Abstract
OBJECTIVE
To systematically evaluate the association between serum uric acid (SUA) level and subsequent development of type 2 diabetes.
RESEARCH DESIGN AND METHODS
We searched Medline (31 March from 1966 to 2009) and Embase (31 March from 1980 to 2009) for observational cohort studies examining the association between SUA and the risk of type 2 diabetes by manual literature search. Relative risks (RRs) for each 1 mg/dl increase in SUA were pooled by using a random-effects model. The studies included were stratified into subgroups representing different study characteristics, and meta-regression analyses were performed to investigate the effect of these characteristics on the association between SUA level and type 2 diabetes risk.
RESULTS
The search yielded 11 cohort studies (42,834 participants) that reported 3,305 incident cases of type 2 diabetes during follow-up periods ranging from 2.0 to 13.5 years. The pooled RR of a 1 mg/dl increase in SUA was 1.17 (95% CI 1.09–1.25). Study results were consistently significant (i.e., >1) across characteristics of participants and study design. Publication bias was both visually and statistically suggested (P = 0.03 for Egger's test, 0.06). Adjustment for publication bias attenuated the pooled RR per mg/dl increase in SUA (RR 1.11 [95% CI 1.03–1.20]), but the association remained statistically significant (P = 0.009).
CONCLUSIONS
The current meta-analysis suggests that SUA level is positively associated with the development of type 2 diabetes regardless of various study characteristics. Further research should attempt to determine whether it is effective to utilize SUA level as a predictor of type 2 diabetes for its primary prevention.
Identifying risk factors for the development of type 2 diabetes is essential for its early screening and prevention. Serum uric acid (SUA) level has been suggested to be associated with risk of type 2 diabetes. Biologically, uric acid (UA) plays an important role in worsening of insulin resistance in animal models by inhibiting the bioavailability of nitric oxide, which is essential for insulin-stimulated glucose uptake (1). However, hyperinsulinemia as a consequence of insulin resistance causes an increase in SUA concentration by both reducing renal UA secretion (2) and accumulating substrates for UA production (3). Therefore, it remains controversial whether SUA is independently associated with the development of type 2 diabetes. The aim of our meta-analysis was to summarize the association between SUA level and risk of type 2 diabetes derived from previously published cohort studies and to examine the effect of study characteristics on this association.
RESEARCH DESIGN AND METHODS
Search strategy
The meta-analysis was fundamentally conducted according to the checklist of the Meta-analysis of Observational Studies in Epidemiology (4). We performed a systematic literature search of Medline (31 March from 1966 to 2009) and Embase (31 March from 1980 to 2009) for observational cohort studies examining the association between SUA level and risk of type 2 diabetes. The key words were related to UA (combined exploded version of the medical subject headings [MeSH] [uric acid] and the following text words: hyperuricemia OR [acid AND uric] OR trioxopurine OR trihydroxypurine OR urate OR gout OR gouts) and type 2 diabetes (combined unexploded version of MeSH [diabetes OR diabetes, type 2] and the following text words [hyperglycemias OR hyperglycemia OR [diabetes mellitus AND {type 2 OR type II OR ketosis resistant OR ketosis-resistant OR maturity onset OR maturity-onset OR noninsulin dependent OR non insulin dependent OR non-insulin-dependent OR slow onset OR slow-onset OR stable OR adult onset OR adult-onset}] OR MODY OR type 2 diabetes).
Included reports had to meet the following criteria: 1) prospective or historical cohort study, 2) inclusion of type 2 diabetes as a specified outcome, 3) baseline assessment of SUA level, and 4) inclusion of data on relative risk (RR), which is generally expressed as the odds ratio in a historical cohort study or the risk ratio in a prospective cohort study, and its corresponding 95% CIs (or data to calculate them) for type 2 diabetes associated with SUA level. When two or more studies were conducted using the same subjects, the study that included the most recently updated data was selected.
Data abstraction
The data that we abstracted included the first author's name, year of publication, country of origin, cohort design (i.e., prospective or historical cohort), methods for ascertaining diabetes, mean follow-up duration, mean or midpoint of participants' age, proportion of men, baseline SUA level, number of participants and events, and adjusted variables. Odds and risk ratios were combined as indicators of RR, based on the assumption that the odds ratio is an approximation of the risk ratio; this assumption has some limitations, however, especially when the outcome of interest is common (5).
If a study provided several RRs, such as unadjusted and adjusted RRs, the most completely adjusted RR was used. Each RR was transformed to its natural logarithm (log RR), and its corresponding 95% CI or P value was used to calculate the SE for each log RR. Two of our investigators independently reviewed each published article and extracted the relevant information. Any disagreement was resolved by consensus.
Data synthesis
To quantify the dose-response relationship between the baseline SUA level and risk of type 2 diabetes, we calculated the RR for each 1 mg/dl increase in SUA in each study. For studies that analyzed SUA level not as a continuous but as a categorical variable (i.e., studies where subjects were categorized based on SUA level and RRs for the development of type 2 diabetes according to SUA level were reported), we used the method for trend estimation supported by Berlin et al. (6) and Orsini et al. (7). This method is particularly useful when the full data are not available. It enables us to correct for covariance between risk estimates from the same study and to estimate the corrected linear trend using generalized least squares if data on the adjusted RR and the number of participants (or person-time) and cases for each category are provided.
When the mean SUA level was not reported, the range's midpoint in each category was used, except for the lowest and highest category, for which the mean SUA level was estimated by assuming normality of SUA distribution, which is the same method as used in a previously published meta-analysis (8). Each log RR was pooled by using a random-effects model (9). The overall RR and its 95% CI could be calculated by exponentiation of the pooled log RR. We assessed heterogeneity of RRs across studies using both I2 and Q statistics (10).
Sensitivity analyses
The studies included were stratified by key factors related to cohort design (i.e., prospective or historical cohort) and other study properties related to study quality and participant characteristics that were identified a priori. Study quality was assessed according to the method of ascertainment of diabetes (whether blood measurements, or reports by participants or physicians, or both), mean follow-up duration (>8 or ≤8 years), and inclusion of adjustment for the following potentially important confounding variables: alcohol intake (yes or no) and metabolic profile (sufficient or insufficient). We regarded the adjustment for metabolic variables as sufficient when the risk estimate was adjusted for more than three factors among obesity, hypertension (or systolic blood pressure), fasting plasma glucose, HDL cholesterol, and triglycerides. We identified country of origin (Asian or Western countries), mean age (>50 or ≤50 years), sex (whether men only, women only, or both men and women), and mean SUA level (>5.5 or ≤5.5 mg/dl) as possible participant characteristics. We calculated the pooled RR within the strata of each study characteristic, and meta-regression analyses were conducted to assess the effects of these study characteristics on the type 2 diabetes risk and incremental increase in SUA level.
The possibility of publication bias was assessed by the Begg's and the Egger's tests (11,12) and visual inspection of a funnel plot. We also performed the Duval and Tweedie “trim-and-fill” procedure (13) to further assess the possible effect of publication bias in our meta-analysis. This method considers the possibility of hypothetical “missing” studies that might exist, imputes their RRs, and recalculates a pooled RR that incorporates the hypothetical missing studies as though they actually existed. Data were analyzed by using STATA software (version 10; Stata, College Station, TX). P < 0.05 was considered as statistically significant except for the test of publication bias, in which the level of significance is P < 0.10 (14).
RESULTS
Literature search
Of 1,258 citations retrieved by the search strategy, 1,225 citations were excluded after a first screening based on titles and abstracts, leaving 33 articles for full-text review. Manual searching of the reference lists of these articles identified 8 additional articles. Of 41 articles for full-text review, 30 articles were excluded for the following reasons: 1) they were case-control studies (6 studies); 2) they were clinical trials (3 studies); 3) risk estimates of the association of type 2 diabetes with SUA level were not reported (9 studies); 4) prespecified outcome did not include type 2 diabetes (5 studies), and sufficient data to estimate the RR of type 2 diabetes and its corresponding SE per incremental increase in UA were not provided (4 studies); and 5) data reported were updated by more recent studies (3 studies). Eleven studies (15–25) met the inclusion criteria. Three studies investigated men and women separately. Finally, 14 cohorts involving a total of 42,834 participants and 3,305 incident cases were included in our analyses.
Study characteristics
Characteristics of the 11 included studies are shown in Table 1. Three studies (17,19,23) were prospective cohort and eight studies (15,16,18,20–22,24,25) were historical cohort. The selected studies were published between 1975 and 2009, and the number of subjects per study ranged from 250 to 8,688. Mean SUA level of subjects ranged from 4.0 to 8.0 mg/dl, and mean age ranged from 41 to 63 years except for one study (23), in which data on mean age (>55 years) were not available. Four studies (15–17,19) included men only, and seven studies (18–21,23–25) included both women and men. Six studies (15,18,19,21,22,24) were conducted in Asian countries and five studies (16,17,20,23,25) in Western countries. Mean follow-up duration ranged from 2.0 to 13.5 years.
Table 1.
Characteristics of studies included in meta-analysis
| First author | Year | Cohort designation | Population | Follow-up (years) | Diabetes ascertainment* | Baseline SUA (mg/dl) | Age (years) | % Men | Number of participants† | Number of cases | Cohort design |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Medalie (15) | 1975 | IIHDS | Israel | 5.0 | Both | 4.8 | 49 | 100 | 8,688 | 344 | H |
| Ohlson (16) | 1988 | SMB | Sweden | 13.5 | Both | 5.3 | 50 | 100 | 766 | 47 | H |
| Perry (17) | 1995 | BRHS | British | 12.8 | Report | 6.0 | 50 | 100 | 7,577 | 194 | P |
| Chou (18) | 1998 | KS | China | 2.0 | Measure | 5.8 | 50 | 52 | 654 | 39 | H |
| Taniguchi (19) | 2001 | OHS | Japan | 9.5 | Measure | 5.2 | 41 | 100 | 6,478 | 639 | P |
| Meisinger (20) | 2002 | MONIKA | Germany | ||||||||
| Men | 7.6 | Report | 5.7 | 52 | 100 | 3,052 | 128 | H | |||
| Women | 4.0 | 51 | 0 | 3,114 | 85 | H | |||||
| Lin (21) | 2004 | KS | China | 7.0 | Both | ||||||
| Men | 8.0 | 49 | 100 | 293 | 27 | H | |||||
| Women | 7.1 | 55 | 0 | 161 | 21 | H | |||||
| Chien (22) | 2008 | CSCCC | China | 9.0 | Measure | 5.6 | 54 | 43 | 2,690 | 548 | H |
| Dehghan (23) | 2008 | RS | the Netherlands | 10.1 | Both | 5.4 | over 55 | NA | 4,536 | 462 | P |
| Nan (24) | 2008 | MNCDS | Mauritius | 8.2 | Both | ||||||
| Men | 6.6 | 41 | 100 | 1,941 | 337 | H | |||||
| Women | 5.0 | 42 | 0 | 2,318 | 379 | H | |||||
| Kramer (25) | 2009 | UC | U.S. | 13.0 | Measure | 5.7 | 63 | 41 | 566 | 55 | H |
*Measure = using blood measurements, report = using reports by participants or physicians, and both = using both blood measurements and reports by participants or physicians.
†Number of participants included in the analysis in each study (not necessarily the number of participants at the beginning of each study). BRHS, British Regional Heart Study; CSCCC, Chin-Shan Community Cardiovascular Center; H, historical cohort; IIHDS, Israel Ischemic Heart Disease Study; JAPF, Japan Arteriosclerosis Prevention Fund; KS, The Kinmen Study; MNCDS, Mauritius Non-Communicable Diseases Surveys; MONIKA, MONIKA-Augsberg Cohort Study; NA, not available; OHS, The Osaka Health Study; P, prospective cohort; RS, The Rotterdam Study; SMB, The Study of Men Born in 1913; UC, University of California.
Regarding methods for ascertaining diabetes, four studies (18,19,22,25) used blood measurements only, two (17,20) used reports by participants and/or physicians only, and five (15,16,21,23,24) used both. Risk measures were adjusted for alcohol intake in five studies (17,19,20,22,24), and the adjustment for sufficient metabolic variables was sufficient in five studies (18,21–24). A few risk estimates were adjusted for smoking status (three studies) (17,19,20), family history of diabetes (four studies) (16,20,22,24), and fasting insulin concentration (three studies) (18,21,24). Only two studies (21,24) considered the effect of serum creatinine, and one study (25) considered the effect of diuretic use. None of risk measurements was adjusted for other drugs that influence SUA level such as alloprinol.
Overall and stratified analyses
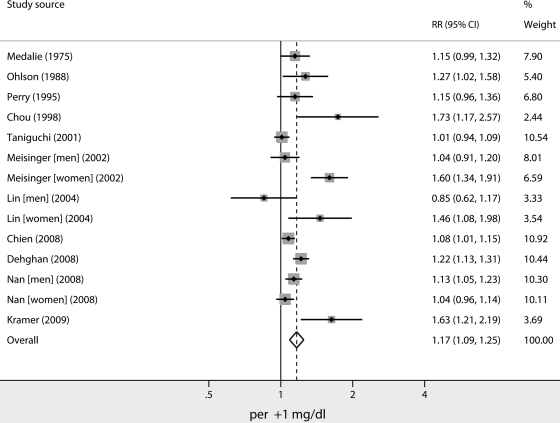
Figure 1 shows a forest plot with RRs and 95% CIs and pooled estimates for the reduction in risk of type 2 diabetes for each mg/dl increase in SUA. The pooled crude RR (95% CI) was 1.17 (1.19–1.25). There was evidence of statistical heterogeneity of RRs across studies (Q statistic, 50.4; I2 statistic, 74.2%; P < 0.001).
Figure 1.
Overall RR (with corresponding 95% CIs) for risk of type 2 diabetes for each mg/dl increase in SUA. The area of each square is proportional to study weight. Diamond indicates overall RR; horizontal lines indicate 95% CIs.
Table 2 shows findings of the stratified and meta-regression analysis to explore the effects of study characteristic. An increased risk of type 2 diabetes associated with an incremental increase in SUA was consistently found within all strata of each study characteristic (i.e., all pooled RRs were >1). There were no significant differences in the pooled risk estimates between cohort design (pooled RR [95% CI] of 1.22 [1.10–1.36] for historical cohort and 1.10 [1.01–1.20] for prospective cohort, P = 0.36). The influence of participant characteristics on the study results was not significant. Adjustment for alcohol intake attenuated the association between SUA and type 2 diabetes risk (P = 0.02), whereas the effect of sufficient adjustment for metabolic variables was not significant (P = 0.46).
Table 2.
Stratified and meta-regression analysis to explore the effects of study characteristics
| Number of cohorts | Pooled RRs (95% CI)* | P value of meta-regression† | |
|---|---|---|---|
| Study design | |||
| Historical cohort | 10 | 1.22 (1.10–1.36) | 0.55 |
| Prospective cohort | 4 | 1.10 (1.01–1.20) | |
| Indicators of participant characteristics | |||
| Country | |||
| Asia | 8 | 1.09 (1.04–1.21) | 0.10 |
| Western | 6 | 1.27 (1.12–1.44) | |
| Mean age (years) | |||
| ≤50 | 8 | 1.12 (1.04–1.19) | 0.14 |
| >50 | 6 | 1.26 (1.11–1.44) | |
| Sex | |||
| Men only | 7 | 1.09 (1.02–1.16) | 0.09 |
| Women only | 4 | 1.28 (1.08–1.51) | 0.31 |
| Both men and women | 3 | 1.40 (0.98–2.00) | |
| Mean SUA level (mg/dl) | |||
| ≤5.5 | 6 | 1.18 (1.15–1.32) | 0.98 |
| >5.5 | 8 | 1.16 (1.05–1.28) | |
| Indicators of study quality | |||
| Study adjustment for | |||
| alcohol intake | |||
| No | 9 | 1.27 (1.13–1.43) | 0.02 |
| Yes | 5 | 1.07 (1.02–1.12) | |
| Metabolic confounders‡ | |||
| Insufficient | 8 | 1.21 (1.09–1.34) | 0.46 |
| Sufficient | 6 | 1.11 (1.02–1.21) | |
| Follow-up duration (years) | |||
| ≤8 | 6 | 1.25 (1.03–1.51) | 0.37 |
| >8 | 8 | 1.13 (1.05–1.20) | |
| Diabetes ascertainment | |||
| Blood measurements only | 4 | 1.18 (1.02–1.37) | 0.81 |
| Report only | 3 | 1.24 (0.96–1.59) | 0.64 |
| Both | 7 | 1.14 (1.06–1.23) |
*Pooled RRs of type 2 diabetes for each 1 mg/dl increase in SUA within the strata of each study characteristic are indicated.
†Represents the test for significance of the effect across strata.
‡If the RRs were adjusted for more than three confounders (among BMI, fasting plasma glucose, hypertension [or systolic blood pressure], HDL cholesterol, and triglycerides), they were regarded as sufficient; otherwise, they were regarded as insufficient.
Test of publication bias
Visual inspection of the funnel plot revealed asymmetry (see online appendix A [available at http://care.diabetesjournals.org/cgi/content/full/dc09-0288/DC1]). This raises the possibility of publication bias, which was statistically supported by the Egger's test (P = 0.06). We decided to adjust for this publication bias using the trim-and-fill method (13). According to this method, it was suggested that there were three hypothetical negative unpublished cohorts that distorted the symmetry of the funnel plot. When these cohorts were incorporated to produce a hypothetically symmetrical funnel plot, the association between SUA and type 2 diabetes was modestly attenuated (RR 1.10 [95% CI 1.03–1.20]) but remained statistically significant (P = 0.009).
CONCLUSIONS
Our meta-analysis is the first to summarize the quantitative relationship between SUA level and risk of type 2 diabetes, indicating that each 1 mg/dl increase in SUA resulted in a 17% increase in the risk of type 2 diabetes. Table 3 compares other risk factors of type 2 diabetes, established from meta-analysis or systematic review (26–29), with SUA. Interestingly, the effect of a 1 mg/dl increment in SUA has been found to be comparable to a 1 kg/m2 increment in BMI.
Table 3.
Comparison of other risk factors of type 2 diabetes with incremental increase in SUA
| Risk factor | RR | To how much of mg/dl in SUA is the RR comparable? |
|---|---|---|
| Obesity (ref. (26) | ||
| BMI (per kg/m2) | 1.16 | 1.0 |
| Waist circumference (per cm) | 1.06 | 0.4 |
| High alcohol intake (ref. (29) | ||
| >3 drinks/day vs. 1 to 3 drinks/day | 1.43 | 2.3 |
| Physical inactivity (ref. (27) | ||
| The lowest vs. the highest level of moderate-intensity physical activity* | 1.20† | 1.2 |
| Smoking (ref. (28) | ||
| Heavy smokers (≥20 cigarettes/day) versus nonsmokers | 1.61 | 3.1 |
| Light smokers (<20 cigarettes/day) versus nonsmokers | 1.29 | 1.7 |
| Former smoker versus nonsmokers | 1.23 | 1.4 |
*Typically, no walking versus ≥2.5 h/week brisk walking.
†This RR is adjusted for BMI.
Pathologically and epidemiologically, it has been indicated that elevated SUA concentration is correlated with lifestyle factors (high alcohol intake [30] in particular) and various metabolic profiles (especially high values of BMI, blood pressure, fasting plasma glucose and triglycerides, and low HDL cholesterol values [31,32], which are typically considered to be diagnostic criteria for metabolic syndrome [33]). Therefore, it is possible to establish whether the observed positive association between SUA level and risk of type 2 diabetes is noncausal. Our sensitivity analysis indicated that a significant association was observed if analyses were limited to studies that included adjustment for alcohol intake or sufficient metabolic confounders (i.e., more than three metabolic confounders among BMI, fasting plasma glucose, hypertension [or systolic blood pressure], HDL cholesterol, and triglycerides), although the adjustment weakened the association. Therefore, the results of this analysis strongly suggest that SUA is an independent predictor of the development of type 2 diabetes. Therefore, these findings suggest that there are both noncausal and causal associations between SUA level and the risk of type 2 diabetes.
The limitations of this meta-analysis must be considered. First, the overall effect estimated by the current analysis might be inaccurate due to the statistically significant publication bias. According to the results of the compensatory trim-and-fill method, the overall RR of type 2 diabetes for each 1 mg/dl SUA increase should be scaled downward by 0.07 to adjust for publication bias. However, this method may overestimate the magnitude of any publication bias (34). Moreover, this method did not change the statistical significance of the association between SUA level and development of type 2 diabetes. Therefore, the effect of adjustment for publication bias was probably modest. Second, the odds and risk ratios were combined as indicators of RR. The odds ratio overestimates the risk ratio, especially when the outcome of interest is common. It is possible that this method could distort the overall and stratified analyses within cohort design. The overestimation is, however, of little practical importance and can be ignored as long as the pooled risk ratio is near to 1 and the total incidence is relatively rare (<10%), as they were in our meta-analysis (5). Third, in the sensitivity analysis, the statistical power might be insufficient to explain the source of the large study heterogeneity because of the small number of data units within strata. For example, there was a substantially larger increase in the risk of elevated SUA for development of type 2 diabetes observed in Western countries (RR 1.27) compared with Asian countries (RR 1.09) and for women (RR 1.28) compared with men (RR 1.09). Although these differences were statistically insignificant, we cannot exclude the possibility of the influence of race or sex on the association between SUA level and type 2 diabetes. This issue might be solved by a patient-level meta-analysis, which would be beyond the current meta-analysis. Fourth, there were few studies that included a consideration of significant confounders influencing SUA level, such as serum creatinine and drugs (e.g., diuretic agents or alloprinol). These confounders could contribute to modification of the association between SUA and risk of type 2 diabetes. Fifth, we thought it was too early to determine whether there is a cutoff level in SUA to increase or reduce the risk of development of type 2 diabetes because of both the limited number of studies that used SUA level as a categorical variable and provided RR data for each category and the variation in methods of how SUA levels in each subject were categorized. Therefore, we cannot rule out the possibility that SUA level has a threshold effect on the risk of type 2 diabetes rather than a dose-response effect.
In conclusion, our meta-analysis suggests that SUA level is independently associated with the development of type 2 diabetes. It is possible that these findings are the first step to utilizing SUA, which has been suggested to be a risk factor for type 2 diabetes, in primary care medical practice. Further research should attempt to investigate whether SUA would be useful for predicting type 2 diabetes with respect to the prevention of type 2 diabetes; for example, studies should aim to specify the population for which the SUA level is especially important and to determine the SUA threshold for increased risk of type 2 diabetes.
Supplementary Material
Acknowledgments
H.S. and S.K. are recipients of a Grant-in-Aid for Scientific Research and Postdoctoral Research Fellowship, respectively, both from the Japan Society for the Promotion of Science (No. 20300227), Japan Cardiovascular Research Foundation, and Ministry of Health Labor and Welfare, Japan. These research organizations providing funding support did not have any role in the design or conduct of the study.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ. Hyperuricemia induces endothelial dysfunction. Kidney Int 2005; 67: 1739– 1742 [DOI] [PubMed] [Google Scholar]
- 2.Quinones Galvan A, Natali A, Baldi S, Frascerra S, Sanna G, Ciociaro D, Ferrannini E. Effect of insulin on uric acid excretion in humans. Am J Physiol 1995; 268: E1– E5 [DOI] [PubMed] [Google Scholar]
- 3.Fox IH. Metabolic basis for disorders of purine nucleotide degradation. Metabolism 1981; 30: 616– 634 [DOI] [PubMed] [Google Scholar]
- 4.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting: Meta-analysis of Observational Studies in Epidemiology (MOOSE) Group. JAMA 2000; 283: 2008– 2012 [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998; 280: 1690– 1691 [DOI] [PubMed] [Google Scholar]
- 6.Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology 1993; 4: 218– 228 [DOI] [PubMed] [Google Scholar]
- 7.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J 2006; 6: 40– 57 [Google Scholar]
- 8.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 1998; 279: 1477– 1482 [DOI] [PubMed] [Google Scholar]
- 9.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177– 188 [DOI] [PubMed] [Google Scholar]
- 10.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539– 1558 [DOI] [PubMed] [Google Scholar]
- 11.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088– 1101 [PubMed] [Google Scholar]
- 12.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629– 634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56: 455– 463 [DOI] [PubMed] [Google Scholar]
- 14.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000; 53: 1119– 1129 [DOI] [PubMed] [Google Scholar]
- 15.Medalie JH, Papier CM, Goldbourt U, Herman JB. Major factors in the development of diabetes mellitus in 10,000 men. Arch Intern Med 1975; 135: 811– 817 [PubMed] [Google Scholar]
- 16.Ohlson LO, Larsson B, Bjorntorp P, Eriksson H, Svardsudd K, Welin L, Tibblin G, Wilhelmsen L. Risk factors for type 2 (non-insulin-dependent) diabetes mellitus: thirteen and one-half years of follow-up of the participants in a study of Swedish men born in 1913. Diabetologia 1988; 31: 798– 805 [DOI] [PubMed] [Google Scholar]
- 17.Perry IJ, Wannamethee SG, Walker MK, Thomson AG, Whincup PH, Shaper AG. Prospective study of risk factors for development of non-insulin dependent diabetes in middle aged British men. BMJ 1995; 310: 560– 564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou P, Li CL, Wu GS, Tsai ST. Progression to type 2 diabetes among high-risk groups in Kin-Chen, Kinmen: exploring the natural history of type 2 diabetes. Diabetes Care 1998; 21: 1183– 1187 [DOI] [PubMed] [Google Scholar]
- 19.Taniguchi Y, Hayashi T, Tsumura K, Endo G, Fujii S, Okada K. Serum uric acid and the risk for hypertension and type 2 diabetes in Japanese men: the Osaka Health Survey. J Hypertens 2001; 19: 1209– 1215 [DOI] [PubMed] [Google Scholar]
- 20.Meisinger C, Thorand B, Schneider A, Stieber J, Doring A, Lowel H. Sex differences in risk factors for incident type 2 diabetes mellitus: the MONICA Augsburg Cohort Study. Arch Intern Med 2002; 162: 82– 89 [DOI] [PubMed] [Google Scholar]
- 21.Lin KC, Tsai ST, Lin HY, Chou P. Different progressions of hyperglycemia and diabetes among hyperuricemic men and women in the kinmen study. J Rheumatol 2004; 31: 1159– 1165 [PubMed] [Google Scholar]
- 22.Chien KL, Chen MF, Hsu HC, Chang WT, Su TC, Lee YT, Hu FB. Plasma uric acid and the risk of type 2 diabetes in a Chinese community. Clin Chem 2008; 54: 310– 316 [DOI] [PubMed] [Google Scholar]
- 23.Dehghan A, van Hoek M, Sijbrands EJ, Hofman A, Witteman JC. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care 2008; 31: 361– 362 [DOI] [PubMed] [Google Scholar]
- 24.Nan H, Qiao Q, Soderberg S, Pitkaniemi J, Zimmet P, Shaw J, Alberti G, Uusitalo U, Pauvaday V, Chitson P, Tuomilehto J. Serum uric acid and incident diabetes in Mauritian Indian and Creole populations. Diabetes Res Clin Pract 2008; 80: 321– 327 [DOI] [PubMed] [Google Scholar]
- 25.Kramer CK, von Muhlen D, Jassal SK, Barrett-Connor E. Serum uric acid levels improve prediction of incident type 2 diabetes in individuals with impaired fasting glucose: the Rancho Bernardo Study. Diabetes Care, 2009. [ Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vazquez G, Duval S, Jacobs DR, Jr, Silventoinen K. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev 2007; 29: 115– 128 [DOI] [PubMed] [Google Scholar]
- 27.Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care 2007; 30: 744– 752 [DOI] [PubMed] [Google Scholar]
- 28.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2007; 298: 2654– 2664 [DOI] [PubMed] [Google Scholar]
- 29.Howard AA, Arnsten JH, Gourevitch MN. Effect of alcohol consumption on diabetes mellitus: a systematic review. Ann Intern Med 2004; 140: 211– 219 [DOI] [PubMed] [Google Scholar]
- 30.Liberopoulos EN, Miltiadous GA, Elisaf MS. Alcohol intake, serum uric acid concentrations, and risk of gout. Lancet 2004; 364: 246– 247 [ author reply, p. 247] [DOI] [PubMed] [Google Scholar]
- 31.Klein BE, Klein R, Lee KE. Components of the metabolic syndrome and risk of cardiovascular disease and diabetes in Beaver Dam. Diabetes Care 2002; 25: 1790– 1794 [DOI] [PubMed] [Google Scholar]
- 32.Yoo TW, Sung KC, Shin HS, Kim BJ, Kim BS, Kang JH, Lee MH, Park JR, Kim H, Rhee EJ, Lee WY, Kim SW, Ryu SH, Keum DG. Relationship between serum uric acid concentration and insulin resistance and metabolic syndrome. Circ J 2005; 69: 928– 933 [DOI] [PubMed] [Google Scholar]
- 33.Tsouli SG, Liberopoulos EN, Mikhailidis DP, Athyros VG, Elisaf MS. Elevated serum uric acid levels in metabolic syndrome: an active component or an innocent bystander? Metabolism 2006; 55: 1293– 1301 [DOI] [PubMed] [Google Scholar]
- 34.High false positive rate for trim and fill method [article online], 2000. Available from http://bmj.bmjjournals.com/cgi/eletters/320/7249/1574#8757 Accessed 7 May 2009
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.