Abstract
Pancreatic pseudocysts develop in 10% to 20% of patients with chronic pancreatitis, and can cause a variety of complications such as infection, bleeding or development of fistulae. However, fistulous communication with the common bile duct is very rare. The present report describes an unusual case of a patient with a large, symptomatic pancreatic pseudocyst with a fistula to the common bile duct that was treated successfully by combined biliary and pancreatic stenting.
Keywords: Fistula, Pancreatitis, Pseudocyst, Stenting
Abstract
De 10 % à 20 % des patients atteints d’une pancréatite chronique acquièrent un pseudokyste pancréatique qui peut causer diverses complications telles qu’une infection, une hémorragie ou l’apparition de fistules. Cependant, la communication fistulaire avec le canal cholédoque est très rare. Le présent rapport décrit le cas inhabituel d’un patient ayant un gros pseudokyste pancréatique symptomatique, accompagné d’une fistule vers le canal cholédoque traitée avec succès par une endoprothèse biliaire et pancréatique combinée.
Pancreatic pseudocysts (PPs) are present in approximately 10% to 20% of patients with chronic pancreatitis, and most commonly occur in alcoholic chronic pancreatitis. Abdominal pain is the most common symptom, with nausea, vomiting, palp-able abdominal mass, bleeding and jaundice being less common findings. Complications include bleeding, common bile duct (CBD) obstruction, duodenal obstruction and external-internal pancreatic fistulae. These fistulae are found in 5% to 41% of cases. Treatment generally consists of percutaneous, endoscopic or surgical drainage for individuals with symptoms, complications or enlarging pseudocysts. We diagnosed a 42-year-old man who had a PP with a fistulous communication to the CBD. This has not been described previously, other than in a small number of case reports.
CASE PRESENTATION
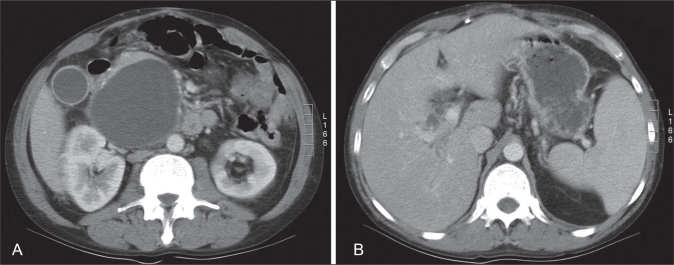
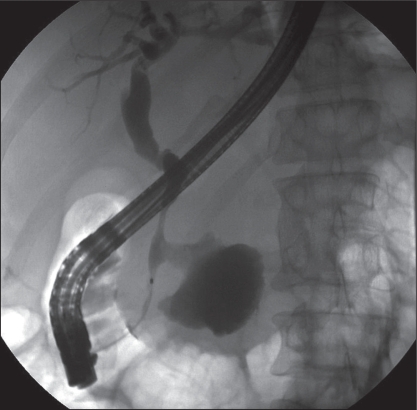
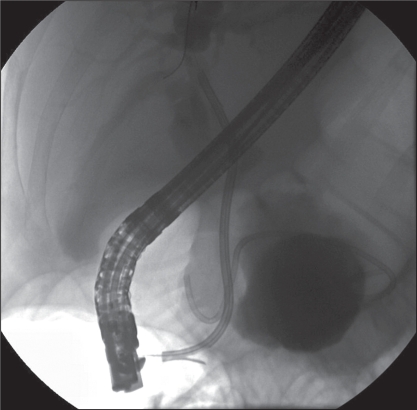
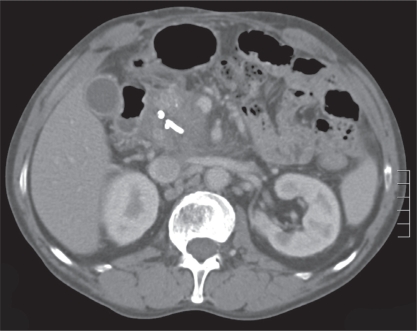
A 42-year-old Caucasian man with a history of deep vein thrombosis, heparin-induced thrombocytopenia, chronic pancreatitis secondary to alcohol abuse and a PP drained surgically five years previously, presented to Vancouver General Hospital, Vancouver, British Columbia. The patient had a two-week history of progressive jaundice, mild-to-moderate dull right upper quadrant pain and a 6 kg to 8 kg weight loss over a one-year period. The physical examination revealed a deeply jaundiced and mildly febrile patient (37.8°C). In addition, his right upper quadrant was tender, with mild hepatomegaly. The initial laboratory investigation revealed a hemoglobin level of 100 g/L, a mean corpuscular volume of 116 fL, gammaglutamyl transferase 387 U/L, alkaline phosphatase 1192 U/L, alanine aminotransferase 120 U/L, aspartate aminotransferase 206 U/L, international normalized ratio 1.27, albumin 24 g/L and direct bilirubin 180 mg/L. Radiological examination, including abdominal ultrasound and computed tomography scan, showed a relatively thin-walled, cystic mass in the region of the pancreatic head measuring 9.6 cm × 8.5 cm × 8.4 cm, causing mild pancreatic duct (4 mm to 5 mm) and CBD dilation (10 mm). In addition, there were multiple punctate pancreatic calcifications (Figure 1). Subsequently, the patient underwent endoscopic retrograde cholangiopancreatography (ERCP) that revealed a long, distal CBD stricture and a large pseudocyst in the head of the pancreas, filling via a fistulous communication with the CBD at the middle of the stricture (Figure 2). A 10 Fr, 10 cm Cotton-Leung stent (Cook Medical, USA) was placed in the CBD, and a 7 Fr, 7 cm double-pigtail stent was placed via the transpapillary route across the fistula into the pseudocyst (Figure 3). The patient was started on antibiotics and discharged home a few days later – after his symptoms dramatically improved and liver enzymes normalized. He remained pain-free without further hospitalization. A follow-up computed tomography scan of his abdomen two months later showed a dramatic resolution of the pseudocyst (Figure 4). The pigtail stent was removed during ERCP two months after the initial presentation and the biliary stent was exchanged in view of the persisting biliary stricture. ERCP performed after one further month revealed no evidence of residual fistulous communication between the CBD and the previous pseudocyst. The patient continued to do well and was scheduled for further stenting of his biliary stricture. The bile duct stricture was presumed secondary to chronic pancreatitis and to compression from the previous pseudocyst. It was unclear at this stage whether he required longer-term biliary stenting or perhaps surgery, or whether this stricture was mainly the result of a relatively inflammatory process.
Figure 1.
A Contrast-enhanced computed tomography scan of the upper abdomen. A relatively thin-walled cystic mass is identified in the region of the pancreatic head measuring 9.6 cm × 8.5 cm × 8.4 cm in size. Multiple punctate pancreatic calcifications are seen, consistent with a history of pancreatitis. B The cyst indents the common bile duct anteriorly, with intrahepatic biliary dilation
Figure 2.
Image from an endoscopic retrograde cholangiopancreatography following injection of contrast into the biliary system. A biliary stricture is seen, with a patulous communication between the common bile duct and the pseudocyst
Figure 3.
A Cotton-Leung stent (Cook Medical, USA) placed in the common bile duct across the site of the communication. A second double-pigtail stent extends from within the cavity across the bile duct and into the duodenum
Figure 4.
Follow-up contrast-enhanced computed tomography scan of the abdomen showing a significant decrease in the size of the pseudocyst with the two stents in position
DISCUSSION
PPs occur in approximately 10% to 20% of patients with chronic pancreatitis, and are most commonly seen in alcoholic chronic pancreatitis (1). PPs are persistent, peripancreatic, enzyme-rich fluid collections with a clearly defined fibrous wall but lacking a true epithelial lining (1). Pseudocysts are typically caused by disruption of the pancreatic duct with extravasation of pancreatic enzymes. Such disruption can be caused either by an acute event (trauma or stone passage) or repeated attacks of pancreatitis. Approximately 30% of acute pseudocysts resolve spontaneously; however, thick-walled chronic pseudocysts can often persist and enlarge. Both acute and chronic pseudocysts can cause serious complications such as sepsis, obstruction, hemorrhage, pancreatic ascites and, rarely, fistula formation to other viscera. The most common organs to which pseudocysts can fistulize are the stomach, duodenum, colon and, less commonly, the esophagus (2). Fistulas to the biliary tract are distinctly rare. In fact, to our knowledge, there have been only 16 cases of PPs with fistula to the CBD reported in the literature (3–15) (Table 1). Among these 16 cases, most were in men. Alcoholic chronic pancreatitis was the most common etiology, with abdominal pain being the most common clinical presentation. Ten of 16 cases were treated surgically, three patients were treated with percutaneous external drainage of the pseudocyst and one patient was observed. Only two cases were successfully treated endoscopically by placing one stent in the biliary tract and another across the fistula (14,15). Fistulous communication of a PP to the CBD is uncommon. We have demonstrated that it can be successfully managed endoscopically. It was believed that biliary stenting alone would not have been adequate because the pseudocyst was quite large, as was the size of the fistulous communication with the CBD.
TABLE 1.
Summary of case reports in the literature
| Author (reference) | Patient sex/age, years | Type and etiology of pancreatitis | Presentation | Treatment |
|---|---|---|---|---|
| Dalton et al (3) | Male/61 | Chronic alcoholic | Abdominal pain | Cystoduodenostomy |
| Sankaran and Walt (4) | Unknown | Alcoholic | Unknown | Unknown |
| Grace and Jordan (5) | Unknown | Unknown | Unknown | Choledochoduodenostomy |
| Ro and Yoon (6) | Male/66 | Chronic | Abdominal pain | Cystoduodenostomy |
| Gadacz et al (7) | Male/59 | Chronic alcoholic | Abdominal pain | Pancreaticoduodenectomy |
| Gadacz et al (7) | Male/40 | Chronic alcoholic | Abdominal pain | Cholecystostomy, external drainage |
| Skellenger et al (8) | Male/47 | Acute alcoholic | Abdominal pain | External drainage |
| Ellenbogen et al (9) | Male/54 | Chronic alcoholic | Abdominal pain | Cystojejunostomy |
| DeVanna et al (10) | Male/2 | Acute | Dehydration, upper respiratory infection | Observation |
| Hauptmann et al (11) | Male/53 | Chronic alcoholic | Abdominal pain | External drainage |
| Bresler et al (12) | Male/66 | Chronic alcoholic | Abdominal pain | T-tube, choledochotomy |
| Raimondo et al (13) | Female/69 | Chronic alcoholic | Abdominal pain | Pancreaticojejunostomy |
| Boulanger et al (14) | Male/52 | Alcoholic | Abdominal pain | Endoscopic internal drainage |
| Carrere et al (15) | Male/74 | Chronic alcoholic | Abdominal pain | Endoscopic internal drainage |
| Carrere et al (15) | Male/67 | Acute | Abdominal pain | Cystojejunostomy, hepaticojejunostomy, cholecystectomy |
| Carrere et al (15) | Male/65 | Acute | Abdominal pain | Cholecystectomy, biliary and pancreatic bypass |
REFERENCES
- 1.Grace PA, Williamson RC. Modern management of pancreatic pseudocysts. Br J Surg. 1993;80:573–81. doi: 10.1002/bjs.1800800508. [DOI] [PubMed] [Google Scholar]
- 2.Clements JL, Jr, Bradley EL, III, Eaton SB., Jr Spontaneous internal drainage of pancreatic pseudocysts. Am J Roentgenol. 1976;126:98–991. doi: 10.2214/ajr.126.5.985. [DOI] [PubMed] [Google Scholar]
- 3.Dalton WE, Lee HM, Williams GM, Hume DM. Pancreatic pseudocyst causing hemobilia and massive gastrointestinal hemorrhage. Am J Surg. 1970;120:106–7. doi: 10.1016/s0002-9610(70)80158-1. [DOI] [PubMed] [Google Scholar]
- 4.Sankaran S, Walt AJ. The natural and unnatural history of pancreatic pseudocysts. Br J Surg. 1975;62:37–44. doi: 10.1002/bjs.1800620110. [DOI] [PubMed] [Google Scholar]
- 5.Grace RR, Jordan PH., Jr Unresolved problems of pancreatic pseudocysts. Ann Surg. 1976;184:16–21. doi: 10.1097/00000658-197607000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ro JO, Yoon BH. Pancreatic pseudocyst as a cause of gastrointestinal bleeding and hemobilia. A case report. Am J Gastroenterol. 1976;66:287–91. [PubMed] [Google Scholar]
- 7.Gadacz TR, Lillemoe K, Zinner M, Merrill W. Common bile duct complications of pancreatitis evaluation and treatment. Surgery. 1983;93:235–42. [PubMed] [Google Scholar]
- 8.Skellenger ME, Patterson D, Foley NT, Jordan PH., Jr Cholestasis due to compression of the common bile duct by pancreatic pseudocysts. Am J Surg. 1983;145:343–8. doi: 10.1016/0002-9610(83)90197-6. [DOI] [PubMed] [Google Scholar]
- 9.Ellenbogen KA, Cameron JL, Cocco AE, Gayler BW, Hutcheon DF. Fistulous communication of a pseudocyst with the common bile duct: Demonstration by endoscopic retrograde cholangiopancreatography. Johns Hopkins Med J. 1981;149:110–1. [PubMed] [Google Scholar]
- 10.DeVanna T, Dunne MG, Haney PJ. Fistulous communication of pseudocyst to the common bile duct: A complication of pancreatitis. Pediatr Radiol. 1983;13:344–5. doi: 10.1007/BF01625964. [DOI] [PubMed] [Google Scholar]
- 11.Hauptmann EM, Wojtowycz M, Reichelderfer M, McDermott JC, Crummy AB. Pancreatic pseudocyst with fistula to the common bile duct: Radiological diagnosis and management. Gastrointest Radiol. 1992;17:151–3. doi: 10.1007/BF01888533. [DOI] [PubMed] [Google Scholar]
- 12.Bresler L, Vidrequin A, Poussot D, et al. Fistulous communication of a pancreatic pseudocyst with the common bile duct: Demonstration by operative cholangiogram. Am J Gastroenterol. 1989;84:835–6. [PubMed] [Google Scholar]
- 13.Raimondo M, Ashby AM, York EA, Derfus GA, Farnell MB, Clain JE. Pancreatic pseudocyst with fistula to the common bile duct presenting with gastrointestinal bleeding. Dig Dis Sci. 1998;43:2622–6. doi: 10.1023/a:1026638908243. [DOI] [PubMed] [Google Scholar]
- 14.Boulanger S, Volpe CM, Ullah A, Lindfield V, Doerr R. Pancreatic pseudocyst with biliary fistula: Treatment with endoscopic internal drainage. South Med J. 2001;94:347–9. [PubMed] [Google Scholar]
- 15.Carrere C, Heyries L, Barthet M, Bernard JP, Grimaud JC, Sahel J. Biliopancreatic fistulas complicating pancreatic pseudocysts: A report of three cases demonstrated by endoscopic retrograde cholangiopancreatography. Endoscopy. 2001;33:91–4. doi: 10.1055/s-2001-11177. [DOI] [PubMed] [Google Scholar]