Abstract
OBJECTIVE
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study found strong associations between higher levels of maternal glucose at 24–32 weeks, within what is currently considered normoglycemia and adverse pregnancy outcomes. Our aim was to evaluate the associations between first-trimester fasting plasma glucose level and adverse pregnancy outcomes.
RESEARCH DESIGN AND METHODS
Charts of all patients who delivered at our hospital between June 2001 and June 2006 were reviewed. Only subjects with singleton pregnancy and a recorded first-trimester fasting glucose level were included. Women with pregestational diabetes, fasting glucose level >105 mg/dl, or delivery <24 weeks were excluded. Fasting glucose levels were analyzed in seven categories, similar to the HAPO study. The main outcomes were development of gestational diabetes mellitus (GDM), large-for-gestational-age (LGA) neonates and/or macrosomia, and primary cesarean section. Multivariate logistic regression analysis was used; significance was <0.05.
RESULTS
A total of 6,129 women had a fasting glucose test at median of 9.5 weeks. There were strong, graded associations between fasting glucose level and primary outcomes. The frequency of GDM development increased from 1.0% in the lowest glucose category to 11.7% in the highest (adjusted odds ratio 11.92 [95% CI 5.39–26.37]). The frequency of LGA neonates and/or macrosomia increased from 7.9 to 19.4% (2.82 [1.67–4.76]). Primary cesarean section rate increased from 12.7 to 20.0% (1.94 [1.11–3.41]).
CONCLUSIONS
Higher first-trimester fasting glucose levels, within what is currently considered a nondiabetic range, increase the risk of adverse pregnancy outcomes. Early detection and treatment of women at high risk for these complications might improve pregnancy outcome.
Women with gestational diabetes mellitus (GDM) are at increased risk for adverse perinatal and maternal outcomes, including macrosomia, cesarean section, birth trauma, and later diabetes. The Toronto Tri-Hospital Gestational Diabetes Project (1) and the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Research Group (2) studied the relationship between plasma glucose levels, less severe than overt GDM, and pregnancy outcomes. They found graded relation between increasing levels of fasting, 1-h, and 2-h plasma glucose obtained on oral glucose tolerance testing at 24–32 weeks and a wide variety of adverse pregnancy outcomes, including increased birth weight, primary cesarean section, neonatal hypoglycemia, and precclampsia.
Using fasting plasma glucose as a screening test for adverse pregnancy outcomes early in gestation offers some advantages compared with glucose challenge tests (GCTs). Glucose tolerance tests are poorly reproducible, are time consuming and expensive, require extensive patient preparation, are inconvenient to administer, and are unpleasant for the patient, and in pregnant women vomiting is a common problem. Fasting plasma glucose is easy to administer, well tolerated, inexpensive, reliable, reproducible, and has been reported to vary little throughout gestation (3). However, there is no universally agreed definition for normal fasting glucose level in pregnancy. In pregnant women, similar to the nonpregnant state, fasting plasma glucose >125 mg/dl is considered diagnostic for diabetes (4). In nonpregnant adults, impaired fasting glucose is diagnosed with fasting glucose levels of 100–125 mg/dl (4). However, there is no definition for impaired fasting glucose during pregnancy: in the 3-h 100-g oral glucose tolerance test (OGTT), a fasting glucose value >105 mg/dl was considered abnormal by the National Diabetes Data Group's criteria (5), whereas the Carpenter and Coustan criteria (6) for the diagnosis of GDM set the normal fasting glucose, in the OGTT, to <95 mg/dl.
Detection of women at higher risk for adverse pregnancy outcomes early in pregnancy is a desirable goal because interventions such as diet, medication, and exercise may be applied earlier and have a positive effect on maternal and fetal outcomes (7–9). Therefore, we wanted to evaluate, retrospectively, the associations between fasting glucose level in the first trimester within what is currently considered normoglycemia and adverse pregnancy outcomes.
RESEARCH DESIGN AND METHODS
The protocol was approved by the institutional review board for human investigation at The Lady Davis Carmel Medical Center, which is part of Clalit HMO. The study was also approved, for computerized laboratory data retrieval, by the committee for health policy research in the central administration of Clalit HMO. This was a retrospective study on deliveries that took place in Carmel Lady Davis Hospital from June 2001 to June 2006. During this period, we used a computerized medical record in the labor-and-delivery ward. The computerized record includes demographic data (including woman's name, identification number, age, and maternal self-report of prepregnancy weight and height), obstetric data (including gravity, parity, previous cesarean section, and estimated last menstrual period based on both date of last menstrual period and early ultrasound examination), delivery data (including gestational age at delivery and mode of delivery), and neonatal data (including neonatal weight and Apgar score). These data were extracted into a computerized database (Access; Microsoft, Seattle, WA).
There is no uniform worldwide guidelines for screening and diagnosis of GDM (10). In Israel, the Ministry of Health recommends universal testing for fasting glucose level at the first prenatal care visit and a universal 50-g GCT at 24–28 weeks of gestation (11). According to these guidelines, women with abnormal GCT of ≥140 mg/dl should undergo a 3-h 100-g OGTT.
The Israeli National Health Insurance Law provides universal health services to every resident. Carmel Lady Davis Hospital is part of the Clalit Health Services, which is the largest HMO in Israel. Using the unique identity number assigned to all Israeli residents and through the central laboratory computer of the Clalit HMO, we extracted the results of all glucose blood tests that were done during the study period for patients who gave birth in our hospital and had Clalit HMO insurance. Gestational age for the various glucose tests was calculated using the date of last menstrual period and the date of glucose test. Venous plasma glucose concentration was determined by the glucose oxidase method in Clalit laboratories. Only subjects with singleton pregnancy and a recorded first-trimester fasting glucose level were included in this study. Women with pregestational diabetes mellitus, fasting glucose level >105 mg/dl, or delivery at <24 weeks of gestation were excluded.
Large for gestational age (LGA), defined as neonatal birth weight >90th percentile for gestational age and sex, was determined according to the published Israeli birth weight standard (12). Macrosomia was defined as birth weight >4,000 g. GDM was diagnosed when an abnormal GCT (≥140 mg/dl) was followed by two or more abnormal values on a 3-h (100-g) glucose tolerance test using the Carpenter and Coustan criteria (6). GDM was also diagnosed with a GCT value of ≥200 mg/dl.
The main outcomes were the development of GDM, LGA and/or macrosomia, and primary cesarean delivery. Secondary outcomes were premature delivery before 37 weeks of gestation and admission to the neonatal intensive care unit. We also analyzed the correlation between first-trimester fasting glucose level and that of GCT and OGTT.
Fasting glucose levels were analyzed in seven categories (<75, 75–79, 80–84, 85–89, 90–94, 95–99, and 100–105 mg/dl), similar to the HAPO study (2). Data were tested for normal distribution (Kolmogorov-Smirnov test). Pearson correlation test and multivariate logistic regression analysis were conducted in SigmaStat version 2.03 and Minitab version 12.23. Statistical significance was set at P < 0.05.
RESULTS
Of 14,550 singleton deliveries at >24 weeks of gestation, 7,126 women were enrollees of Clalit Health Care Services. A total of 145 (2.0%) were excluded because they had pregestational diabetes or a first-trimester fasting glucose level of ≥105 mg/dl. A total of 852 (12%) were excluded because they had a recorded fasting glucose level after the first trimester or prior to pregnancy. Among the 852 women who did not have a first-trimester fasting glucose level and those who had it, the median maternal age was 28.7 vs. 29.3 years, neonatal weight was 3,269 vs. 3,246 g, and the rate of LGA and/or macrosomia was 10.8 vs. 10.3%, respectively.
The characteristics of the mothers, newborns, and pregnancy outcomes are summarized in Table 1. The median fasting glucose level was 79 mg/dl at a median of 9.5 weeks of gestation. More than 95% of women had a GCT done at a median gestational age of 24.8 weeks; 781 women had a confirmatory 100-g OGTT at a median gestational age of 26.7 weeks. A total of 634 (or 10.3%) of neonates were LGA and/or macrosomic, and 173 (2.8%) of women developed GDM. A total of 5,679 women did not have a prior cesarean section, 843 (14.8%) of whom had a primary cesarean delivery. A total of 436 deliveries occurred prior to 37 weeks of gestation, and 317 neonates were admitted to the neonatal intensive care unit.
Table 1.
Characteristics of women, newborns and frequency of pregnancy outcomes
| Median (interquartile range) | n (%) | |
|---|---|---|
| Maternal and neonatal characteristics | ||
| Parity | 1 (0–2) | 6,096 (99.5) |
| Maternal age (years) | 29.4 (26.2–33.1) | 6,129 (100) |
| Neonatal birth weight (g) | 3,246 (2,924–3,552) | 6,128 (100) |
| Gestational age at delivery (weeks) | 39.7 (38.6–40.6) | 6,129 (100) |
| Gestational age at fasting glucose (weeks) | 9.5 (7.6–11.6) | 6,129 (100) |
| Fasting glucose level (mg/dl) | 79 (75–85) | 6,129 (100) |
| Gestational age at GCT (weeks) | 24.8 (24.1–25.9) | 5,827 (95.1) |
| GCT (mg/dl) | 100 (83–121) | 5,827 (95.1) |
| Maternal and neonatal outcomes | Percent | n with outcomes |
| LGA and or macrosomia | 10.3 | 634 |
| GDM | 2.8 | 173 |
| Primary cesarean delivery* | 14.8 | 843 |
| Preterm delivery (<37 weeks) | 7.1 | 436 |
| Neonatal intensive care unit admission | 5.2 | 317 |
*There were 5,679 women without previous cesarean section.
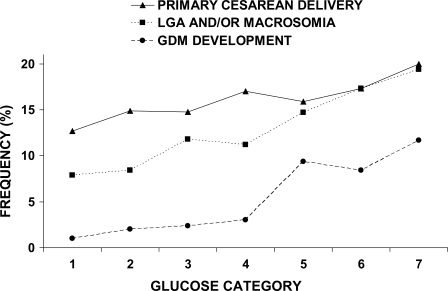
The frequency of each primary outcome across the seven glucose categories is shown in Fig. 1. With increasing fasting maternal glucose levels, the frequency of GDM development increased from 1.0% in the lowest category to 11.7% in the highest; the frequency of LGA and/or macrosomia increased from 7.9 to 19.4%, and for primary cesarean section it increased from 12.7 to 20.0%.
Figure 1.
The relationship between maternal first-trimester fasting glucose level and frequency of primary outcomes. Fasting glucose categories are defined as follows: category 1, <75 mg/dl; category 2, 75–79 mg/dl; category 3, 80–84 mg/dl; category 4, 85–89 mg/dl; category 5, 90–94 mg/dl; category 6, 95–99 mg/dl; and category 7, 100–105 mg/dl.
Table 2 shows the associations of maternal fasting glucose as a categorical variable with each primary outcome, including adjusted odds ratios (AORs) and 95% CIs for each category, as compared with the lowest glucose category. There was strong association between the development of GDM and first-trimester maternal fasting glycemia, with the association increasing with increasing fasting glycemia category (AOR 11.92 [95% CI 5.39–26.37] for the highest category of fasting plasma glucose). There was also strong association with LGA and/or macrosomia, which increased across the increasing fasting glycemia categories (Table 2). This association was maintained almost the same even after excluding the women who developed GDM (1.51 [1.18–1.95] for fasting glucose level 80–84 mg/dl and it increased to 2.41 [1.35–4.29] in the highest category of the fasting plasma glucose compared with the lowest glucose category [P < 0.0001]). The AOR for primary cesarean section increased across categories of maternal glycemia and was 1.94 in the highest category of fasting plasma glucose. This association was no longer significant after the exclusion of women with GDM.
Table 2.
AORs for associations between maternal glucose as a categorical variable and primary outcomes*
| Fasting glucose | n (%) | GDM [n (% outcome)] | GDM [OR (95% CI)] | LGA and/or macrosomia [n (% outcome)] | LGA and/or macrosomia [OR (95% CI)] | Primary cesarean section [n (% outcome)] | Primary cesarean section [OR (95% CI)]† |
|---|---|---|---|---|---|---|---|
| 1 <75 mg/dl | 1,525 (24.9) | 15 (1.0) | 1.0 | 120 (7.9) | 1.0 | 182 (12.7) | 1.0 |
| 2 75–79 mg/dl | 1,587 (25.9) | 31 (2.0) | 1.95 (1.05–3.62) | 134 (8.4) | 1.08 (0.84–1.40) | 222 (14.9) | 1.21 (0.98–1.5) |
| 3 80–84 mg/dl | 1,427 (23.3) | 34 (2.4) | 2.39 (1.30–4.42) | 168 (11.8) | 1.56 (1.22–2.00) | 195 (14.8) | 1.22 (0.98–1.52) |
| 4 85–89 mg/dl | 893 (14.6) | 27 (3.0) | 3.04 (1.60–5.75) | 100 (11.2) | 1.48 (1.12–1.95) | 141 (17.0) | 1.43 (1.12–1.82) |
| 5 90–94 mg/dl | 415 (6.8) | 39 (9.4) | 9.32 (5.07–17.14) | 61 (14.7) | 2.02 (1.45–2.80) | 58 (15.9) | 1.45 (1.04–2.00) |
| 6 95–99 mg/dl | 179 (2.9) | 15 (8.4) | 8.63 (4.13–18.04) | 31 (17.3) | 2.45 (1.60–3.77) | 28 (17.3) | 1.56 (1.00–2.42) |
| 7 100–105 mg/dl | 103 (1.7) | 12 (11.7) | 11.92 (5.39–26.37) | 20 (19.4) | 2.82 (1.67–4.76) | 17 (20.0) | 1.94 (1.11–3.41) |
*Associations were adjusted for parity and maternal age.
†Data for women who had a previous cesarean section were excluded.
BMI is a known confounding factor for GDM risk. Thus, we performed a subgroup analysis including 4,876 women in whom we had pregestational BMI data. After controlling for BMI, maternal age, and parity, there was similar strong, graded association between first-trimester fasting glucose level and GDM, with the association increasing with increasing fasting glycemia category (AOR 2.01 [95% CI 1.02–4.05] for fasting glucose level 80–84 mg/dl and it increased to 9.49 [3.87–23.26] in the highest category of fasting plasma glucose compared with the lowest glucose category [P < 0.0001]).
We also found a strong, graded association between first-trimester fasting glucose level and abnormal GCT (GCT >140 mg/dl), with the association increasing with increasing fasting glycemia category (AOR increased from 1.86 [95% CI 1.41–2.45] in the lowest glucose category to 6.85 [4.08–11.48] in the highest category of the fasting plasma glucose [P < 0.0001]). A total of 781 women had an OGTT. There was fair correlation between first-trimester fasting glucose level and the fasting glucose at the time of OGTT (Pearson correlation coefficient 0.365, P < 0.0001).
There were no significant associations between fasting glucose level category and either preterm delivery <37 weeks of gestation or neonatal intensive care unit admission (OR 0.73–1.35 [95% CI across one and P > 0.1 in all fasting glucose categories for both preterm delivery and neonatal intensive care unit admissions]). Importantly, there was no clear threshold for fasting glucose level that puts pregnant women at a significantly increased risk for adverse pregnancy outcome.
CONCLUSIONS
Our results indicate associations between fasting first-trimester maternal plasma glucose level, below those diagnostic of diabetes, and adverse pregnancy outcome including development of GDM, LGA and/or macrosomia, and primary cesarean delivery. GDM risk remained almost unchanged even after controlling for pregestational BMI, and the risk for LGA and/or macrosomia was maintained even after excluding women with GDM. We also found a strong, graded association between first-trimester fasting glucose level and abnormal GCT and fair correlation between first-trimester fasting glucose level and the fasting glucose at the time of OGTT.
Traditionally, GDM screen is recommended in the beginning of the third trimester in order to maximize the metabolic effects of pregnancy. However, many protocols for GDM screening use a two-step process, thus leaving only a brief window for implementing therapeutic interventions designed to improve outcome. The benefits of screening for and treating GDM have been a matter of considerable debate (13). Advocates point to an association of GDM with maternal and neonatal morbidity. Critics argue that some past studies have failed to show that potential adverse outcomes are markedly improved by diagnosis and treatment. One potential explanation for past difficulty in identifying a benefit from screening for and treating GDM is that such screening as typically implemented does not occur until the third trimester, a point late in pregnancy, thus allowing only a brief period for intervention. Indeed, ∼20% of fetuses already had signs of macrosomia (abdominal circumference above the 90% percentile in ultrasound exam) at the time the women were first referred for GDM treatment (14). Several recent studies (15–17) provide contemporary evidence that screening for and treating GDM is beneficial, and this evidence has bolstered the arguments of those who favor universal GDM testing. Nevertheless, outcomes found in the treatment arms of these studies as well as those observed in general clinical practice suggest that there is room for improvement even within the recommended testing paradigm. Earlier detection of women at risk for GDM might allow earlier intervention, in order to reduce either the later diagnosis of GDM or its associated morbidities. For example, first-trimester testing and identification of high-risk women allow proper diet and exercise guidance from the beginning of the second trimester, at a time when the vomiting period ends and usually the women's appetite is greatly increased. Such guidance from early pregnancy might reduce the rate of excessive weight gain that greatly increases the risk of pregnancy complications including macrosomia, cesarean section, shoulder dystocia, etc. (18–21).
GDM is similar to type 2 diabetes in many aspects including treatment. It is well documented that we can prevent or delay the development of type 2 diabetes in nonpregnant populations at risk for type 2 diabetes by lifestyle intervention, including diet modification, weight reduction, and exercise (22). It is thus possible that earlier recognition of women at risk for the development of GDM and other adverse pregnancy outcomes might benefit from earlier detection and intervention. Indeed, a small study (9) found that first-trimester screening and therapy in women at high risk for GDM result in appropriate-for-gestational-age newborns. Another study (7) has showed that early glucose tolerance screening could prevent some diabetes-related complications in women with GDM. Also, several large population studies (18–21) found that excessive weight gain during pregnancy, especially in overweight and obese pregnant women, greatly increases their risks for adverse pregnancy outcomes, including LGA infants and cesarean delivery. Furthermore, recreational physical activity performed before and/or during pregnancy is associated with a reduced risk of GDM (23). Physically active women are also less likely to develop preeclampsia and excessive gestational weight gain (24).
In the current study, we found that higher first-trimester fasting plasma glucose levels, below those diagnostic of diabetes, were associated with higher risk for GDM development later in pregnancy. Interestingly, a large observational study among young men have found that higher fasting plasma glucose levels, within the normoglycemic range (i.e., <100 mg/dl), constitute an independent risk factor for the development of type 2 diabetes within a few years (25).
Our results for first-trimester fasting glucose level are similar to the HAPO study; for example, in the HAPO study a fasting glucose level of ≥90 mg/dl was found in 11.9% of pregnant women in the beginning of the third trimester. This cutoff detected 22.1% of LGA neonates and 15.1% of primary cesarean deliveries. In the current study, fasting glucose level of ≥90 mg/dl was found in 11.4% of pregnant women during the first trimester. It identified 17.7% of LGA neonates, 12.2% of women who had a primary cesarean delivery, and 38.1% of women who later developed GDM.
First-trimester screening by fasting glucose level also offers the opportunity to detect and treat undiagnosed pregestational diabetes, which becomes a major problem as the prevalence of diabetes increases rapidly. Otherwise, these high-risk women would not receive any special treatment until the beginning of the third trimester. Also, unrecognized and untreated pregestational diabetes has increased risk for congenital malformations, intrauterine fetal deaths, etc., that would not get appropriate attention if the diagnosis was not made in early pregnancy.
There are several limitations to our study. Due to the retrospective nature of the study, we cannot be sure that all the glucose tests were done as they should (i.e., after appropriate fasting or after the appropriate GCT). The 12% of women who did not have a recorded fasting glucose test during the first trimester were excluded. We believe that this is unlikely to materially affect our results because the differences in maternal age, neonatal weight, and LGA rate were small between those who had a first-trimester fasting glucose and those who did not. GDM data are somewhat incomplete given that only 95.1% of women had a GCT and 9% of women with an abnormal GCT did not have a diagnostic 100-g OGTT; however, it is unlikely that this might have changed the results substantially. Our analysis was restricted to women who are insured by the Clalit HMO. The Israeli National Health Insurance Law provides universal health services to every resident at the same cost. Thus, it is unlikely that this had a significant bias on our results. The study was conducted in a single center, and this might influence the results, mainly in regard to mode of delivery and neonatal intensive care unit admission; however, the main outcomes of the study, LGA risk and the development of GDM, are not influenced by hospital policy. Also, because it is a single-center study, we cannot be sure about the relevance of the study in other parts of the world. Some confounders, such as previous GDM or previous macrosomia, may have influenced clinical decisions such as the choice of route of delivery.
In conclusion, we found that fasting first-trimester glucose level lower than what is considered impaired fasting glucose in the nonpregnant state is associated with adverse pregnancy outcome. It may help identify and treat, early in pregnancy, apparently healthy women in order to improve pregnancy outcome. A large, prospective multicenter study on maternal and neonatal outcome is needed to better evaluate the association of first-trimester fasting glucose levels and the usefulness of timely interventions on pregnancy outcome.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in poster form at the 5th International Symposium on Diabetes and Pregnancy (DIP 2009), Sorrento, Italy, 26–28 March 2009.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Sermer M, Naylor CD, Farine D, Kenshole AB, Ritchie JW, Gare DJ, Cohen HR, McArthur K, Holzapfel S, Biringer A. The Toronto Tri-Hospital Gestational Diabetes Project: a preliminary review. Diabetes Care 1998; 21( Suppl. 2): B33– B42 [PubMed] [Google Scholar]
- 2.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358: 1991– 2002 [DOI] [PubMed] [Google Scholar]
- 3.Siegmund T, Rad NT, Ritterath C, Siebert G, Henrich W, Buhling KJ. Longitudinal changes in the continuous glucose profile measured by the CGMS in healthy pregnant women and determination of cut-off values. Eur J Obstet Gynecol Reprod Biol 2008; 139: 46– 52 [DOI] [PubMed] [Google Scholar]
- 4.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003; 26: 3160– 3167 [DOI] [PubMed] [Google Scholar]
- 5.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979; 28: 1039– 1057 [DOI] [PubMed] [Google Scholar]
- 6.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol 1982; 144: 768– 773 [DOI] [PubMed] [Google Scholar]
- 7.Bartha JL, Martinez-Del-Fresno P, Comino-Delgado R. Early diagnosis of gestational diabetes mellitus and prevention of diabetes-related complications. Eur J Obstet Gynecol Reprod Biol 2003; 109: 41– 44 [DOI] [PubMed] [Google Scholar]
- 8.Super DM, Edelberg SC, Philipson EH, Hertz RH, Kalhan SC. Diagnosis of gestational diabetes in early pregnancy. Diabetes Care 1991; 14: 288– 294 [DOI] [PubMed] [Google Scholar]
- 9.Seshiah V, Cynthia A, Balaji V, Balaji MS, Ashalata S, Sheela R, Thamizharasi M, Arthi T. Detection and care of women with gestational diabetes mellitus from early weeks of pregnancy results in birth weight of newborn babies appropriate for gestational age. Diabetes Res Clin Pract 2008; 80: 199– 202 [DOI] [PubMed] [Google Scholar]
- 10.Agarwal MM, Dhatt GS, Punnose J, Koster G. Gestational diabetes: dilemma caused by multiple international diagnostic criteria. Diabet Med 2005; 22: 1731– 1736 [DOI] [PubMed] [Google Scholar]
- 11.Guidelines for the management of pregnant women. Israel ministry of health 2001. Available at http://www.health.gov.il/download/forms/a234_a234_woman_preg.pdf Accessed 16 December 2008
- 12.Dollberg S, Haklai Z, Mimouni FB, Gorfein I, Gordon ES. Birth weight standards in the live-born population in Israel. Isr Med Assoc J 2005; 7: 311– 314 [PubMed] [Google Scholar]
- 13.Hanna FW, Peters JR. Screening for gestational diabetes; past, present and future. Diabet Med 2002; 19: 351– 358 [DOI] [PubMed] [Google Scholar]
- 14.Schaefer-Graf UM, Kjos SL, Kilavuz O, Plagemann A, Brauer M, Dudenhausen JW, Vetter K. Determinants of fetal growth at different periods of pregnancies complicated by gestational diabetes mellitus or impaired glucose tolerance. Diabetes Care 2003; 26: 193– 198 [DOI] [PubMed] [Google Scholar]
- 15.Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: the consequences of not treating. Am J Obstet Gynecol 2005; 192: 989– 997 [DOI] [PubMed] [Google Scholar]
- 16.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005; 352: 2477– 2486 [DOI] [PubMed] [Google Scholar]
- 17.Landon MB, Thom E, Spong CY, Carpenter M, Mele L, Johnson F, Tillinghast J, Anderson G. The National Institute of Child Health and Human Development Maternal-Fetal Medicine Unit Network randomized clinical trial in progress: standard therapy versus no therapy for mild gestational diabetes. Diabetes Care 2007; 30( Suppl. 2): S194– S199 [DOI] [PubMed] [Google Scholar]
- 18.Cedergren MI. Optimal gestational weight gain for body mass index categories. Obstet Gynecol 2007; 110: 759– 764 [DOI] [PubMed] [Google Scholar]
- 19.Hillier TA, Pedula KL, Vesco KK, Schmidt MM, Mullen JA, LeBlanc ES, Pettitt DJ. Excess gestational weight gain: modifying fetal macrosomia risk associated with maternal glucose. Obstet Gynecol 2008; 112: 1007– 1014 [DOI] [PubMed] [Google Scholar]
- 20.Kiel DW, Dodson EA, Artal R, Boehmer TK, Leet TL. Gestational weight gain and pregnancy outcomes in obese women: how much is enough? Obstet Gynecol 2007; 110: 752– 758 [DOI] [PubMed] [Google Scholar]
- 21.DeVader SR, Neeley HL, Myles TD, Leet TL. Evaluation of gestational weight gain guidelines for women with normal prepregnancy body mass index. Obstet Gynecol 2007; 110: 745– 751 [DOI] [PubMed] [Google Scholar]
- 22.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393– 403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dempsey JC, Butler CL, Sorensen TK, Lee IM, Thompson ML, Miller RS, Frederick IO, Williams MA. A case-control study of maternal recreational physical activity and risk of gestational diabetes mellitus. Diabetes Res Clin Pract 2004; 66: 203– 215 [DOI] [PubMed] [Google Scholar]
- 24.Weissgerber TL, Wolfe LA, Davies GA, Mottola MF. Exercise in the prevention and treatment of maternal-fetal disease: a review of the literature. Appl Physiol Nutr Metab 2006; 31: 661– 674 [DOI] [PubMed] [Google Scholar]
- 25.Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T, Kochba I, Rudich A. Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med 2005; 353: 1454– 1462 [DOI] [PubMed] [Google Scholar]