Abstract
OBJECTIVE
Microalbuminuria is associated with cardiovascular mortality, particularly among individuals with type 2 diabetes, but the mechanisms underlying this association are not completely understood. Microalbuminuria is known to be associated with cardiovascular autonomic dysfunction (C-AD), and C-AD in turn is associated with cardiovascular mortality. The purpose of this study, therefore, was to investigate whether C-AD can explain the relationship between microalbuminuria and cardiovascular mortality.
RESEARCH DESIGN AND METHODS
We studied 490 individuals from a population-based cohort of individuals aged 50–75 years who were followed for a median period of 13.6 years. Microalbuminuria was defined as an albumin-to-creatinine ratio ≥2.0 mg/mmol in an early-morning spot-urine sample. Ten parameters reflecting different aspects of cardiovascular autonomic function were measured and compiled into a total score of C-AD (mean of separate z scores). The association between C-AD and microalbuminuria was estimated by multiple linear regression, and relative risks (RRs) for cardiovascular mortality were estimated by Cox proportional hazards analyses.
RESULTS
After adjustments for age, sex, glucose tolerance status, and other risk factors, C-AD was associated with microalbuminuria (β = 0.16 [95% CI 0.01–0.33]), and both microalbuminuria (RR 2.09 [1.07–4.08]) and C-AD (1.74 [1.04–2.89]) were associated with cardiovascular mortality. These associations did not change after further mutual adjustment for C-AD (2.13 [1.09–4.17]) or microalbuminuria (1.76 [1.05–2.94]), respectively.
CONCLUSIONS
Both microalbuminuria and C-AD are independently associated with cardiovascular mortality, and the excess mortality attributable to microalbuminuria cannot be explained by C-AD.
Microalbuminuria is associated with an increased risk of cardiovascular disease and mortality (1). This association is independent of other known cardiovascular risk factors such as hypertension, dyslipidemia, obesity, smoking, and impaired renal function (1,2). Several mechanisms, notably endothelial dysfunction and low-grade inflammation, have been proposed to explain, at least in part, the increased risk of cardiovascular mortality in individuals with microalbuminuria (3). Cardiovascular autonomic dysfunction (C-AD) could potentially constitute another such mechanism.
Indeed, we as well as others have previously shown that C-AD is associated with microalbuminuria, especially in individuals with impaired glucose metabolism (IGM) and type 2 diabetes (4–6). Two proposed mechanisms explaining this association are, first, a disturbance in glomerular arteriolar autoregulation, which in turn may result in an inability to counteract glomerular hypertension (7), and, second, a reduced drop in nightly blood pressure due to C-AD, both of which may result in microalbuminuria (8). In addition, C-AD is associated with cardiovascular mortality (9,10) and can potentially link microalbuminuria to cardiovascular mortality by arrhythmogenic or atherogenic effects, for example, by promoting vascular calcification and arterial stiffness (11).
In view of these considerations, we investigated, in a prospective cohort study, whether C-AD can explain the relationship between microalbuminuria and cardiovascular mortality or, alternatively, whether both microalbuminuria and C-AD are independently associated with cardiovascular mortality. These hypotheses have never been investigated in the general population and could have clinical relevance because the first hypothesis suggests that C-AD should be targeted to decrease mortality risk in individuals with microalbuminuria and, conversely, the second hypothesis suggests that both microalbuminuria and C-AD can be used for estimating the risk of cardiovascular mortality.
RESEARCH DESIGN AND METHODS
We used data from the Hoorn Study, a population-based cohort study on glucose metabolism and other cardiovascular risk factors in a general Caucasian population, which has been described in detail previously (12). In brief, men and women aged 50–75 years were randomly selected from the population register of the town of Hoorn, the Netherlands; 2,484 subjects participated (response rate 71%). Baseline examinations were conducted from October 1989 until February 1992. All subjects had a 75-g oral glucose tolerance test, except those in whom type 2 diabetes had previously been diagnosed. An extensive metabolic and cardiovascular investigation was performed in an age-, sex-, and glucose tolerance–stratified, random subsample of 631 participants (89% of those invited), which is used in the present study (12).
Participants without representative urine samples available (n = 45) and/or who did not complete at least 7 of the 10 autonomic function tests (n = 31), had a history of neurological disease (n = 5), and were using ACE inhibitors (n = 33) and/or drugs known to influence autonomic nerve function (namely antiparkinson drugs, phenytoin, antihistamines, or parasympatholytic, parasympathomimetic, and sympathomimetic drugs) (n = 49) were excluded from the analyses. The present study, therefore, consisted of 490 individuals: 305 with normal glucose metabolism, 71 with IGM (including those with impaired fasting glucose and/or impaired glucose tolerance), and 114 with type 2 diabetes.
The Hoorn Study was approved by the ethical review committee of VU University Medical Centre, Amsterdam, the Netherlands. Informed consent was obtained from all participants.
Baseline measurements
Urinary albumin concentration was measured in a first voided sample by rate nephelometry (Array Protein System, Beckman Coulter, Galway, Ireland) with a detection threshold of 6.2 mg/l (intra- and interassay coefficients of variation of 5 and 8%, respectively). Urinary creatinine was measured with a modified Jaffé method. Microalbuminuria was present if the albumin-to-creatinine ratio (ACR) was in the range of 2.0–30 mg/mmol (5). The average value of the ACR was used if two representative samples (n = 154) were available. Excluding subjects with an ACR >30 mg/mmol (i.e., macroalbuminuria, n = 4) did not materially affect any of the results, which are therefore presented for the entire group.
Cardiovascular autonomic function tests were performed as described in detail elsewhere (9). In brief, participants were asked to refrain from smoking and drinking coffee for at least 2 h before the assessments. A light meal >1 h before the measurements was allowed. Tests took place between 8:30 a.m. and 4:00 p.m. in a quiet location with room temperature of 19–22°C after a resting period of at least 10 min. Ten parameters of cardiac autonomic function were derived from the R-R interval and finger systolic blood pressure (SBP) continuous recordings by a computerized data analyses system developed locally by the Department of Medical Physics, VU University, Amsterdam, the Netherlands (13). Tests were performed under three conditions: during spontaneous breathing over 3 min in the supine position (mean of all normal to normal R-R intervals [MeanNN], SD of all normal to normal R-R intervals [SDNN], low-frequency [LF] power, high-frequency [HF] power, and LF/(LF + HF)), during six deep breaths over 1 min in the supine position (expiration-inspiration [EI] difference and baroreflex sensitivity [BRS]), and during an active change from lying to standing (SBP difference, R-R interval maximum [RRmax], and R-R interval maximum divided by minimum [RRmax/min]) (for further explanation of measurements, see supplementary Table A1, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc08-1544/DC1). We thus obtained results in four tests that reflect heart rate or blood pressure changes due to certain “maneuvers,” in this case deep breathing or standing up (i.e., EI difference, RRmax, RRmax/min, and SBP difference) (14), five tests of spectral analyses of heart rate variability (HRV) [i.e., MeanNN, SDNN, LF power, HF power, and LF/(LF + HF)] (15), and one BRS measurement (16). In addition, these tests were grouped into those representing predominantly parasympathetic (i.e., EI difference, RRmax, and HF power), sympathetic (i.e., SBP difference), or both functions (i.e., Mean-NN, SDNN, LF power, LF/(LF + HF), BRS, and RRmax/min) (15,16).
BMI, waist-to-hip ratio (WHR), SBP and diastolic blood pressure (DBP), levels of fasting plasma glucose; A1C; insulin; total, HDL, and LDL cholesterol; triglycerides; creatinine; and smoking status were measured as described elsewhere (5,9,12). Glomerular filtration rate was estimated by the short Modification of Diet in Renal Disease equation. Hypertension was defined as SBP ≥140 mmHg and/or DBP ≥90 mmHg and/or the use of antihypertensive drugs. Prior cardiovascular disease (CVD) was defined when individuals had (any of the following): a history of myocardial infarction, abnormalities on a resting electrocardiogram (Minnesota codes 1.1–1.3, 4.1–4.3, 5.1–5.3, or 7.1), previous coronary bypass surgery or angioplasty, peripheral arterial bypass, or nontraumatic amputation, or an ankle-brachial index of <0.9 in either leg.
Follow-up
Data on the participants' vital status up to 1 January 2005 were collected from the mortality register of the municipality of Hoorn. Information on cause of death was extracted from the medical records of the general practitioners and the local hospital and coded according to the ICD-9. Cardiovascular mortality, including sudden death, was defined by ICD-9 codes 390–459 and 798. Information on cause of death could not be obtained for 20 of the deceased individuals. All subjects were followed until death or end of follow-up, at which time they were censored.
Statistical analyses
Baseline characteristics between survivors and nonsurvivors were compared with the use of Student's t or χ2 tests. We computed z scores ([individual's observed value − population mean]/SD) for each of the 10 measurements of C-AD. All z scores (except SBP difference) were inverted and averaged into a total score (“C-AD total score”), so that higher values reflect greater C-AD.
The association between microalbuminuria and C-AD total score was investigated with multiple linear regression analyses. Kaplan-Meier survival curves were plotted for cardiovascular mortality among individuals with normoalbuminuria versus microalbuminuria and similarly across tertiles of the C-AD total score, and differences between groups were tested with a log-rank test. Cox proportional hazards regression models were used to calculate the crude and adjusted relative risks (RRs) and respective 95% CI of microalbuminuria (versus normoalbuminuria) and of the C-AD total score (per SD [= 0.605] increase) for cardiovascular and all-cause mortality. Two-sided P < 0.05 was considered statistically significant. All analyses were performed with SPSS (version 15.0).
RESULTS
Subjects excluded from the analyses (n = 141) were older, more often had type 2 diabetes, and had a worse risk profile than the subjects included (n = 490) (data not shown). Median duration of the follow-up was 13.6 (range 0.52–15.19) years. During the follow-up period, 141 (28.8%) participants died, of whom 53 (37.6%) died of CVD. At baseline, individuals who died had a worse C-AD total score, more often had microalbuminuria and were older, more often were men and had type 2 diabetes and hypertension, and had a higher A1C and WHR as compared with the survivors (Table 1).
Table 1.
Baseline characteristics of survivors versus nonsurvivors
| Survivors | Nonsurvivors |
||
|---|---|---|---|
| All-cause mortality | Cardiovascular mortality | ||
| n | 349 | 141 | 53 |
| Age (years) | 62.4 ± 6.8 | 67.1 ± 6.4* | 67.7 ± 6.7* |
| Sex (% men) | 43.6 | 53.9* | 50.9 |
| Glucose tolerance status | |||
| NGM | 66.5 | 51.8 | 43.4 |
| IGM | 14.0 | 15.6 | 17.0 |
| Type 2 diabetes | 19.5 | 32.6* | 39.6* |
| A1C (%) | 5.7 ± 1.1 | 6.2 ± 1.4* | 6.4 ± 1.6* |
| SBP (mmHg) | 136.2 ± 18.5 | 142.8 ± 19.6* | 147.2 ± 21.6* |
| DBP (mmHg) | 82.0 ± 9.3 | 83.3 ± 11.2 | 84.4 ± 10.8 |
| Use of blood pressure–lowering drugs (%)† | 20.9 | 31.9* | 32.1* |
| Hypertension (%) | 46.4 | 65.2* | 69.8* |
| History of CVD (%) | 19.8 | 27.0 | 30.2 |
| Ever smokers (%) | 59.6 | 71.6* | 73.6 |
| WHR | 0.90 ± 0.08 | 0.94 ± 0.08* | 0.95 ± 0.08* |
| BMI (kg/cm2) | 26.8 ± 3.7 | 27.2 ± 3.9 | 27.4 ± 3.8 |
| Serum creatinine (μmol/l) | 89.5 ± 16.1 | 95.0 ± 23.0* | 97.6 ± 30.6 |
| eGFR (ml/min) | 69 ± 11 | 66 ± 13* | 64 ± 15* |
| Cholesterol (mmol/l) | 6.61 ± 1.13 | 6.73 ± 1.31 | 6.86 ± 1.19 |
| HDL cholesterol (mmol/l) | 1.31 ± 0.37 | 1.26 ± 0.34 | 1.23 ± 0.31 |
| LDL cholesterol (mmol/l) | 4.51 ± 0.99 | 4.60 ± 1.13 | 4.77 ± 1.05 |
| Use of lipid-lowering drugs (%) | 1.7 | 1.4 | 1.9 |
| Triglycerides (mmol/l) | 1.50 (1.10–2.10) | 1.70 (1.20–2.40)* | 1.80 (1.25–2.40) |
| EI difference (ms) | 162 (107–237) | 131 (83–213)* | 113 (79–183)* |
| RRmax (ms) | 254 ± 96 | 217 ± 88* | 223 ± 91* |
| RRmax/min | 1.27 ± 0.16 | 1.19 ± 0.14* | 1.21 ± 0.13* |
| HF power (ms2) | 183 (85–438) | 151 (67–363)* | 145 (77–330) |
| LF power (ms2) | 239 (123–525) | 160 (68–390)* | 152 (63–307)* |
| LF/(LF + HF) | 0.56 ± 0.19 | 0.52 ± 0.21 | 0.50 ± 0.21* |
| BRS (ms/mmHg) | 9.01 ± 4.96 | 7.91 ± 4.20* | 7.84 ± 4.14 |
| SDNN (ms) | 36 ± 16 | 33 ± 19* | 32 ± 19 |
| MeanNN (ms) | 966 ± 147 | 927 ± 156* | 935 ± 157 |
| SBP difference (mmHg) | −6.42 ± 14.82 | −6.32 ± 15.17 | −6.45 ± 16.51 |
| C-AD total score | −0.08 ± 0.58 | 0.22 ± 0.61* | 0.22 ± 0.65* |
| Microalbuminuria (%) | 7.7 | 16.3* | 24.5* |
Data are presented as frequencies (%), means ± SD, or medians (interquartile range). For a detailed explanation of autonomic function tests, see supplementary Table A1.
*P < 0.05 vs. survivors.
†These include diuretics, α-blockers, β-blockers, calcium channel blockers, and other blood pressure–lowering drugs but exclude ACE inhibitors (because subjects using these drugs were excluded from the analyses). eGFR, estimated glomerular filtration rate; NGM, normal glucose metabolism.
Association between microalbuminuria and C-AD
C-AD total score was associated with microalbuminuria (β = 0.33 [95% CI 0.16–0.51]) (Table 2, model 1). After adjustment for potential confounding factors, C-AD total score was still borderline associated with microalbuminuria (0.16 [−0.01 to 0.33]) (Table 2, model 3). Comparable results were obtained when tests were combined on the basis of their methodology or on the part of the autonomic nervous system they predominately represent. All individual C-AD tests, except LF/(LF + HF), were positively associated with microalbuminuria (although not all statistically significantly so) (data not shown).
Table 2.
Association between microalbuminuria and C-AD
| Model* | β† | 95% CI | P | |
|---|---|---|---|---|
| C-AD total score | 1 | 0.33 | 0.16 to 0.51 | <0.001 |
| 2 | 0.21 | 0.04 to 0.38 | 0.016 | |
| 3 | 0.16 | −0.01 to 0.33 | 0.067 | |
| C-ADmaneuvers | 1 | 0.34 | 0.15 to 0.53 | 0.001 |
| 2 | 0.17 | −0.01 to 0.35 | 0.061 | |
| 3 | 0.12 | −0.07 to 0.30 | 0.209 | |
| C-ADBRS | 1 | 0.40 | 0.08 to 0.71 | 0.014 |
| 2 | 0.23 | −0.08 to 0.54 | 0.149 | |
| 3 | 0.21 | −0.11 to 0.53 | 0.194 | |
| C-ADHRV | 1 | 0.31 | 0.12 to 0.51 | 0.001 |
| 2 | 0.22 | 0.03 to 0.41 | 0.025 | |
| 3 | 0.16 | −0.04 to 0.35 | 0.110 | |
| C-ADparasympathetic | 1 | 0.45 | 0.22 to 0.69 | <0.001 |
| 2 | 0.28 | 0.05 to 0.51 | 0.017 | |
| 3 | 0.23 | −0.01 to 0.46 | 0.055 | |
| C-ADsympathetic | 1 | 0.14 | −0.18 to 0.44 | 0.394 |
| 2 | 0.13 | −0.19 to 0.44 | 0.433 | |
| 3 | 0.11 | −0.22 to 0.44 | 0.503 | |
| C-ADboth | 1 | 0.32 | 0.14 to 0.49 | <0.001 |
| 2 | 0.23 | 0.06 to 0.41 | 0.001 | |
| 3 | 0.18 | 0.01 to 0.36 | 0.041 |
*Model 1: univariate analysis; model 2: adjusted for sex, age, and GTS; model 3: model 2 plus adjustments for hypertension, WHR, estimated glomerular filtration rate, LDL, HDL, triglycerides, smoking, and prior CVD.
†β indicates the difference in C-AD score between individuals with microalbuminuria vs. normoalbuminuria.
Association of microalbuminuria and C-AD total score with cardiovascular and all-cause mortality
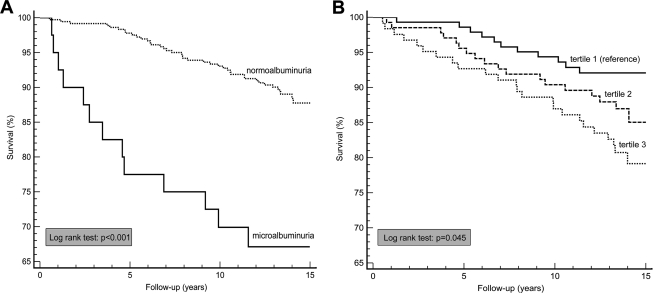
Both microalbuminuria (RR 3.49 [95% CI 1.87–6.53]) and C-AD total score (2.54 [1.60–4.04]) were associated with cardiovascular mortality (Table 3, model 1; Fig. 1). These associations were attenuated after adjustment for age, sex, and glucose tolerance status (GTS) (2.23 [1.16–4.29] and 1.81 [1.11–2.94], respectively; model 2). Further adjustment for other cardiovascular risk factors only slightly further attenuated these RRs (model 3), and when the C-AD total score or microalbuminuria was added to these models, the RR attributable to microalbuminuria remained practically unchanged and both microalbuminuria (2.13 [1.09–4.17]) and C-AD total score (1.76 [1.05–2.94]) were independently associated with cardiovascular mortality (model 4).
Table 3.
Association of microalbuminuria or C-AD with cardiovascular and all-cause mortality
| Model* | RR (95% CI)† |
||
|---|---|---|---|
| Cardiovascular mortality | All-cause mortality | ||
| Microalbuminuria | 1 | 3.49 (1.87–6.53) | 2.12 (1.36–3.21) |
| 2 | 2.23 (1.16–4.29) | 1.46 (0.92–2.31) | |
| 3 | 2.09 (1.07–4.08) | 1.32 (0.83–2.12) | |
| 4 | 2.13 (1.09–4.17) | 1.33 (0.83–2.13) | |
| C-AD total score | 1 | 2.54 (1.60–4.04) | 2.11 (1.58–2.81) |
| 2 | 1.81 (1.11–2.94) | 1.62 (1.20–2.20) | |
| 3 | 1.74 (1.04–2.89) | 1.52 (1.11–2.08) | |
| 4 | 1.76 (1.05–2.94) | 1.52 (1.11–2.09) | |
*Model 1: univariate analysis; model 2: adjusted for sex, age, and GTS; model 3: model 2 plus adjustments for hypertension, WHR, estimated glomerular filtration rate, LDL, HDL, triglycerides, smoking and prior CVD; model 4: model 3, with additional adjustment for, respectively, C-AD or microalbuminuria.
†RR of microalbuminuria vs. normoalbuminuria or per SD (=0.605) increase in C-AD total score.
Figure 1.
Kaplan-Meier survival plots for cardiovascular mortality. Among individuals with normo- versus microalbuminuria (A) and across tertiles of C-AD total score (B).
Both microalbuminuria and C-AD total score were also associated with all-cause mortality (Table 3, model 1). However, after adjustment for age, sex, GTS, and other cardiovascular risk factors, microalbuminuria (RR 1.33 [95% CI 0.83–2.13]), in contrast to C-AD total score (1.52 [1.11–2.09]), was not independently associated with all-cause mortality (model 3).
Additional analyses
The associations described above were also investigated by examining autonomic function tests combined on the basis of their methodology or on the part of the autonomic nervous system they predominantly represent (supplementary Table A2, available in an online appendix). These additional analyses showed that overall all different groups of tests were associated with increased mortality risk, that these associations were attenuated when further adjusted for age, sex, GTS, and other risk factors; and that none of the specific group of tests explained the association between microalbuminuria and mortality.
The associations reported in Table 3 did not materially change after additional adjustment for use of lipid- or blood pressure–lowering drugs and the presence of leukocytes in urine (tested by microscopy and scored as positive whenever there were more than five leukocytes per high-power field) or when the dichotomous variable hypertension used in those models was replaced by either SBP or pulse pressure (a marker of arterial stiffness) or when analyses were adjusted for prior coronary heart disease instead of prior CVD (data not shown). Finally, we found no evidence of effect modification by GTS in the association between cardiovascular mortality and C-AD total score or microalbuminuria (Pinteraction > 0.4 in both cases).
CONCLUSIONS
In this prospective study we found that both microalbuminuria and C-AD (estimated from the mean of 10 standardized tests) were independently associated with cardiovascular mortality in a cohort of elderly, Caucasian subjects with and without diabetes. Therefore, C-AD does not explain why microalbuminuria is related to cardiovascular mortality in either the general population or in diabetic subjects.
Recently, HRV was found not to predict the decline of GFR but was independently associated with cardiovascular mortality in middle-aged type 1 diabetic subjects with overt nephropathy (17). In another study, investigating the independent predictive role of GFR, microalbuminuria, and cardiovascular autonomic neuropathy showed the latter to also be associated with all-cause and cardiovascular mortality in subjects with diabetes (18). These findings are in line with those reported herein. Hitherto, no other prospective studies examining the relation of microalbuminuria and C-AD with cardiovascular mortality have been reported. The independent association of both microalbuminuria and C-AD with cardiovascular mortality reported herein indicates that they are linked to cardiovascular mortality by different biological pathways. Several mechanisms linking C-AD to cardiovascular mortality have been proposed (e.g., QT interval prolongation and silent myocardial ischemia) (19). Microalbuminuria may be linked to CVD by a common pathophysiological process (such as endothelial dysfunction or chronic low-grade inflammation) (3). However, the possibility that microalbuminuria reflects the presence of a set of undiscovered factors that are causally related to CVD remains to be further elucidated. Our study shows that C-AD is an unlikely candidate in this regard.
Microalbuminuria is an established risk indicator for CVD. Furthermore, aggressive treatment of microalbuminuria by inhibiting the renin-angiotensin-aldosterone system has been shown to decrease renal and cardiovascular risk in individuals with diabetes (20). There is some evidence that C-AD is also a useful risk indicator for CVD, especially in subjects at high risk (type 2 diabetes, hypertension, or prior CVD) (9,21), and this study reinforces that observation. However, whether the improvement in C-AD, e.g., with exercise training or blood pressure– or lipid-lowering drugs (22–24), leads to a better prognosis is unknown. In our study, additional adjustment for use of β-blockers or lipid-lowering drugs had no effect on the associations reported. Therefore, the clinical relevance of treating C-AD in the general population remains unclear and needs to be further examined.
The strengths of our study are the relatively large, truly population-based and prospective design with a long follow-up period. In addition, we characterized subjects' C-AD as comprehensively as possible by conducting 10 autonomic function tests, which are thought to reflect all aspects of cardiovascular autonomic function. Previous cross-sectional studies evaluating the relationship between microalbuminuria and C-AD used less extensive autonomic function tests (4,6). Because we included all three groups of GTS, we were able to analyze possible effect modification of GTS in the relationship between the main determinants, C-AD and microalbuminuria and cardiovascular mortality (but found none).
There are several limitations to our study. First, each of the test parameters of autonomic function was measured with moderate levels of reproducibility (reliability coefficient ∼50%) (13). Therefore, the results were combined into a C-AD total score, which enabled us to circumvent, at least partially, this problem. Specifically, the results herein reported with the C-AD total score were more powerful than results obtained on the basis of each individual test (separately), because these results would be more strongly affected by misclassification (likely to be random), causing an underestimation of the strength of the associations. Indeed, the associations between each individual test result with cardiovascular mortality were in accordance, although with lower strength, with those as reported herein with the C-AD total score (data not shown). In addition, this approach had the advantage of circumventing the problem of multiple testing (type I errors) that would have occurred when the associations with each test were analyzed separately. However, because each test may represent different parts of autonomic function, some overlapping but others complementary, our C-AD total score does not allow the distinction of potential different contributions of sympathetic and parasympathetic functions to cardiovascular mortality. Therefore, we examined the autonomic function tests combined on the basis of their methodology or on the part of the autonomic nervous system they predominantly represent in the additional analyses. The results of these alternative combinations of autonomic function tests were comparable to those of the C-AD total score (supplementary Table A2), but we acknowledge that these analyses may not have been specific enough, as most authors agree that a clear distinction between parasympathetic or sympathetic dysfunction cannot be made on the basis of these tests. Altogether, we feel that the advantages (i.e., reduced misclassification and avoidance of type I errors without impairment of etiological validity) of the approach used by us outweigh the disadvantages. Second, the spectral analyses were performed during 3 min. Measurement of HRV during a longer period may be more reliable, although measurements obtained in time periods as short as 2 min correlate highly with 24-h measurements (25). Third, evaluation of subjects' C-AD was conducted at baseline only, and, therefore, we were not able to evaluate deterioration of autonomic function during follow-up. Fourth, for most participants microalbuminuria was defined on an ACR measured in one urine sample only. Because microalbuminuria is quite variable from day to day we cannot fully exclude the possibility that some subjects were misclassified. However, we tried to avoid this problem by collecting urine overnight and expressing albumin excretion as ACR. In addition, analyses in the 154 participants for whom two samples were available yielded essentially similar results (data not shown). Finally, we studied an elderly, Caucasian population, and it is not known whether our results can be generalized to other ethnic groups or to younger individuals.
In summary, we have shown that both microalbuminuria and C-AD are independently associated with cardiovascular mortality in an elderly, Caucasian population of individuals with normal glucose metabolism, IGM, and diabetes. These results suggest that microalbuminuria and C-AD are related to cardiovascular mortality by different biological pathways. Therefore, it may be useful to treat not only microalbuminuria but also C-AD in populations at high risk of cardiovascular mortality.
Supplementary Material
Acknowledgments
I.F. is supported by a postdoctoral research grant (2006T050) from the Netherlands Heart Foundation.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286: 421– 426 [DOI] [PubMed] [Google Scholar]
- 2.Borch-Johnsen K, Feldt-Rasmussen B, Strandgaard S, Schroll M, Jensen JS. Urinary albumin excretion: an independent predictor of ischemic heart disease. Arterioscler Thromb Vasc Biol 1999; 19: 1992– 1997 [DOI] [PubMed] [Google Scholar]
- 3.Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol 2006; 17: 2106– 2111 [DOI] [PubMed] [Google Scholar]
- 4.Moran A, Palmas W, Field L, Bhattarai J, Schwartz JE, Weinstock RS, Shea S. Cardiovascular autonomic neuropathy is associated with microalbuminuria in older patients with type 2 diabetes. Diabetes Care 2004; 27: 972– 977 [DOI] [PubMed] [Google Scholar]
- 5.Smulders YM, Jager A, Gerritsen J, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD. Cardiovascular autonomic function is associated with (micro-)albuminuria in elderly Caucasian subjects with impaired glucose tolerance or type 2 diabetes: the Hoorn Study. Diabetes Care 2000; 23: 1369– 1374 [DOI] [PubMed] [Google Scholar]
- 6.Wirta OR, Pasternack AI, Mustonen JT, Laippala PJ, Reinikainen PM. Urinary albumin excretion rate is independently related to autonomic neuropathy in type 2 diabetes mellitus. J Intern Med 1999; 245: 329– 335 [DOI] [PubMed] [Google Scholar]
- 7.Ritz E, Stefanski A. Diabetic nephropathy in type II diabetes. Am J Kidney Dis 1996; 27: 167– 194 [DOI] [PubMed] [Google Scholar]
- 8.Jermendy G, Ferenczi J, Hernandez E, Farkas K, Nadas J. Day-night blood pressure variation in normotensive and hypertensive NIDDM patients with asymptomatic autonomic neuropathy. Diabetes Res Clin Pract 1996; 34: 107– 114 [DOI] [PubMed] [Google Scholar]
- 9.Gerritsen J, Dekker JM, TenVoorde BJ, Kostense PJ, Heine RJ, Bouter LM, Heethaar RM, Stehouwer CD. Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension, or a history of cardiovascular disease: the Hoorn Study. Diabetes Care 2001; 24: 1793– 1798 [DOI] [PubMed] [Google Scholar]
- 10.Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care 2003; 26: 1895– 1901 [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama H, Yokota Y, Tada J, Kanno S. Diabetic neuropathy is closely associated with arterial stiffening and thickness in type 2 diabetes. Diabet Med 2007; 24: 1329– 1335 [DOI] [PubMed] [Google Scholar]
- 12.Beks PJ, Mackaay AJ, de Neeling JN, de Vries H, Bouter LM, Heine RJ. Peripheral arterial disease in relation to glycaemic level in an elderly Caucasian population: the Hoorn Study. Diabetologia 1995; 38: 86– 96 [DOI] [PubMed] [Google Scholar]
- 13.Gerritsen J, TenVoorde BJ, Dekker JM, Kingma R, Kostense PJ, Bouter LM, Heethaar RM. Measures of cardiovascular autonomic nervous function: agreement, reproducibility, and reference values in middle age and elderly subjects. Diabetologia 2003; 46: 330– 338 [DOI] [PubMed] [Google Scholar]
- 14.Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 1985; 8: 491– 498 [DOI] [PubMed] [Google Scholar]
- 15.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996; 93: 1043– 1065 [PubMed] [Google Scholar]
- 16.TenVoorde BJ, Faes TJC, Jansen TJW, Scheffer GJ, Rompelman O. Respiratory modulation of blood pressure and heart rate studied with a computer model of baroreflex control. In Computer Analysis of Cardiovascular Signals Di Rienzo M. Ed. Amsterdam, IOS Press, 1995, p. 119– 134 [Google Scholar]
- 17.Astrup AS, Tarnow L, Rossing P, Hansen BV, Hilsted J, Parving HH. Cardiac autonomic neuropathy predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Diabetes Care 2006; 29: 334– 339 [DOI] [PubMed] [Google Scholar]
- 18.Wirta O, Pasternack A, Mustonen J, Laippala P. Renal and cardiovascular predictors of 9-year total and sudden cardiac mortality in non-insulin-dependent diabetic subjects. Nephrol Dial Transplant 1997; 12: 2612– 2617 [DOI] [PubMed] [Google Scholar]
- 19.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care 2003; 26: 1553– 1579 [DOI] [PubMed] [Google Scholar]
- 20.Araki S, Haneda M, Koya D, Hidaka H, Sugimoto T, Isono M, Isshiki K, Chin-Kanasaki M, Uzu T, Kashiwagi A. Reduction in microalbuminuria as an integrated indicator for renal and cardiovascular risk reduction in patients with type 2 diabetes. Diabetes 2007; 56: 1727– 1730 [DOI] [PubMed] [Google Scholar]
- 21.Wichterle D, Simek J, La Rovere MT, Schwartz PJ, Camm AJ, Malik M. Prevalent low-frequency oscillation of heart rate: novel predictor of mortality after myocardial infarction. Circulation 2004; 110: 1183– 1190 [DOI] [PubMed] [Google Scholar]
- 22.Carnethon MR, Prineas RJ, Temprosa M, Zhang ZM, Uwaifo G, Molitch ME. The association among autonomic nervous system function, incident diabetes, and intervention arm in the Diabetes Prevention Program. Diabetes Care 2006; 29: 914– 919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebbehoj E, Poulsen PL, Hansen KW, Knudsen ST, Molgaard H, Mogensen CE. Effects on heart rate variability of metoprolol supplementary to ongoing ACE-inhibitor treatment in type I diabetic patients with abnormal albuminuria. Diabetologia 2002; 45: 965– 975 [DOI] [PubMed] [Google Scholar]
- 24.Pehlivanidis AN, Athyros VG, Demitriadis DS, Papageorgiou AA, Bouloukos VJ, Kontopoulos AG. Heart rate variability after long-term treatment with atorvastatin in hypercholesterolaemic patients with or without coronary artery disease. Atherosclerosis 2001; 157: 463– 469 [DOI] [PubMed] [Google Scholar]
- 25.Bigger JT, Fleiss JL, Rolnitzky LM, Steinman RC. The ability of several short-term measures of RR variability to predict mortality after myocardial infarction. Circulation 1993; 88: 927– 934 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.