Abstract
Sentinel lymph node biopsy (SLNB), a less invasive alternative to axillary lymph node dissection (ALND), has become the standard of care for patients with clinically node-negative breast cancer. In SLNB, lymphatic mapping with radio-labeled sulfur colloid and/or blue dye helps identify the sentinel lymph node (SLN), which is most likely to contain metastatic breast cancer. Even though SLNB, using both methylene blue and radioactive tracers, has a high identification rate, it still relies on an invasive surgical procedure, with associated morbidity. In this study, we have demonstrated a non-invasive single-walled carbon nanotube (SWNT)-enhanced photoacoustic (PA) identification of SLN in a rat model. We have successfully imaged the SLN in vivo by PA imaging (793 nm laser source, 5 MHz ultrasonic detector) with high contrast-to-noise ratio (= 89) and good resolution (~500 μm). The SWNTs also show a wideband optical absorption, generating PA signals over an excitation wavelength range of 740–820 nm. Thus, by varying the incident light wavelength to the near infrared region, where biological tissues (hemoglobin, tissue pigments, lipids, and water) show low light absorption, the imaging depth is maximized. In the future, functionalization of the SWNTs with targeting groups should allow the molecular imaging of breast cancer.
1. Introduction
For the majority of invasive breast cancers, the surgical removal of primary breast tumor and level I and level II axillary lymph node dissections (ALND) are widely performed (NIH Consensus Statement 1990). However, the common side effects after ALND include upper-extremity lymphedema, arm numbness, impaired shoulder mobility, arm weakness, and infections in the breast, chest, or arm (Swenson et al 2002). A less invasive, more accurate alternative to ALND is sentinel lymph node biopsy (SLNB). For patients with clinically node-negative breast cancer, SLNB has rapidly become the standard of care (Krag et al 1998, McMasters et al 2000). The concept of a sentinel lymph node biopsy assumes that the primary draining or sentinel node will be the first to contain metastases. The hypothesis is that both the mammary gland and overlying skin share a common lymphatic pathway to the same axillary sentinel node. Therefore, intradermal injection of blue dye will lead to the accumulation of the dye in the sentinel lymph nodes (Borgstein et al 1997). In this surgery, a special blue dye and/or a radioactive substance is first injected into the breast to determine which lymph nodes are the first to receive drainage from the breast. These nodes are potentially the first to be invaded by cancer cells. One to three sentinel nodes are usually removed and tested for cancer. If cancerous, then all the lymph nodes are removed. This surgery has fewer complications than axillary node dissection, but the physicians performing the procedure must have special training. The identification rate and sensitivity of this technique are less than 95%, even in experienced hands (Krag et al 1998, McMasters et al 2000, Ung 2004). Furthermore, the complications associated with the SLNB procedure include seroma formation, lymphedema, sensory nerve injury, and limitation in the range of motion (Purushotham et al 2005). These limitations of SLNB strongly suggest that alternative strategies to stage the axilla should be explored.
Axillary ultrasound (AUS) has been proposed as a potential non-invasive technique for identifying axillary metastases (Krishnamurthy et al 2002, Deurloo et al 2003, Brancato et al 2004). AUS can visualize the lymph node’s size, shape, and contour, as well as changes in cortical morphology and texture that appear to be associated with the presence of axillary metastases. However, the ability of AUS alone to stage the axilla accurately is limited because the sonographic signs of metastatic disease may overlap with those of benign reactive changes. On the other hand, in vivo identification of a sentinel lymph node (SLN) using photoacoustic (PA) imaging would allow non-invasive axillary staging, in conjunction with either percutaneous fine needle aspiration biopsy (FNAB) or other emerging molecular techniques.
Photoacoustic tomography (PAT) (Hoelen et al 1998, Ku and Wang 2005, Ku et al 2005, Zhang et al 2006, Song and Wang 2007, Song et al 2008) that combines the advantages of optical absorption contrast with ultrasonic spatial resolution has emerged as an excellent technique for non-invasive imaging of biological tissues, offering high spatial resolution and high soft tissue contrast. In vivo PA imaging of SLN in a rat model was also reported using methylene blue (Song et al 2008). However, methylene blue does not permit molecular imaging. Recently, single-walled carbon nanotube (SWNT)-based contrast agents have shown promise for a variety of imaging techniques. The prospect of SWNTs as a contrast agent for PA imaging was also reported (Pramanik et al 2009). In this study, we exploited the intrinsic optical absorbance (Huges et al 2007, Berciaud et al 2007) of carbon nanotubes to develop them as contrast agents, and used them to perform non-invasive imaging of SLN in a rat model in vivo.
2. Methods and Materials
2.1. Photoacoustic Imaging
A reflection-mode PA imaging system (Song and Wang 2007) was used to get the PA spectroscopy of the SWNTs. A tunable Ti:sapphire laser (LT-2211A, LOTIS TII) pumped by Q-switched Nd:YAG (LS-2137, LOTIS II) laser was the light source, providing <15 ns pulse duration and a 10-Hz pulse repetition rate. A dark-field ring-shaped illumination was used (Maslov et al 2005). The light energy on the sample surface was controlled to conform to the American National Standards Institute (ANSI) standard for maximum permissible exposure (MPE) (ANSI 2000). A 3.5 MHz/5 MHz central frequency, spherically focused (4.95 cm/2.54 cm focus length, 1.91 cm diameter active area element, and 70%/72% bandwidth) ultrasonic transducer (V380/V308, Panametrics-NDT) was used to acquire the generated PA signals. The signal was then amplified by a low-noise amplifier (5072PR, Panametrics-NDT), and recorded using a digital oscilloscope (TDS 5054, Tektronix) with a 50 mega-sampling rate. PA signal fluctuations due to pulse-to-pulse energy variation were compensated by signals from a photodiode (DET110, Thorlabs), which sampled the energy of each laser pulse.
A linear translation stage (XY-6060, Danaher Motion) was used for raster scanning to obtain three-dimensional (3-D) PA data. A computer controlled the stage and synchronized it with the data acquisition. To shorten the data acquisition time, a continuous scan was used without signal averaging. An A-line (A-scan) was the PA signal obtained along the depth direction at a single point. Multiple A-lines (acquired by a one-dimensional (1-D) scan) gave a two-dimensional (2-D) B-scan. A 3-D image was acquired with a 2-D scan. A 1-D depth-resolved image was obtained by multiplying the time axis of the initial A-scan (resolved in time along the depth direction) by the speed of sound in soft tissue (~1500 m/s).
The scanning time depends on the laser pulse repetition rate (PRR), the scanning step size, and the field of view (FOV). Typical values are a scanning step size for a 1-D scan = 0.1 mm, for a 2-D scan = 0.2 mm, a laser PRR = 10 Hz, and a FOV = 20 mm × 20 mm. The acquisition time = ~25 sec for a B-scan, and = ~42 min for a 3-D image. The transducer was located inside a water container with an opening of 5 cm × 5 cm at the bottom, sealed with a thin, clear membrane. The object was placed under the membrane, and ultrasonic gel was used for coupling the sound.
2.2. SWNTs synthesis
Iron (Fe) was coated onto a silicon wafer using the diblock copolymer template method (Fu et al 2004). SWNTs were synthesized using the carbon vapor deposition (CVD) method with Fe as the metal catalyst (Easy Tube 2000, First Nano). The wafer was placed into a 3 inch quartz reaction chamber that was heated with Ar at 900°C, hydrogen gas was added for 2 minutes, and methane was fed into the reactor for 20 minutes to grow SWNTs over the Fe catalyst. The SWNTs were further dispersed in 1 wt% Pluronic® F127 surfactant (1 g of surfactant in 100 g of deionized water) at the appropriate concentration and sonicated rigorously to obtain a homogeneous dispersion. To visualize the SWNT structure, transmission electron microscopy (TEM) was performed (Tecnai12 BioTwinG2, FEI, Hillsboro, OR) at 80 kV on SWNT samples mounted on a 400 mesh copper grid with formvar coating. High resolution TEM (hrTEM) (JEOL 2000 FX) was also performed at 200 kV for SWNT samples mounted on a copper grid that was coated with amorphous carbon-holey film. In addition, atomic force microscopy (AFM) was also performed (MFD-3D-BIO, Asylum Research, Santa Barbara, CA). A Raman spectrum of the SWNTs was also taken at 633 nm (LabRam Aramis, Horiba JvonYvon).
2.3. Animal and Drug Information
Guidelines on the care and the use of laboratory animals at Washington University in St. Louis were followed for all animal experiments. Adult Sprague Dawley rats with various body weights (250 – 350 g) were used for the experiments. Initial anesthetization of the rat was done using a mixture of ketamine (85 mg/kg) and xylazine (15 mg/kg). The hair on the region of interest of the rat was gently removed before imaging, using a commercial hair-removal lotion. Intradermal injection of 0.075 ml of 0.5 mg/ml SWNTs was performed on a left/right forepaw pad, depending on which side was imaged. PA images were acquired after the administration of SWNTs. During the image acquisition, anesthesia was maintained using vaporized isoflurane (1 L/min oxygen and 0.75% isoflurane, Euthanex Corp.), and a pulse oximeter (NONIN Medical INC., 8600V) was used to monitor the vitals. If needed, 8 ml of 0.9% saline was administered to the rat for hydration. After image acquisition, the animal was euthanized by pentobarbital overdose.
3. Results and Discussions
3.1. Characterization of SWNTs
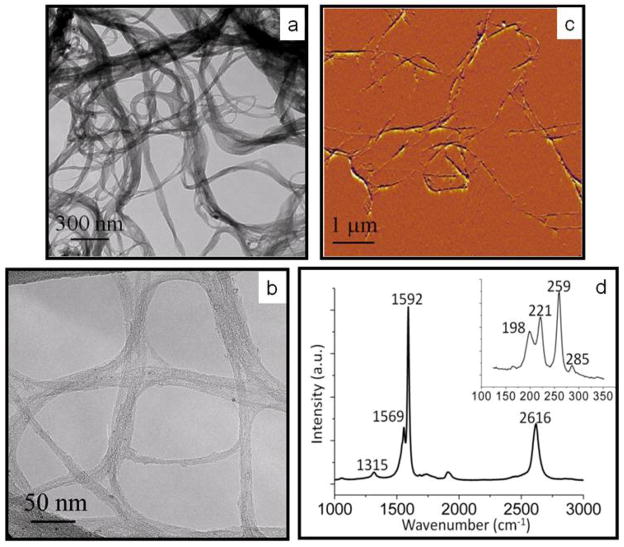
Figure 1 shows the TEM, hrTEM, and Raman spectroscopy of the synthesized SWNTs used in this study. These images were used to characterize the SWNTs. Figures 1(a) and 1(b) show low-resolution bright field TEM and the hrTEM images of the SWNTs, respectively. These two figures confirm the growth of SWNTs with an approximate diameter of 2 nm. Figure 1(c) shows an AFM image, which confirms that the SWNT diameters were consistent with the diameters observed in the hrTEM image. Figure 1(d) shows the Raman spectrum of the SWNTs at 633 nm. The Raman spectrum shows the G band at 1592 cm−1 and the D band at 1315 cm−1. The G band is a peak specific to graphene, while the D band indicates defects on the graphene sheet. The low D band to G band ratio signifies few defects in the SWNT sample. The radial breathing modes, which occur in the presence of small diameter tubes of a single graphene layer, can be viewed within the inset, and further confirm that these samples were SWNTs (Dresselhaus et al 2005).
Figure 1.
SWNT characterization. (a) TEM and (b) hrTEM image of the SWNTs produced by the Fe catalyst. (c) AFM image of the SWNTs produced by the Fe catalyst, confirming the nanotube diameter of 2 nm. (d) A Raman spectrum of the SWNT obtained at 633 nm. The G band is at 1592 cm−1, while the D band is found at 1315 cm−1. The radial breathing modes occur between 100 and 350 cm−1 (see inset).
3.2. Photoacoustic spectroscopy of SWNTs
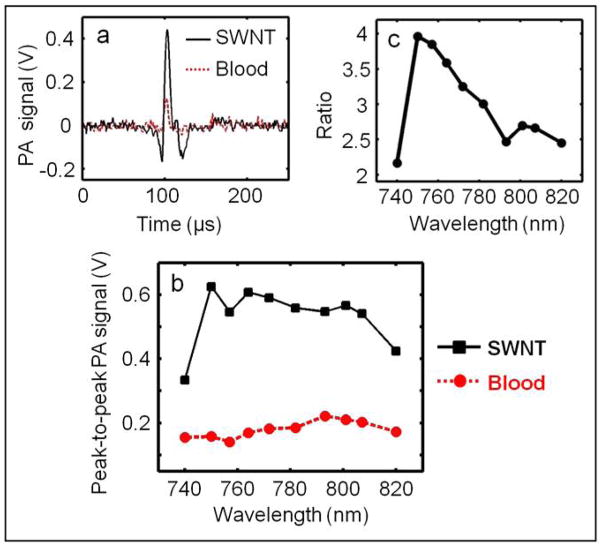
Blood is a dominant optical absorber in the human body and produces strong PA signals. Therefore, the PA signal from SWNTs was compared with that from blood to show that, SWNTs are capable of generating PA signals comparable to or stronger than that of a known absorber in the body in the near infrared (NIR) wavelength window. Figure 2(a) shows the PA signals obtained from a tygon tube (I.D. 250 μm, O.D. 500 μm) filled with SWNTs (0.25 mg/ml) and rat blood. The laser was tuned to 764 nm wavelength. At this excitation wavelength, the peak-to-peak PA signal amplitude obtained from SWNTs was ~600 mV, compared to a ~170 mV peak-to-peak PA signal amplitude from blood alone. Figure 2(b) shows the PA spectrum (peak-to-peak PA signal amplitude versus excitation light wavelength) of the SWNTs (in black) for an excitation wavelength range of 740–820 nm. The PA spectrum of rat blood (in red) is also shown in the same figure. It is evident that the PA signal obtained from SWNTs is much stronger than that of blood over the entire wavelength range. Therefore, one can choose a specific light wavelength for imaging within a broad range. Figure 2(c) plots the ratio of the peak-to-peak PA signal amplitude of SWNTs to that of blood between 740 and 820 nm. The PA signal from the tygon tube filled with SWNTs is more than four times stronger than that from blood at 750 nm. Over the entire 740–820 nm window, the PA signal from SWNTs is more than two times stronger than that from blood. Due to the weak blood absorption, the NIR window is well known for providing deep tissue PA imaging at the expense of blood contrast. The strong PA signal from SWNTs in the NIR region implies that they could be used as contrast agents to boost the signal strength for PA imaging in this region.
Figure 2.
(a) PA signals generated from a tygon tube (I.D. 250 μm, O.D. 500 μm) filled with SWNTs (0.25 mg/ml) and rat blood. The excitation optical wavelength is 764 nm. (b) PA spectra of SWNTs and blood over a 740–820 nm range of NIR wavelengths. (c) Ratio of the peak-to-peak PA signal amplitudes generated from SWNTs to those generated from blood.
3.3. Deep tissue imaging
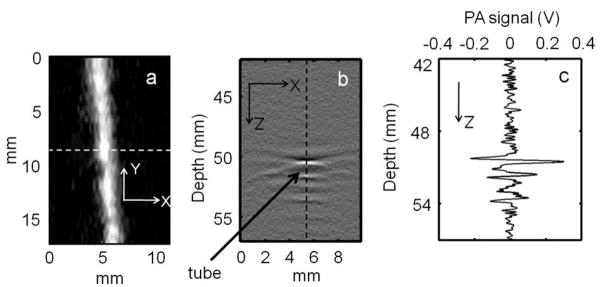
The sensitivity of the PA imaging system was determined from chicken tissue phantom experiments, used to mimic human breast tissue (Song et al 2008). The light source was tuned to 793 nm wavelength. A tube (Silastic® laboratory tubing, Dow Corning Corp.) with 1.47 mm I.D. was placed between two layers of chicken breast tissue. The tube was filled with 1 mg/ml SWNTs. The thickness of the tissue layer on top of the tube was ~20 mm. Figure 3(a) shows the maximum amplitude projection (MAP) (Zhang et al 2006) image of the tube. The tube is clearly seen in the image, with a high contrast-to-noise ratio (CNR) of 25. Figure 3(b) shows the B-scan PA image (along the dotted line in figure 3(a)). The bright spot in the B-scan represents the PA signal generated from the tube filled with SWNTs. Figure 3(c) shows the A-line PA signal (along the dotted line in figure 3(b)), clearly showing the strong PA signal from the tube filled with SWNTs compared to the weak signal from the surrounding chicken breast tissue. These results prove that use of SWNTs as a contrast agent enables tissue imaging more than 20 mm deep. For human studies, the depth of imaging is very important, as the mean depth of SLNs is 12±5 mm (distance from the skin surface to the top surface of SLN) (Margenthaler 2007). Therefore, the ability to image more than 20 mm deep with high CNR and high resolution is a key for mapping SLN non-invasively.
Figure 3.
(a) Maximum Amplitude Projection (MAP) image of a tube (Silastic® laboratory tubing, Dow Corning Corp., with 1.47 mm I.D.) filled with SWNTs (1 mg/ml) placed inside chicken breast tissue. The tube was placed 20 mm below the top surface of the tissue. The PA image clearly shows the tube, with a CNR of 25. (b) PA B-scan image along the dotted line in figure 3(a), with the bright spot showing the PA signal originating from the tube filled with SWNTs. (c) Photoacoustic A-line along the dotted line in figure 3(b).
3.4. Sentinel lymph node imaging non-invasively in a rat in vivo
Adult Sprague Dawley rats with various body weights (250 – 350 g) were used for the experiments. The hair on the axillary region of the rat was removed and a PA image was taken. This image was a control image for comparison with future images. An intradermal injection of 0.075 ml of 0.5 mg/ml SWNTs was performed on the forepaw pad. Four PA images were acquired at intervals of 25–30 minutes after the SWNTs injection. For all the PA images, the following parameters were used: FOV = 25 mm × 30 mm, step size along the X direction = 0.2 mm, step size along the Y direction = 0.4 mm, total scan time = ~23 minutes. Please note that no signal averaging was done for any of these images. The images shown here are cropped to a FOV of 16 mm × 24 mm, since the outside region was not of interest.
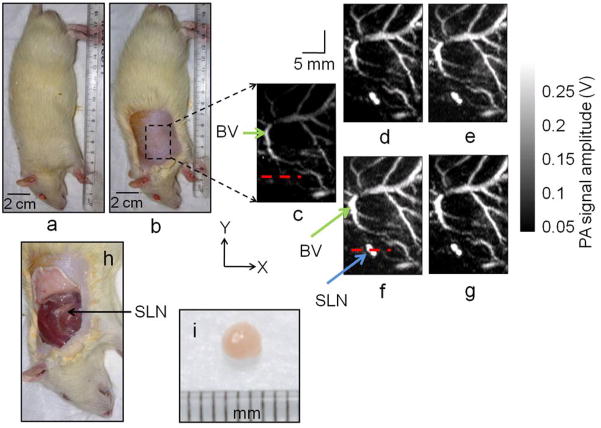
Figure 4(a) shows a representative digital photograph of a rat taken prior to image acquisition, and figure 4(b) shows the shaved axillary surface where the PA imaging was performed. Before the SWNTs injection, a PA control image was obtained, which is shown in the form of a MAP (Zhang et al 2006) in figure 4(c). The vasculature near an axial node (one blood vessel is marked as BV) was clearly imaged, with a high CNR of ~79 and good resolution of ~500 μm. Note that no lymph nodes are visible in the control image, since there is no intrinsic optical absorption in lymph nodes to produce any PA signal to image. Figure 4(d) shows the PA image (MAP) of the same area immediately after the SWNTs were injected, approximately 30 min after the control image was taken. Figures 4(e), 4(f), and 4(g) are the post-injection PA images (MAP) of the same area 30 min, 55 min, and 85 min after the SWNTs injection. The SLN appears at the left lower quadrant, marked as SLN in figure 4(f), and is clearly visible in all the post-injection PA images (Figures 4(d), 4(e), 4(f), and 4(g)). The images show high CNRs (74 in figure 4(d), 67 in figure 4(e), 84 in figure 4(f), and 89 in figure 4(g)). The contrast of the SLN to the surrounding blood vessel was up to 1.8 (ratio of the peak-to-peak PA signal amplitude obtained from SLN and BV) after SWNTs injection. The signal amplitude of the surrounding blood vessels was also increased by up to ~124% compared to that in the control image (94% in figure 4(d), 110% in figure 4(e), 124% in figure 4(f), and 105% in figure 4(g)), although the CNR remained almost the same. This increase in signal amplitude suggests that the SWNTs have traveled into the blood stream and the nearby tissues boosting the blood vessel signal as well as the background signal. Figure 4(h) is a digital photograph of the same rat with the skin removed after the completion of the PA imaging. Figure 4(i) is a digital photograph of the SLN removed from the rat (arrow in figure 4(i)). The photograph shows the SLN size to be 2–3 mm, matching the size obtained from PA images (Figures 4(d) to 4(g)).
Figure 4.
Non-invasive in vivo PA images (MAP) of the SLN in a rat. For all PA images, the laser was tuned to 793 nm wavelength. (a) Photograph of the rat. (b) Photograph of the rat after the hair was removed from the scanning region before taking the PA images. The scanning region is marked with a black dotted square. (c) Control PA image acquired before SWNTs injection. Bright parts represent optical absorption, here, from blood vessels (BV). (d) PA image (MAP) acquired immediately after the SWNTs injection. (e) 30 min post-injection PA image. (f) 55 min post-injection PA image. (g) 85 min post-injection PA image. Blood vessel (BV) and sentinel lymph node (SLN) are marked with arrows, and the SLN is visible in all images except the control image. (h) Photograph of the rat with the skin removed after PA imaging. (i) The excised lymph node. For Figures 4(c) to 4(g): FOV = 25 mm × 30 mm, step size along the X direction = 0.2 mm, step size along the Y direction = 0.4 mm, total scan time = ~23 minutes. No signal averaging was used. Only a FOV of 16 mm × 24 mm is shown.
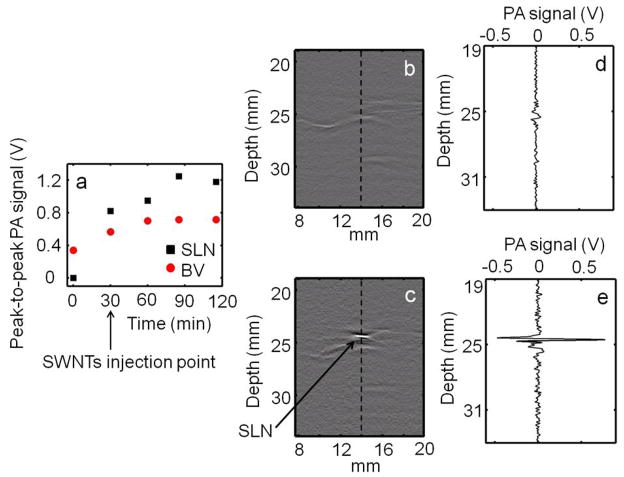
Figure 5(a) shows the uptake kinetics of the SWNTs. An increased PA signal was observed in both blood vessels and SLN during the first 30 minutes post injection, with the SLN showing a greater increase than blood vessels at all time points. Subsequently, the PA signal in the blood reached a plateau while the PA signal in the SLN kept increasing. These results further corroborate the imaging data presented in figure 4, where increase in signal intensities were observed post injection from both SLN and BV, but the intensity from SLN was stronger compared to the nearby blood vessels. The stronger signal is due to the strong light absorption of SWNTs compared to blood, and to the greater accumulation of SWNTs in the lymph node than in the surrounding blood vessels. Figures 5(b) and 5(c) show the B-scan PA images corresponding to the dotted lines marked in figures 4(c) and 4(f), respectively. The bright spot in figure 5(c) represents the strong PA signal from the SLN, which is not present in the pre-injection B-scan (Figure 5(b)). Figures 5(d) and 5(e) show the A-line PA signals corresponding to the dotted lines in figures 5(b) and 5(c), respectively. The strong PA signal obtained from the SLN is clearly seen in figure 5(e), whereas there is no such signal in the control image (Figure 5(d)). We used a 0.5 mg/ml concentration of SWNTs for our in vivo study (the average molecular weight of SWNTs is ~106 Da or g/mol; 0.5 mg/ml = 0.5 mg/ml/106 g/ml = 500 nM). However, that choice does not limit the use of SWNTs at other lower concentrations (De La Zerda A et al 2008). Our system is capable of detecting SWNTs on the order of nM concentration (Pramanik et al 2009).
Figure 5.
(a) Peak-to-peak PA signal amplitude obtained from SLN and blood vessel (BV) over time. The SWNTs were injected 30 min after the control scan. (b) PA B-scan image corresponding to the dotted line in figure 4(c), the control image. (c) PA B-scan image corresponding to the dotted line in figure 4(f), image showing the SLN. The bright spot represents the PA signal generated from the SLN. (d) A-line PA signal corresponding to the dotted line in (b). (e) A-line PA signal corresponding to the dotted line in (c), showing the strong PA signal generated from the SLN.
Our results show that non-invasive in vivo PA imaging for SLN identification with the use of SWNTs as a contrast agent is highly feasible in a small animal model. Moreover, the PA imaging technique used here to image rat SLN can easily be translated to humans. Although, SLN mapping using different contrast agents, e.g., carbon nanotubes, gold nanocages, gold nanorods, and methylene blue dye, has been done successfully, we believe SWNTs have many advantages over the other materials. The broad absorption spectrum of SWNTs will provide us a wide range of light wavelengths for imaging. Most of the other contrast agents have narrow absorption spectra, restricting their use to a particular wavelength. SWNTs also show good efficacy as contrast agents for thermoacoustic imaging (Pramanik et al 2009). In the future, thermoacoustic tomography can also be combined with PA imaging system for SLN mapping. Targeted SWNTs could also be used for more specific identification. Our previous PA studies demonstrated that SWNTs produce a PA signal at concentrations as low as 0.1 mg/ml (Pramanik et al. 2009). Using a 0.5 mg/ml concentration of SWNTs, we have obtained very high CNR sentinel lymph node images (CNR = ~90), thus it can be inferred that we could detect SWNTs at concentrations as low as 0.01 mg/ml. Assuming the SWNTs have an average diameter of 2 nm and average length of 1 μm, there should be ~ 105 carbon atoms in one SWNT, as derived by Yamamoto et al 2005, and a SWNT has a molecular weight of ~1.2 × 106 g/mol. Using these values, SWNTs should act as contrast agents in human blood at concentrations as low as 8 nM (0.1/1.2 × 106). SWNTs can also be functionalized in order to target specific tissues and to increase the SWNTs blood circulation time. This would prevent the SWNTs from being diluted in the human bloodstream for periods long enough to perform PA imaging.
Currently, this imaging system is limited by its slow scanning speed. Employing a higher pulse-repetition-frequency laser and an ultrasound array system could accelerate acquisition, potentially allowing real-time PA imaging (Yin et al 2004, Zeng et al 2004, Yang et al 2005). The in vivo biocompatibility of SWNTs needs to be thoroughly examined before its translation for clinical use. Nevertheless, since SLN identification by PA imaging is totally non-invasive and safe, it shows potential future clinical applications without the limitations of current invasive and minimally-invasive techniques.
4. Conclusions
We have shown that the PA signal from SWNTs is stronger than that of blood over the NIR wavelength window (740–820 nm) and demonstrated a non-invasive SWNTs-enhanced PA identification of SLN in a rat model in vivo with a high contrast-to-noise ratio (CNR = 89) and good resolution (~500 μm). We have also shown the possibility of deep tissue imaging (20 mm deep) using SWNTs as contrast agent. Our results suggest that this technology could be a useful pre-clinical and possibly clinical tool to identify SLNs non-invasively in vivo. In the future, the identification rate of node-negative breast cancer could be improved by functionalization of the SWNTs with targeting groups.
Acknowledgments
This work was supported by National Institutes of Health grants (R01 EB000712, R01 NS46214 (Bioengineering Research Partnerships), R01 EB008085, and U54 CA136398 (Network for Translational Research) - LVW) and the Office of the Vice President of Research at Stony Brook University, Carol M. Baldwin fund (SB). L.W. has a financial interest in Endra, Inc., which, however, did not support this work. The authors would like to thank Dr. Oleg Gang and Dr. Huming Xiong at the Center for Functional Nanomaterials, Brookhaven National Laboratory for access to the AFM, Mr. Tom Salagaj and Mr. Christopher Jensen at FirstNano/CVD Equipment Corporation for access to their CVD facilities and Dr. Eunah Lee at Horiba JvonYvon, Edison, NJ for the Raman Spectroscopy measurements.
References
- ANSI 2000 American National Standard for Safe Use of Lasers ANSI Standard Z136.1
- Berciaud S, Cognet L, Poulin P, Weisman RB, Lounis B. Absorption spectroscopy of individual single-walled carbon nanotubes. Nano Letters. 2007;7:1203–1207. doi: 10.1021/nl062933k. [DOI] [PubMed] [Google Scholar]
- Borgstein PJ, Meijer S, Pijpers R. Intradermal blue dye to identify sentinel lymph-node in breast cancer. Lancet. 1997;349:1668–9. doi: 10.1016/s0140-6736(05)62634-7. [DOI] [PubMed] [Google Scholar]
- Brancato B, et al. Role of ultrasound-guided fine needle cytology of axillary lymph nodes in breast carcinoma staging. Radiol Med (Torino) 2004;108:345–355. [PubMed] [Google Scholar]
- De La Zerda A, et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nature Nanotechnology. 2008;3:557–562. doi: 10.1038/nnano.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deurloo EE, Tanis PJ, Gilhuijs KGA, Muller SH, Kroger R, Peterse JL, Rutgers EJT, Olmos RV, Kool LJS. Reduction in the number of sentinel lymph node procedures by preoperative ultrasonography of the axilla in breast cancer. European Journal of Cancer. 2003;39:1068–1073. doi: 10.1016/s0959-8049(02)00748-7. [DOI] [PubMed] [Google Scholar]
- Dresselhaus MS, Dresselhaus G, Saito R, Jorio A. Raman spectroscopy of carbon nanotubes. Physics Reports-Review Section of Physics Letters. 2005;409:47–99. [Google Scholar]
- Fu Q, Huang SM, Liu J. Chemical vapor depositions of single-walled carbon nanotubes catalyzed by uniform Fe2O3 nanoclusters synthesized using diblock copolymer micelles. Journal of Physical Chemistry B. 2004;108:6124–6129. doi: 10.1021/jp049483+. [DOI] [PubMed] [Google Scholar]
- Hoelen CGA, De Mul FFM, Pongers R, Dekker A. Three-dimensional photoacoustic imaging of blood vessels in tissue. Optics Letters. 1998;23:648–650. doi: 10.1364/ol.23.000648. [DOI] [PubMed] [Google Scholar]
- Hughes ME, Brandin E, Golovchenko JA. Optical absorption of DNA-carbon nanotube structures. Nano Letters. 2007;7:1191–1194. doi: 10.1021/nl062906u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krag D, et al. The sentinel node in breast cancer - A multicenter validation study. New England Journal of Medicine. 1998;339:941–946. doi: 10.1056/NEJM199810013391401. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy S, Sneige N, Bedi DG, Edieken BS, Fornage BD, Kuerer HM, Singletary SE, Hunt KK. Role of ultrasound-guided fine-needle aspiration of indeterminate and suspicious axillary lymph nodes in the initial staging of breast carcinoma. Cancer. 2002;95:982–988. doi: 10.1002/cncr.10786. [DOI] [PubMed] [Google Scholar]
- Ku G, Wang LHV. Deeply penetrating photoacoustic tomography in biological tissues enhanced with an optical contrast agent. Optics Letters. 2005;30:507–509. doi: 10.1364/ol.30.000507. [DOI] [PubMed] [Google Scholar]
- Ku G, Wang XD, Xie XY, Stoica G, Wang LHV. Imaging of tumor angiogenesis in rat brains in vivo by photoacoustic tomography. Applied Optics. 2005;44:770–775. doi: 10.1364/ao.44.000770. [DOI] [PubMed] [Google Scholar]
- Margenthaler JA. Depth measurement of sentinel lymph nodes in human. 2007 Unpublished data. [Google Scholar]
- Maslov K, Stoica G, Wang LVH. In vivo dark-field reflection-mode photoacoustic microscopy. Optics Letters. 2005;30:625–627. doi: 10.1364/ol.30.000625. [DOI] [PubMed] [Google Scholar]
- McMasters KM, et al. Sentinel lymph node biopsy for breast cancer: A suitable alternative to routine axillary dissection in multi-institutional practice when optimal technique is used. Journal of Clinical Oncology. 2000;18:2560–2566. doi: 10.1200/JCO.2000.18.13.2560. [DOI] [PubMed] [Google Scholar]
- NIH Consensus Statement. Treatment of early stage breast cancer. NIH Consensus Statement. 1990;8:1–19. [Google Scholar]
- Pramanik M, Swierczewska M, Green D, Sitharaman B, Wang LHV. Carbon nanotubes as a multimodal—thermoacoustic and photoacoustic—contrast agent. Journal of Biomedical Optics. 2009;14(3) doi: 10.1117/1.3147407. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushotham AD, Upponi S, Klevesath MB, Bobrow L, Millar K, Myles JP, Duffy SW. Morbidity after sentinel lymph node biopsy in primary breast cancer: Results from a randomized controlled trial. Journal of Clinical Oncology. 2005;23:4312–4321. doi: 10.1200/JCO.2005.03.228. [DOI] [PubMed] [Google Scholar]
- Song KH, Wang LHV. Deep reflection-mode photoacoustic imaging of biological tissue. Journal of Biomedical Optics. 2007;12:060503. doi: 10.1117/1.2818045. [DOI] [PubMed] [Google Scholar]
- Song KH, Stein EW, Margenthaler JA, Wang LHV. Noninvasive photoacoustic identification of sentinel lymph nodes containing methylene blue in vivo in a rat model. Journal of biomedical Optics. 2008;13:054033. doi: 10.1117/1.2976427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson KK, Nissen MJ, Ceronsky C, Swenson L, Lee MW, Tuttle TM. Comparison of side effects between sentinel lymph node and axillary lymph node dissection for breast cancer. Annals of Surgical Oncology. 2002;9:745–753. doi: 10.1007/BF02574496. [DOI] [PubMed] [Google Scholar]
- Ung OA. Australasian experience and trials in sentinel lymph node biopsy: the RACS SNAC trial. Asian J Surg. 2004;27:284–290. doi: 10.1016/S1015-9584(09)60052-X. [DOI] [PubMed] [Google Scholar]
- Wang XD, Pang YJ, Ku G, Xie XY, Stoica G, Wang LHV. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nature Biotechnology. 2003;21:803–806. doi: 10.1038/nbt839. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Kamimura T, Matsumoto K. Nitrogen doping of single-walled carbon nanotube by using mass-separated low-energy ion beams. Japanese Journal of Applied Physics Part 1-Regular Papers Short Notes & Review Papers. 2005;44:1611–1614. [Google Scholar]
- Yang DW, Xing D, Gu HM, Tan Y, Zeng LM. Fast multielement phase-controlled photoacoustic imaging based on limited-field-filtered back-projection algorithm. Applied Physics Letters. 2005;87:194101. [Google Scholar]
- Yin BZ, Xing D, Wang Y, Zeng YG, Zeng YG, Tan Y, Chen Q. Fast photoacoustic imaging system based on 320-element linear transducer array. Physics in Medicine and Biology. 2004;49:1339–1346. doi: 10.1088/0031-9155/49/7/019. [DOI] [PubMed] [Google Scholar]
- Zeng YG, Xing D, Wang Y, Yin BZ, Chen Q. Photoacoustic and ultrasonic coimage with a linear transducer array. Optics Letters. 2004;29:1760–1762. doi: 10.1364/ol.29.001760. [DOI] [PubMed] [Google Scholar]
- Zhang HF, Maslov K, Stoica G, Wang LHV. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nature Biotechnology. 2006;24:848–851. doi: 10.1038/nbt1220. [DOI] [PubMed] [Google Scholar]