Abstract
OBJECTIVE
To summarize the efficacy of metformin in reducing BMI and cardiometabolic risk in obese children and adolescents without diabetes.
RESEARCH DESIGN AND METHODS
We performed a systematic review and meta-analysis of randomized controlled trials (RCTs). Double-blind RCTs of ≥6 months duration in obese subjects age ≤19 years without diabetes were included. Our primary outcomes of interest include changes in BMI and measures of insulin sensitivity.
RESULTS
Five trials met inclusion criteria (n = 320 individuals). Compared with placebo, metformin reduced BMI by 1.42 kg/m2 (95% CI 0.83–2.02) and homeostasis model assessment insulin of resistance (HOMA-IR) score by 2.01 (95% CI 0.75–3.26).
CONCLUSIONS
Metformin appears to be moderately efficacious in reducing BMI and insulin resistance in hyperinsulinemic obese children and adolescents in the short term. Larger, longer-term studies in different populations are needed to establish its role in the treatment of overweight children.
Metformin has been shown to reduce weight gain, hyperinsulinemia, and hyperglycemia in adults with type 2 diabetes (1,2) and to reduce progression from impaired glucose tolerance to diabetes in those without diabetes (3). These benefits have led to an increase in the use of metformin in obese children with hyperinsulinemia. However, obesity is not a licensed indication for metformin in the U.K. or the U.S., and its use has proceeded faster than the evidence of its benefits. We undertook a systematic review of randomized controlled trials (RCTs) investigating the efficacy of metformin for reducing BMI and cardiometabolic risk in obese children without diabetes.
RESEARCH DESIGN AND METHODS
We searched Ovid MEDLINE, EMBASE, the Cochrane Register of Controlled Trials, the metaRegister of Controlled Trials, and key journals published before December 2008 (online appendix Tables 1 and 2 available at http://care.diabetesjournals.org/cgi/content/full/dc09-0258/DC1). We included double-blind RCTs of ≥6 months duration with obese subjects age ≤19 years without diabetes and without secondary or syndromic causes of obesity. Primary outcomes of interest were BMI (weight in kilograms divided by the square of height in meters) and measures of insulin sensitivity. Secondary outcomes included fat mass, blood pressure, fasting lipids, and adverse effects.
Where three or more studies reported a common outcome, treatment effect was explored in a meta-analysis (Stata Statistical Software 10.1; StataCorp, College Station, TX), pooling data from the end of the follow-up period for trial completers. A random-effects model was selected. Sensitivity analyses were performed using fixed-effects models and by dose of metformin (1,000 vs. 2,000 mg), age of participants (12–19 vs. <12 years), co-intervention (metformin vs. metformin + co-intervention), baseline BMI (mean ≥35 vs. <35 kg/m2), and by excluding one study reporting greater treatment effects than the other studies (4).
RESULTS
Five studies published between 2001 and 2008 met the inclusion criteria (4–8). This included one crossover trial (5).
Three studies took place in the U.S. (6–8), and one each in Australia (5) and Turkey (4). All trials lasted 6 months with metformin doses from 1,000–2,000 mg/day. Three studies used lifestyle co-interventions in either trial arms (4,7,8). Two studies included adolescents (ages 12–19 years) (6,7), one looked at younger children (ages 6–12 years) (6), and the others spanned ages 9–18 years. In the U.S. and Australian studies, a large proportion of participants (45–90%) were from ethnic backgrounds with high prevalence of metabolic syndrome (African American, Hispanic, or Asian). All participants were hyperinsulinemic or insulin resistant. Sample size ranged from 28–120 participants at randomization; in total there were 365 participants and 320 trial completers. Mean attrition rates were 11% in metformin groups and 16% in placebo groups.
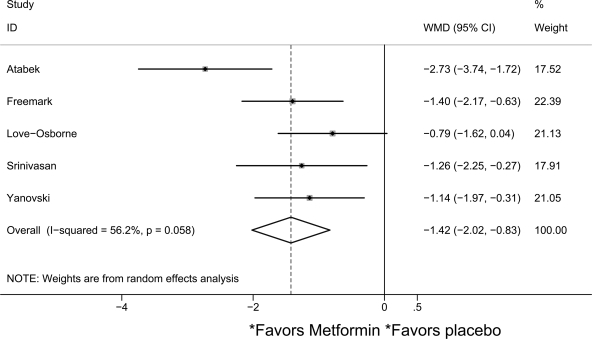
In the pooled analysis, metformin reduced BMI by a mean of 1.42 kg/m2 (95% CI 0.83–2.02) compared with placebo (I2 = 56.2%; n = 342) (Fig. 1). Sensitivity analyses did not reveal notable differences by age, dose, or baseline BMI. When the outlier result was excluded, metformin reduced BMI by 1.15 kg/m2 (0.73–1.57, I2 = 0%). Reduction in fasting insulin was greater in metformin than placebo groups in three studies, but evidence for a treatment effect was weak (−5.30 μU/ml [95% CI −11.96 to 1.36], I2 = 78.7%; n = 257) (4–7). Pooled metformin effect on the homeostasis model assessment of insulin resistance (HOMA-IR) score was −2.01 (95% CI −3.26 to −0.75, I2 = 49.5%; n = 234) (4,6,8) and −1.28 (−2.55 to −0.21, I2 = 0%) if the Turkish study was excluded.
Figure 1.
Forest plot comparing change in BMI (kg/m2) in metformin and placebo groups.
Pooled mean metformin effect on total cholesterol was −0.19 mmol/l (95% CI −0.38 to −0.01, I2 = 0%; n = 234) (4,6,7). Analyses did not provide strong evidence for a treatment effect on fasting glucose, HDL cholesterol, triglyceride levels, or blood pressure. There was insufficient data to comment on body fat outcomes. Gastrointestinal problems were the most common reported side effect (in 20–30%) and were more frequently reported in metformin than in placebo groups (risk difference 10–14%) (6,7). Only one participant reported gastrointestinal problems as the reason for leaving a study (7).
CONCLUSIONS
Our meta-analysis provides some support for a beneficial metformin effect on obesity outcomes among hyperinsulinemic children and adolescents. Treatment over 6 months may be efficacious in reducing BMI by 1.42 kg/m2 (equivalent to 0.4 SD, based on SD for BMI in U.K. and U.S. adolescents) and HOMA-IR score by 2.01 (∼0.6 SD) (9). Metformin use was also associated with a small reduction in total cholesterol level (∼0.26 SD) (10), but these are unadjusted measures, and it is not possible to determine whether the effects are secondary to reductions in BMI and HOMA-IR or attributable to other factors. To our knowledge, the effects of metformin on BMI in obese children without diabetes have been synthesized in only one published review based on three studies (11), which identified no treatment effect at 6 months (−0.17 kg/m2 [95% CI −0.62 to −0.28]).
Metformin may not be as effective as behavioral interventions in reducing BMI: a meta-analysis of behavioral interventions in obese adolescents reported an effect of −3.04 kg/m2 (95% CI −3.14 to −2.94) at 6 months, which was maintained at 12 months follow-up (12). When compared with drugs that are licensed for obesity, metformin has moderate effect: meta-analyses of RCTs reported an orlistat effect of −0.76 kg/m2 (−1.07 to −0.44) and a sibutramine effect of −1.66 kg/m2 (−1.89 to −1.43) at 6 months (12).
The results of this review must be interpreted with caution: the studies were short-term and based on small samples; participants were mainly from the U.S., and large portions were from ethnic backgrounds known to be at increased risk of metabolic disorders, limiting the generalizability of findings; and the studies presented unadjusted measures without intention-to-treat analyses, which may have overestimated treatment effects.
Metformin may be efficacious in reducing BMI and insulin resistance among obese hyperinsulinemic children and adolescents in the short term. Larger, long-term studies across different populations are needed to establish the role of metformin as therapy for obesity and cardiometabolic risk in young people.
Supplementary Material
Acknowledgments
M.H.P. is funded by a studentship from the Economic and Social Research Council, U.K.
No potential conflicts of interest relevant to this article were reported.
Data were presented in abstract form at the 17th European Congress on Obesity (ECO 2009), Amsterdam, The Netherlands, 6–9 May 2009.
We thank the authors of the original studies who provided additional data to enable us to perform meta-analyses. M.H.P. contributed to the protocol, search strategy, literature searches, study selection, data extraction, data synthesis, and manuscript writing. R.V. and S.K. conceived the review and contributed to the protocol, study selection, and manuscript writing. K.J.W. contributed to the protocol, search strategy, data extraction, and data synthesis. B.W. contributed to data extraction. All authors commented on the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Golay A. Metformin and body weight. Int J Obes 2007; 32: 61– 72 [DOI] [PubMed] [Google Scholar]
- 2.UKPDS Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352: 854– 865 [PubMed] [Google Scholar]
- 3.Diabetes Prevention Program Research Group: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393– 403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atabek ME, Pirgon O. Use of metformin in obese adolescents with hyperinsulinemia: a 6-month, randomized, double-blind, placebo-controlled clinical trial. J Pediatr Endocrinol 2008; 21: 339– 348 [DOI] [PubMed] [Google Scholar]
- 5.Srinivasan S, Ambler GR, Baur LA, Garnett SP, Tepsa M, Yap F, Ward GM, Cowell CT. Randomized, controlled trial of metformin for obesity and insulin resistance in children and adolescents: improvement in body composition and fasting insulin. J Clin Endocrinol Metab 2006; 91: 2074– 2080 [DOI] [PubMed] [Google Scholar]
- 6.Freemark M, Bursey D. The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics 2001; 107: E55. [DOI] [PubMed] [Google Scholar]
- 7.Love-Osborne K, Sheeder J, Zeitler P. Addition of metformin to a lifestyle modification program in adolescents with insulin resistance. J Pediatr 2008; 152: 817– 822 [ See comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanovski JA, Sorg RA, Krakoff J, Kozlosky M, Sebring NG, Salaita CG, Keil M, McDuffie JR, Calis KA. A randomized, placebo-controlled trial of the effects of metformin on body weight and body composition in children with insulin resistance. Abstract presented at the 90th Annual Meeting of The Endocrine Society, 16 June 2008, Moscone Center, San Francisco, California [Google Scholar]
- 9.Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. Prevalence and determinants of insulin resistance among U.S. adolescents. Diabetes Care 2006; 29: 2427– 2432 [DOI] [PubMed] [Google Scholar]
- 10.Whincup PH, Cook DG, Adshead F, Taylor S, Papacosta O, Walker M, Wilson V. Cardiovascular risk factors in British children from towns with widely differing adult cardiovascular mortality. BMJ 1996; 313: 79– 84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGovern L, Johnson JN, Paulo R, Hettinger A, Singhal V, Kamath C, Erwin PJ, Montori VM. Clinical review: treatment of pediatric obesity: a systematic review and meta-analysis of randomized trials. J Clin Endocrinol Metab 2008; 93: 4600– 4605 [DOI] [PubMed] [Google Scholar]
- 12.Oude Luttikhuis H, Baur L, Jansen H, Shrewsbury VA, O'Malley C, Stolk RP, Summerbell CD. Interventions for treating obesity in children. Cochrane Database Syst Rev 2009;( 1): CD001872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.