Abstract
OBJECTIVE
The antidiabetic properties of metformin are mediated through its ability to activate the AMP-activated protein kinase (AMPK). Activation of AMPK can suppress tumor formation and inhibit cell growth in addition to lowering blood glucose levels. We tested the hypothesis that metformin reduces the risk of cancer in people with type 2 diabetes.
RESEARCH DESIGN AND METHODS
In an observational cohort study using record-linkage databases and based in Tayside, Scotland, U.K., we identified people with type 2 diabetes who were new users of metformin in 1994–2003. We also identified a set of diabetic comparators, individually matched to the metformin users by year of diabetes diagnosis, who had never used metformin. In a survival analysis we calculated hazard ratios for diagnosis of cancer, adjusted for baseline characteristics of the two groups using Cox regression.
RESULTS
Cancer was diagnosed among 7.3% of 4,085 metformin users compared with 11.6% of 4,085 comparators, with median times to cancer of 3.5 and 2.6 years, respectively (P < 0.001). The unadjusted hazard ratio (95% CI) for cancer was 0.46 (0.40–0.53). After adjusting for sex, age, BMI, A1C, deprivation, smoking, and other drug use, there was still a significantly reduced risk of cancer associated with metformin: 0.63 (0.53–0.75).
CONCLUSIONS
These results suggest that metformin use may be associated with a reduced risk of cancer. A randomized trial is needed to assess whether metformin is protective in a population at high risk for cancer.
Recent research suggests that the antidiabetic drug metformin, which exerts its effects by activating the AMP-activated protein kinase (AMPK), may have potential for the treatment of cancer in humans (1). The hypothesis that metformin may have anticancer effects is supported by laboratory studies showing that metformin is associated with reduced incidence of pancreatic cancer in hamsters (2) and delays onset of mammary (3) and other tumors (4) in tumor-prone mice. Metformin also inhibits growth of human breast cancer cells (5). Although the potential for prevention of cancer in humans using metformin has not been explored, we previously reported the results of a pilot case-control study that identified a reduced risk of cancer among patients with type 2 diabetes who had used metformin (6). However, the outcome was limited to hospital admissions for cancer, and the date of diagnosis was assumed to be date of first hospital admission.
Other diabetic drugs may also have cancer-related effects. An independent epidemiological study found that users of sulfonylureas were at higher risk of cancer-related mortality than metformin users (7). Sulfonylureas (and insulin) increase circulating insulin levels, and hyperinsulinemia may promote carcinogenesis (8). Treatments such as metformin and glitazones reduce insulin resistance, with insulin resistance possibly associated with increased risk of cancer (9). The objective of this study was to test the hypothesis that metformin use is associated with a reduced risk of cancer in people with type 2 diabetes using a national cancer registry to ensure valid diagnoses of cancer with precise dates of diagnosis. We also adjusted results for the effects of exposure to other diabetic drugs.
RESEARCH DESIGN AND METHODS
This observational historical cohort study was carried out using anonymous patient data for the resident population of Tayside Health Board in Scotland, U.K. (∼400,000 people). Data were provided by the Health Informatics Centre (HIC), University of Dundee, which has developed the record linkage of multiple routinely collected datasets for research. Scottish Care Information–Diabetes Collaboration (SCI-DC; formerly known as the Diabetes Audit and Research in Tayside Scotland [DARTS]) is a validated population-based diabetes register with detailed clinical information (10). A pharmacoepidemiological database (formerly known as the Tayside Medicines Monitoring Unit [MEMO] drug safety database) holds computerized records of every diabetic drug dispensed to residents of Tayside, Scotland, U.K., since 1993 (11). Scottish Morbidity Record 6 (SMR6) is a national database of all diagnoses of cancer (12). Computerized death certification records from the Registrar General with ICD9/ICD10-coded causes of death (13,14) were also available. All HIC data are anonymized prior to analysis to maintain confidentiality and conform to data protection legislation.
The DARTS database was used to identify patients who were diagnosed with type 2 diabetes in Tayside, Scotland, U.K., before 2004 (patients diagnosed over the age of 35 years and younger patients with no insulin requirement are classified as having type 2 diabetes). We identified patients who received a first metformin prescription any time between 1 January 1994 and 31 December 2003 (excluding patients who received metformin in 1993 or before diabetes diagnosis). We classified these patients as metformin users and defined the index date as date of first metformin prescription. Patients who had a record of cancer in SMR6 at any time between 1980 and the index date were excluded.
Comparator patients individually matched to the metformin users were generated at random from a pool of patients with type 2 diabetes who had no record of metformin use. We used a computer algorithm that identified comparators for each metformin user (listed in random order). For each metformin user, a potential comparator was identified with the same year of diagnosis and assigned the same index date. Year of diagnosis was chosen as a matching variable to control for effects of treatment patterns that could vary over time but not be measured directly. However, if there was a record of cancer in SMR6 prior to the index date, or if they had died, the comparator was discarded (but was potentially available for a different metformin user). This process was repeated until suitable comparators were identified. The comparators who were identified for metformin users were therefore diagnosed with diabetes in the same year and survived until the index date without cancer (the date that the corresponding metformin user started metformin). Any potential survival bias (metformin users surviving to go on to metformin) was thus eliminated.
Baseline data were collated for all metformin users and comparators: age at index date, age at diagnosis of diabetes, sex, smoking status, mean BMI and A1C during the study period, and use of sulfonylureas or insulin within 3 months or 1 year of the index date, respectively. An area-based measure of material deprivation (Carstairs score [15]) based on four variables from the national census (car ownership, unemployment, overcrowding, and head-of-household job classification) was also used.
Main outcome measures
We followed-up all patients from the index dates for predefined outcomes. The primary outcome was diagnosis of cancer (as recorded in SMR6). Time to outcome was defined as the period from index date to 1) date of diagnosis of cancer in SMR6, 2) date of death if no cancer diagnosis, or 3) end of the study (31 December 2003) if no cancer diagnosis.
Secondary outcomes
We evaluated the risks of the following secondary outcomes: diagnosis with bowel cancer (ICD9 153–154, ICD10 C18–C20), lung cancer (ICD9 162, ICD10 C33–C34), or breast cancer in women (ICD9 174, ICD10 C50) as well as all-cause mortality and mortality from cancer (any mention on death certificate).
Statistical methods
Time from index date to outcome in the cohorts was shown using Kaplan-Meier plots. The proportional hazards assumption was examined using log (−log) survival plots, with parallel lines indicating that the assumption was reasonable. The relationship between metformin use and diagnosis of cancer was assessed in an unadjusted Cox regression analysis and then in a multivariable model adjusted for age at index date, sex, smoking status, deprivation, mean BMI and A1C during the study period, and use of sulfonylureas and insulin. These were all treated as categorical variables (as defined in Table 1) with the exception of A1C and BMI, which were treated as continuous variables. The analyses were stratified by year of diagnosis (the matching variable).
Table 1.
Baseline characteristics and results of Cox regression analysis for incidence of cancer among metformin users and comparators
| Metformin users | Comparators | Diagnosed cancer | Unadjusted | Adjusted* | |
|---|---|---|---|---|---|
| Comparators | 4,085 | — | 474 | 1.00 | 1.00 |
| Metformin users | — | 4,085 | 297 | 0.46 (0.40–0.53) | 0.63 (0.53–0.75) |
| Sex | |||||
| Female | 1,848 (45.9) | 1,875 (45.2) | 315 | 1.00 | 1.00 |
| Male | 2,237 (54.1) | 2,210 (54.8) | 456 | 1.26 (1.09–1.45) | 1.37 (1.18–1.59) |
| Age (years) | |||||
| 35–55 | 1,001 (24.5) | 533 (13.1) | 51 | 1.00 | 1.00 |
| 56–63 | 964 (23.6) | 647 (15.8) | 138 | 2.72 (1.97–3.75) | 2.66 (1.92–3.68) |
| 64–69 | 865 (21.2) | 691 (16.9) | 160 | 3.40 (2.48–4.67) | 3.13 (2.27–4.32) |
| 70–76 | 781 (19.1) | 939 (23.0) | 205 | 4.61 (3.39–6.28) | 4.09 (2.98–5.61) |
| 77–100 | 474 (11.6) | 1,275 (31.2) | 217 | 5.95 (4.37–8.12) | 4.86 (3.51–6.73) |
| Smoking status | |||||
| Current | 577 (14.1) | 558 (13.7) | 109 | 1.00 | 1.00 |
| Ex-smoker | 1,015 (24.9) | 866 (21.2) | 177 | 0.99 (0.78–1.26) | 0.77 (0.60–0.98) |
| Never | 1,637 (40.1) | 1,411 (34.5) | 271 | 0.87 (0.69–1.09) | 0.75 (0.60–0.94) |
| Not known | 856 (21.0) | 1,250 (30.6) | 214 | 1.19 (0.94–1.51) | 0.91 (0.72–1.16) |
| Carstairs deprivation category | |||||
| 1 (least deprived) | 224 (5.5) | 206 (5.0) | 46 | 1.00 | 1.00 |
| 2 | 805 (19.7) | 902 (22.1) | 162 | 0.75 (0.56–1.01) | 0.74 (0.55–0.99) |
| 3 | 1,129 (27.6) | 1,171 (28.7) | 223 | 0.80 (0.61–1.07) | 0.82 (0.62–1.09) |
| 4 | 458 (11.2) | 488 (12.0) | 99 | 0.78 (0.56–1.08) | 0.81 (0.58–1.12) |
| 5 | 521 (12.8) | 457 (11.2) | 77 | 0.66 (0.47–0.92) | 0.69 (0.49–0.97) |
| 6 | 603 (14.8) | 579 (14.2) | 106 | 0.71 (0.52–0.98) | 0.79 (0.58–1.09) |
| 7 (most deprived) | 345 (8.5) | 282 (6.9) | 58 | 0.72 (0.50–1.04) | 0.83 (0.58–1.19) |
| BMI | 30.7 ± 3.5 | 28.6 ± 3.1 | — | 0.93 (0.91–0.95) | 0.98 (0.96–1.00) |
| A1C (%) | 7.9 ± 1.0 | 7.2 ± 1.2 | — | 0.77 (0.72–0.82) | 0.91 (0.84–0.98) |
| Insulin use | |||||
| No use | 3,833 (93.8) | 3,512 (86.0) | 696 | 1.00 | 1.00 |
| Use within 1 year | 252 (6.2) | 573 (14.0) | 75 | 0.99 (0.85–1.15) | 1.13 (0.97–1.33) |
| Sulphonylurea use | |||||
| No use | 2,196 (53.8) | 1,996 (73.3) | 483 | 1.00 | 1.00 |
| Use within 3 months | 1,889 (46.2) | 1,089 (26.7) | 288 | 1.00 (0.78–1.28) | 1.12 (0.87–1.47) |
Data are n, n (%), means ± SD, or HR (95% CI).
*Adjusted for all covariates.
Dose-response analyses
For each metformin user, we identified the maximum metformin dose prescribed during follow-up. We then categorized these as low dose (<50% of maximum prescribable dose), medium dose (50–80% of maximum prescribable dose), and high dose (>80%). The risks associated with each dose level were determined in a stratified analysis with adjustments for all covariates. However, in case of confounding by duration of follow-up, we further stratified patients according to length of time in the study.
Ethical approval was obtained from the multicenter research and ethics committee for Scotland.
RESULTS
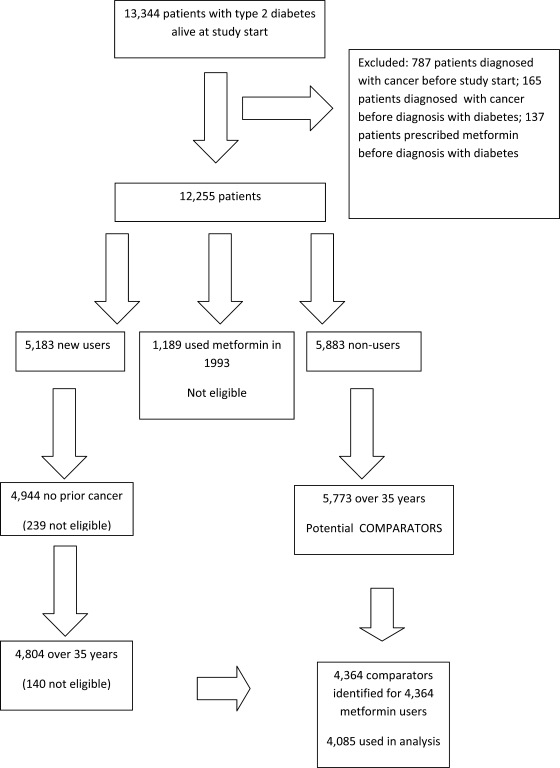
There were 13,344 patients alive in January 1993 who were diagnosed with type 2 diabetes in Tayside, Scotland, U.K., before 2004, of which 12,255 were eligible for the study. We identified 5,183 patients who received a first prescription for metformin after 1994 and selected 4,944 for whom there was no cancer diagnosis prior to metformin use. The remaining patients were 5,883 patients who received no metformin prescriptions between 1993 and 2004 and 1,189 patients who received a prescription for metformin in 1993. These latter patients were excluded as the date of starting metformin was unknown (no prescribing data available prior to 1993). Figure 1 shows how patients were selected for the study.
Figure 1.
Flowchart showing how metformin users and comparators were selected for the study.
Metformin users and comparators
The computer algorithm was used to identify comparators for the 4,804 metformin users who were aged ≥35 years at their index date. The comparators were identified from the 5,773 patients who were aged ≥35 years and had no record of metformin use. A comparator was successfully identified for 4,364 users (the remaining 440 metformin users for whom comparators could not be found were excluded). However, all further analyses were restricted to the 4,085 metformin users who had more than one prescription for metformin and their respective comparators.
The baseline characteristics of the two groups are presented in Table 1. Metformin users were younger than their comparators and slightly more likely to be current smokers (although smoking status was unavailable for about one-quarter of study subjects). BMI and A1C values were also unavailable for 3 and 9% of study subjects, respectively, who were therefore assigned the mean values. Metformin users had higher mean values of BMI and A1C. There was a much higher proportion of metformin users who were treated with sulfonylureas within 3 months but a lower proportion who used insulin within a year. These differences were all statistically significant.
Main outcome measures
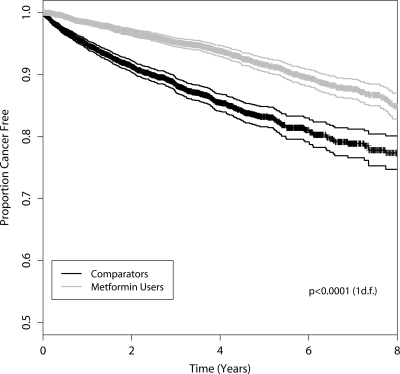
Cancer was diagnosed among 297 (7.3%) of the metformin users during follow-up, compared with 474 (11.6%) of the comparators. Median time to cancer was 3.5 years (interquartile range [IQR] 2.1–5.8) for metformin users, compared with 2.6 years (1.2–4.1) for comparators. Figure 2 shows the Kaplan-Meier plot for diagnosis of cancer (log-rank test P < 0.001). The proportional hazards assumption was met for all study subjects.
Figure 2.
Kaplan-Meier plot with 95% CIs showing time to cancer among metformin users and comparators.
The unadjusted Cox regression analysis showed a statistically significant reduction in the risk of cancer in the new metformin users with a hazard ratio (HR) (95% CI) 0.46 (0.40–0.53) (Table 1). Increased cancer risk was observed among men and with increasing age. It also appeared that higher BMI and A1C were associated with a reduced risk of cancer. This is difficult to explain but may be a diagnostic bias, with high BMI and A1C indicative of less frequent health care–seeking behavior.
In the multivariable analysis, the adjusted HR (95% CI) increased to 0.63 (0.53–0.75) for metformin users. Never and ex-smokers were at reduced risk of cancer, but the reduced risks associated with increased BMI and A1C were less marked. No statistically significant effects were observed for use of sulfonylureas or insulin.
Secondary outcomes
The unadjusted and adjusted risks of the secondary outcomes are presented in Table 2. Metformin users were at much lower risk of overall mortality and cancer-related mortality than their comparators. Overall, 14.9% of metformin users died, compared with 34.8% of the comparators. The median survival times were 3.6 years (IQR 2.2–5.9) and 2.8 years (1.4–4.3), respectively. Additionally, 3.0% of metformin users died from cancer, compared with 6.1% of comparators. Reduced risks of magnitude similar to that for all cancers were observed for bowel cancer, lung cancer, and breast cancer, although the results were not all statistically significant.
Table 2.
Unadjusted and adjusted HRs for secondary outcomes with adjusted HRs for incidence of cancer stratified by maximum prescribed dose and duration of follow-up with comparators as the reference category
| n (%) | Unadjusted | Adjusted* | |
|---|---|---|---|
| Incidence of bowel cancer | |||
| Comparators | 76 (1.9) | 1.00 | 1.00 |
| Users | 40 (1.0) | 0.41 (0.28–0.61) | 0.60 (0.38–0.94) |
| Incidence of lung cancer | |||
| Comparators | 58 (1.4) | 1.00 | 1.00 |
| Users | 35 (0.9) | 0.49 (0.32–0.74) | 0.70 (0.43–1.15) |
| Incidence of breast cancer in women | |||
| Comparators | 41 (2.2) | 1.00 | 1.00 |
| Users | 24 (1.3) | 0.44 (0.26–0.73) | 0.60 (0.32–1.10) |
| Overall mortality | |||
| Comparators | 1,422 (34.8) | 1.00 | 1.00 |
| Users | 609 (14.9) | 0.32 (0.29–0.35) | 0.42 (0.38–0.47) |
| Mortality from cancer | |||
| Comparators | 248 (6.1) | 1.00 | 1.00 |
| Users | 123 (3.0) | 0.48 (0.39–0.60) | 0.63 (0.49–0.81) |
| Incidence of cancer | <2 years follow-up* | 2–4 years follow-up* | >4 years follow-up* |
| Maximum prescribed dose during follow-up (n) | |||
| Low (1,017) | 3.15 (1.92–5.18) | 0.99 (0.44–2.25) | 0.16 (0.06–0.44) |
| Medium (2,090) | 1.94 (1.20–3.13) | 0.51 (0.31–0.82) | 0.40 (0.27–0.60) |
| High (978) | 2.76 (0.56–13.45) | 0.28 (0.12–0.70) | 0.15 (0.09–0.25) |
Data are HR (95% CI) unless otherwise indicated.
*Adjusted for age, sex, smoking, deprivation, BMI, A1C, insulin use, and sulphonylurea use.
Dose-response analysis
The stratified analysis for maximum dose is also presented in Table 2. Although none of the risks for low, medium, and high doses stratified by length of follow-up were significantly different from each other (as indicated by overlapping CIs), there was a clear trend for metformin users to have a higher risk of cancer in the first 2 years of follow-up. After this, among patients with the same duration of follow-up, the risk appeared to be lower with the highest metformin doses.
CONCLUSIONS
This study supports the hypothesis that users of metformin are at lower risk of cancer compared with people with type 2 diabetes on other treatments. Fewer than 8% of a cohort of metformin users were diagnosed with cancer during a maximum of 10 years of follow-up, compared with 11% of a comparator cohort of nonusers. The median time to cancer was 3.6 years among metformin users, compared with 2.5 years among comparators, and they also had reduced overall and cancer-related mortality.
This was an observational study; therefore, we could not control for all differences between study groups. Metformin users could have been at lower baseline risk of cancer than the comparators. Indeed, they were younger than their comparators (but mean BMI and A1C were higher). Metformin users did seem to be a different group clinically from nonusers, with a much lower rate of mortality (some of which could be explained by lower risk of cardiovascular mortality [16]). Although this limitation is inherent in the observational nature of the study, we adjusted results for known potential confounders and there were sizable changes to the risk estimates. There may still have been residual confounding or unknown confounders, but it is unlikely that this could account for the entire 37% reduced risk of cancer observed.
Adjusting for use of other diabetic drugs was necessary because there was a higher proportion of metformin users who were treated with sulfonylureas compared with the comparators but a lower proportion treated with insulin. This probably reflects the heterogeneity of the pool of potential comparators. Patients not treated with metformin will encompass those who do not yet require oral therapy as well as those who have progressed to insulin after treatment with sulfonylureas only. However, we found no statistically significant independent effects of sulfonylureas and insulin on risk of cancer in the Cox regression analysis. In contrast, men and older people were at increased risk of cancer, as might be expected. The results were similar for specific cancer types.
In a dose-response analysis, metformin users appeared to have a higher risk of cancer during the first 2 years of follow-up. This may be because patients who begin treatment with metformin are more likely to have cancer diagnosed because they have increasing contact with health care professionals. In later years of follow-up, high maximum doses of metformin were associated with the greatest reduction in risk of cancer. Metformin dose usually increases with increasing duration of use; therefore, dose variables can be confounded by duration. This could produce a survival bias, with higher doses spuriously associated with reduced cancer because patients have survived to receive a higher dose. This is the reason for stratification by length of follow-up (although residual confounding may still be present).
Within the known limitations of observational data, we are confident in our study design and data sources. The data sources used were independent of each other, and they provided objective measures of exposure and outcome. The diabetic population of Tayside, Scotland, U.K., is well defined, and the MEMO database used to identify metformin users has been widely used for drug safety research (11). The likelihood of misclassification of metformin exposure due to data error is low because we ensured that all patients had multiple metformin prescriptions. We were otherwise unable to judge whether patients actually took the metformin as prescribed, although we know that the drug was collected from the pharmacies (11). We are confident that we eliminated survival bias in our choice of comparators. The national cancer registry (SMR6) was used to identify cancer diagnoses. Specificity is likely to be higher than sensitivity in this register, but if any cancer diagnoses were missed, this would not occur differentially with respect to metformin status.
This study has produced sufficient epidemiological evidence that metformin reduces the risk of cancer to make further investigation a high priority. A plausible biological mechanism hinges on the discovery that the upstream LKB1 regulator of AMPK is a tumor suppressor and that activation of AMPK by LKB1 plays an important role in inhibiting cell growth when cellular energy levels are low (17). Metformin activates AMPK by inhibiting mitochondrial respiration and increasing 5′-AMP, which enhances activation of AMPK by LKB1 (1). The blood glucose–lowering properties of metformin are mediated through AMPK restoring cellular energy levels by phosphorylating regulatory proteins that lead to stimulation of glucose uptake into muscle tissues as well as inhibition of gluconeogenesis in the liver. The anticancer properties of metformin are likely to be mediated by AMPK's ability to preserve cellular energy levels by phosphorylating proteins such as p27KIP and TSC2 that lead to inhibition of cell growth and proliferation signaling networks (18,19).
Prior to the discovery that the LKB1 tumor suppressor activated AMPK, there was little interest in the role of AMPK in cancer. However, the ability of AMPK to gauge and control cellular energy places it in an ideal position to ensure that cell growth and proliferation is coupled to the availability of a sufficient supply of nutrients and energy. Recent laboratory evidence showing that three distinct drugs activate AMPK-delayed tumorigenesis in tumor-prone mice suggests that activators of AMPK could have therapeutic benefit for the treatment of cancer in humans (4). The protective effects of metformin on cancer development could potentially be rapid and may occur at quite a late stage of cancer development. Treatment of animal cells with metformin significantly activates the AMPK pathway within 30 min (20–22). Metformin also inhibits growth of cancer cells (5) or mouse embryonic stem cells (4) within 1–2 days. We believe that there is now a strong case to conduct a randomized trial to establish whether metformin is protective in a population at high risk for cancer.
Acknowledgments
We thank Tenovus, Scotland, U.K., for financial support. The researchers were independent. The funding source had no involvement in study design or analysis.
No potential conflicts of interest relevant to this article were reported.
We thank the staff of HIC who provided data and John Kernthaler who assisted with programming.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying editorial, p. 1748.
References
- 1.Hardie DG: AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol 2007; 47: 185– 210 [DOI] [PubMed] [Google Scholar]
- 2.Schneider MB, Matsuzaki H, Haorah J, Ulrich A, Stabdop J, Ding XZ, Adrian TE, Pour PM: Prevention of pancreatic induction in hamsters by metformin. Gastroenterology 2001; 120: 1263– 1270 [DOI] [PubMed] [Google Scholar]
- 3.Anisimov VN, Egormin PA, Bershtein LM, Zabezhinskii MA, Piskunova TS, Popovich IG, Semenchenko IV: Metformin decelerates aging and development of mammary tumors in HER-2/neu transgenic mice. Bull Exp Biol Med 2005; 139: 721– 723 [DOI] [PubMed] [Google Scholar]
- 4.Huang X, Wellschleger S, Shapiro N, McGuire VA, Sakamoto K, Woods YL, McBurnie W, Fleming S, Alessi DR: Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J 2008; 412: 211– 221 [DOI] [PubMed] [Google Scholar]
- 5.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M: Metformin is an AMP kinase–dependent growth inhibitor for breast cancer cells. Cancer Res 2006; 66: 10267– 10273 [DOI] [PubMed] [Google Scholar]
- 6.Evans JMM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD: Metformin and reduced risk of cancer in diabetic patients. BMJ 2005; 330: 1304– 1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowker SL, Majumdar SR, Veugelers P, Johnson JA: Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006; 29: 254– 258 [DOI] [PubMed] [Google Scholar]
- 8.Moore MA, Park CB, Tsuda H: Implications of the hyperinsulinaemia-diabetes-cancer link for preventive efforts. Eur J Cancer Prev 1998; 7: 89– 107 [PubMed] [Google Scholar]
- 9.Garmendia ML, Pereira A, Alvarado ME, Atalah E: Relation between insulin resistance and breast cancer among Chilean women. Ann Epidemiol 2007; 17: 403– 409 [DOI] [PubMed] [Google Scholar]
- 10.Morris AD, Boyle DIR, MacAlpine R, Emslie-Smith A, Jung RT, Newton RW, MacDonald TM: for the DARTS/MEMO Collaboration The diabetes audit and research in Tayside Scotland (DARTS) study: electronic record linkage to create a diabetes register. BMJ 1997; 315: 524– 528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans JMM, McDevitt DG, MacDonald TM: The Tayside Medicines Monitoring Unit (MEMO): a record-linkage system for pharmacovigilance. Pharmaceut Med 1995; 9: 177– 184 [Google Scholar]
- 12.Information and Statistics Division Scotland Scottish Cancer Registry [Internet]. Information Services Division, NHS National Services Scotland. Available from http://www.isdscotland.org/isd/3535.html Accessed 1 November 2008
- 13.World Health Organization Manual of the International Statistical Classification of Diseases, Injuries and Causes of Death 9th revision Geneva, World Health Org., 1978 [Google Scholar]
- 14.World Health Organization International Statistical Classification of Diseases and Related Health Problems 10th revision Geneva, World Health Org., 1994 [Google Scholar]
- 15.Carstairs V: Deprivation and health in Scotland. Health Bull (Edinb) 1990; 48: 162– 175 [PubMed] [Google Scholar]
- 16.Evans JMM, Ogston SA, Emslie-Smith A, Morris AD: Risks of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulphonylureas and metformin. Diabetologia 2006; 49: 930– 936 [DOI] [PubMed] [Google Scholar]
- 17.Alessi DR, Sakamoto K, Bayascas JR: LKB1-dependent signaling pathways. Annu Rev Biochem 2006; 75: 137– 163 [DOI] [PubMed] [Google Scholar]
- 18.Inoki K, Guan K-L: Complexity of the TOR signalling network. Trends Cell Biol 2006; 16: 206– 212 [DOI] [PubMed] [Google Scholar]
- 19.Carling D: The role of the AMP-activated protein kinase in the regulation of energy homeostasis. Novartis Found Symp 2007; 286: 72– 81 [DOI] [PubMed] [Google Scholar]
- 20.Fryer LG, Parbu-Patel A, Carling D: The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem 2002; 277: 25226– 25232 [DOI] [PubMed] [Google Scholar]
- 21.Hardie DG: Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 2003; 144: 5179– 5183 [DOI] [PubMed] [Google Scholar]
- 22.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE: Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001; 108: 1167– 1174 [DOI] [PMC free article] [PubMed] [Google Scholar]