Abstract
OBJECTIVE
Continuously administered insulin is limited by the need for frequent blood glucose measurements, dose adjustments, and risk of hypoglycemia. Regimens based on glucagon-like peptide 1 (GLP-1) could represent a less complicated treatment alternative. This alternative might be advantageous in hyperglycemic patients hospitalized for acute critical illnesses, who benefit from near normoglycemic control.
RESEARCH DESIGN AND METHODS
In a prospective open randomized crossover trial, we investigated eight clinically stable type 2 diabetic patients during intravenous insulin or GLP-1 regimens to normalize blood glucose after a standardized breakfast.
RESULTS
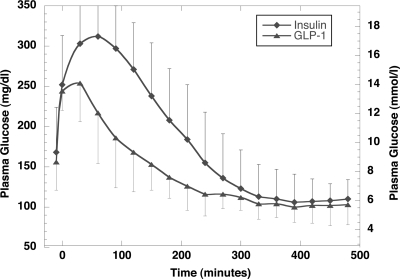
The time to reach a plasma glucose below 115 mg/dl was significantly shorter during GLP-1 administration (252 ± 51 vs. 321 ± 43 min, P < 0.01). Maximum glycemia (312 ± 51 vs. 254 ± 48 mg/dl, P < 0.01) and glycemia after 2 h (271 ± 51 vs. 168 ± 48 mg/dl, P = 0.012) and after 4 h (155 ± 51 vs. 116 ± 27 mg/dl, P = 0.02) were significantly lower during GLP-1 administration.
CONCLUSIONS
GLP-1 infusion is superior to an established insulin infusion regimen with regard to effectiveness and practicability.
Admission hyperglycemia is associated with an increased morbidity and mortality in diabetic and nondiabetic patients hospitalized for acute critical conditions (1,2). Several intervention studies of patients with acute myocardial infraction or cardiac surgery that used intravenously administered regular human insulin suggest that normalization of hyperglycemia reduces morbidity as well as mortality in these patients (3–6). Insulin-based regimens, however, require frequent blood glucose measurements and adjustments of infusion rate to achieve this goal. In addition, hypoglycemia is a frequent and important side effect that has been shown to be associated with a worse outcome in patients hospitalized with acute coronary syndromes (7). Hypoglycemia was also discussed as a reason for the worse outcome in the intensive group in the recent National Investigators Collaboration on Enoxaparin (NICE) trial (8).
Glucagon-like peptide 1 (GLP-1) is an insulinotropic glucagonostatic gastrointestinal hormone that lowers glucose at fixed rates of administration in a glycemia-dependent manner and, therefore, does not cause hypoglycemia (9). The aim of our study was to compare for the first time the efficacy and safety of intravenously administered GLP-1 with an established intravenous insulin regimen in clinically stable hyperglycemic type 2 diabetic patients as a pilot trial for possible future investigations in patient populations with acute, critical conditions.
RESEARCH DESIGN AND METHODS
We performed a prospective open randomized cross-over trial in eight patients with type 2 diabetes. Self-measured fasting glucose levels were required to be >150 mg/dl for inclusion into the study. Patients with New York Heart Association class III or IV heart failure, uncontrolled hypertension, impaired kidney function (creatinine >3 mg/dl), or acute infection were excluded. The study was approved by the local ethics committee and conducted following good clinical practices, and signed informed consent was obtained from all the participants. Six patients had histories of coronary artery disease (two strokes, two myocardial infarctions, two coronary artery bypass graftings, and two coronary artery revascularizations). All the patients were treated with oral antidiabetic drugs.
Investigations took place on two occasions separated by 7 ± 3 days. Patients were admitted for a 1-day stay at the Metabolism and Vascular Research Unit. After an overnight fast, patients received a standardized breakfast (634 kcal, 100 g carbohydrates, 35 g fat, and 13.6 g protein). Treatment started 30 min after the end of the test meal. Patients were randomized to either the insulin infusion protocol as used in the Munich registry (10) or a continuous GLP-1 infusion (Clinalfa; Laeufelingen, Switzerland) at a dose of 1.2 pmol · kg−1 · min−1 for 8 h. Both groups received a concomitant glucose (10%) infusion at a rate of 30 ml/h; blood glucose measurements were performed every 30 min or at symptoms of hypoglycemia. The primary outcome was the time taken to reach a plasma glucose level below 115 mg/dl, and the secondary outcome parameters were plasma glucose after 2 and 4 h, as well as maximum glycemia and the number of hypoglycemic episodes. Differences of study variables were tested by using ANOVA for repeated measurements or paired Student's t test.
RESULTS
We investigated eight patients (five male) with a mean age of 58.2 ± 2.3 years, a BMI of 24.4 ± 1.0 kg/m2, and an A1C of 7.3 ± 0.7%. Glucose levels at the start of infusion therapy were comparable on both days of investigation (insulin 252 ± 42 mg/dl, GLP-1 244 ± 24 mg/dl).
The primary end point (the time to reach plasma glucose below 115 mg/dl) was significantly shorter during GLP-1 administration (252 ± 51 vs. 321 ± 43 min, P < 0.01) (Fig. 1). Maximum glycemia (312 ± 51 vs. 254 ± 48 mg/dl, P < 0.01), glycemia after 2 h (271 ± 51 vs. 168 ± 48 mg/dl, P = 0.012), and glycemia after 4 h (155 ± 51 vs. 116 ± 27 mg/dl, P = 0.02) were significantly higher during insulin administration in comparison with GLP-1. Glycemia after 8 h — at the end of the intervention — was comparable between both regimens (insulin 110 ± 24 mg/dl, GLP-1 103 ± 22 mg/dl, P = NS). Serum insulin levels were generally lower during GLP-1 treatment (data not shown). One symptomatic hypoglycemia occurred during insulin infusion (48 mg/dl), whereas no hypoglycemia was noted in the GLP-1 regimen. Nausea was observed in one patient during GLP-1 infusion.
Figure 1.
Plasma glucose course by using the insulin regimen in comparison with the GLP-1 regimen.
CONCLUSIONS
Our study compared for the first time an established insulin infusion regimen with a GLP-1–infusion regimen in nonfasted type 2 diabetic patients regarding the efficacy to normalize hyperglycemia.
We clearly showed that glucose targets could be achieved faster with the GLP-1–based regimen in comparison with the insulin regimen, and that maximal glycemic excursions were markedly reduced. Beside the advantage in time course of lowering hyperglycemia, there is no need for frequent blood glucose measurements and subsequent dose adaptations as is required when using intravenous insulin. Our pilot study, thus, indicates that GLP-1–based regimens should be further tested in acute clinical settings (e.g., in hyperglycemic patients with acute myocardial infarction or undergoing vascular surgery where hyperglycemia was shown to predict a worse outcome) (1–6).
Until now, blood glucose lowering in this setting was performed by variable insulin infusion protocols that may cause hypoglycemia. High rates of hypoglycemia, in turn, were discussed as a possible explanation for the worse outcome of the intensive control arm (6.8 vs. 0.5% in the conventional arm) in the NICE trial (8). In addition, Kosiborod et al. (7) recently showed that the relation between mean in-hospital blood glucose and mortality rate is J-shaped, indicating that a low mean blood glucose or recurring hypoglycemic episodes are associated with a worse outcome. In that regard, a GLP-1 regimen has the clear advantage not to cause hypoglycemia.
Preserved capacity of insulin secretion is important for adequate GLP-1 action, thus type 1 diabetic subjects as well as insulin-treated type 2 diabetic patients might not respond sufficiently to GLP-1 infusion. Since postprandial hyperglycemia is the main target for GLP-1 due to additional inhibitory effects on gastrointestinal motility, our study might overestimate the therapeutic potential (11). Previous studies, however, could also demonstrate a clear beneficial effect of GLP-1 on fasting glycemia (12).
In summary, the results of our pilot trial indicate that for hyperglycemic clinically stable type 2 diabetic patients, a GLP-1–based infusion regimen is superior to an insulin-based regimen in effectiveness and practicability for reaching normoglycemia. We suggest that GLP-1–based treatment strategies should be further tested in hyperglycemic patients under conditions of acute illness with regard to effectiveness as well as clinical end points.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
Clinical trial registry no. NCT00859079, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 2000; 355: 773– 778 [DOI] [PubMed] [Google Scholar]
- 2.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc 2003; 78: 1471– 1478 [DOI] [PubMed] [Google Scholar]
- 3.Malmberg K, Rydén L, Efendic S, Herlitz J, Nicol P, Waldenström A, Wedel H, Welin L. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol 1995; 26: 57– 65 [DOI] [PubMed] [Google Scholar]
- 4.Schmeltz LR, DeSantis AJ, Thiyagarajan V, Schmidt K, O'Shea-Mahler E, Johnson D, Henske J, McCarthy PM, Gleason TG, McGee EC, Molitch ME. Reduction of surgical mortality and morbidity in diabetic patients undergoing cardiac surgery with a combined intravenous and subcutaneous insulin glucose management strategy. Diabetes Care 2007; 30: 823– 828 [DOI] [PubMed] [Google Scholar]
- 5.Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation 2004; 109: 1497– 1502 [DOI] [PubMed] [Google Scholar]
- 6.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med 2001; 345: 1359– 1367 [DOI] [PubMed] [Google Scholar]
- 7.Kosiborod M, Inzucchi SE, Krumholz HM, Xiao L, Jones PG, Fiske S, Masoudi FA, Marso SP, Spertus JA. Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk. Circulation 2008; 117: 1018– 1027 [DOI] [PubMed] [Google Scholar]
- 8.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360: 1283– 1297 [DOI] [PubMed] [Google Scholar]
- 9.Meier JJ, Nauck MA. Glucagon-like peptide 1 (GLP-1) in biology and pathology. Diabetes Metab Res Rev 2005; 21: 91– 117 [DOI] [PubMed] [Google Scholar]
- 10.Schnell O, Schäfer O, Kleybrink S, Doering W, Standl E, Otter W. Intensification of therapeutic approaches reduces mortality in diabetic patients with acute myocardial infarction: the Munich registry. Diabetes Care 2004; 27: 455– 460 [DOI] [PubMed] [Google Scholar]
- 11.Schirra J, Houck P, Wank U, Arnold R, Göke B, Katschinski M. Effects of glucagon-like peptide-1(7–36)amide on antro-pyloro-duodenal motility in the interdigestive state and with duodenal lipid perfusion in humans. Gut 2000; 46: 622– 631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nauck MA, Sauerwald A, Ritzel R, Holst JJ, Schmiegel W. Influence of glucagon-like peptide 1 on fasting glycemia in type 2 diabetic patients treated with insulin after sulfonylurea secondary failure. Diabetes Care 1998; 21: 1925– 1931 [DOI] [PubMed] [Google Scholar]