Abstract
OBJECTIVE
We studied tubular and glomerular damage in type 1 diabetic patients by measuring urinary–liver fatty acid binding protein (U-LFABP) and albuminuria. Subsequently, we evaluated the effect of ACE inhibition on U-LFABP in patients with diabetic nephropathy.
RESEARCH DESIGN AND METHODS
We studied Caucasians with type 1 diabetes: 58 with normoalbuminuria (urinary albumin <30 mg/24 h), 45 with persistent microalbuminuria (30–300 mg/24 h), and 45 with persistent macroalbuminuria (≥300 mg/24 h). A control group consisted of 57 healthy individuals. The groups were matched by sex and duration of diabetes. In addition, U-LFABP was measured in 48 type 1 diabetic patients with diabetic nephropathy in a randomized crossover trial consisting of 2 months of treatment with 20, 40, and 60 mg lisinopril once daily in random order.
RESULTS
In the cross-sectional study, levels of U-LFABP were significantly higher in normoalbuminuric patients versus those in the control group (median 2.6 [interquartile range 1.3–4.1] vs. 19 [0.8–3.0] μg/g creatinine, P = 0.02) and increased with increasing levels of albuminuria (microalbuminuric group 4.2 [1.8–8.3] μg/g creatinine and nephropathy group 71.2 [8.1–123.4], P < 0.05 for all comparisons). U-LFABP correlates with the urinary albumin-to-creatinine ratio (R2 = 0.54, P < 0.001). In the intervention study, all doses of lisinopril significantly reduced urinary albumin excretion rate and U-LFABP from baseline. The reductions in U-LFABP were 43, 46, and 40% with increasing doses of lisinopril (NS).
CONCLUSIONS
An early and progressive increase in tubulointerstitial damage as reflected by increased U-LFABP levels occurs in type 1 diabetic patients and is associated with albuminuria. Furthermore, ACE inhibition reduces the tubular and glomerular damage and dysfunction.
Diabetic nephropathy is a serious and common complication in diabetic patients. Although studies from selected centers suggest a declining incidence of diabetic nephropathy (1), still 30–40% of all patients with diabetes develop this complication (2). Diabetic nephropathy is associated with a higher risk of other complications such as cardiovascular disease, retinopathy, and neuropathy. It is the most common cause of end-stage renal failure in Western countries. Therefore, it is of great interest to predict and prevent the development of diabetic nephropathy.
Persistent microalbuminuria is the established predictor of development of diabetic nephropathy and progressive renal insufficiency. However, use of the urinary albumin excretion rate (UAER), as an indicator of renal damage, has some limitations. Several patients with microalbuminuria do not progress to macroalbuminuria but continue having microalbuminuria (30%) or even regress to normoalbuminuria (15–30% after 7.5 years of follow-up) (3). As reviewed previously, glomerular damage and tubulointerstitial damage are important factors in the pathology of diabetic nephropathy (2,4).
Liver fatty acid binding protein (LFABP) is an intracellular carrier protein that is expressed in the proximal tubules in the human kidney and the liver (5). By immunohistochemical staining of renal biopsy specimens, it has been shown that urinary LFABP (U-LFABP) excretion is highly associated with structural and functional tubular kidney damage. This was confirmed in patients with chronic kidney disease including minimal-change nephritic syndrome, nephrosclerosis, lupus nephritis, and diabetic nephropathy (6). A previous study in chronic kidney disease has shown that serum LFABP levels do not affect the U-LFABP level, which suggests that there is no transglomerular passage of LFABP in chronic kidney disease and that the LFABP measured in urine originates primarily from tubular cells (7). It has been hypothesized that LFABP is a protective protein; however, previous studies have, to our knowledge, not been able to confirm this hypothesis (8).
In patients with diabetic nephropathy, a reduction in albuminuria is a predictor of renoprotection (9). It is suggested that the combination of the two parameters, albuminuria and U-LFABP, would be useful in monitoring chronic kidney disease more than either alone.
The relationship between albuminuria and U-LFABP has, to our knowledge, not been studied in type 1 diabetic patients. Furthermore, whether U-LFABP could be used to monitor the renoprotective effect on the decline in glomerular filtration rate (GFR) or the effect on urinary albumin excretion in short-term studies in type 1 diabetic patients with diabetic nephropathy has not been studied.
The aim of this study was to investigate the levels of U-LFABP in a cross-sectional study of type 1 diabetic patients with different levels of albuminuria compared with those in a nondiabetic control group. In addition, we wanted to explore the short-term effect of increasing doses of the ACE inhibitor lisinopril on U-LFABP levels in patients with type 1 diabetes and diabetic nephropathy from a randomized, double-blind crossover study.
RESEARCH DESIGN AND METHODS
The cross-sectional study was based on data from a cohort used to identify biomarkers of diabetic nephropathy by proteomic analyses (10). The population consisted of Caucasian patients with type 1 diabetes and different levels of albuminuria recruited from the outpatient clinic at Steno Diabetes Center in 2004. Based on albumin excretion in 24-h urine samples that were collected as part of the routine care of the patients before the present study, patients were divided into three groups: 58 with normoalbuminuria (UAER <30 mg/24 h), 45 with persistent microalbuminuria (UAER between 30 and 300 mg/24 h in at least two of three consecutive samples) and 45 with persistent macroalbuminuria (UAER >300 mg/24 h in at least two of three consecutive samples). The control group consisted of 57 nondiabetic healthy individuals. The 24-h urine samples were only used for assessing inclusion criteria. Groups were matched by sex and duration of diabetes.
Investigations were performed in the morning. Arterial blood pressure was measured three times with an appropriate-sized cuff after at least a 10-min rest. Urinary albumin concentration was measured by an enzyme immunoassay from early morning spot urine samples and expressed as the urinary albumin-to-creatinine ratio (UACR). Serum and urine creatinine concentrations were assessed by a kinetic Jaffé method. Estimated GFR (eGFR) was calculated using the reexpressed four-variable Modification of Diet in Renal Disease study equation (eGFR = 175 × plasma creatinine−1.154 × age−0.203 × 1.212 [if black] or × 0.742 [if female]) as defined previously (11) where eGFR is expressed as milliliters per minute per 1.73 m2 and plasma creatinine is expressed as milligrams per deciliter. U-LFABP was measured in a two-step sandwich enzyme-linked immunosorbent assay (12) and expressed as the U-LFABP-to-creatinine ratio. The inter- and intra-assay variations were 6.8 and 8.2%, respectively. Plasma samples were stored at −80°C, and urine samples were stored at −20°C until analysis.
The second study was a randomized double-masked crossover trial performed in 2005 (13). Patients were treated in random order with 20, 40, and 60 mg lisinopril, with each period lasting 2 months. A total of 56 patients with type 1 diabetes, hypertension (>135/85 mmHg), and diabetic nephropathy were randomly assigned in the study.
The primary end point was changes in UAER (micrograms per 24 h). Albuminuria was determined in three consecutive 24-h urine collections completed immediately before the end of each treatment period. Among the secondary end points measured at the end of each treatment period were 24-h ambulatory blood pressure and eGFR. U-LFABP was also measured in 24-h urine collections (micrograms per 24 h). For safety reasons, blood pressure, plasma potassium, plasma sodium, and plasma creatinine were determined 3 weeks after the beginning of each treatment period. GFR (baseline) was measured at baseline after a single intravenous injection of 3.7 MBq 51Cr-EDTA at 8:30 a.m. by determining the radioactivity in venous blood samples taken 180, 200, 220, and 240 min after injection. The results were standardized for 1.73 m2 body surface area. Blood pressure was measured by a 24-h ambulatory blood pressure device (Takeda TM2421; A & D Medical, Tokyo, Japan).
The study was initiated by a 2-month washout period during which all antihypertensive drugs were withdrawn, except for slow-release furosemide in individual doses to prevent fluid retention and hyperkalemia and control blood pressure. Thereafter, patients were treated in random order with 20, 40, and 60 mg lisinopril, with each period lasting 2 months. Dietary intake of protein and salt was not restricted.
Both studies were performed according to the principles of the Declaration of Helsinki and the intervention study was approved by the ethics committee of Copenhagen County. Written informed consent was obtained from all patients.
Statistical analysis
Normally distributed variables are expressed as means ± SD (baseline characteristics) and means ± SEM. U-LFABP and UACR are given as medians (interquartile range [IQR]). All comparisons between groups of normally or log-normally distributed parameters were performed with a one-way ANOVA, and ordinal data were compared using a Kruskall-Wallis and a Mann-Whitney U test. Changes in variables between visits during the intervention study are expressed as mean differences (95% CI). Comparisons of log-U-LFABP between each treatment period were performed using linear mixed models. The adapted model was one with fixed effects of treatment level, visit, and carryover (i.e., treatment level in the previous period) and a random effect of person included to account for the person independence in data. Linear regression analysis was used to analyze for correlations between the change from baseline in U-LFABP based on differences in log-transformed values and changes in UAER, ambulatory blood pressure, and eGFR, respectively. P < 0.05 was considered significant (two-tailed test). Data were evaluated using SPSS (version 14.0; SPSS, Chicago, IL).
RESULTS
Cross-sectional study
The clinical characteristics of the patients are shown in Table 1. The patients were well matched regarding the duration of diabetes and sex. Patients with normoalbuminuria and microalbuminuria were slightly older than control subjects and patients with macroalbuminuria.
Table 1.
Clinical data of control group and type 1 diabetic patients differentiated according to the level of albuminuria in cross-sectional study
| Control group | Normoalbuminuria | Microalbuminuria | Macroalbuminuria | P | |
|---|---|---|---|---|---|
| n (male/female) | 57 (37/20) | 58 (30/28) | 45 (24/21) | 45 (27/18) | 0.273 |
| Age (years) | 51 ± 11.0 | 56 ± 10.8 | 54 ± 11.1 | 49 ± 9.3 | 0.004 |
| Diabetes duration (years) | — | 37 ± 11 | 35 ± 11 | 34 ± 11 | 0.411 |
| Systolic blood pressure (mmHg) | 132 ± 16 | 138 ± 21 | 142 ± 23 | 145 ± 19 | 0.009 |
| Diastolic blood pressure (mmHg) | 81 ± 11 | 75 ± 11 | 74 ± 12 | 78 ± 10 | 0.002 |
| A1C (%) | 5.5 ± 0.3 | 8.2 ± 1.1 | 8.8 ± 1.2 | 8.8 ± 1.1 | <0.001 |
| eGFR (ml/min per 1.73m2) | 70.5 ± 9.6 | 70.3 ± 10.3 | 71.0 ± 13.3 | 48.7 ± 19.2 | <0.001 |
| Creatinine (μmol/l)* | 95 (73–146) | 91 (69–121) | 89 (70–143) | 127 (84–144) | <0.001 |
| UACR (mg/g)† | 2 (1–5) | 5 (3–9) | 27 (11–68) | 461 (173–1,172) | — |
| U-LFABP (μg/g creatinine)† | 1.9 (0.8–3.0) | 2.6 (1.3–4.1) | 4.2 (1.8–8.3) | 71.2 (8.1–123.4) | <0.05 |
Data are means ± SD, *median (range), or †median (IQR). P value refers to allover difference between groups (ANOVA).
We found a significant difference in systolic blood pressure among the groups (P = 0.009) because of a difference between control subjects and patients with microalbuminuria (P = 0.017) and macroalbuminuria (P = 0.001). Diastolic blood pressure was significantly higher in control subjects, whereas there was no difference among diabetic groups. eGFR was significantly lower in the macroalbuminuric group than in all other groups. The significant difference in serum creatinine was due to differences between the macroalbuminuric group and all other groups.
U-LFABP and urinary albumin excretion
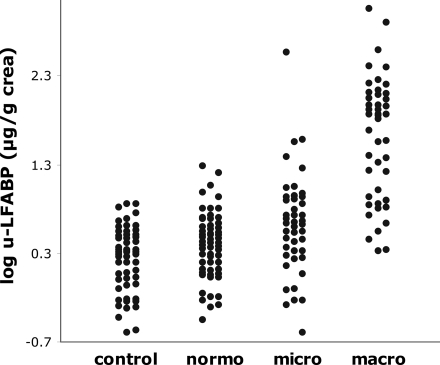
U-LFABP levels are shown in Table 1 and illustrated in Fig. 1. U-LFABP ratios were significantly higher in the normoalbuminuric group versus the control group (median 2.6 [IQR 1.3–4.1] vs. 1.9 [0.8–3.0] μg/g creatinine, P = 0.02) and increased with increasing levels of albuminuria (microalbuminuric group 4.2 [1.8–8.3] μg/g creatinine and macroalbuminuric group 71.2 [8.1–123.4] μg/g creatinine, P < 0.05 for all comparisons.
Figure 1.
U-LFABP in control group and three diabetic groups with different levels of albuminuria.
U-LFABP correlated with UACR (R2 = 0.54, P < 0.001) in the combined group of all diabetic patients. In the normoalbuminuric group, there was also a significant but weak association between UACR and U-LFABP (R2 = 0.07, P = 0.04).
In patients with macroalbuminuria, U-LFABP levels correlated with UACR (R2 = 0.50, P < 0.001) and eGFR (R2 = 0.34, P < 0.001). When adjusted for eGFR, there was still a significant difference in U-LFABP for comparisons between patients with macroalbuminuria and the other groups (P < 0.01).
There was a significant correlation between U-LFABP and systolic blood pressure (R2 = 0.07, P < 0.001). However, this may be explained by the association between U-LFABP and renal function because it disappeared after adjustment for eGFR and UACR. There were no significant associations between U-LFABP and sex, age, BMI, diastolic blood pressure, cholesterol, or A1C.
Effect of ACE inhibition on U-LFABP: intervention study
A total of 56 patients were randomly assigned in the study, and 49 patients completed the study. One patient did not have U-LFABP measured at baseline; results are given for the remaining 48.
Age at baseline was (mean ± SD) 50 ± 10 years and the duration of diabetes was 33 ± 10 years. Baseline UAER was (geometric mean) 365 mg/24 h (95% CI 240–554). Baseline GFR (51Cr-EDTA) was 73 ± 28 ml/min per 1.73 m2.
All doses of lisinopril significantly reduced UAER, U-LFABP, and arterial blood pressure compared with baseline (Table 2). At baseline, U-LFABP was median 12.69 μg/24 h (IQR 3.88–49.82). Reductions from baseline in U-LFABP were 43% (95% CI 15–62), 46% (19–64), and 40% (11–60) with increasing doses of lisinopril (no significant difference between doses). The reduction in U-LFABP was associated with the changes in 24-h systolic ambulatory blood pressure (R2 = 0.22, P < 0.01) (similar for diastolic ambulatory blood pressure) and UAER (R2 = 0.38, P < 0.001) but not with changes in eGFR. (Data are given for change from baseline to 40 mg of lisinopril, which gave the largest response, but the same results were found for the other doses of lisinopril.) The decline in U-LFABP was still significant when adjusted for the decline in UAER and for 24-h systolic blood pressure (P = 0.011).
Table 2.
Laboratory data during treatment with 20, 40, and 60 mg lisinopril in random order compared with baseline in 48 type 1 diabetic patients with diabetic nephropathy
| Baseline | Lisinopril |
|||
|---|---|---|---|---|
| 20 mg | 40 mg | 60 mg | ||
| 24-h systolic blood pressure (mmHg), reduction from baseline | — | 10 (6–14)* | 13 (8–18)*† | 12 (8–17)*† |
| 24-h diastolic blood pressure (mmHg), reduction from baseline | — | 5 (3–7)* | 7 (5–10)*† | 7 (5–10)*† |
| Reduction in UAER compared with baseline | — | 63 (55–69)* | 71 (66–76)* | 70 (64–75)* |
| eGFR (ml/min per 1.73 m2) | 75 ± 4 | 69 ± 4* | 68 ± 4* | 67 ± 4* |
| A1C (%) | 8.6 ± 0.1 | 8.7 ± 0.2 | 8.8 ± 0.2 | 8.9 ± 0.1* |
| Plasma potassium (mmol/l) | 3.9 ± 0.1 | 4.3 ± 0.1* | 4.4 ± 0.1* | 4.4 ± 0.1* |
| U-LFABP reduction compared with baseline | — | 43 (15–62)* | 46 (19–64)* | 40 (11–60)* |
Data are means ± SEM or mean difference (95% CI). The Friedman test for several related samples was used followed by a paired-samples t test if significant.
*P < 0.05 vs. baseline;
†P < 0.05 vs. 20 mg.
CONCLUSIONS
In our cross-sectional study, we have shown that the marker of tubulointerstitial damage, U-LFABP, is elevated in type 1 diabetic patients compared with nondiabetic healthy control subjects. In addition, we have shown that U-LFABP is further increased in type 1 diabetic patients with micro- and macroalbuminuria, reflecting increased tubular damage with increasing levels of albuminuria. There were no significant correlations between U-LFABP and sex, age, or A1C.
In our randomized, double-masked crossover study, ACE inhibition with lisinopril reduced U-LFABP. There was no significant difference in effect between doses of lisinopril from 20 to 60 mg daily.
Previous studies of U-LFABP in diabetes were cross-sectional studies on U-LFABP in type 2 diabetic patients. Suzuki et al. (14) performed a cross-sectional study in 356 adult type 2 diabetic patients. They divided the patients into four groups: normoalbuminuric, microalbuminuric, macroalbuminuric, and renal failure, but no control group was included. They reported a significant association between the stage of diabetic nephropathy and U-LFABP, although no significant difference between the normoalbuminuric and microalbuminuric groups was seen.
The results from our cross-sectional study show that patients with normoalbuminuria and type 1 diabetes had higher U-LFABP than the healthy control subjects (Table 1). The normoalbuminuric group had a significantly higher level of albuminuria than the healthy control subjects, but even with adjustment for this result, there was still a significant difference in U-LFABP between the two groups (P = 0.014). One possible explanation is that having diabetes elevates U-LFABP. However, this possibility is not likely because U-LFABP is not correlated with A1C. Another hypothesis is that some of the patients in the normoalbuminuric group had higher levels of U-LFABP as a predictor of future development of microalbuminuria and diabetic nephropathy. The significant association between UACR and U-LFABP in the normoalbuminuric group also indicated this. However, to test this hypothesis we need prospective follow-up studies in normoalbuminuric type 1 diabetic patients.
We also saw that with increasing levels of albuminuria, from normoalbuminuria to microalbuminuria and macroalbuminuria, U-LFABP is increasing. Part of this association can be explained by the transport with albumin of fatty acids to the proximal tubules. Here the fatty acids are absorbed into the proximal tubular cells, where the role of LFABP is to transport the fatty acids to the mitochondria. Therefore, when albuminuria increases, the LFABP gene is upregulated and more LFABP is excreted into the urine (15). U-LFABP is also elevated independently of albuminuria because of increased tubular production due to tubular hypoxia and oxidative stress (16), which is seen in diabetes (17). Our study is cross-sectional, and therefore we cannot draw conclusions on the time perspective between elevation in U-LFABP and development of nephropathy. However, from earlier studies in nondiabetic chronic renal disease, the potential of U-LFABP as an early predictor of nephropathy is supported. Kamijo et al. (18) performed a multicenter observational trial in 48 patients with nondiabetic chronic kidney disease. Retrospectively, they divided the patients into progressors and nonprogressors in their chronic kidney disease based on changes in creatinine clearance during 1 year of follow-up. They found that U-LFABP had a higher sensitivity (94%) than urinary albumin (69%) but a lower specificity (63%) than urinary albumin (94%) in predicting progression in chronic kidney disease.
U-LFABP is different from other suggested biomarkers, e.g., α1- and β2-microglobulins: U-LFABP is produced in the tubular cells, whereas α1- and β2-microglobulins are freely filtered through the glomerular basement membrane. During poor glycemic control, urinary excretion of β2-microglobulin is increased (19); this is a result of a lack of reabsorption in the damaged tubular cell (20,21). β2-Microglobulin is unstable at low pH, causing an underestimate of the tubular damage (22).
In our randomized, crossover study, we saw that 2 months of ACE inhibition reduces U-LFABP by ∼40%. There was no significant difference in the decline in U-LFABP among doses of lisinopril, suggesting an optimal effect with 20 mg lisinopril daily, which was in contrast to the increased decline in UAER when doses of lisinopril were increased from 20 to 40 mg.
We observed that the decrease in U-LFABP is associated with a decrease in albuminuria, but the relatively weak association (R2 = 0.38, P < 0.001) supports the fact that the decline in U-LFABP is not only explained by reduced albuminuria but also suggests, as mentioned earlier, that the reduction also reflects reduced tubular damage. The decrease in U-LFABP indicates that the tubular damage and upregulation of the U-LFABP gene are reversible.
Our finding is in accordance with earlier studies in type 2 diabetic patients (23), which showed a significant decrease in U-LFABP when these patients were treated with an angiotensin II receptor antagonist. Experimental studies in diabetic rats have shown that renin-angiotensin-aldosterone system blockade reduces antiapoptotic factors (24) and that oxidative stress in tubular cells is reduced and chronic hypoxia is corrected independent of the blood pressure–lowering effect (25) and thereby preserves tubular function.
In summary, an early and progressive rise in tubulointerstitial damage as reflected by increased U-LFABP levels occurs in type 1 diabetic patients and is associated with albuminuria. Furthermore, ACE inhibition reduces the tubular and glomerular damage and dysfunction. Our studies indicate that U-LFABP is a new marker of tubular damage and a potential supplement to the glomerular damage marker albuminuria for prognosis, diagnosis, and treatment of kidney injury, although further longitudinal studies are needed.
Acknowledgments
The studies were financed out of local funds and were not supported by the pharmaceutical industry. T.S. is the director and senior scientist of CMIC, Tokyo, Japan, the company that produced the kits for LFABP analysis. No other potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 44th annual meeting of the European Association for the Study of Diabetes, Rome, Italy, 11 September 2008.
We thank B.R. Jensen, T.R. Juhl, B.V. Hansen, U.M. Smidt, and L. Pietraszek for their help with collecting the data.
Footnotes
Clinical trial reg. no. NCT00118976, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Hovind P, Tarnow L, Rossing K, Rossing P, Eising S, Larsen N, Binder C, Parving HH. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care 2003; 26: 1258– 1264 [DOI] [PubMed] [Google Scholar]
- 2.Parving H-H, Mauer M, Ritz E. Diabetic nephropathy. In Brenner & Rector's The Kidney 8th ed.Brenner BM. Ed. Boston, WB Saunders, 2008, p. 1265– 1298 [Google Scholar]
- 3.Hovind P, Tarnow L, Rossing P, Jensen BR, Graae M, Torp I, Binder C, Parving HH. Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. Br Med J 2004; 328: 1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int 1999; 56: 1627– 1637 [DOI] [PubMed] [Google Scholar]
- 5.Maatman RG, van de Westerlo EM, van Kuppevelt TH, Veerkamp JH. Molecular identification of the liver- and the heart-type fatty acid-binding proteins in human and rat kidney. Use of the reverse transcriptase polymerase chain reaction. Biochem J 1992; 288( Pt. 1): 285– 290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamijo A, Sugaya T, Hikawa A, Okada M, Okumura F, Yamanouchi M, Honda A, Okabe M, Fujino T, Hirata Y, Omata M, Kaneko R, Fujii H, Fukamizu A, Kimura K. Urinary excretion of fatty acid-binding protein reflects stress overload on the proximal tubules. Am J Pathol 2004; 165: 1243– 1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamijo A, Sugaya T, Hikawa A, Yamanouchi M, Hirata Y, Ishimitsu T, Numabe A, Takagi M, Hayakawa H, Tabei F, Sugimoto T, Mise N, Omata M, Kimura K. Urinary liver-type fatty acid binding protein as a useful biomarker in chronic kidney disease. Mol Cell Biochem 2006; 284: 175– 182 [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman AW, Veerkamp JH. Fatty-acid-binding proteins do not protect against induced cytotoxicity in a kidney cell model. Biochem J 2001; 360: 159– 165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossing P, Hommel E, Smidt UM, Parving H-H. Reduction in albuminuria predicts a beneficial effect on diminishing the progression of human diabetic nephropathy during antihypertensive treatment. Diabetologia 1994; 37: 511– 516 [DOI] [PubMed] [Google Scholar]
- 10.Rossing K, Mischak H, Dakna M, Zurbig P, Novak J, Julian BA, Good DM, Coon JJ, Tarnow L, Rossing P. Urinary proteomics in diabetes and CKD. J Am Soc Nephrol 2008; 19: 1283– 1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van LF. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145: 247– 254 [DOI] [PubMed] [Google Scholar]
- 12.Nakamura T, Sugaya T, Kawagoe Y, Ueda Y, Osada S, Koide H. Effect of pitavastatin on urinary liver-type fatty acid-binding protein levels in patients with early diabetic nephropathy. Diabetes Care 2005; 28: 2728– 2732 [DOI] [PubMed] [Google Scholar]
- 13.Schjoedt KJ, Astrup A-S, Persson F, Frandsen E, Boomsma F, Rossing K, Tarnow L, Rossing P, Parving H-H. Optimal dose of lisinopril for renoprotection in type 1 diabetic patients with diabetic nephropathy. Diabetologia 2009; 52: 46– 49 [DOI] [PubMed] [Google Scholar]
- 14.Suzuki K, Babazono T, Murata H, Iwamoto Y. Clinical significance of urinary liver-type fatty acid-binding protein in patients with diabetic nephropathy. Diabetes Care 2005; 28: 2038– 2039 [DOI] [PubMed] [Google Scholar]
- 15.Mayer G, Cerritto l, Sugaya T. Urinary-LFABP: a novel biomarker for renal disease and its role in the diagnosis and prognosis of chronic and acute kidney disease. Fats Life 2008; 10: 4– 11 [Google Scholar]
- 16.Kamijo A, Kimura K, Sugaya T, Yamanouchi M, Hikawa A, Hirano N, Hirata Y, Goto A, Omata M. Urinary fatty acid-binding protein as a new clinical marker of the progression of chronic renal disease. J Lab Clin Med 2004; 143: 23– 30 [DOI] [PubMed] [Google Scholar]
- 17.Singh DK, Winocour P, Farrington K. Mechanisms of disease: the hypoxic tubular hypothesis of diabetic nephropathy. Nat Clin Pract Nephrol 2008; 4: 216– 226 [DOI] [PubMed] [Google Scholar]
- 18.Kamijo A, Sugaya T, Hikawa A, Yamanouchi M, Hirata Y, Ishimitsu T, Numabe A, Takagi M, Hayakawa H, Tabei F, Sugimoto T, Mise N, Kimura K. Clinical evaluation of urinary excretion of liver-type fatty acid-binding protein as a marker for the monitoring of chronic kidney disease: a multicenter trial. J Lab Clin Med 2005; 145: 125– 133 [DOI] [PubMed] [Google Scholar]
- 19.Parving H-H, Noer I, Deckert T, Evrin P-E, Nielsen SL, Lyngsøe J, Mogensen CE, Rørth M, Svendsen PA, Trap-Jensen J, Lassen NA. The effect of metabolic regulation on microvascular permeability to small and large molecules in short-term juvenile diabetics. Diabetologia 1976; 12: 161– 166 [DOI] [PubMed] [Google Scholar]
- 20.Ekstrom B, Peterson PA, Berggard I. Urinary and plasma α-1-glycoprotein of low-molecular weight: isolation and some properties. Biochem Biophys Res Commun 1975; 65: 1427– 1433 [DOI] [PubMed] [Google Scholar]
- 21.Maack T, Johnson V, Kau ST, Figueiredo J, Sigulem D. Renal filtration, transport, and metabolism of low-molecular-weight proteins—review. Kidney Int 1979; 16: 251– 270 [DOI] [PubMed] [Google Scholar]
- 22.Yu H, Yanagisawa Y, Forbes MA, Cooper EH, Crockson RA, Maclennan ICM. α-1-Microglobulin—an indicator protein for renal tubular function. J Clin Pathol 1983; 36: 253– 259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura T, Sugaya T, Koide H. Angiotensin II receptor antagonist reduces urinary liver-type fatty acid-binding protein levels in patients with diabetic nephropathy and chronic renal failure. Diabetologia 2007; 50: 490– 492 [DOI] [PubMed] [Google Scholar]
- 24.Kelly DJ, Cox AJ, Tolcos M, Cooper ME, Wilkinson-Berka JL, Gilbert RE. Attenuation of tubular apoptosis by blockade of the renin-angiotensin system in diabetic Ren-2 rats. Kidney Int 2002; 61: 31– 39 [DOI] [PubMed] [Google Scholar]
- 25.Izuhara Y, Nangaku M, Inagi R, Tominaga N, Aizawa T, Kurokawa K, van Ypersele de SC, Miyata T. Renoprotective properties of angiotensin receptor blockers beyond blood pressure lowering. J Am Soc Nephrol 2005; 16: 3631– 3641 [DOI] [PubMed] [Google Scholar]