Abstract
OBJECTIVE
We studied the incidence of dysglycemia and its prediction of the development of type 1 diabetes in islet cell autoantibody (ICA)-positive individuals. In addition, we assessed whether dysglycemia was sustained.
RESEARCH DESIGN AND METHODS
Participants (n = 515) in the Diabetes Prevention Trial–Type 1 (DPT-1) with normal glucose tolerance who underwent periodic oral glucose tolerance tests (OGTTs) were followed for incident dysglycemia (impaired fasting glucose, impaired glucose tolerance, and/or high glucose levels at intermediate time points of OGTTs). Incident dysglycemia at the 6-month visit was assessed for type 1 diabetes prediction.
RESULTS
Of 515 participants with a normal baseline OGTT, 310 (60%) had at least one episode of dysglycemia over a maximum follow-up of 7 years. Dysglycemia at the 6-month visit was highly predictive of the development of type 1 diabetes, both in those aged <13 years (P < 0.001) and those aged ≥13 years (P < 0.01). Those aged <13 years with dysglycemia at the 6-month visit had a high cumulative incidence (94% estimate by 5 years). Among those who developed type 1 diabetes after a dysglycemic OGTT and who had at least two OGTTs after the dysglycemic OGTT, 33 of 64 (52%) reverted back to a normal OGTT. However, 26 (79%) of the 33 then had another dysglycemic OGTT before diagnosis.
CONCLUSIONS
ICA-positive individuals with normal glucose tolerance had a high incidence of dysglycemia. Incident dysglycemia in those who are ICA positive is strongly predictive of type 1 diabetes. Children with incident dysglycemia have an especially high risk. Fluctuations in and out of the dysglycemic state are not uncommon before the onset of type 1 diabetes.
There is increasing evidence that impaired glucose tolerance (IGT) is a predictor and common precursor of type 1 diabetes (1–3). Still, little is known about the incidence of IGT and other forms of dysglycemia in individuals who have pancreatic autoantibodies and normal glucose tolerance. In addition, there is no information about the risk of type 1 diabetes when dysglycemia occurs in those individuals. Moreover, it is not known whether dysglycemia is sustained once it occurs.
We used data from the Diabetes Prevention Trial–Type 1 (DPT-1) (4,5) to examine these questions. In addition to IGT, impaired fasting glucose (IFG) and high glucose values at intermediate times (between fasting and 2 h) during oral glucose tolerance tests (OGTTs), termed indeterminate glycemia (INDET), were included as other forms of dysglycemia in the analyses. Glucose levels at intermediate times have been shown to be predictive of type 1 diabetes (6,7).
Information regarding the incidence of these various forms of dysglycemia and their prediction of type 1 diabetes should be helpful for understanding the pathogenesis and natural history of type 1 diabetes. Such information should also be useful for improving type 1 diabetes prevention trials.
RESEARCH DESIGN AND METHODS
There were a total of 711 participants in the parenteral (n = 339) and oral (n = 372) insulin DPT-1 trials. All were islet cell autoantibody (ICA)-positive relatives of type 1 diabetic patients. Greater than a 50% 5-year risk of developing type 1 diabetes was required for eligibility for the parenteral insulin trial. Individuals were deemed to have a >50% 5-year risk if the first-phase insulin response (FPIR) on an intravenous glucose tolerance test was below a defined threshold and/or there were OGTT abnormalities (IFG, INDET, or IGT). Those without metabolic criteria but positive for insulin autoantibodies were considered to have a 26–50% 5-year risk and were eligible for the oral insulin trial. There was no overall treatment effect in either trial. The analyses included 515 participants in the parenteral (n = 168) and oral trials (n = 347). All had normal OGTTs before trial entry, at least one nondiabetic OGTT after randomization, and no missing values (n = 6). Individuals excluded were somewhat older (15.4 ± 10.6 vs. 13.3 ± 9.1 years, P < 0.01). There was almost no difference in sex (excluded: 58% male; included: 56% male).
Procedures
Participants in the parenteral insulin trial intervention group received recombinant human ultralente insulin, whereas those in the oral insulin trial intervention group received recombinant human insulin crystals. OGTTs were performed at 6-month (±3 months) intervals in both trials. The dose of oral glucose was 1.75 g/kg (maximum, 75 g carbohydrate). Blood samples were obtained for plasma glucose in the fasting state and at 30, 60, 90, and 120 min. In most individuals, type 1 diabetes was diagnosed at routine visits. Those with OGTTs in the diabetic range were asked to return for confirmation by another OGTT unless this was clinically contraindicated. If the second OGTT was not confirmatory, participants continued to be followed at 6-month intervals.
Laboratory measures
Methodologies for assessing autoantibody positivity in DPT-1 have been described previously (8). ICAs were determined by indirect immunofluorescence, and insulin autoantibodies were measured by a competitive fluid-phase radioassay. Plasma glucose levels were measured by the glucose oxidase method. Insulin was measured by radioimmunoassay.
Data analysis
The t test and χ2 test were used for simple comparisons, and the log-rank test was used to compare the distributions of event times between groups. The Cox proportional hazards regression model was used for assessing type 1 diabetes associations over time. Kaplan-Meier curves were used to obtain cumulative incidence estimates of type 1 diabetes over time. Incident dysglycemia was defined as the first dysglycemic OGTT that occurred.
Glucose tolerance abnormalities were defined as follows: IFG, fasting glucose value of 100–125 mg/dl; INDET, 30-, 60-, and/or 90-min glucose value ≥200 mg/dl; and IGT, 2-h glucose value 140–199 mg/dl. The thresholds for diabetes were a fasting glucose value ≥126 mg/dl and/or a 2-h glucose value ≥200 mg/dl. Unconfirmed OGTTs in the diabetic range were excluded (n = 81 [2.8%] of all OGTTs performed during follow-up) from the analysis. The FPIR was defined as the sum of insulin levels at the 1st and 3rd min of the intravenous glucose tolerance test. An FPIR less than the 10th percentile according to age norms was considered below threshold. Of those analyzed, 35% were below this threshold.
SAS (version 9.1.3) was used for the analyses. All P values are two-sided.
RESULTS
Of the 515 DPT-1 participants with normal glucose tolerance at baseline who were studied, 56% were male. The mean ± SD age at baseline was 13.3 ± 9.1 years.
Over a maximum follow-up of 7.0 years (mean ± SD 2.3 ± 1.6 years), dysglycemia occurred in 310 (60%) of the 515 participants, with 2- and 5-year estimates of ∼41 and ∼73%. In proportional hazards models, there were no associations of dysglycemia with either age or sex. Dysglycemia occurred in 199 of 330 (60%) of those aged <13 years, with 2- and 5-year estimates of 44 and 73%, respectively. Of those aged ≥13 years, 111 of 185 (60%) developed dysglycemia, with 2- and 5-year estimates of 36 and 72%, respectively. In a proportional hazards model, there was no association between incident dysglycemia and an FPIR below threshold.
Distributions of the specific abnormalities for the first occurrence of dysglycemia are shown in Table 1, overall and according to <13 and ≥13 years age categories. Overall, IGT alone (43%) occurred much more frequently than either IFG alone (16%) or INDET alone (17%). Sixty-five percent of the participants developed IGT alone or in combination. There were no significant differences in the proportions of IFG, INDET, and IGT between those aged <13 and ≥13 years. Whereas the proportions of IFG and INDET were similar between female and male participants (IFG 24 vs. 25% and INDET 34 vs. 41%, respectively), the proportion with IGT was higher in female participants (75 vs. 58%, P < 0.01).
Table 1.
Distribution of glucose tolerance abnormalities for the first occurrence of dysglycemia among participants
| All | <13 years | ≥13 years | |
|---|---|---|---|
| IFG alone | 50 (16.1) | 27 (13.6) | 23 (20.7) |
| INDET alone | 51 (16.5) | 33 (16.6) | 18 (16.2) |
| IGT alone | 133 (42.9) | 87 (43.7) | 46 (41.4) |
| IFG and INDET | 7 (1.9) | 5 (2.5) | 2 (1.7) |
| IFG and IGT | 9 (2.9) | 8 (4.0) | 1 (0.9) |
| IGT and INDET | 49 (16.1) | 35 (17.6) | 14 (12.6) |
| IFG, IGT, and INDET | 11 (3.5) | 4 (2.0) | 7 (6.3) |
| Total | 310 | 199 | 111 |
Data are n (%).
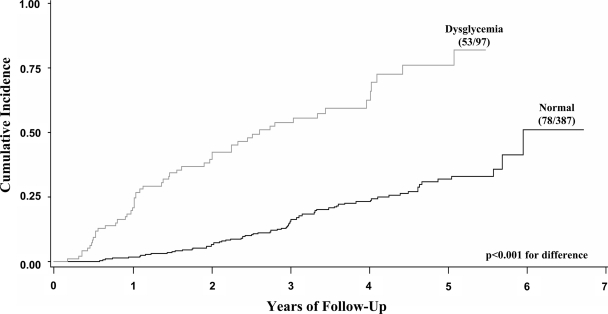
The risk for the development of subsequent type 1 diabetes after the occurrence of dysglycemia was examined among those individuals (n = 484) who had a nondiabetic OGTT at the 6-month visit (6 ± 3 months). Over a maximum follow-up of 6.7 years (2.9 ± 1.6 years) from the 6-month visit, 131 (27%) developed type 1 diabetes. In proportional hazards models (Table 2), those with a dysglycemic OGTT at the 6-month visit had a much greater risk for the subsequent development of type 1 diabetes than those with a normal OGTT (53 of 97 vs. 78 of 387; P < 0.001). When the data were stratified according to age <13 and ≥13 years, the association between type 1 diabetes and dysglycemia at 6 months was apparent in both groups (P < 0.001 for age <13 years and P < 0.01 for age ≥13 years). The increased progression to type 1 diabetes among those with dysglycemia at the 6-month visit is evident in the cumulative incidence curves in Fig. 1. When IGT was used as a marker, 40 of 63 (63%) developed type 1 diabetes. The 4-year estimate for IGT was 72%, whereas that for dysglycemia was 65%.
Table 2.
Prediction of type 2 diabetes according to the presence of incident dysglycemia at the 6-month visit
| Age | n | Type 1 diabetes/total |
HR (95% CI)* | |
|---|---|---|---|---|
| Dysglycemic | Normal | |||
| All | 484 | 53/97 | 78/387 | 5.2 (3.7–7.5)† |
| <13 years | 312 | 44/67 | 60/245 | 5.4 (3.6–8.1)† |
| ≥13 years | 172 | 9/30 | 18/142 | 4.1 (1.8–9.3)‡ |
*With an adjustment for age.
†P < 0.001;
‡P < 0.01.
Figure 1.
Shown are cumulative incidence curves for the subsequent development of type 1 diabetes according to whether dysglycemia occurred at the 6-month visit. The actual proportion of those developing type 1 diabetes is shown for each curve. The cumulative incidence was significantly greater when dysglycemia occurred at the 6-month visit.
Among those who also had a normal OGTT at 6 months, there was still a strong association between type 1 diabetes and dysglycemia occurring at 1 year (19 of 43 vs. 48 of 291; P < 0.001), even with the shorter follow-up (maximum: 5.5 years). Sex was not predictive of type 1 diabetes either at 6 months or at 1 year.
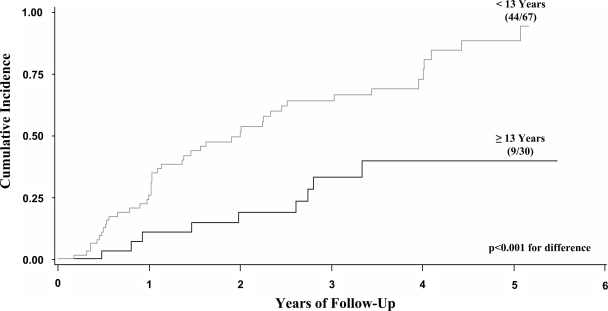
In an analysis limited to only those individuals with dysglycemia at 6 months, type 1 diabetes was inversely related to age (P < 0.001) in a proportional hazards model. This finding is evident in Fig. 2, in which the risk estimate for type 1 diabetes was much higher for those aged <13 years (94 vs. 40% by 5 years). The hazard ratio (HR) was 3.3 (95% CI 1.6–6.7; P = 0.001). There were 24 participants aged <13 years with incident dysglycemia at 6 months that persisted at 1 year. Of these, 18 developed type 1 diabetes (75%) with a maximum follow-up of 4.5 years. Of the 15 children aged <13 years with incident IGT that persisted at 1 year, 14 (93%) developed type 1 diabetes.
Figure 2.
Shown are cumulative incidence curves for the development of type 1 diabetes according to whether participants were aged <13 or ≥13 years among those who were dysglycemic at the 6-month visit. The cumulative incidence was significantly higher in the younger age-group, with an estimate of 94% by 5 years in those children.
Among the 515 individuals studied, 136 developed type 1 diabetes. Of the 136, 78 (57%) had a minimum of three visits before diagnosis. Of those 78, 74 (95%) had at least one dysglycemic OGTT. Among the 275 participants with a minimum of three visits who did not develop type 1 diabetes, 152 (55%) had at least one dysglycemic OGTT.
The occurrence of a single glucose tolerance abnormality at 6 months was assessed for the prediction of type 1 diabetes. Type 1 diabetes did not occur significantly more frequently in individuals with IFG (5 of 15; P = 0.216) when they were compared with those who had a normal OGTT at the 6-month visit (78 of 387). However, in a proportional hazards model with age included as a covariate, IFG was predictive (P = 0.009). Type 1 diabetes occurred significantly more frequently in individuals with INDET alone (8 of 17; P = 0.008) or with IGT alone (19 of 36; P < 0.001) at the 6-month visit when they were compared with those with normal OGTTs. In proportional hazards models with age as a covariate, the associations persisted (P = 0.002 for INDET and P < 0.001 for IGT).
To assess whether dysglycemia was sustained once it occurred, we studied 64 participants who developed type 1 diabetes after dysglycemia had occurred and who had at least two OGTTs after the dysglycemic OGTT. Of these, 33 (52%) reverted back to a normal OGTT. However, 26 of the 33 (79%) then had another dysglycemic OGTT before diagnosis.
CONCLUSIONS
This study is unique in that it examined the occurrence of dysglycemia in autoantibody-positive individuals who had preexisting normal glucose tolerance. The incidence of dysglycemia was very high in both the younger and older age-groups. The distribution of the forms of dysglycemia was similar between the age-groups, and IGT was the most common type. The data also showed that incident dysglycemia was strongly predictive of type 1 diabetes, in both younger and older individuals.
There is no prior information available regarding the incidence of dysglycemia and its prediction of type 1 diabetes in autoantibody-positive individuals with antecedent normal glucose tolerance. However, IGT has been found to be a predictor and precursor of type 1 diabetes (1–3). In addition, fasting glucose levels and glucose levels at various OGTT time points have been found to be predictive of type 1 diabetes (7,9).
The data in this report indicate that among autoantibody-positive individuals there is a high likelihood of dysglycemia occurring at some point before the onset of type 1 diabetes. The occurrence of at least one episode of dysglycemia in those who developed type 1 diabetes was very high. Although the occurrence of dysglycemia was much lower among those who did not develop type 1 diabetes, it was still substantial. This finding suggests that some of the latter could have developed type 1 diabetes with more extended follow-up.
IGT occurring alone appears to be highly predictive of type 1 diabetes. Although the extent to which either IFG or INDET occurring alone predicts type 1 diabetes is difficult to gauge because of the small numbers, each of those dysglycemia abnormalities occurring singly at 6 months was predictive of type 1 diabetes with age as a covariate. The data suggest that INDET can indeed be used as a predictor of type 1 diabetes in addition to the more traditional indicators of dysglycemia.
The risk for type 1 diabetes at 4 years was somewhat higher for IGT than for dysglycemia. However, the number who developed dysglycemia at 6 months was much greater (97 vs. 63). In choosing criteria for entry into prevention trials, both of these findings need to be taken into account. It appears that the ultimate decision for the criteria to be used rests on the nature of the specific prevention trial.
In the overall analyses there was a lack of influence of age on the occurrence of dysglycemia. However, among those with dysglycemia, age was a strong predictor of type 1 diabetes. There is no previous information regarding these specific associations. Although the cutoff at age 13 was arbitrary, it served to demonstrate the influence of age.
The data indicate that even among those who ultimately develop type 1 diabetes, dysglycemia is not necessarily sustained. Moreover, it appears that even after glucose levels normalize, dysglycemia tends to recur before the diagnosis of diabetes. This finding suggests that there are fluctuations at an undetermined frequency between the normal and dysglycemic states before the onset of type 1 diabetes. The recurrence of dysglycemia suggests the possibility that dysglycemia could have occurred before study entry in some individuals.
Because this study was based on a population of ICA-positive relatives, some selected on the basis of an FPIR below threshold and some selected on the basis of insulin autoantibody positivity, the findings may not necessarily fully generalize to other populations. In addition, there was limited information regarding IFG and INDET occurring alone.
The findings in this report have significant implications with regard to increasing the efficiency of prevention trials for type 1 diabetes. Because the data show that dysglycemia will occur in an appreciable percentage of autoantibody-positive individuals whose initial screening is negative for dysglycemia, repeating OGTTs in those individuals should increase the yield of potential high-risk participants. Moreover, because children aged <13 years with incident dysglycemia have a very high risk for type 1 diabetes (94% 5-year estimate despite the variability of dysglycemia), dysglycemia could possibly be used as an early indicator of efficacy in prevention trials for those with normal glucose tolerance at baseline.
The pathogenetic development of type 1 diabetes appears to be an ongoing process (10,11) with an initial immunologic insult to β-cells followed by progressive metabolic deterioration before and after diagnosis (12–15). Therefore, from both clinical and research perspectives, it may be advantageous to identify individuals as early as possible in this process. The very high likelihood that autoantibody-positive children will develop type 1 diabetes within 5 years after the occurrence of dysglycemia suggests that the earlier identification of the disease is a distinct possibility.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Rosenbloom AL, Hunt SS, Rosenbloom EK, Maclaren NK: Ten-year prognosis of impaired glucose tolerance in siblings of patients with insulin-dependent diabetes. Diabetes 1982; 31: 385– 387 [DOI] [PubMed] [Google Scholar]
- 2.Beer SF, Heaton DA, Alberti KGMM, Pyke A, Leslie RDG: Impaired glucose tolerance precedes but does not predict insulin-dependent diabetes mellitus: a study of identical twins. Diabetologia 1990; 33: 497– 502 [DOI] [PubMed] [Google Scholar]
- 3.Sosenko J, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS: Diabetes Prevention Trial-Type 1 Study Group. Patterns of metabolic progression to type 1 diabetes in the diabetes prevention trial-type 1. Diabetes Care 2006; 29: 643– 649 [DOI] [PubMed] [Google Scholar]
- 4.Diabetes Prevention Trial-Type 1 Diabetes Study Group Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002; 346: 1685– 1691 [DOI] [PubMed] [Google Scholar]
- 5.Diabetes Prevention Trial-Type 1 Diabetes Study Group Effects of oral insulin in relatives of patients with type 1 diabetes. Diabetes Care 2005; 28: 1068– 1076 [DOI] [PubMed] [Google Scholar]
- 6.Barker JM, McFann K, Harrison LC, Fourlanos S, Krischer J, Cuthbertson D, Chase HP, Eisenbarth GS: DPT-1 Study Group Pre-type 1 diabetes dysmetabolism: maximal sensitivity achieved with both oral and intravenous glucose tolerance testing. J Pediatr 2007; 150: 31– 36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sosenko J, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS: Diabetes Prevention Trial-Type 1 Study Group Increasing the accuracy of oral glucose tolerance testing and extending its application to individuals with normal glucose tolerance for the prediction of type 1 diabetes. Diabetes Care 2007; 30: 38– 42 [DOI] [PubMed] [Google Scholar]
- 8.Krischer JP, Cuthbertson DD, Yu L, Orban T, Maclaren N, Jackson R, Winter WE, Schatz DA, Palmer JP, Eisenbarth GS: Screening strategies for the identification of multiple antibody-positive relatives of individuals with type 1 diabetes. J Clin Endocrinol Metab 2003; 88: 103– 108 [DOI] [PubMed] [Google Scholar]
- 9.Bingley PJ, Gale EAM: European Nicotinamide Diabetes Intervention Trial (ENDIT) Group. Progression to type 1 diabetes in islet cell antibody-positive relatives in the European Nicotinamide Diabetes Intervention Trial: the role of additional immune, genetic and metabolic markers of risk. Diabetologia 2006; 49: 881– 890 [DOI] [PubMed] [Google Scholar]
- 10.Skyler JS: Prediction and prevention of type 1 diabetes. Clin Pharmacol Ther 2007; 81: 768– 771 [DOI] [PubMed] [Google Scholar]
- 11.Eisenbarth GS: Update in type 1 diabetes. J Clin Endocrinol Metab 2007; 92: 2403– 2407 [DOI] [PubMed] [Google Scholar]
- 12.Snorgaard O, Lassen LH, Binder C: Homogeneity in pattern of decline of β-cell function in IDDM: prospective study of 204 consecutive cases followed for 7.4 yr. Diabetes Care 1992; 15: 1009– 1013 [DOI] [PubMed] [Google Scholar]
- 13.Steele C, Hagopian WA, Gitelman S, Masharani U, Cavaghan M, Rother KI, Donaldson D, Harlan DM, Bluestone J, Herold KC: Insulin secretion in type 1 diabetes. Diabetes 2004; 53: 426– 433 [DOI] [PubMed] [Google Scholar]
- 14.Sherry NA, Tsai EB, Herold KC: Natural history of β-cell function in type 1 diabetes. Diabetes 2005; 54( Suppl. 2): S32– S39 [DOI] [PubMed] [Google Scholar]
- 15.Sosenko JM, Palmer JP, Rafkin-Mervis L, Krischer JP, Cuthbertson D, Matheson D, Skyler JS: Glucose and C-peptide changes in the peri-onset period of type 1 diabetes in the diabetes prevention trial type 1. Diabetes Care 2008; 31: 2188– 2192 [DOI] [PMC free article] [PubMed] [Google Scholar]