Abstract
OBJECTIVE
There are conflicting data regarding relationships of systemic biomarkers of inflammation, hemostasis, and homocysteine with diabetic retinopathy. We examined these relationships in the Multi-Ethnic Study of Atherosclerosis.
RESEARCH DESIGN AND METHODS
A total of 921 participants with diabetes were included. Diabetic retinopathy was graded from retinal photographs. We defined two outcomes: any diabetic retinopathy and vision-threatening diabetic retinopathy (severe nonproliferative diabetic retinopathy or worse). Systemic markers analyzed were C-reactive protein, homocysteine, fibrinogen, plasmin-α2-antiplasmin complex (PAP), interleukin-6, d-dimer, factor VIII, serum creatinine, and urinary albumin-to-creatinine (UAC) ratio.
RESULTS
Prevalence of diabetic retinopathy was 33.2% and vision-threatening diabetic retinopathy 7.1%. After adjusting for established risk factors (diabetes duration, A1C, systolic blood pressure, waist-to-hip ratio, and use of diabetes medications), fibrinogen (odds ratio 1.14 [95% CI 1.01–1.32], P = 0.05) and PAP (1.25 [1.05–1.50], P = 0.01) were associated with any diabetic retinopathy, while PAP (1.54 [1.13–2.11], P = 0.007) and homocysteine (1.57 [1.16–2.11], P = 0.003) were associated with vision-threatening diabetic retinopathy. Only PAP remained significant after additional adjustment for serum creatinine and UAC ratio. Area under receiver-operator characteristic curve (AUROC) for diabetic retinopathy was constructed for established and novel risk factors. Established risk factors accounted for a 39.2% increase of the AUROC, whereas novel markers (fibrinogen, PAP, homocysteine, serum creatinine, and UAC ratio) only accounted for an additional 2.2%.
CONCLUSIONS
There were few associations of novel markers of inflammation, hemostasis, and homocysteine with diabetic retinopathy after controlling for established risk factors. These data suggest that there is limited clinical use of these biomarkers for prediction of diabetic retinopathy.
Diabetic retinopathy is the leading cause of blindness in working-age individuals (1). There is increasing evidence that established risk factors for diabetic retinopathy (2,3), including duration of diabetes, hyperglycemia, and hypertension, only explain a limited amount of the variance in the risk of diabetic retinopathy (1). Furthermore, the underlying pathogenesis of diabetic retinopathy remains inadequately understood (4). This has resulted in examination of the relation of novel risk markers such as inflammation (e.g., C-reactive protein [CRP]), markers of hemostatic disturbances (e.g., fibrinogen levels), and hyperhomocysteinemia to diabetic retinopathy. However, to date, the relations of these factors to diabetic retinopathy have not been consistent (5–17). The reasons for these inconsistencies may be due, in part, to differences in study sample and definitions of diabetic retinopathy (e.g., clinical versus photograph grading) and failure in some studies to make adequate adjustments for traditional risk factors such as glycemic control and hypertension. Thus, it remains unclear if there is a role for the use of these systemic markers as additional clinical tests to identify individuals at high risk of diabetic retinopathy. In this study, we evaluated the relationship of a range of inflammatory, hemostatic, and novel vascular markers with diabetic retinopathy, while controlling for traditional risk factors, in a large multiethnic population.
RESEARCH DESIGN AND METHODS
The Multi-Ethnic Study of Atherosclerosis (MESA) is a population-based study of men and women aged 45–84 years comprising four racial/ethnic groups (whites, blacks, Hispanics, and Chinese). Participants have no history of clinical cardiovascular disease at baseline and are residents of six U.S. communities (18). Tenets of the Declaration of Helsinki were followed, and institutional review board approval was granted at each study site. Written informed consent was obtained from all participants.
There were 6,814 participants at the first examination (from July 2000 to August 2002). Retinal photography was done at the second examination, which immediately followed the baseline examination (from August 2002 to January 2004). A total of 6,237 participants returned for retinal photography, of whom 6,147 had digital images gradable for retinopathy. Of these, 921 participants had diabetes, defined as fasting glucose ≥7.0 mmol/l (≥126 mg/dl) and/or use of insulin and/or oral hypoglycemic medication.
Definition of diabetic retinopathy
Diabetic retinopathy assessment has been previously published (19). For each eye, a diabetic retinopathy severity score was assigned based on modification of the Airlie House classification system (20). We defined “any diabetic retinopathy” as level 14 (any combination of definite hard exudates, cotton wool spots, intraretinal microvascular abnormalities, and/or venous loops in the absence of definite microaneurysms) and above and “vision-threatening diabetic retinopathy” as levels 51 (microaneurysms and one or more of the following: venous beading, hemorrhages or microaneurysms more than or equal to the Early Treatment Diabetic Retinopathy Study standard photograph 2A [>20 retinal hemorrhages], or intraretinal microvascular abnormalities more than or equal to the Early Treatment Diabetic Retinopathy Study standard photograph 8A [prominent]) to 80 (total vitreous hemorrhage) or presence of macular edema. A subject's diabetic retinopathy level was based on the score of the worse eye. Interobserver variation for exact agreement for the 17-step diabetic retinopathy severity scale κ score varied from 0.68 to 0.86, and for intraobserver variation for 100% agreement the κ score varied from 0.68 to 0.91.
Assessment of other risk factors
A detailed questionnaire was used to obtain participant information, including past medical history, current cigarette smoking, and current alcohol consumption status. Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or current use of antihypertensive medications. Resting blood pressure was measured three times in the seated position using a Dinamap Model Pro 100 automated oscillometric sphygmomanometer (Critikon, Tampa, FL). The average of the last two measurements was used in analysis. Height and weight were measured to determine BMI.
Fasting (>8 h) blood samples were drawn from participants, and aliquots were prepared for central analysis and for storage (∼65 aliquots per participant at the first examination) at the University of Vermont and the University of Minnesota (18). Standardized protocols were designed to allow several domains of study to be addressed, including measurements of lipids and lipoproteins, systemic inflammation, and endothelial cell function (21). Details of these methods, including coefficients of variation, are provided elsewhere (21). The following were analyzed in this report: plasma total and HDL cholesterol, plasma triglycerides, plasma A1C, plasma plasmin-α2-antiplasmin complex (PAP), plasma d-dimer, plasma factor VIII, plasma total homocysteine, serum glucose, serum high-sensitive CRP, serum fibrinogen, serum interleukin (IL)-6, serum creatinine, and the urinary albumin-to-creatinine (UAC) ratio.
Statistical analysis
Baseline characteristics of participants with and without diabetic retinopathy were compared using χ2 test for proportions, t test, or Mann-Whitney U test for means. Logistic regression models were constructed and initial model was adjusted for age, sex, race, and study center. The risk factors that were significant in the initial model were further adjusted for confounders previously found to be independently associated with diabetic retinopathy in the MESA (19) (SBP, use of diabetes medications, duration of diabetes, A1C, and waist-to-hip ratio [model 1]). Further adjustments were made for UAC ratio alone (model 2) and then UAC ratio and serum creatine combined (model 3). Area under the receiver-operator characteristic curve (AUROC) for diabetic retinopathy were constructed for each of the established and novel risk factors, and the percentage of the incremental changes in AUROC (in addition to age and sex) were presented to determine the use of the various traditional and novel risk factors for diabetic retinopathy prediction. Analyses were performed using SPSS version 16.0.1 (SPSS, Chicago, IL).
RESULTS
Among participants with diabetes, the prevalence of any diabetic retinopathy was 33.2% and the prevalence of vision-threatening diabetic retinopathy 7.9%. Participants with diabetic retinopathy were more likely to have a history of use of insulin and/or oral hypoglycemic medications, higher SBP, higher serum glucose, higher A1C, and longer duration of diabetes than those without diabetic retinopathy (Table 1). There were also higher proportions of African Americans and Hispanics with diabetic retinopathy than Caucasians and Chinese Americans (we have previously published the detailed analysis of prevalence of diabetic retinopathy in the MESA population [19]).
Table 1.
Characteristics of 921 participants with diabetes, the MESA
| No retinopathy | Diabetic retinopathy | P* | |
|---|---|---|---|
| n | 643 | 278 | |
| Sex (male) | 51.9 | 52.1 | 0.96 |
| Race | 0.01 | ||
| White | 24.6 | 16.2 | |
| African American | 33.7 | 40.3 | |
| Hispanic | 28.6 | 33.1 | |
| Chinese | 13.1 | 10.4 | |
| History of alcohol consumption | 38.4 | 34.7 | 0.26 |
| Current cigarette smoker | 11.4 | 10.1 | 0.56 |
| Hypertension | 71.7 | 76.8 | 0.09 |
| Use of oral diabetes medication | 47.2 | 55.5 | <0.001 |
| Use of insulin | 5.9 | 21.5 | <0.001 |
| Age (years) | 65.3 ± 9.2 | 65.0 ± 9.2 | 0.60 |
| Serum glucose (mg/dl) | 148.5 ± 49.0 | 166.1 ± 63.4 | <0.001 |
| SBP (mmHg) | 129.3 ± 20.0 | 133.9 ± 24.9 | 0.003 |
| Diabetes duration (years)† | 0 (5) | 7 (15) | <0.001 |
| A1C (%) | 7.03 ± 1.44 | 7.78 ± 1.85 | <0.001 |
| BMI (kg/m2) | 30.9 ± 6.12 | 30.6 ± 5.96 | 0.43 |
| Plasma total cholesterol (mg/dl) | 181.5 ± 36.4 | 182.6 ± 38.6 | 0.70 |
| HDL cholesterol (mg/dl) | 46.2 ± 12.9 | 47.2 ± 12.7 | 0.25 |
| Triglycerides (mg/dl) | 163.5 ± 119.6 | 148.2 ± 101.2 | 0.06 |
| CRP (mg/dl)† | 2.6 (4.7) | 2.5 (4.7) | 0.66 |
| Plasma fibrinogen (mg/dl) | 360 ± 79.2 | 370 ± 81.1 | 0.006 |
| PAP (nmol/l)† | 4.0 (1.9) | 4.4 (2.4) | <0.001 |
| IL-6 (pg/ml)† | 1.5 (1.3) | 1.5 (1.4) | 0.83 |
| d-Dimer (ug/ml)† | 0.2 (0.3) | 0.2 (0.2) | 0.96 |
| Factor VIII (%) | 184 ± 72.5 | 187 ± 80.9 | 0.60 |
| Creatinine (mg/dl)† | 0.9 (0.2) | 0.9 (0.4) | 0.24 |
| UAC ratio (mg/dl)† | 8.6 (16.3) | 14.9 (48.5) | <0.001 |
| Homocysteine (μmol/l)† | 8.8 (3.7) | 8.9 (3.4) | 0.58 |
Data are percent, means ± SD, or median (interquartile range). Data were obtained during the first examination (from July 2000 to August 2002), except for retinopathy, which was collected during the second examination (from August 2002 to January 2004).
*P value based on χ2 (categorical), t test (quantitative and normal), or Mann-Whitney U test (quantitative and skewed), comparing diabetes participants with and without retinopathy.
†Results are shown as median (interquartile range) for skewness.
Table 1 also shows that individuals with diabetic retinopathy had higher levels of plasma fibrinogen (370 ± 81.1 vs. 360 ± 79.2 mg/dl, P = 0.006), PAP (4.4 ± 2.4 vs. 4.0 ± 1.9 nmol/l, P < 0.001), and UAC ratio (14.9 ± 48.5 vs. 8.6 ± 16.3, P < 0.001) than those without diabetic retinopathy. CRP, IL-6, d-dimer, factor VIII, creatinine, and homocysteine were not associated with the presence of diabetic retinopathy.
After adjustment for age, sex, race, study center, SBP, use of diabetes medications, duration of diabetes, A1C, and waist-to-hip ratio, fibrinogen (odds ratio 1.14 [95% CI 1.01–1.32], P = 0.05) and PAP (1.25 [1.05–1.50], P = 0.01) were associated with any diabetic retinopathy, while PAP (1.54 [1.13–2.11], P = 0.007) and homocysteine (1.57 [1.16–2.11], P = 0.003) were associated with vision-threatening diabetic retinopathy (Table 2). These associations remained after additional adjustment for UAC ratio. However, after further adjustment of serum creatinine level, only PAP remained associated with any diabetic retinopathy (1.28 [1.07–1.54], P = 0.008) or vision-threatening diabetic retinopathy (1.51 [1.10–2.08], P = 0.01). Further stratification by above/below 65 years of age did not change the associations observed in the two age-groups (data not shown).
Table 2.
Relationship of inflammatory and hemostatic factors with any diabetic retinopathy and vision-threatening retinopathy
| Per SD change in risk factors | Any retinopathy |
Vision-threatening retinopathy |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1* | P | Model 2† | P | Model 3‡ | P | Model 1* | P | Model 2† | P | Model 3‡ | P | |
| Plasma fibrinogen | 1.14 | 0.05 | 1.14 | 0.05 | 1.16 | 0.05 | 1.30 | 0.05 | 1.25 | 0.11 | 1.23 | 0.15 |
| 79.5 mg/dl increase | (1.01–1.32) | (1.0–1.32) | (1.00–1.35) | (1.00–1.72) | (0.94–1.66) | (0.93–1.62) | ||||||
| PAP complex | 1.25 | 0.01 | 1.25 | 0.01 | 1.28 | 0.008 | 1.54 | 0.007 | 1.52 | 0.009 | 1.51 | 0.01 |
| 2.45 nmol/l increase | (1.05–1.50) | (1.0–1.50) | (1.07–1.54) | (1.13–2.11) | (1.11–2.08) | (1.10–2.08) | ||||||
| Creatinine | 0.99 | 0.76 | 0.89 | 0.16 | — | — | 1.39 | 0.006 | 1.38 | 0.006 | — | — |
| 0.42 mg/dl increase | (0.90–1.08) | (0.7–1.05) | (1.10–1.75) | (1.09–1.74) | ||||||||
| UAC ratio | 1.19 | 0.006 | — | — | — | — | 1.42 | 0.002 | — | — | — | — |
| 216 mg/dl increase | (1.05–1.35) | (1.14–1.78) | ||||||||||
| Homocysteine | 0.93 | 0.44 | 0.92 | 0.40 | 0.98 | 0.89 | 1.57 | 0.003 | 1.50 | 0.01 | 1.27 | 0.22 |
| 3.3 μmol/l increase | (0.78–1.12) | (0.7–1.12) | (0.80–1.22) | (1.16–2.11) | (1.10–2.05) | (0.87–1.85) | ||||||
Data are odds ratio (95% CI) and were obtained during the first examination (from July 2000 to August 2002) except for retinopathy, which was collected during the second examination (from August 2002 to January 2004). Each risk factor is in a separate model.
*Model 1: adjusted for age, sex, race, study center, SBP, use of diabetes medications, duration of diabetes, A1C, and waist-to-hip ratio.
†Model 2: model 1 plus UAC ratio.
‡Model 3: model 2 plus creatinine.
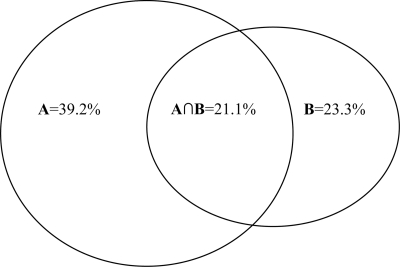
Table 3 shows the AUROC for the prediction of diabetic retinopathy with traditional and novel risk factors for diabetic retinopathy. Each variable was added to age and sex separately to determine the AUROC for that variable (and age and sex). The total change (%) in the AUROC for the traditional risk factors is 39.2%, while for novel risk factors it is 23.3%. Figure 1 is a Venn diagram used to illustrate the relationship of the change in the AUROC for diabetic retinopathy between the traditional and novel risk factors. The change in the AUROC for the traditional risk factors alone is 18.1% and the novel risk factors alone is 2.2%, while the inseparable (or “overlap”) change of traditional and novel risk factors is 21.1%.
Table 3.
AUROC for diabetic retinopathy of the predictive models that include traditional and novel risk factors
| AUROC for diabetic retinopathy |
||
|---|---|---|
| AUROC | Change (%) in AUROC* | |
| Model 1: age/sex adjusted | 0.541 | — |
| Model 2† | ||
| Duration of diabetes | 0.717 | 32.5 |
| Diabetes medications | 0.668 | 23.5 |
| A1C | 0.640 | 18.3 |
| SBP | 0.574 | 6.1 |
| Waist-to-hip ratio | 0.569 | 5.2 |
| Traditional/established risk factors† | 0.753 | 39.2 |
| Model 2† | ||
| UAC ratio | 0.643 | 18.9 |
| PAP | 0.596 | 10.2 |
| Fibrinogen | 0.574 | 6.1 |
| Homocysteine | 0.543 | 0.4 |
| Serum creatinine | 0.543 | 0.4 |
| Novel risk factors‡ | 0.667 | 23.3 |
| Traditional and novel risk factors§ | 0.765 | 41.4 |
Data were obtained during the first examination (from July 2000 to August 2002) except for retinopathy, which was collected during the second examination (from August 2002 to January 2004).
*Percent increase of AUROC = AUROC of model 2 − AUROC model 1 × 100 AUROC model 1.
†Each variable was added separately to age and sex. The AUROC of each row is that of the variable, age, and sex in the model only.
‡The combined change of the AUROC for the traditional/established risk factors (i.e., duration of diabetes, use of diabetes medications, A1C, SBP, and waist-to-hip ratio) or the novel risk factors (i.e., UAC ratio, PAP, fibrinogen, homocysteine, and serum creatinine).
§The combined change of the AUROC for the traditional/established and novel risk factors.
Figure 1.
Venn diagram to illustrate the relationship of the change (%) in the AUROC for diabetic retinopathy of the predictive models between traditional and novel risk factors. Circle A: Traditional/established risk factors: 39.2%. Circle B: Novel risk factors: 23.3%. A∩B intersection of circles A and B: Inseparable effect of traditional/established and novel risk factors: 21.1%. Traditional/established risk factors alone: 18.1%. Novel risk factors alone: 2.2%.
CONCLUSIONS
Our study demonstrated an association of plasma fibrinogen, PAP, and homocysteine with diabetic retinopathy; however, only the association of PAP with diabetic retinopathy was independent of the traditional risk factors for diabetic retinopathy, such as duration of diabetes, SBP, A1C, serum creatinine, and UAC ratio. Furthermore, the incremental contribution of these novel risk factors (fibrinogen, PAP, homocysteine, and the UAC ratio) to diabetic retinopathy risk is small. The AUC with traditional risk factors accounted for 36.8% of the variation of diabetic retinopathy, and the addition of novel markers only accounted for an additional 1.4%. Thus, these analyses suggest there is limited clinical use with the addition of these systemic biomarkers for diabetic retinopathy prediction.
We found an association of homocysteine with vision-threatening diabetic retinopathy, but this was attenuated and no longer significant after further adjustment for serum creatinine and UAC ratio. This is consistent with data from some other studies (9–11,16). In studies in which an association between presence of diabetic retinopathy and elevated homocysteine has been reported previously, serum creatinine and UAC ratio were not included in the statistical analyses (7,8,15). Our study therefore suggests that part of the association of homocysteine with diabetic retinopathy was related to concurrent diabetic nephropathy.
Our study found a significant relationship of PAP with any diabetic retinopathy and vision-threatening diabetic retinopathy. Le et al. (17) did find increased PAP with diabetic retinopathy, but the result was no longer significant after multivariable adjustment. The relationship in our study could be a consequence of increased sample size (921 vs. 104) and/or older age of the MESA participants (mean 52.0 vs. 32.0 years). d-Dimer and PAP are markers of fibrinolysis. Procoagulant reactions producing fibrin activate fibrinolysis to produce plasmin, which degrades fibrin to produce d-dimer. PAP is formed by the binding to and inactivation of free plasmin by its inhibitor, α2-antiplasmin; therefore, the PAP level measures recent plasmin production (22). In addition, d-dimer and PAP appear to measure different aspects of fibrinolysis, as their predictive abilities of myocardial infarction and coronary deaths have previously been shown to be independent of each other (23). Similarly, d-dimer, unlike PAP, was not elevated in those with diabetic retinopathy in our study. However, further confirmation of our finding, as well as its significance, is needed.
While inflammation has been considered to be a pathogenic factor in the development and progression of diabetic retinopathy (24), associations of systemic markers of inflammation, such as serum CRP, with diabetic retinopathy have been inconsistently reported in some studies (12,13) but not others (5,6,17). Our study also showed that common systemic markers of inflammation, such as CRP and IL-6, were not associated with diabetic retinopathy. Possible differences in the findings among studies are likely related to differences in type of diabetes, sample sizes, or inadequately controlling for confounding factors. For example, Schram et al. (6) examined 543 subjects with type 1 diabetes but did not control for presence of hypertension and nephropathy, while Van Hecke et al.(5) examined 192 subjects with type 2 diabetes and did not control for presence of hypertension, nephropathy, duration of diabetes, and A1C. In our study, we were able to adjust for not only traditional risk factors for diabetic retinopathy such as duration of diabetes, SBP, and A1C but also serum creatinine, although no distinction was made between type 1 and type 2 diabetes in the MESA. In addition, while there is evidence that inflammatory changes may be involved with the pathogenesis of diabetic retinopathy in the eye, the lack of finding such a relation between systemic markers of inflammation and diabetic retinopathy in our study and other studies may reflect the fact that due to the presence of the retinal-blood barrier, higher levels of inflammatory markers such as IL-6 found in the vitreous in patients with diabetic retinopathy are not seen in the systemic circulation.
In addition, our finding of a lack of association between diabetic retinopathy and fibrinogen levels that was independent of traditional risk factors should be compared with two previous studies (14,25) that have also found no association. Of these studies, one involved 92 subjects with type 2 diabetes (25), while the other studied 909 subjects with type 1 diabetes from the Diabetes Control and Complication Trial/Early Treatment Diabetic Retinopathy Study cohort (14). In a recent study of 104 Pima Indian participants, an association was found between fibrinogen level and diabetic retinopathy (17). However, the participants in this study were younger (mean age 32 years), and the statistical analysis did not account for glycemic control, blood pressure, and serum creatinine.
Analysis by using the AUROC shows that there is an “overlapping” of the effects of the predictive models for diabetic retinopathy using traditional/established and novel risk factors. This illustrates the complex relationship between the factors and diabetic retinopathy. It is conceivable that the novel risk markers can also be the underlying pathological mechanisms of the traditional risk factors of diabetic retinopathy, such as abnormal hemostasis and elevated homocysteinemia may be due to prolonged hyperglycemia (i.e., longer duration of diabetes and poor diabetes and blood pressure control).
The strengths of this study include a large population-based sample and the assessment of diabetic retinopathy by standardized grading protocols. Limitations of this study should also be noted. First, the cross-sectional nature of the study limits ability to judge temporal sequence of associations. Second, the failure to find associations with these novel markers may be due to survival bias. We obtained 45° nonstereoscopic, nonmydriatic photographs to grade diabetic retinopathy, which is less sensitive than grading from seven fields of stereoscopic fundus photographs, and therefore we could have underestimated the proportion with diabetic retinopathy in our study population. Finally, the key to predicting tissue injury in diabetes is testing for the correct molecules, and therefore future knowledge gained from, for example, proteomic expression studies of inflammatory markers may yield new insights.
In conclusion, this study shows that novel systemic biomarkers of inflammation and hemostasis and homocysteine are not consistently or strongly related to diabetic retinopathy independently of established risk factors. These data suggest limited clinical use of these biomarkers for diabetic retinopathy prediction.
Acknowledgments
This research was supported by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute. Additional support was provided by National Institutes of Health (NIH) Grants R01HL69979-03 (to K.R. and W.T.Y.) and the NIH Intramural Program Award Z01EY403 (to M.F.C.).
R.K. is a paid consultant for Pfizer, Eli Lilly and Company, and Astra-Zeneca and has received honoraria from these commercial entities. No other potential conflicts of interest relevant to this article were reported.
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA Investigators and Institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA 2007; 298: 902– 916 [DOI] [PubMed] [Google Scholar]
- 2.Nguyen TT, Wang JJ, Wong TY. Retinal vascular changes in pre-diabetes and prehypertension: new findings and their research and clinical implications. Diabetes Care 2007; 30: 2708– 2715 [DOI] [PubMed] [Google Scholar]
- 3.Nguyen TT, Wong TY. Retinal vascular manifestations of metabolic disorders. Trends Endocrinol Metab 2006; 17: 262– 268 [DOI] [PubMed] [Google Scholar]
- 4.Frank RN. Diabetic retinopathy. N Engl J Med 2004; 350: 48– 58 [DOI] [PubMed] [Google Scholar]
- 5.van Hecke MV, Dekker JM, Nijpels G, Moll AC, Heine RJ, Bouter LM, Polak BC, Stehouwer CD. Inflammation and endothelial dysfunction are associated with retinopathy: the Hoorn Study. Diabetologia 2005; 48: 1300– 1306 [DOI] [PubMed] [Google Scholar]
- 6.Schram MT, Chaturvedi N, Schalkwijk CG, Fuller JH, Stehouwer CD. Markers of inflammation are cross-sectionally associated with microvascular complications and cardiovascular disease in type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetologia 2005; 48: 370– 378 [DOI] [PubMed] [Google Scholar]
- 7.Brazionis L, Rowley K, Sr, Itsiopoulos C, Harper CA, O'Dea K. Homocysteine and diabetic retinopathy. Diabetes Care 2008; 31: 50– 56 [DOI] [PubMed] [Google Scholar]
- 8.Hoogeveen EK, Kostense PJ, Eysink PE, Polak BC, Beks PJ, Jakobs C, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD. Hyperhomocysteinemia is associated with the presence of retinopathy in type 2 diabetes mellitus: the Hoorn Study. Arch Intern Med 2000; 160: 2984– 2990 [DOI] [PubMed] [Google Scholar]
- 9.Looker HC, Fagot-Campagna A, Gunter EW, Pfeiffer CM, Narayan KM, Knowler WC, Hanson RL. Homocysteine as a risk factor for nephropathy and retinopathy in type 2 diabetes. Diabetologia 2003; 46: 766– 772 [DOI] [PubMed] [Google Scholar]
- 10.Smulders YM, Rakic M, Slaats EH, Treskes M, Sijbrands EJ, Odekerken DA, Stehouwer CD, Silberbusch J. Fasting and post-methionine homocysteine levels in NIDDM: determinants and correlations with retinopathy, albuminuria, and cardiovascular disease. Diabetes Care 1999; 22: 125– 132 [DOI] [PubMed] [Google Scholar]
- 11.Van Hecke MV, Dekker JM, Nijpels G, Teerlink T, Jakobs C, Stolk RP, Heine RJ, Bouter LM, Polak BC, Stehouwer CD. Homocysteine, S-adenosylmethionine and S-adenosylhomocysteine are associated with retinal microvascular abnormalities: the Hoorn Study. Clin Sci (Lond) 2008; 114: 479– 487 [DOI] [PubMed] [Google Scholar]
- 12.Spijkerman AM, Gall MA, Tarnow L, Twisk JW, Lauritzen E, Lund-Andersen H, Emeis J, Parving HH, Stehouwer CD. Endothelial dysfunction and low-grade inflammation and the progression of retinopathy in type 2 diabetes. Diabet Med 2007; 24: 969– 976 [DOI] [PubMed] [Google Scholar]
- 13.Izuora KE, Chase HP, Jackson WE, Coll JR, Osberg IM, Gottlieb PA, Rewers MJ, Garg SK. Inflammatory markers and diabetic retinopathy in type 1 diabetes. Diabetes Care 2005; 28: 714– 715 [DOI] [PubMed] [Google Scholar]
- 14.Klein RL, Hunter SJ, Jenkins AJ, Zheng D, Semler AJ, Clore J, Garvey WT. Fibrinogen is a marker for nephropathy and peripheral vascular disease in type 1 diabetes: studies of plasma fibrinogen and fibrinogen gene polymorphism in the DCCT/EDIC cohort. Diabetes Care 2003; 26: 1439– 1448 [DOI] [PubMed] [Google Scholar]
- 15.Chiarelli F, Pomilio M, Mohn A, Tumini S, Vanelli M, Morgese G, Spagnoli A, Verrotti A. Homocysteine levels during fasting and after methionine loading in adolescents with diabetic retinopathy and nephropathy. J Pediatr 2000; 137: 386– 392 [DOI] [PubMed] [Google Scholar]
- 16.Soedamah-Muthu SS, Chaturvedi N, Teerlink T, Idzior-Walus B, Fuller JH, Stehouwer CD. Plasma homocysteine and microvascular and macrovascular complications in type 1 diabetes: a cross-sectional nested case-control study. J Intern Med 2005; 258: 450– 459 [DOI] [PubMed] [Google Scholar]
- 17.Le DS, Miles R, Savage PJ, Cornell E, Tracy RP, Knowler WC, Krakoff J. The association of plasma fibrinogen concentration with diabetic microvascular complications in young adults with early-onset of type 2 diabetes. Diabetes Res Clin Pract 2008; 82: 317– 323 [DOI] [PubMed] [Google Scholar]
- 18.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002; 156: 871– 881 [DOI] [PubMed] [Google Scholar]
- 19.Wong TY, Klein R, Islam FM, Cotch MF, Folsom AR, Klein BE, Sharrett AR, Shea S. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol 2006; 141: 446– 455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diabetic Retinopathy Study Research Group. Design, methods, and baseline results: a modification of the Airlie House classification of diabetic retinopathy (DRS Report #7). Invest Ophthalmol Vis Sci 21: 1b– 226b, 1981 [PubMed] [Google Scholar]
- 21.MESA Coordinating Center. Multi-Ethnic Study of Atherosclerosis Field Center Manual of Operations Seattle, Washington, University of Washington, 2001 [Google Scholar]
- 22.Holvoet P, de Boer A, Verstreken M, Collen D. An enzyme-linked immunosorbent assay (ELISA) for the measurement of plasmin-alpha 2-antiplasmin complex in human plasma–application to the detection of in vivo activation of the fibrinolytic system. Thromb Haemost 1986; 56: 124– 127 [PubMed] [Google Scholar]
- 23.Cushman M, Lemaitre RN, Kuller LH, Psaty BM, Macy EM, Sharrett AR, Tracy RP. Fibrinolytic activation markers predict myocardial infarction in the elderly: the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol 1999; 19: 493– 498 [DOI] [PubMed] [Google Scholar]
- 24.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, Kern TS, Adamis AP. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J 2004; 18: 1450– 1452 [DOI] [PubMed] [Google Scholar]
- 25.Erem C, Hacihasanoglu A, Celik S, Ovali E, Ersoz HO, Ukinc K, Deger O, Telatar M. Coagulation and fibrinolysis parameters in type 2 diabetic patients with and without diabetic vascular complications. Med Princ Pract 2005; 14: 22– 30 [DOI] [PubMed] [Google Scholar]