Abstract
Complement effectors are known to contribute to host cell injury in several inflammatory diseases. Contrary to this paradigm, in this study utilizing surgical liver resection (partial hepatectomy) in various complement-deficient mice as a model, we have demonstrated that complement anaphylatoxins C3a and C5a are required for the survival of liver cells during regeneration. The mechanisms of these cytoprotective functions of complement were related to the regulation of IL-6 and TNF production or release after liver resection. Disturbances in the cytokine milieu, induced by a loss of complement activity, were found to alter prosurvival signaling, including the IL-6/STAT3 and PI3K/Akt/mammalian target of rapamycin pathways. In conclusion, this study documents functions of complement proteins as prosurvival factors that, through their interactions with cytokines, inhibit apoptotic signaling in proliferating cells of epithelial origin.
The complement system, which is an important contributor to innate and adaptive immune responses, is composed of cell membrane regulators and receptors and several circulating plasma proteins, forming a proteolytic cascade that is immediately activated when rapid initiation of the inflammatory reaction is needed (1). Activation of complement leads to the generation of several complement effectors, including the anaphylatoxins C3a and C5a. Anaphylatoxins can enhance inflammation, either by directly binding to their receptors on target cells or by acting indirectly through interactions with other contributors to the inflammatory response (1). Although the function of inflammation is to eliminate pathogens, under certain clinical conditions it may cause more damage than the pathogens themselves (2). Inflammation-mediated tissue damage is a major pathogenetic mechanism in several inflammatory disorders, including systemic lupus erythematosus (3, 4) and ischemia/reperfusion-induced tissue injury (5-7). In these diseases complement enhances tissue injury through its contribution to inflammation (8, 9).
Previous work has shown the involvement of the complement proteins C3a and C5a in the priming phase of liver regeneration (10-12). A lack of complement signaling in complement-deficient mice resulted in impaired liver regeneration. Intriguingly, various complement deficiencies were also associated with significant liver injury after partial hepatectomy (PHx)5 (11). The presence of such severe damage to liver parenchyma in mice lacking complement components unexpectedly points to the importance of these proteins for prosurvival signaling triggered during liver regeneration.
Liver regeneration is a compensatory hyperplasia occurring as a response to the loss of liver mass caused by injury or surgical resection, and it is regulated by the interplay between cytokines and growth factors (13). Dividing and enlarging liver cells require factors that regulate cell growth and proliferation. Additionally, several mechanisms that inhibit death signaling are activated in these cells. Recent investigations have shown that some of the factors implicated in the regulation of liver cell proliferation are also important for the survival of regenerating liver cells (13, 14). Antiapoptotic signaling triggered by these factors includes the PI3K/Akt/mammalian target of rapamycin (mTOR) pathway. The PI3K, Akt, and mTOR proteins comprise one of the best characterized prosurvival signaling pathways and are also involved in the regulation of several other crucial cellular processes, including transcription, translation, metabolism, and cell cycle progression (15). It has been also suggested that IL-6, produced and secreted by activated Kupffer cells, is a major activator of this prosurvival signaling in regenerating liver cells (16).
We now present evidence that complement anaphylatoxins generated through activation of the complement cascade contribute to the activation of this signaling by regulating the cytokine milieu during liver regeneration, and that this contribution is essential for the survival of liver cells during regeneration.
Materials and Methods
Animal studies
Complement-deficient mice lacking C3 (C3−/−), the C3a receptor (C3aR−/−), the C5a receptor (C5aR−/−), or both C3 and C5 (C3/C5−/−) have been previously described (11, 17-19). Complement-deficient mice were backcrossed for at least nine generations onto a C57BL/6 background, and their wild-type littermates or C57BL/6J mice (The Jackson Laboratory) were used as controls.
For PHx studies, male mice 12−16 wk of age were anesthetized and subjected to midventral laparotomy with ∼70% liver resection (PHx) (20). Control animals were subjected to midventral laparotomy without manipulation of the liver (sham). Histological slides from the resected liver lobes were analyzed to exclude preexisting pathology. One hour before sacrifice, at 0 h or after 12 h, BrdU (Sigma-Aldrich) was injected i.p. at a single dose of 50 mg/kg animal weight. At the time of sacrifice, clinical status was assessed, mice were anesthetized, blood was harvested from the inferior vena cava, and the remaining liver lobes and other internal organs were removed, weighed, and processed for protein or histological analysis. Histological slides prepared from lungs, kidneys, spleen, intestine, and pancreas of sacrificed mice were analyzed for the presence of any morphological abnormalities that could indicate infection. Mice with an inflammatory infiltrate in any of the internal organs were excluded from further analysis. To assess complement activation or the levels of cytokines secreted during liver regeneration, 30 μl of blood was collected with EDTA (for plasma) or without anticoagulant (for serum) from the tail vein before and 0.5, 1, 2, 3, 6, 12, 24, 48, and 72 h after PHx. Plasma and serum were separated by gentle centrifugation (1000 × g) at 4°C for 10 min and then stored at −70°C until analyzed.
For reconstitution studies, C3/C5−/− mice were injected i.p. with three successive doses of synthetic mouse C3a and/or C5a 20 min before and at two 6-h intervals after PHx (15 μg/mouse/injection). Synthesis of mouse C3a and expression of C5a has been described previously (11). C3−/− mice were reconstituted i.p. with C3-sufficient and -deficient mouse sera (freshly collected) 20 min before or 8 h after PHx.
Mice were housed in an animal facility of the University of Pennsylvania, within a barrier, on a 12-h light/dark cycle. Before the engagement of mice into the experimental groups, sera and feces were tested for the most common rodent infections, including Helicobacter hepaticus. Water and standard rodent diet were provided ad libitum. All mice were used with the approval of the University of Pennsylvania Institutional Animal Care and Use Committee and under National Institutes of Health guidelines.
Extract preparation
Whole-cell protein extracts from the liver were prepared in the presence of protease and phosphatase inhibitors as previously described (21).
Liver histology and immunohistochemical staining
Liver morphology was assessed by light microscopy (Olympus BX 60) of H&E-stained 5-μm paraffin sections in a blinded fashion. Staining for BrdU, quantification of the proliferative fraction of hepatocytes, and analysis of the presence of apoptotic cells in paraffin liver sections were performed as described previously (12).
ELISA for measuring C3b/iC3b/C3c
The levels of C3 cleavage products, reflecting the activation of complement, were determined in mouse plasma as described (12). The magnitude of complement activation is expressed as the percentage of C3 cleavage products relative to that measured in sera activated with cobra venom factor, which is considered to cause 100% complement activation.
Monitoring of the cytokine response in mouse sera
The multiplex bead-based assay system (Bio-Plex; Bio-Rad Laboratories) was used to determine the levels of IL-6 and TNF in mouse sera before and after PHx. The assay was conducted according to the manufacturer's instructions.
Immunoblots
Samples of whole-cell protein extracts (100 μg) were electrophoresed on polyacrylamide gels and transferred to polyvinylidene difluoride membranes. Membranes were incubated overnight at 4°C with the following Abs: rabbit anti-human phospho-JAK1 (Tyr1022/Tyr1023), phospho-ERK1/2 (Thr202/Tyr204), or phospho-S6; rabbit anti-mouse phospho-STAT3 (Tyr705), STAT3, phospho-Akt (Ser473), or Akt; rabbit anti-rat ERK1/2; or mouse monoclonal β-actin. All Abs were obtained from Cell Signaling Technology, except phospho-JAK1 (Biosource/Invitrogen) and β-actin (Abcam). Primary Ab binding was detected using HRP-conjugated anti-rabbit IgG (Bio-Rad Laboratories) and chemiluminescence (Amersham Pharmacia Biotech). Protein loading was normalized according to the level of β-actin expression, with Ponceau S-stained membranes used for verification. A control sample was run on all gels for use in normalization to allow comparisons between different immunoblots. Protein levels were measured by densitometry (ImageQuant software; Molecular Dynamics).
Data analysis
Statistical analyses were performed using the unpaired t test (Microsoft Excel), two-way ANOVA (GraphPad Prism; GraphPad Software), and the χ2 test. A p value of 0.05 or less was considered to indicate a significant difference between groups.
Results
Complement prevents the death of proliferating liver cells
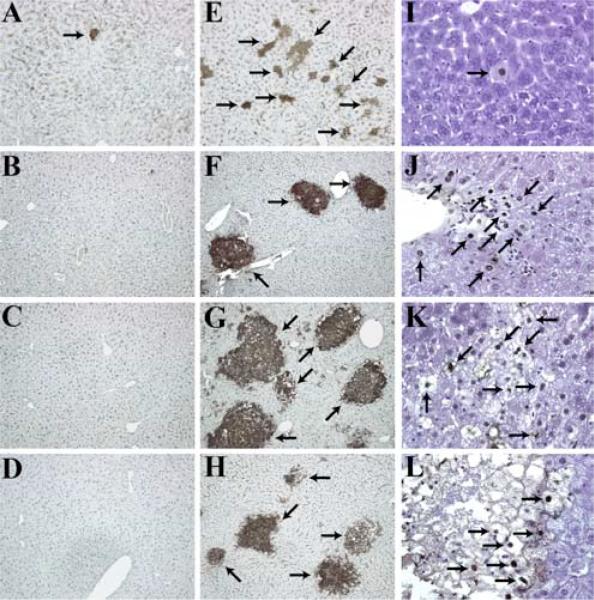
Previous results showed that C3 and C5 complement deficiencies, as well as pharmacological blockade of the C5aR, were associated with impaired proliferation of hepatocytes and severe injury to the liver parenchyma after PHx (11). The presence of tissue injury 44 h after liver resection in these mice suggested that complement, in addition to regulating the proliferation of regenerating liver cells, is also involved in protection of these cells from damage. Therefore, we examined the mode and kinetics of cell death after PHx in complement-deficient mice. A deficiency of C3, the major complement component, eliminates most of the activities of the complement system. We found that the absence of C3 was associated with the presence of apoptotic hepatocytes, either dispersed or in small groups, as soon as 2 h after PHx in significantly higher numbers compared with littermate controls (Fig. 1, A, E, and I). The larger number of apoptotic hepatocytes demonstrated by staining for the cleavage product of caspase 3 (Fig. 1E), in comparison to TUNEL (Fig. 1I), suggested that liver cells are in the early stages of apoptotic cell death 2 h after PHx (TUNEL staining for wild-type livers is not shown, as no apoptotic cells were detected).
FIGURE 1.
Increased liver injury in C3−/− mice following PHx. A–H, Liver sections of wild-type (A–D) or C3−/− (E–H) mice at various time points after PHx, showing staining for the activated caspase-3 site on cytokeratin 18. Magnification, ×100. I–L, TUNEL staining in C3−/− livers after PHx. Magnification, ×400. In E–L, arrows point to areas of tissue damage. Post-PHx time points for shown histology are as follows: A, E, and I, Two hours (n ≥ 5 mice/cohort); B, F, and J, 36 h (n ≥ 6 mice/cohort); C, G, and K, 48 h (n ≥ 22 mice/cohort); D, H, and L, 72 h (n ≥ 13 mice/cohort).
During the observation period, the magnitude of liver injury increased in C3−/− mice, reaching its maximum at 36 h (Fig. 1, F and J) and remaining at that same level at 48 h (Fig. 1, G and K) and 72 h (Fig. 1, H and L) after PHx. At these time points, large confluent areas of damaged liver parenchyma were observed in C3−/− mice, whereas the liver parenchyma of control mice was free of damage (Fig. 1, B–D). The distribution of apoptotic areas did not show any relationship to histological liver structures such as portal triads or central veins, suggesting that the presence of apoptosis in C3−/− mice was not associated with ischemia or portal tract pathology, but was likely directly linked to complement deficiency. These results suggested an early requirement for complement components for the protection of parenchymal liver cells from injury.
Liver regeneration is associated with complement activation
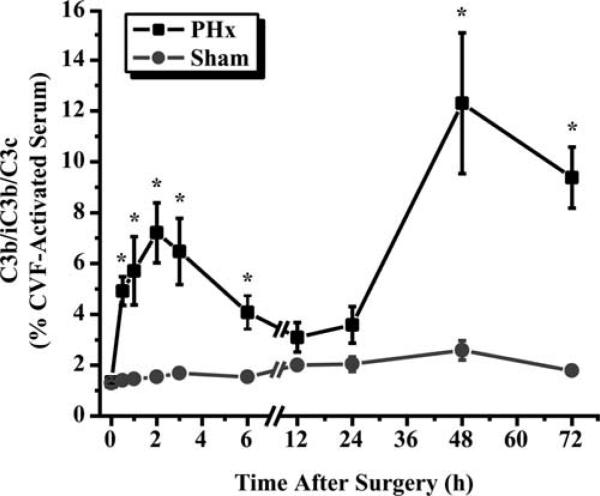
To exert their functions, complement proteins need to be activated by enzymatic cleavage. Therefore, we examined whether complement activation occurs during liver regeneration after PHx. As C3 cleavage is a common event for all pathways of complement activation, we analyzed the presence of C3 cleavage products in plasma samples collected from mice subjected to PHx. This analysis showed a pattern of complement activation in hepatectomized mice composed of two distinct peaks. The first peak occurred 2 h after surgery; the second, more pronounced peak was observed between 24 and 72 h, reaching a maximum at 48 h (Fig. 2). This activation was related to liver regeneration, since sham-operated mice did not show any significant increase in C3 cleavage products in their plasma throughout the observation time (Fig. 2).
FIGURE 2.
Complement activation after PHx. Levels of complement cleavage products (C3b/iC3b/C3c) in plasma of wild-type mice after PHx or sham surgery. Values are expressed as a percentage compared with cobra venom factor (CVF)-activated serum, which was considered to cause 100% complement activation. Data are presented as mean ± SEM (n = 4−6 (PHx) and 7 (sham) mice/time point). ■, PHx; •, sham; *, p ≤ 0.05, unpaired t test.
Early complement activation is essential for liver regeneration
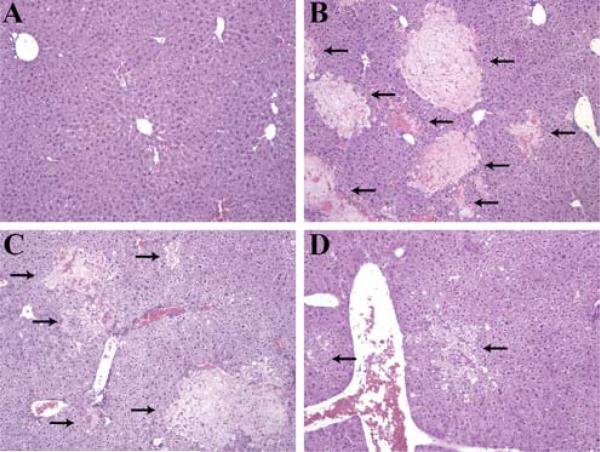
Given that we found a higher number of apoptotic hepatocytes just 2 h after PHx in C3−/− mice, we hypothesized that early complement activation contributes to the survival of regenerating cells. To check this hypothesis, we reconstituted C3−/− mice with wild-type mouse sera, containing physiological amounts of C3, 20 min before and 8 h after PHx. Seventy-five percent of C3−/− mice that received C3-sufficient sera before PHx had no liver injury 48 h after the surgery (Fig. 3A). Conversely, only 25% of C3−/− mice that, before surgery, received sera lacking C3 had noninjured livers, with the rest showing severe parenchymal injury (Fig. 3B). The presence of a low number of C3−/− mice with intact liver parenchyma after injection of C3-deficient sera can be attributed to the particular phenotype of C3−/− mice after PHx, as we have observed that ∼40% of these mice do not show any signs of clinical deterioration related to liver damage after surgical resection. Conversely, all of the C3−/− mice injected with sera (both C3-sufficient and C3-deficient) 8 h after PHx had injured livers 48 h after surgery (Fig. 3, C and D). The inability of this later C3 reconstitution to protect livers from injury demonstrated that early activation of the complement cascade is important for the survival of regenerating liver cells.
FIGURE 3.
Lack of hepatoprotection after late C3 reconstitution. A and B, H&E-stained liver sections taken 48 h after PHx from C3−/− mice injected with C3-sufficient (A) or C3-deficient (B) sera 20 min before PHx (n = 4 mice/cohort; p ≤ 0.05, χ2 test). C and D, Corresponding sections as described in A and B, but for mice injected with sera 8 h after PHx (n = 3 mice/cohort). For A–D, arrows point to areas of tissue damage. Magnification, ×100.
Complement contributes to the survival of regenerating liver cells through anaphylatoxin signaling
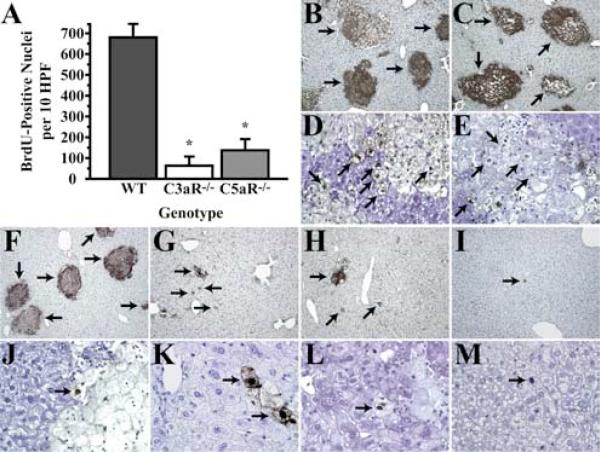
Previous studies suggested that the anaphylatoxins C3a and C5a were involved in the priming phase of liver regeneration (11). Therefore, to examine which of the complement components downstream of C3 are required for the survival of regenerating liver cells, C3aR−/− or C5aR−/− mice were subjected to PHx, and livers were harvested at the time at which peak hepatocyte proliferation was observed in wild-type controls (44−48 h). Livers from both groups of receptor-deficient mice showed impaired regeneration, as demonstrated by a lack of hepatocyte proliferation (Fig. 4A), therefore confirming previous findings. Importantly, we found that livers from these mice revealed massive apoptosis of liver parenchyma after PHx (Fig. 4, B–E). These data suggested that the signaling triggered by both anaphylatoxins, through binding to their reciprocal receptors, contributes to both the regulation of liver cell proliferation and hepatoprotection.
FIGURE 4.
Impaired recovery and liver regeneration in complement receptor-deficient livers and amelioration of injury in C3/C5−/− livers after PHx by reconstitution with anaphylatoxins. A, BrdU incorporation into hepatocytes of wild-type (WT), C3aR−/−, and C5aR−/− livers 44 h after surgery. Data are represented as mean ± SEM (n = 11 (WT), 4 (C3aR−/−), and 6 (C5aR−/−)). *, p = 0.0001, unpaired t test. B–M, Liver sections 44 h following PHx, showing staining for the activated caspase-3 site on cytokeratin 18 (B, C, and F–I; magnification, ×100) or TUNEL staining (D, E, and J–M; magnification, ×400) in C3aR−/− mice (B and D), C5aR−/− mice (C and E), C3/C5−/− mice (F and J), C3/C5−/− mice treated with C3a (G and K), C3/C5−/− mice treated with C5a (H and L), and C3/C5−/− mice treated with both C3a and C5a (I and M). Images are representative of experiments with six (C3aR−/−), seven (C5aR−/−), eight (C3/C5−/−), and three (C3/C5−/− plus C3a and/or C5a) mice.
Synergistic activity of both anaphylatoxins is required for full protection of liver cells from death
If both C3a and C5a are prosurvival factors required for proliferating parenchymal cells, as suggested by the experiments with mice deficient in the anaphylatoxin receptors, then mice missing C3a, C5a, or both ligands should display injury to the liver parenchyma after PHx. To test this hypothesis, we generated mice deficient in both C3 and C5 (C3/C5−/−), as described previously (11). The lack of C3 and C5 prevents the formation of C3a and C5a. As expected, mice missing both anaphylatoxins displayed significant apoptosis in the liver parenchyma after PHx (Fig. 4, F and J). This parenchymal injury was attenuated when C3/C5−/− mice were reconstituted with C3a (Fig. 4, G and K) or C5a (Fig. 4, H and L), whereas the reconstitution of these mice with both anaphylatoxins, injected simultaneously before and soon after surgery, resulted in almost complete protection from liver damage (Fig. 4, I and M). The complete restoration of the wild-type phenotype in C3/C5−/− livers achieved through the reconstitution of deficient animals with both anaphylatoxins (C3a and C5a), as compared with the partial restoration seen after treatment with only one anaphylatoxin (C3a or C5a), suggests that the synergistic activity of both complement mediators protects liver cells from injury during regeneration.
Lack of complement impairs cytokine responses during regeneration
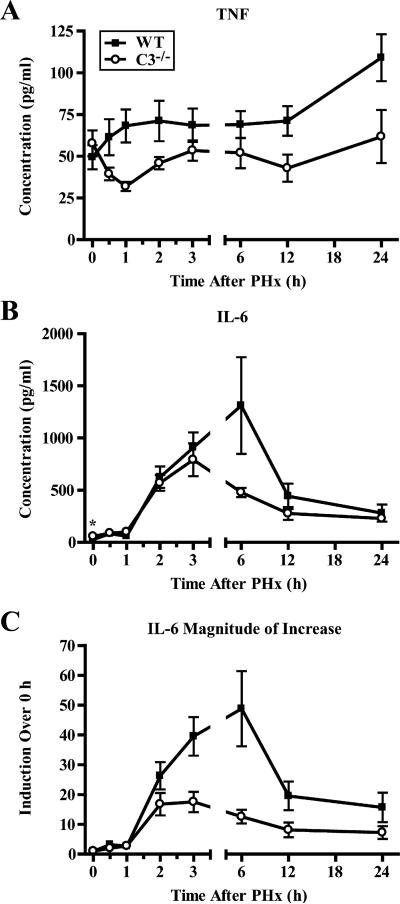
IL-6 and TNF are key players in the priming phase of liver regeneration (16). It has been previously demonstrated that pharmacological blockade of C5aR resulted in the decreased expression of genes for IL-6 and TNF 3−6 h after PHx (11). Additionally, IL-6 has recently been postulated to have a hepatoprotective role (22, 23). Therefore, we hypothesized that prosurvival complement properties are attributed to the regulation of the cytokine response, in particular IL-6, to liver resection. To test this hypothesis, we evaluated the protein levels of IL-6 and TNF in sera of C3−/− mice at multiple time points after PHx, since mRNA does not always correlate with the amounts of active proteins. C3 deficiency resulted in a significant reduction in the amounts of TNF at several time points after PHx and an alteration of the overall pattern of this cytokine response to liver resection (Fig. 5A). As the amount of IL-6 was significantly lower in C3+/+ mice compared with C3−/− mice before surgery (Fig. 5B), in addition to absolute protein concentrations (Fig. 5B), we analyzed the data expressed as a ratio between the levels at baseline and specific time points, that is, the magnitude of increase (Fig. 5C). We found a significant difference in the pattern of cytokine induction for IL-6, with a smaller increase over baseline levels seen in C3−/− mice.
FIGURE 5.
Changes in serum levels and induction of TNF and IL-6 after liver resection. A and B, Changes in levels of TNF (A; p < 0.0001, two-way ANOVA) and IL-6 (B; *, p = 0.0233, unpaired t test) measured in sera from wild-type (WT) or C3−/− mice after PHx. C, Magnitude of the increase in levels (compared with baseline) for IL-6 (p < 0.0001, two-way ANOVA). Data are presented as mean ± SEM (n ≥ 9 (WT) and 7 (C3−/−) mice/time point). ■, Wild-type; ○, C3−/−.
Complement regulates prosurvival signaling
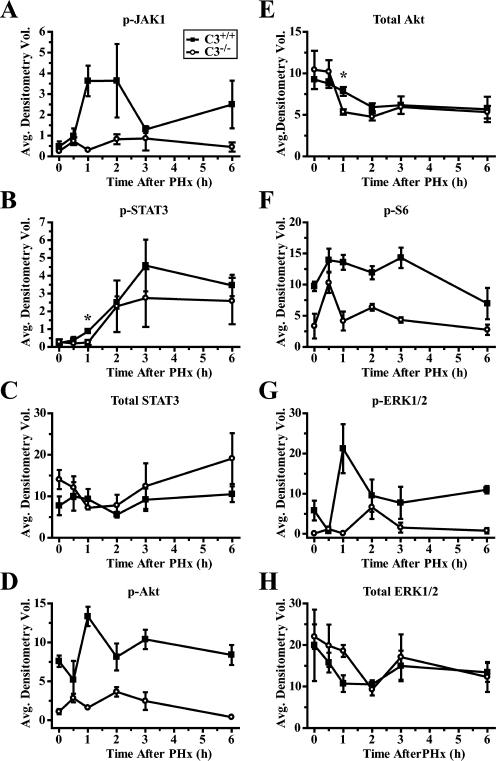
To prove a functional link between complement deficiency and apoptosis in regenerating liver, we examined intracellular signaling, which is known to contribute to the survival of regenerating liver cells after PHx. We focused this analysis on intracellular pathways regulated by IL-6, as the induction of this cytokine was significantly affected in complement-deficient mice and IL-6 is a major prosurvival factor for regenerating liver cells. The phosphorylation of JAK1, which is a direct result of IL-6 binding to its receptor (16), was lower in C3−/− livers at early time points after PHx (Fig. 6A). As expected, the phosphorylation of STAT3 at Tyr705, which is a consequence of JAK1 activity (16), was also decreased (Fig. 6B), although a statistically significant difference was demonstrated only 1 h after PHx. The amount of total STAT3 protein increased slightly immediately after surgery in wild-type livers, whereas an analogous increase was not observed in C3−/− livers (Fig. 6C). However, these changes were minimal when compared with the induction of phosphorylated protein, indicating that the increased phosphorylation was not simply related to an increase in the amount of total protein, but rather depended on JAK1 tyrosine kinase activity. The lack of complement activity also resulted in an alteration in the PI3K/Akt pathway, as illustrated by markedly lower phosphorylation of Akt at all the time points examined (Fig. 6D). The level of total Akt tended to decline in both C3−/− and control livers; however, a statistically significant decrease in total Akt was observed in C3−/− livers only at 1 h after PHx (Fig. 6E). The PI3K/Akt pathway is linked to the mTOR pathway (24), which is known to be involved in the hypertrophy of regenerating hepatocytes (25). mTOR signaling leads to the activation of p70S6 kinase, which phosphorylates S6 ribosomal protein (24). Therefore, we examined S6 phosphorylation as an indicator of whether the alteration of PI3K/Akt signaling led to similar changes in downstream mTOR signaling. The pattern of S6 phospohorylation we observed was similar to that for Akt, with lower levels of phosphorylated protein in C3−/− livers (Fig. 6F). Finally, to elucidate the mechanism of impaired hepatocyte proliferation in complement-deficient mice, we examined the phosphorylation of ERK. This analysis showed that, as was true for prosurvival signaling, complement also affected pro-proliferative factors, with a lower induction of ERK being observed in C3−/− livers (Fig. 6G) and no significant differences in total ERK protein levels (Fig. 6H).
FIGURE 6.
Altered phosphorylation and expression of proteins involved in proliferation and hepatoprotection in post-PHx C3−/− livers. Densitometry of immunoblots for protein expression or phosphorylation using whole-cell liver extracts from C3+/+ or C3−/− mice. Results are shown for phosphorylation of Tyr1022/Tyr1023 of JAK1 (A; p = 0.0008, two-way ANOVA), phosphorylation of Tyr705 of STAT3 (B; *, p = 0.0172, unpaired t test), total STAT3 protein (C), phosphorylation of Ser473 of Akt (D; p < 0.0001, two-way ANOVA), total Akt protein (E), phosphorylation of Ser235/Ser236 of S6 ribosomal protein (F; p = 0.0026, two-way ANOVA), phosphorylation of Thr202/Tyr204 of ERK1 (top band) and ERK2 (bottom band) (G; p = 0.0130, two-way ANOVA), and total ERK1 and ERK2 protein (H). Graphs represent mean ± SEM (n = 3 mice/time point/cohort). ■, C3+/+; ○, C3−/−.
Discussion
This study provides evidence that complement components, despite contributing to host tissue injury in several clinical conditions (9), protect proliferating liver cells from apoptosis. To elucidate prosurvival properties of complement components, we chose the PHx model, as the mammalian liver is the only parenchymal organ with the capacity to regenerate through the hyperplasia and hypertrophy of mature parenchymal cells. Therefore, surgical resection of this organ and its subsequent regeneration is a valid experimental setting for studying biological processes related to cell proliferation in vivo (14). Additionally, it has recently been postulated that regenerating liver cells require protection from death signaling, and factors regulating the process of regeneration can also contribute to hepatoprotection (13). For example, a hepatoprotective role for IL-6 has been suggested (22). As previous work has shown that complement anaphylatoxins participate in the regulation of the initial phase of liver regeneration (priming) through cross-talk with IL-6 and TNF (11), and several complement deficiencies have been associated with injury to the liver parenchyma after PHx, we hypothesized that complement activity is required to protect liver cells when they regenerate.
We showed that complement is activated during liver regeneration. All three pathways of complement activation converge at C3 and lead to the formation of the C3 convertases that are required for the activation of remaining complement proteins (26). Therefore, the elimination of C3 prevents the generation of complement effectors. Thus, the considerable apoptosis of the liver parenchyma that we observed in mice deficient in C3 suggested that activated complement proteins are required for hepatoprotection.
Cleavage of C3 by C3 convertases produces the anaphylatoxin C3a, which has proinflammatory properties (27, 28). Cleavage of C3 also leads to the formation of the C5 convertase, which produces C5a, an even more potent inflammatory mediator than C3a (26, 29). The requirement for C3a and C5a for the protection of regenerating liver cells was demonstrated by our studies utilizing mice deficient in the receptors for C3a and C5a, which revealed the presence of severe apoptosis in livers from these mice after PHx. Further confirmation of the cytoprotective functions of the C3a and C5a anaphylatoxins was indicated by the presence of liver damage after PHx in mice lacking both ligands (C3/C5−/− mice). Nearly full protection of these mice against liver injury, after simultaneous reconstitution with both C3a and C5a, confirmed that the synergistic activity of both anaphylatoxins is required for the inhibition of death signaling in regenerating liver cells.
The absence of complement activity in C3−/− mice altered the induction of TNF and IL-6 after PHx. Both of these cytokines are crucial regulators of the priming phase of liver regeneration, while, importantly, IL-6 is also involved in hepatoprotection (22). Notably, Kupffer cells that produce and secrete these cytokines express anaphylatoxin receptors (30). Reciprocal interactions between complement anaphylatoxins and cytokines have been demonstrated in the context of the immune response (31). However, our observations suggesting that complement effectors act as upstream regulators of the cytokine network, which in turn may influence the fate of proliferating epithelial cells, revealed an unrecognized function of complement. Previous work showed that blocking the C5aR affected mRNA levels of TNF and IL-6 at single time points after liver resection (11). Currently, by monitoring serum amounts of cytokines at multiple points after PHx, we found that lack of complement activities in C3−/− mice resulted in a lower induction of IL-6 3−6 h after surgery. This time window corresponded to the early complement activation that was observed in wild-type mice. Additionally, the importance of this early activation for regeneration was proven by testing pre- and post-PHx reconstitution of C3−/− mice with sera containing C3. Importantly, only C3 delivered before surgery prevented liver injury. Thus, these experiments demonstrated that early complement activation is essential for the proliferation and survival of hepatocytes, and that this corresponds to the peak of IL-6 secretion after liver resection. The lack of an effect of C3 reconstitution on the C3−/− phenotype after PHx, when C3 was administered 8 h after surgery, ruled out the contribution of “late” complement activation to hepatoprotection. Therefore, we hypothesize that the second peak of complement activation can be attributed to the removal of necrotic tissue at the site of resection, based on previous study showing the contribution of C3 to the clearance of damaged liver parenchyma (12). Although PHx in wild-type mice is not associated with damage to the regenerating portion of the liver, the tissue located distally to the sutures undergoes necrosis due to an interrupted blood supply. Most of this tissue is removed during surgery; however, a small remaining portion gradually undergoes necrosis and likely activates complement through mechanisms involving danger-associated molecular patterns (32).
IL-6 signaling is currently the best characterized pathway thought to be associated with hepatoprotection (22). The binding of IL-6 to its receptor induces the phosphorylation of JAK1, which is an upstream activator of STAT3, a transcription factor required for hepatoprotection (22). The phosphorylation of Tyr705 of STAT3 leads to dimerization of this protein and its translocation into the nucleus, where it activates immediate-early phase genes involved in the regenerative response (16). IL-6 is also involved in the activation of the PI3K/Akt pathway, a well-recognized antiapoptotic signaling pathway in several biological systems, including liver regeneration (33, 34). PI3K/Akt signaling interacts with the mTOR pathway, which is fundamentally important for the growth of benign and malignant cells (35). The alterations in intracellular prosurvival signaling that we observed in the livers of C3−/− mice after PHx included a decreased phosphorylation of STAT3, JAK1, and Akt. Additionally, we found lower phosphorylation of the S6 ribosomal protein in these livers, highlighting a potential link between complement and the mTOR pathway. Based on these changes seen in C3−/− mice in proteins related to IL-6 prosurvival signaling, we postulate that prosurvival complement activities are attributed to regulating the IL-6 response to PHx. Additionally, the kinetics of signaling alterations for these proteins correlated with the observed peaks of IL-6 secretion and early complement activation, further confirming functional associations between complement, IL-6, and intracellular hepatoprotective signaling. Finally, the presence of early apoptosis in C3−/− livers strengthens this hypothesis and indicates that the phenotype observed in mice lacking complement activities is a result of the described molecular alterations.
Thus, disturbances in extracellular events such as a lack of complement activity and an impairment of the induction of IL-6 correlated with alterations in intracellular prosurvival signaling that is triggered in the liver to prevent cell death. These correlations point to a consequential link between complement deficiencies, alterations in the cytokine milieu, and inhibition of intracellular prosurvival signaling in the liver, all of which led to the parenchymal damage observed in the livers of complement-deficient mice after PHx.
Overall, this study demonstrates that the prosurvival functions of the complement system in proliferating parenchymal cells are exerted through cross-talk with the cytokine network. Although the experiments described herein were performed using a model of liver regeneration, these complement functions can potentially apply to other biological systems, including proliferating tumor cells, since the molecular mechanisms underlying complement activity in the regenerating liver are applicable to cell survival and proliferation in general. These findings also have clinical implications. The results suggest that anticomplement and antiinflammatory therapies that are widely used for patients suffering from inflammatory diseases should be applied with particular care, taking into consideration these significant interconnections between complement, cytokine networks, and antiapoptotic pathways.
Acknowledgments
We thank D. Ricklin, S. Ricklin, and A. Koutoulaki for critical review of the manuscript and D. McClellan for editorial assistance.
Footnotes
This work was supported by National Institutes of Health Grants AI068730 (to J.D.L.) and AI025011 and AI074333 (to R.A.W.).
Abbreviations used in this paper: PHx, partial hepatectomy; mTOR, mammalian target of rapamycin; C3aR, C3a receptor; C5aR, C5a receptor.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am. J. Pathol. 2007;171:715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 3.Cook HT, Botto M. Mechanisms of disease: the complement system and the pathogenesis of systemic lupus erythematosus. Nat. Clin. Pract. Rheumatol. 2006;2:330–337. doi: 10.1038/ncprheum0191. [DOI] [PubMed] [Google Scholar]
- 4.Tsokos GC, Fleming SD. Autoimmunity, complement activation, tissue injury and reciprocal effects. Curr. Dir. Autoimmun. 2004;7:149–164. doi: 10.1159/000075691. [DOI] [PubMed] [Google Scholar]
- 5.Stahl GL, Xu Y, Hao L, Miller M, Buras JA, Fung M, Zhao H. Role for the alternative complement pathway in ischemia/reperfusion injury. Am. J. Pathol. 2003;162:449–455. doi: 10.1016/S0002-9440(10)63839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh MC, Bourcier T, Takahashi K, Shi L, Busche MN, Rother RP, Solomon SD, Ezekowitz RA, Stahl GL. Mannose-binding lectin is a regulator of inflammation that accompanies myocardial ischemia and reperfusion injury. J. Immunol. 2005;175:541–546. doi: 10.4049/jimmunol.175.1.541. [DOI] [PubMed] [Google Scholar]
- 7.Fleming SD. Natural antibodies, autoantibodies and complement activation in tissue injury. Autoimmunity. 2006;39:379–386. doi: 10.1080/08916930600739381. [DOI] [PubMed] [Google Scholar]
- 8.Walport MJ. Complement: second of two parts. N. Engl. J. Med. 2001;344:1140–1144. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 9.Holers VM. Complement as a regulatory and effector pathway in human diseases. In: Lambris JD, Holers VM, editors. Therapeutic Intervention in the Complement System. Humana; Totowa, NJ: 2000. pp. 12–21. [Google Scholar]
- 10.Mastellos D, Papadimitriou JC, Franchini S, Tsonis PA, Lambris JD. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J. Immunol. 2001;166:2479–2486. doi: 10.4049/jimmunol.166.4.2479. [DOI] [PubMed] [Google Scholar]
- 11.Strey CW, Markiewski M, Mastellos D, Tudoran R, Spruce LA, Greenbaum LE, Lambris JD. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J. Exp. Med. 2003;198:913–923. doi: 10.1084/jem.20030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markiewski MM, Mastellos D, Tudoran R, DeAngelis RA, Strey CW, Franchini S, Wetsel RA, Erdei A, Lambris JD. C3a and C3b activation products of the third component of complement (C3) are critical for normal liver recovery after toxic injury. J. Immunol. 2004;173:747–754. doi: 10.4049/jimmunol.173.2.747. [DOI] [PubMed] [Google Scholar]
- 13.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 14.Michalopoulos GK, DeFrances M. Liver regeneration. Adv. Biochem. Eng. Biotechnol. 2005;93:101–134. doi: 10.1007/b99968. [DOI] [PubMed] [Google Scholar]
- 15.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 16.Taub R. Liver regeneration: from myth to mechanism. Nat. Rev. Mol. Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 17.Circolo A, Garnier G, Fukuda W, Wang X, Hidvegi T, Szalai AJ, Briles DE, Volanakis JE, Wetsel RA, Colten HR. Genetic disruption of the murine complement C3 promoter region generates deficient mice with extrahepatic expression of C3 mRNA. Immunopharmacology. 1999;42:135–149. doi: 10.1016/s0162-3109(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 18.Kildsgaard J, Hollmann TJ, Matthews KW, Bian K, Murad F, Wetsel RA. Cutting edge: targeted disruption of the C3a receptor gene demonstrates a novel protective anti-inflammatory role for C3a in endotoxin-shock. J. Immunol. 2000;165:5406–5409. doi: 10.4049/jimmunol.165.10.5406. [DOI] [PubMed] [Google Scholar]
- 19.Hopken UE, Lu B, Gerard NP, Gerard C. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature. 1996;383:86–89. doi: 10.1038/383086a0. [DOI] [PubMed] [Google Scholar]
- 20.Higgins GM, Anderson RM. Experimental pathology of the liver: I. Restoration of the liver of the white rat following partial surgical removal. Arch. Pathol. 1931;12:186–202. [Google Scholar]
- 21.DeAngelis RA, Kovalovich K, Cressman DE, Taub R. Normal liver regeneration in p50/nuclear factor •B1 knockout mice. Hepatology. 2001;33:915–924. doi: 10.1053/jhep.2001.23192. [DOI] [PubMed] [Google Scholar]
- 22.Taub R. Hepatoprotection via the IL-6/Stat3 pathway. J. Clin. Invest. 2003;112:978–980. doi: 10.1172/JCI19974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bansal MB, Kovalovich K, Gupta R, Li W, Agarwal A, Radbill B, Alvarez CE, Safadi R, Fiel MI, Friedman SL, et al. Interleukin-6 protects hepatocytes from CCl4-mediated necrosis and apoptosis in mice by reducing MMP-2 expression. J. Hepatol. 2005;42:548–556. doi: 10.1016/j.jhep.2004.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin KA, Blenis J. Coordinate regulation of translation by the PI 3-kinase and mTOR pathways. Adv. Cancer Res. 2002;86:1–39. doi: 10.1016/s0065-230x(02)86001-8. [DOI] [PubMed] [Google Scholar]
- 25.Haga S, Ogawa W, Inoue H, Terui K, Ogino T, Igarashi R, Takeda K, Akira S, Enosawa S, Furukawa H, et al. Compensatory recovery of liver mass by Akt-mediated hepatocellular hypertrophy in liver-specific STAT3-deficient mice. J. Hepatol. 2005;43:799–807. doi: 10.1016/j.jhep.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 26.Walport MJ. Complement: first of two parts. N. Engl. J. Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 27.Mollnes TE, Song WC, Lambris JD. Complement in inflammatory tissue damage and disease. Trends Immunol. 2002;23:61–64. doi: 10.1016/s1471-4906(01)02129-9. [DOI] [PubMed] [Google Scholar]
- 28.Lambris JD, Sahu A, Wetsel RA. C3, C4 and C5:genes, structure, and functions. In: Volanakis JE, Frank M, editors. The Human Complement System in Health and Disease. Marcel Dekker; New York: 1998. pp. 83–118. [Google Scholar]
- 29.Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu. Rev. Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 30.Puschel GP, Nolte A, Schieferdecker HL, Rothermel E, Gotze O, Jungermann K. Inhibition of anaphylatoxin C3a- and C5a- but not nerve stimulation- or noradrenaline-dependent increase in glucose output and reduction of flow in Kupffer cell-depleted perfused rat livers. Hepatology. 1996;24:685–690. doi: 10.1002/hep.510240335. [DOI] [PubMed] [Google Scholar]
- 31.DeAngelis RA, Markiewski MM, Lambris JD. Liver regeneration: a link to inflammation through complement. Adv. Exp. Med. Biol. 2006;586:17–34. doi: 10.1007/0-387-34134-X_2. [DOI] [PubMed] [Google Scholar]
- 32.Markiewski MM, DeAngelis RA, Lambris JD. Complexity of complement activation in sepsis. J. Cell. Mol. Med. 2008;12:2245–2254. doi: 10.1111/j.1582-4934.2008.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong F, Nguyen VA, Shen X, Kunos G, Gao B. Rapid activation of protein kinase B/Akt has a key role in antiapoptotic signaling during liver regeneration. Biochem. Biophys. Res. Commun. 2000;279:974–979. doi: 10.1006/bbrc.2000.4044. [DOI] [PubMed] [Google Scholar]
- 34.Testa JR, Tsichlis PN. AKT signaling in normal and malignant cells. Oncogene. 2005;24:7391–7393. doi: 10.1038/sj.onc.1209100. [DOI] [PubMed] [Google Scholar]
- 35.Corradetti MN, Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene. 2006;25:6347–6360. doi: 10.1038/sj.onc.1209885. [DOI] [PubMed] [Google Scholar]