, Pauwels R, Praet M, Siragy HM, de Gasparo M. Distribution of type-1 and type-2 angiotensin receptors in the normal human lung and in lungs from patients with chronic obstructive pulmonary disease. Histochem Cell Biol. 2001;115:117–124. doi: 10.1007/s004180000235. [DOI] [PubMed] [Google Scholar]
- 9.Lefebvre F, Préfontaine A, Calderone A, Caron A, Jasmin JF, Villeneuve L, Dupuis J. Modification of the pulmonary renin-angiotensin system and lung structural remodelling in congestive heart failure. Clin Sci (Lond) 2006;111:217–224. doi: 10.1042/CS20060027. [DOI] [PubMed] [Google Scholar]
- 10.DeMarco VG, Habibi J, Whaley-Connell AT, Schneider RI, Heller RL, Bosanquet JP, Hayden MR, Delcour K, Cooper SA, Andresen BT, Sowers JR, Dellsperger KC. Oxidative stress contributes to pulmonary hypertension in the transgenic (mRen2)27 rat. Am J Physiol Heart Circ Physiol. 2008;294:H2659–H2668. doi: 10.1152/ajpheart.00953.2007. [DOI] [PubMed] [Google Scholar]
- 11.Morrell NW, Morris KG, Stenmark KR. Role of angiotensin-converting enzyme and angiotensin II in development of hypoxic pulmonary hypertension. Am J Physiol. 1995;269:H1186–H1194. doi: 10.1152/ajpheart.1995.269.4.H1186. [DOI] [PubMed] [Google Scholar]
- 12.Orte C, Polak JM, Haworth SG, Yacoub MH, Morrell NW. Expression of pulmonary vascular angiotensin-converting enzyme in primary and secondary plexiform pulmonary hypertension. J Pathol. 2000;192:379–384. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH715>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 13.Cassis L, Shenoy U, Lipke D, Baughn J, Fettinger M, Gillespie M. Lung angiotensin receptor binding characteristics during the development of monocrotaline-induced pulmonary hypertension. Biochem Pharmacol. 1997;54:27–31. doi: 10.1016/s0006-2952(97)00142-1. [DOI] [PubMed] [Google Scholar]
- 14.Molteni A, Ward WF, Ts'ao CH, Solliday NH. Monocrotaline-induced cardiopulmonary damage in rats: amelioration by the angiotensin-converting enzyme inhibitor CL242817. Proc Soc Exp Biol Med. 1986;182:483–493. doi: 10.3181/00379727-182-42370. [DOI] [PubMed] [Google Scholar]
- 15.Jeffery TK, Wanstall JC. Pulmonary vascular remodeling: a target for therapeutic intervention in pulmonary hypertension. Pharmacol Ther. 2001;92:1–20. doi: 10.1016/s0163-7258(01)00157-7. [DOI] [PubMed] [Google Scholar]
- 16.Mascitelli L, Pezzetta F. Inhibition of the renin-angiotensin system in patients with COPD and pulmonary hypertension. Chest. 2007;131:938. doi: 10.1378/chest.06-2018. [DOI] [PubMed] [Google Scholar]
- 17.Reid JL. ACE inhibitors: future perspectives. J Cardiovasc Pharmacol. 1993;22:541–543. [PubMed] [Google Scholar]
- 18.Seist G, Jeannesson E, Visvikis-Seist S. Enzymes and pharmacogenomics of cardiovascular drugs. Clin Chim Acta. 2007;381:26–31. doi: 10.1016/j.cca.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Shah AD, Arora RR. Do all patients with coronary artery disease benefit from angiotensin converting enzyme inhibitors? J Cardiovasc Pharmacol Ther. 2005;10:281–283. doi: 10.1177/107424840501000408. [DOI] [PubMed] [Google Scholar]
- 20.Dandona P, Dhindsa S, Ghanim H, Chaudhuri A. Angiotensin II and inflammation: the effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. J Hum Hypertens. 2007;21:20–27. doi: 10.1038/sj.jhh.1002101. [DOI] [PubMed] [Google Scholar]
- 21.Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol. 2006;20:953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- 22.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 23.Der Sarkissian S, Huentelman MJ, Stewart J, Katovich MJ, Raizada MK. ACE2: A novel therapeutic target for cardiovascular diseases. Prog Biophys Mol Biol. 2006;91:163–198. doi: 10.1016/j.pbiomolbio.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Raizada MK, Ferreira AJ. ACE2: A new target for cardiovascular disease therapeutics. J Cardiovasc Pharmacol. 2007;50:112–119. doi: 10.1097/FJC.0b013e3180986219. [DOI] [PubMed] [Google Scholar]
- 25.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imai Y, Kuba K, Penninger JM. Angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Cell Mol Life Sci. 2007;64:2006–2012. doi: 10.1007/s00018-007-6228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto K, Ohishi M, Katsuya T, Ito N, Ikushima M, Kaibe M, Tatara Y, Shiota A, Sugano S, Takeda S, Rakugi H, Ogihara T. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertens. 2008;47:718–726. doi: 10.1161/01.HYP.0000205833.89478.5b. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Molina-Molina M, Abdul-Hafez A, Uhal V, Xaubet A, Uhal BD. Angiotensin converting enzyme-2 is protective but downregulated in human and experimental lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L178–L185. doi: 10.1152/ajplung.00009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zisman LS, Keller RS, Weaver B, Lin Q, Speth R, Bristow MR, Canver CC. Increased angiotensin – (1–7)-forming activity in failing human heart ventricles: evidence for upregulation of angiotensin-converting enzyme homologue ACE2. Circ. 2003;108:1707–1712. doi: 10.1161/01.CIR.0000094734.67990.99. [DOI] [PubMed] [Google Scholar]
- 30.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grobe JL, Mecca AP, Mao H, Katovich MJ. Chronic angiotensin-(1–7) prevents cardiac fibrosis in the DOCA-salt model of hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H2417–H2423. doi: 10.1152/ajpheart.01170.2005. [DOI] [PubMed] [Google Scholar]
- 32.Hessel MHM, Steendijk P, den Adel B, Schutte CI, van der Laarse A. Characterization of right ventricular function after monocrotaline-induced pulmonary hypertension in the intact rat. Am J Physiol Heart Circ Physiol. 2006;291:H2424–H2430. doi: 10.1152/ajpheart.00369.2006. [DOI] [PubMed] [Google Scholar]
- 33.Tsutsumi Y, Matsubara H, Ohkubo N, Mori Y, Nozawa Y, Murasawa S, Kijima K, Maruyama K, Masaki H, Moriguchi Y, Shibasaki Y, Kamihata H, Inada M, Iwasaka T. Angiotensin II type 2 receptor is upregulated in human heart with interstitial fibrosis, and cardiac fibroblasts are the major cell type for its expression. Circ Res. 1998;83(10):1035–1046. doi: 10.1161/01.res.83.10.1035. [DOI] [PubMed] [Google Scholar]
- 34.Zhu YZ, Chimon GN, Zhu YC, Lu Q, Li B, Hu HZ, Yap EH, Lee HS, Wong PT. Expression of Angiotensin II AT2 receptor in the acute phase of stroke in rats. Neuroreport. 2000;11:1191–1204. doi: 10.1097/00001756-200004270-00009. [DOI] [PubMed] [Google Scholar]
- 35.Ferreira AJ, Shenoy V, Yamazato Y, Sriramula S, Francis J, Yuan L, Castellano RK, Ostrov DA, Oh SP, Katovich MJ, Raizada MK. Evidence for Angiotensin Converting Enzyme 2 as a Therapeutic Target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med. 2009 doi: 10.1164/rccm.200811-1678OC. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shenoy V, Ferreira AJ, Qi Y, Dooies KA, Raizada MK, Katovich MJ. Lenti-viral mediated overexpression of ACE2 or Angiotensin-(1–7) prevents bleomycin-induced pulmonary fibrosis. FASEB J. 2009;23:770.7. [Google Scholar]
- 37.Booz GW, Baker KM. Role of type 1 and type 2 angiotensin receptors in angiotensin II-induced cardiomyocyte hypertrophy. Hypertens. 1996;28:635–640. doi: 10.1161/01.hyp.28.4.635. [DOI] [PubMed] [Google Scholar]
- 38.Feng Y, Yue X, Xia H, Bindom SM, Hickman PJ, Filipeanu CM, Wu G, Lazartigues E. Angiotensin-converting enzyme 2 overexpression in the subfornical organ prevents the angiotensin II-mediated pressor and drinking responses and is associated with angiotensin II type 1 receptor downregulation. Circ Res. 2008;102:729–736. doi: 10.1161/CIRCRESAHA.107.169110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schober A, Zernecke A. Chemokines in vascular remodeling. Thromb Haemost. 2007;97:730–737. [PubMed] [Google Scholar]
- 40.Ferrario CM, Strawn WB. Role of the renin-angiotensin system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;58:81–88. doi: 10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 41.Huentelman MJ, Grobe JL, Vazquez J, Steward JM, Mecca AP, Katovich MJ, Ferrario CM, Raizada MK. Protection from angiotension II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol. 2005;90:783–790. doi: 10.1113/expphysiol.2005.031096. [DOI] [PubMed] [Google Scholar]
- 42.Packer M. Vasodilator therapy for primary pulmonary hypertension. Limitations and hazards. Ann Intern Med. 1985;103:258–270. doi: 10.7326/0003-4819-103-2-258. [DOI] [PubMed] [Google Scholar]
- 43.Rubin LJ, Badesch DB. Evaluation and management of the patient with pulmonary arterial hypertension. Ann Intern Med. 2005;143:282–292. doi: 10.7326/0003-4819-143-4-200508160-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.