Abstract
OBJECTIVE
Interleukin (IL)-1 impairs insulin secretion and induces β-cell apoptosis. Pancreatic β-cell IL-1 expression is increased and interleukin-1 receptor antagonist (IL-1Ra) expression reduced in patients with type 2 diabetes. Treatment with recombinant IL-1Ra improves glycemia and β-cell function and reduces inflammatory markers in patients with type 2 diabetes. Here we investigated the durability of these responses.
RESEARCH DESIGN AND METHODS
Among 70 ambulatory patients who had type 2 diabetes, A1C >7.5%, and BMI >27 kg/m2 and were randomly assigned to receive 13 weeks of anakinra, a recombinant human IL-1Ra, or placebo, 67 completed treatment and were included in this double-blind 39-week follow-up study. Primary outcome was change in β-cell function after anakinra withdrawal. Analysis was done by intention to treat.
RESULTS
Thirty-nine weeks after anakinra withdrawal, the proinsulin-to-insulin (PI/I) ratio but not stimulated C-peptide remained improved (by −0.07 [95% CI −0.14 to −0.02], P = 0.011) compared with values in placebo-treated patients. Interestingly, a subgroup characterized by genetically determined low baseline IL-1Ra serum levels maintained the improved stimulated C-peptide obtained by 13 weeks of IL-1Ra treatment. Reductions in C-reactive protein (−3.2 mg/l [−6.2 to −1.1], P = 0.014) and in IL-6 (−1.4 ng/l [−2.6 to −0.3], P = 0.036) were maintained until the end of study.
CONCLUSIONS
IL-1 blockade with anakinra induces improvement of the PI/I ratio and markers of systemic inflammation lasting 39 weeks after treatment withdrawal.
Type 2 diabetes is caused by inability of the functional β-cell mass to compensate for increased insulin needs due to insulin resistance (1). During the course of the disease, β-cell function progressively declines irrespective of treatment with glucose-lowering drugs (2–4). β-Cell mass is reduced through apoptosis (5) and type 2 diabetes is associated with a low-grade systemic inflammation (6), but the mechanisms underlying β-cell failure and destruction in type 2 diabetes remain elusive.
In vitro, long-term exposure to high glucose and the peptide hormone leptin secreted by adipose tissue induce β-cell apoptosis and production of the proinflammatory cytokine interleukin (IL)-1 in β-cells and pancreatic islets, respectively (7,8). IL-1 inhibits the function and induces apoptosis of β-cells (9) and has been implicated as a mediator of the β-cell destruction leading to type 1 diabetes (10). Exogenous addition of interleukin-1 receptor antagonist (IL-1Ra), a naturally occurring competitive inhibitor of IL-1 signaling, protects the β-cells from the deleterious effects of high glucose and leptin exposure (7,8).
Both β-cell expression and serum levels of IL-1Ra are reduced in patients with type 2 diabetes (8,11). This inadequate IL-1 antagonism seems to be a genetic trait because genetic polymorphisms in the gene encoding IL-1Ra are associated with altered serum levels of IL-1Ra (12–15).
We showed previously that 13 weeks of IL-1Ra treatment improved β-cell function and reduced A1C and markers of systemic inflammation in patients with type 2 diabetes (16). The aim of this 39-week follow-up study was to investigate the durability of these effects.
RESEARCH DESIGN AND METHODS
This study was a 52-week investigator-initiated, placebo-controlled, double-blind, parallel-group, randomized proof-of-concept clinical trial conducted in Switzerland (University Hospital Zurich) and Denmark (Steno Diabetes Center) between January 2004 and March 2006. The protocol for the study was in accordance with the Declaration of Helsinki and was approved by the local ethics committees. Written informed consent was provided by all patients before entering the study.
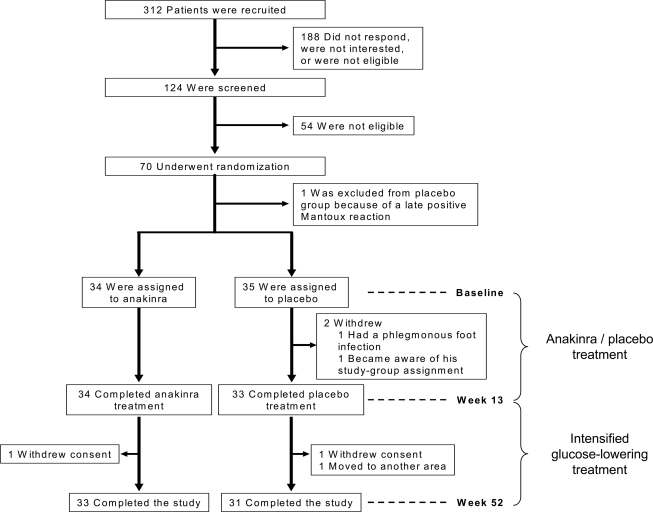
The study was designed a priori in two parts (Fig. 1). The first part was a 13-week intervention study to test the efficacy and safety of recombinant human IL-1Ra (anakinra, [Kineret]) in patients with type 2 diabetes, with metabolic control as the primary outcome and β-cell function, insulin sensitivity, and inflammatory markers as secondary outcomes, as reported previously (16). The second part of the study reported here (supplementary consort information, available in an online appendix http://care.diabetesjournals.org/cgi/content/full/dc09-0533/DC1) was a 39-week follow-up study commencing at the time of withdrawal of study drug to test the durability of the intervention on β-cell function, inflammatory markers, insulin requirement, and insulin sensitivity. In the follow-up study, glucose-lowering therapy was intensified if indicated, and insulin treatment was initiated if A1C was >8.0% or fasting plasma glucose exceeded 8 mmol/l. Other medications were added or increased in dose at the discretion of the investigator. The study was unblinded after the last patient's final visit (week 52).
Figure 1.
Enrollment and outcome. Of the 70 patients who underwent randomization, 67 completed 13 weeks of anakinra or placebo treatment and were included in the present 39-week follow-up study. Of the former anakinra- and placebo-treated patients, 33 and 31, respectively, completed the study.
Inclusion and exclusion criteria have been described previously (16). Inclusion criteria were age ≥20 years, type 2 diabetes according to criteria from the American Diabetes Association (17) for >3 months, BMI >27 kg/m2, A1C >7.5%, and no change in either type or doses of medications in 3 months preceding the study. In brief, exclusion criteria were autoantibodies to GAD65 or islet cell antibody 512, A1C >12%, fasting C-peptide <400 pmol/l, and current treatment with anti-inflammatory drugs (low-dose aspirin was allowed).
Study outcomes
The primary outcome of the follow-up study was change between baseline and 52 weeks in β-cell secretory function measured by the fasting ratio of proinsulin to insulin (PI/I) and the area under the concentration-time curve (AUC) for stimulated C-peptide during an oral glucose tolerance test; an intravenous stimulation test with glucose, glucagon, and arginine; and the two tests combined. Secondary outcomes included change between baseline and 52 weeks in IL-6, C-reactive protein (CRP), insulin requirements, A1C, and the homeostasis model assessment–insulin sensitivity index (HOMA-Si) (18). In addition, IL-1Ra genotypes were determined.
Study procedures
In the follow-up study patients were seen at week 13 on the last day of anakinra or placebo treatment and at weeks 26, 39, and 52. A1C was measured at every visit, and glucose-lowering therapy was intensified if indicated. At baseline and at 13 and 52 weeks, β-cell secretory function was assessed by the fasting PI/I ratio and by a 2-h oral glucose tolerance test directly followed by a 12-min intravenous glucose, glucagon, and arginine stimulation test as described previously (16). Physical examination, funduscopy, routine safety blood tests, and analysis of urinary albumin excretion were performed at baseline and after 13 and 52 weeks. At baseline whole blood was sampled for DNA analysis of the IL-1Ra gene (IL1RN).
Laboratory values
C-peptide, insulin, and proinsulin were determined at the Steno Diabetes Center. Insulin and proinsulin were assessed by enzyme-linked immunosorbent assays, and C-peptide levels were determined by a time-resolved fluoroimmunoassay. IL-1Ra, IL-6, and CRP were measured at the University Hospital Zurich by enzyme-linked immunosorbent assays. Measurements of A1C levels and routine clinical laboratory tests were performed locally in the central laboratory units of the two participating centers. DNA was extracted from whole-blood buffy coats by the Maxwell 16 System (Promega). Genotyping of the variable number tandem repeat (VNTR) polymorphism in intron 2 of IL1RN was analyzed as described previously (19) with use of (forward) 5′-CTC AGC AAC ACT CCTAT-3′ and (reverse) 5′-TCC TGG TCT GCA GGTAA-3′ primers and separation of the PCR products on 2% agarose gel. Genotyping of the single nucleotide polymorphism (SNP) tagged by rs4251961 near 5′ of IL1RN (13) was performed with TaqMan SNP Genotyping Assays (c-32060323-10; Applied Biosystems) according to the manufacturer's description.
Statistical analysis
All end points were analyzed in the intention-to-treat population, defined as patients completing 13 weeks of anakinra or placebo treatment. Missing data were imputed by last observation carried forward. Differences in continuous variables were compared by a two-sample t test. The Mann-Whitney U test was used in the case of nonnormal distribution. For categorical variables, Fisher's exact test was used. Correlations were analyzed by regression analysis. P < 0.05 was considered to indicate statistical significance.
RESULTS
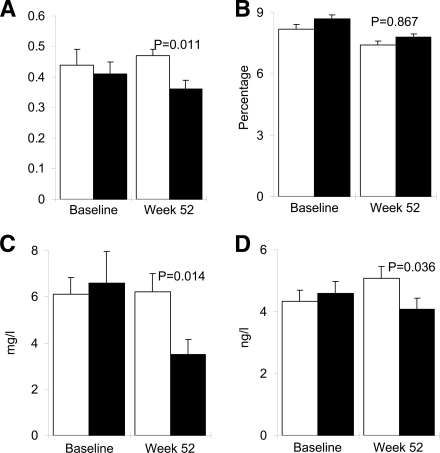
Thirty-three of 34 patients who finished anakinra treatment and 31 of 33 patients who finished placebo treatment completed the study (Fig. 1). At the end of the study, 39 weeks after withdrawal of anakinra or placebo treatment, the PI/I ratio was lower in the former anakinra-treated patients than in the former placebo-treated patients with a baseline adjusted between-group difference of −0.07 (95% CI −0.14 to −0.02, P = 0.011) (Fig. 2A). No differences in AUC for C-peptide during the oral glucose tolerance test, the intravenous stimulation test, and the combined test at study end between former anakinra- and placebo-treated patients were observed with baseline adjusted between-group differences of 11.0 (−13.4 to 35.3, P = 0.224), 2.9 (−3.1 to 9.0, P = 0.333), and 11.1 (−18.3 to 40.6, P = 0.282) nmol/l × min for the oral, intravenous, and combined test, respectively.
Figure 2.
Change in glycemic and inflammatory markers at study end. Data for PI/I ratio (A), A1C (B), C-reactive protein (C), and IL-6 (D) at baseline and end of study (week 52) in patients treated with anakinra (■) or placebo (□) from baseline until week 13. Data are means ± SEM.
After 39 weeks of anakinra withdrawal, CRP (−3.2 mg/l [95% CI −6.2 to −1.1, P = 0.014]) (Fig. 2C) and IL-6 (−1.4 ng/l [−2.6 to −0.3, P = 0.036]) (Fig. 2D) were reduced compared with values in placebo group patients. No correlation between the reduction in PI/I ratio and the reductions in CRP (r2 = 0.007, P = 0.670) or IL-6 (r2 = 0.012, P = 0.556) was observed in the anakinra-treated patients.
At 52 weeks the adjusted mean difference in A1C from baseline between the former anakinra- and placebo-treated patients was −0.05% (95% CI −0.62 to 0.52, P = 0.867) (Fig. 2B). The absolute means ± SEM reductions in A1C were from 8.7 ± 0.2 to 7.8 ± 0.2% and from 8.2 ± 0.2 to 7.4 ± 0.1% in the anakinra and placebo groups, respectively. Insulin sensitivity at study end assessed by HOMA-Si was unchanged with a baseline adjusted between-group difference (anakinra vs. placebo) of −0.04 l2/mM × mU (−0.20 to 0.13, P = 0.485). The average daily insulin dose from withdrawal of anakinra or placebo treatment until week 52 increased by 18.9 ± 4.0 and 28.5 ± 7.9 IU (P = 0.670) in the anakinra and placebo groups, respectively. The daily metformin dose was increased by 69 ± 50 and 308 ± 115 mg (P = 0.257) in the anakinra and placebo groups, respectively. Other oral glucose–lowering medications were unchanged during the study.
Statin therapy was initiated in seven and eight patients during the follow-up of anakinra- and placebo-treated patients, respectively. Three anakinra and four placebo group patients started aspirin during the follow-up. The average weight gains during the study for anakinra- and placebo-treated patients were 1.3 ± 0.6 and 1.7 ± 0.8 kg (P = 0.678), respectively. Three symptomatic self-reported hypoglycemic episodes were noted: two in the placebo group and one in the anakinra group. No change in blood pressure, lipids, 24-h urinary albumin excretion, or retinal fundus photographs was observed during the study.
As reported previously (16) 21 of 34 patients responded to treatment defined as any reduction in A1C after 13 weeks of anakinra treatment (responders to anakinra), whereas 10 of 33 patients receiving placebo treatment experienced reductions in A1C (P < 0.0001). To characterize the responders to anakinra treatment, a subgroup analysis was performed. At baseline patients responding to anakinra (n = 21) were older (P = 0.015) and had a higher rate of cardiovascular disease (P = 0.009) compared with nonresponders to anakinra (n = 13) (supplementary Table A1, available in an online appendix).
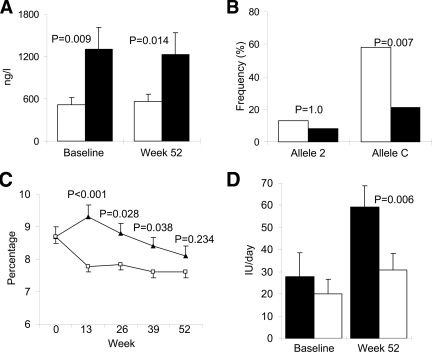
Furthermore, the baseline IL-1Ra serum level was lower in responders to anakinra than in nonresponders (519 ± 104 vs. 1,299 ± 314 ng/l, P = 0.009) and remained unchanged at the end of the study (Fig. 3A). Because polymorphisms of the IL1RN gene encoding IL-1Ra have been associated to serum levels of the protein, two IL1RN gene polymorphisms were investigated. Allele 2 of the VNTR polymorphism in intron 2 of the gene has been associated with an increased serum IL-1Ra level (12,15). An overall correlation between allele 2 carrier status and baseline serum IL-1Ra levels was found (r2 = 0.18, P = 0.021); however, there was no difference between responders and nonresponders with respect to allele 2 frequency (P = 1.0) (Fig. 3B). Allele C of the SNP rs4251961 tagging near 5′ of IL1RN has been associated with a lower serum IL-1Ra level and higher serum CRP and IL-6 levels (13,14). In this study the correlation between allele C carrier status and baseline serum IL-1Ra levels was confirmed (r2 = 0.46, P < 0.001); however, no correlation to baseline CRP (r2 = 0.11, P = 0.124) or IL-6 (r2 = 0.06, P = 0.254) was found. Allele C frequency was higher (58%) in the responders to anakinra treatment than in the nonresponders (21%, P = 0.007) (Fig. 3B).
Figure 3.
IL-1Ra serum levels and genotypes; A1C and insulin requirements. A: Serum IL-1Ra levels at baseline and end of study (week 52) in patients with (□, responders) or without (■, nonresponders) any reduction in A1C after 13 weeks of anakinra treatment. B: Allele frequencies of allele 2 and C of the VNTR tandem repeat polymorphism in intron 2 and the SNP tagged by rs4251961, respectively, of the IL1-Ra gene in responders (□) and nonresponders (■) to anakinra treatment. C: A1C at baseline (week 0), and 13, 26, 39, and 52 weeks in responders (□) and nonresponders (▲) to anakinra treatment. D: Daily insulin dose at baseline and end of study (week 52) in responders (□) and nonresponders (■) to anakinra treatment. Data are means ± SEM or frequencies where indicated.
Identical target A1C at 52 weeks (Fig. 3C) was reached with lower increases in insulin dose (10.8 ± 2.6 IU/day) in the patients responding to anakinra compared with anakinra-unresponsive patients (31.4 ± 6.0 IU/day, P = 0.006) (Fig. 3D).
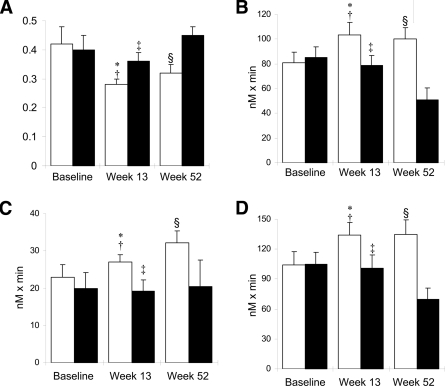
At the end of anakinra treatment (week 13) responders to anakinra showed improved β-cell function as assessed by the PI/I ratio (P = 0.041) and AUC for C-peptide during the stimulation tests (oral test P = 0.006, intravenous test P = 0.048, and combined tests P = 0.025) compared with that in patients not responding to anakinra treatment (Fig. 4). The improved β-cell function in responders to anakinra treatment was maintained 39 weeks after anakinra withdrawal, whereas a reduction in β-cell function in nonresponders to anakinra treatment was seen 39 weeks after anakinra withdrawal (Fig. 4), with the exception of the AUC for C-peptide of the intravenous stimulation test, which was unchanged (P = 0.793) (Fig. 4C). The inflammatory markers CRP and IL-6 were reduced compared with baseline both at the time of anakinra withdrawal and at the end of the study irrespective of whether the patients were responsive (reduction in A1C) or unresponsive to anakinra treatment. No correlation between the changes in CRP and IL-6 and any measurement of β-cell function in either of the two subgroups was found (data not shown). No subgroup differences in insulin sensitivity measured by HOMA-Si were observed.
Figure 4.
β-Cell function after anakinra withdrawal. β-Cell function assessed by PI/I (A); and AUC for C-peptide during an oral glucose-tolerance test (B); an intravenous stimulation with glucose, glucagon, and arginine (C); and the oral and intravenous test combined (D) at baseline, anakinra withdrawal (week 13), and end of study (week 52) in patients with (□, responders) or without (■, nonresponders) any reduction in A1C after 13 weeks of anakinra treatment. A: *P = 0.041 vs. nonresponders at week 13, †P = 0.435 vs. responders week 52, ‡P = 0.016 vs. nonresponders week 52, and §P = 0.005 vs. nonresponders week 52. B: *P = 0.006 vs. nonresponders at week 13, †P = 0.750 vs. responders week 52, ‡P = 0.021 vs. nonresponders week 52, and §P = 0.008 vs. nonresponders week 52. C: *P = 0.048 vs. nonresponders at week 13, †P = 0.039 vs. responders week 52, ‡P = 0.793 vs. nonresponders week 52, and §P = 0.092 vs. nonresponders week 52. D: *P = 0.025 vs. nonresponders at week 13, †P = 0.947 vs. responders week 52, ‡P = 0.021 vs. nonresponders week 52, and §P = 0.001 vs. nonresponders week 52. Data are means ± SEM.
CONCLUSIONS
In the first part of this clinical trial, we reported that 13 weeks of IL-1Ra treatment with anakinra reduced A1C, improved β-cell function, and reduced inflammatory markers (16). In the present protocolled follow-up study, we show that the reduced PI/I ratio and CRP and IL-6 serum levels were maintained 39 weeks after anakinra withdrawal, indicating that at least 39 weeks of remission of these parameters were caused by 13 weeks of anakinra treatment.
Furthermore, a subgroup analysis showed that the 21 (62%) patients who experienced any reduction in A1C after 13 weeks of IL-1Ra treatment with anakinra (responders) maintained their anakinra-induced improved β-cell function assessed by both the PI/I ratio and stimulatory testing 39 weeks after anakinra withdrawal, whereas the β-cell function of the patients treated with placebo and those unresponsive to anakinra treatment further deteriorated after cessation of placebo and anakinra therapy, respectively. The superior β-cell function of the anakinra-responsive patients was reflected by a lower insulin requirement to obtain target glycemia in this group.
The preserved β-cell function 39 weeks after anakinra withdrawal indicates that modulation of β-cell function or increased β-cell mass occurred during IL-1Ra therapy. Apart from one study using short-term intensive insulin treatment (20), in which the 45–51% who responded had maintained an acute insulin response to intravenous glucose testing, no other therapy has been shown to maintain β-cell function in type 2 diabetes after treatment withdrawal.
Both leptin and hyperglycemia induce IL-1 production in the pancreatic islets (7,8). Reduction of hyperglycemia improves β-cell function (20). However, the improvement in β-cell function observed in this study does not seem to be a consequence of improved glycemia per se, because declining β-cell function was observed in patients not responsive to anakinra and placebo group patients, irrespective of the improved glycemic control obtained during the follow-up phase. This finding suggests that the inhibition of IL-1 signaling was the mechanism behind the improved β-cell function.
Elevated IL-1Ra serum levels are found in nondiabetic individuals prone to develop type 2 diabetes (21), whereas patients with overt type 2 diabetes have reduced β-cell expression and serum levels of IL-1Ra (8,11), indicating that a downregulation of IL-1Ra expression is a consequence of the diabetic state. In this study, low baseline IL-1Ra serum levels predicted the glycemic and β-cell secretory responses to anakinra treatment. The increased frequency of the C allele of SNP rs4251961, associated with lower IL-1Ra and higher IL-1 serum levels (13,14) in the responders to anakinra treatment, indicates that the observed 62% response rate to anakinra treatment may be genetically determined. In accordance with this notion, we have previously shown that β-cells from 6 of 10 patients with type 2 diabetes expressed IL-1 mRNA (22). The observed frequencies of the T and C alleles of SNP rs4251961 in this study did not differ from the frequencies in populations without type 2 diabetes (13,14), indicating that the C allele is not predisposing to diabetes development per se but is a pharmacogenetic biomarker for a glycemic response to anakinra treatment.
The lack of association between changes in CRP and IL-6, surrogate markers of systemic IL-1 activity, and the PI/I ratio or any reduction in A1C after anakinra therapy indicates that systemic inflammation is not likely to affect β-cell function and that the improved β-cell function caused by anakinra treatment is due to inhibition of IL-1 produced in the vicinity of the β-cell. IL-1 production could thus originate from the β-cell (7) or from macrophages infiltrating the islets (23) as the IL1B gene is overexpressed in blood monocytes from patients with type 2 diabetes (24).
The sustained reduction of CRP and IL-6 levels at 39 weeks after anakinra withdrawal is notable and indicates that transient IL-1 blockade is capable of inducing not only a metabolic but also a 39-week-long inflammatory remission. In this study, the inflammatory remission was not related to insulin sensitivity. This finding is in line with recent findings from the Whitehall II study (25).
There are some limitations to this follow-up study. First, multiple hypotheses were tested; second, a subgroup analysis was performed.
In summary, this study suggests that 13 weeks of anakinra treatment is capable of inducing a 39-week-long preservation of β-cell function and a similar inflammatory remission in patients with type 2 diabetes. Low baseline serum IL-1Ra levels predicted the metabolic response, and this phenotypic trait was associated with the C allele of an IL1RN SNP. Future studies will have to be conducted to confirm these results and to test higher doses and longer treatment and follow-up periods in patients with type 2 diabetes selected on the basis of their IL1RN SNP genotype. If the results are confirmed, the perspective of these findings is that anakinra treatment could be used as substitution therapy in patients with type 2 diabetes with genetically determined IL-1Ra deficiency and as induction therapy during flares of hyperglycemia in the course of the disease.
Supplementary Material
Acknowledgments
M.Y.D. is listed as the inventor of a patent filed in 2003 for the use of an interleukin-1 receptor antagonist for the treatment or prophylaxis against type 2 diabetes. No other potential conflicts of interest relevant to this article were reported.
Footnotes
Clinical trial reg. no. NCT00303394, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Cerasi E. Insulin deficiency and insulin resistance in the pathogenesis of NIDDM: is a divorce possible? Diabetologia 1995; 38: 992– 997 [DOI] [PubMed] [Google Scholar]
- 2.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355: 2427– 2443 [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. UK Prospective Diabetes Study Group. Diabetes 1995; 44: 1249– 1258 [PubMed] [Google Scholar]
- 4.Alvarsson M, Sundkvist G, Lager I, Berntorp K, Fernqvist-Forbes E, Steen L, Orn T, Holberg MA, Kirksaether N, Grill V. Effects of insulin vs. glibenclamide in recently diagnosed patients with type 2 diabetes: a 4-year follow-up. Diabetes Obes Metab 2008; 10: 421– 429 [DOI] [PubMed] [Google Scholar]
- 5.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003; 52: 102– 110 [DOI] [PubMed] [Google Scholar]
- 6.Kolb H, Mandrup-Poulsen T. An immune origin of type 2 diabetes? Diabetologia 2005; 48: 1038– 1050 [DOI] [PubMed] [Google Scholar]
- 7.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-induced β-cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J Clin Invest 2002; 110: 851– 860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maedler K, Sergeev P, Ehses JA, Mathe Z, Bosco D, Berney T, Dayer JM, Reinecke M, Halban PA, Donath MY. Leptin modulates β cell expression of IL-1 receptor antagonist and release of IL-1β in human islets. Proc Natl Acad Sci USA 2004; 101: 8138– 8143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendtzen K, Mandrup-Poulsen T, Nerup J, Nielsen JH, Dinarello CA, Svenson M. Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science 1986; 232: 1545– 1547 [DOI] [PubMed] [Google Scholar]
- 10.Mandrup-Poulsen T. The role of interleukin-1 in the pathogenesis of IDDM. Diabetologia 1996; 39: 1005– 1029 [DOI] [PubMed] [Google Scholar]
- 11.Marculescu R, Endler G, Schillinger M, Iordanova N, Exner M, Hayden E, Huber K, Wagner O, Mannhalter C. Interleukin-1 receptor antagonist genotype is associated with coronary atherosclerosis in patients with type 2 diabetes. Diabetes 2002; 51: 3582– 3585 [DOI] [PubMed] [Google Scholar]
- 12.Hurme M, Santtila S. IL-1 receptor antagonist (IL-1Ra) plasma levels are co-ordinately regulated by both IL-1Ra and IL-1β genes. Eur J Immunol 1998; 28: 2598– 2602 [DOI] [PubMed] [Google Scholar]
- 13.Rafiq S, Stevens K, Hurst AJ, Murray A, Henley W, Weedon MN, Bandinelli S, Corsi AM, Guralnik JM, Ferruci L, Melzer D, Frayling TM. Common genetic variation in the gene encoding interleukin-1-receptor antagonist (IL-1RA) is associated with altered circulating IL-1RA levels. Genes Immun 2007; 8: 344– 351 [DOI] [PubMed] [Google Scholar]
- 14.Reiner AP, Wurfel MM, Lange LA, Carlson CS, Nord AS, Carty CL, Rieder MJ, Desmarais C, Jenny NS, Iribarren C, Walston JD, Williams OD, Nickerson DA, Jarvik GP. Polymorphisms of the IL1-receptor antagonist gene (IL1RN) are associated with multiple markers of systemic inflammation. Arterioscler Thromb Vasc Biol 2008; 28: 1407– 1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strandberg L, Lorentzon M, Hellqvist A, Nilsson S, Wallenius V, Ohlsson C, Jansson JO. Interleukin-1 system gene polymorphisms are associated with fat mass in young men. J Clin Endocrinol Metab 2006; 91: 2749– 2754 [DOI] [PubMed] [Google Scholar]
- 16.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 2007; 356: 1517– 1526 [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2006; 29: S43– S48 [PubMed] [Google Scholar]
- 18.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004; 27: 1487– 1495 [DOI] [PubMed] [Google Scholar]
- 19.Mandrup-Poulsen T, Pociot F, Molvig J, Shapiro L, Nilsson P, Emdal T, Roder M, Kjems LL, Dinarello CA, Nerup J. Monokine antagonism is reduced in patients with IDDM. Diabetes 1994; 43: 1242– 1247 [DOI] [PubMed] [Google Scholar]
- 20.Weng J, Li Y, Xu W, Shi L, Zhang Q, Zhu D, Hu Y, Zhou Z, Yan X, Tian H, Ran X, Luo Z, Xian J, Yan L, Li F, Zeng L, Chen Y, Yang L, Yan S, Liu J, Li M, Fu Z, Cheng H. Effect of intensive insulin therapy on β-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 2008; 371: 1753– 1760 [DOI] [PubMed] [Google Scholar]
- 21.Herder C, Brunner EJ, Rathmann W, Strassburger K, Tabak AG, Schloot NC, Witte DR. Elevated levels of the anti-inflammatory interleukin-1 receptor antagonist precede the onset of type 2 diabetes: the Whitehall II study. Diabetes Care 2009; 32: 421– 423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boni-Schnetzler M, Thorne J, Parnaud G, Marselli L, Ehses JA, Kerr-Conte J, Pattou F, Halban PA, Weir GC, Donath MY. Increased interleukin (IL)-1β messenger ribonucleic acid expression in β-cells of individuals with type 2 diabetes and regulation of IL-1β in human islets by glucose and autostimulation. J Clin Endocrinol Metab 2008; 93: 4065– 4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, Gueripel X, Ellingsgaard H, Schneider MK, Biollaz G, Fontana A, Reinecke M, Homo-Delarche F, Donath MY. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 2007; 56: 2356– 2370 [DOI] [PubMed] [Google Scholar]
- 24.Kaizer EC, Glaser CL, Chaussabel D, Banchereau J, Pascual V, White PC. Gene expression in peripheral blood mononuclear cells from children with diabetes. J Clin Endocrinol Metab 2007; 92: 3705– 3711 [DOI] [PubMed] [Google Scholar]
- 25.Brunner EJ, Kivimaki M, Witte DR, Lawlor DA, Davey SG, Cooper JA, Miller M, Lowe GD, Rumley A, Casas JP, Shah T, Humphries SE, Hingorani AD, Marmot MG, Timpson NJ, Kumari M. Inflammation, insulin resistance, and diabetes: Mendelian randomization using CRP haplotypes points upstream. PLoS Med 2008; 5: e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.