Abstract
Restless leg syndrome (RLS) is a sensorimotor disorder. Clinical studies have implicated the dopaminergic system in RLS, while others have suggested that it is associated with insufficient levels of brain iron. To date, alterations in brain iron status have been demonstrated but, despite suggestions from the clinical literature, there have been no consistent findings documenting a dopaminergic abnormality in RLS brain tissue. In this study, the substantia nigra and putamen were obtained at autopsy from individuals with primary RLS and a neurologically normal control group. A quantitative profile of the dopaminergic system was obtained. Additional assays were performed on a catecholaminergic cell line and animal models of iron deficiency. RLS tissue, compared with controls, showed a significant decrease in D2R in the putamen that correlated with severity of the RLS. RLS also showed significant increases in tyrosine hydroxylase (TH) in the substantia nigra, compared with the controls, but not in the putamen. Both TH and phosphorylated (active) TH were significantly increased in both the substantia nigra and putamen. There were no significant differences in either the putamen or nigra for dopamine receptor 1, dopamine transporters or for VMAT. Significant increases in TH and phosphorylated TH were also seen in both the animal and cell models of iron insufficiency similar to that from the RLS autopsy data. For the first time, a clear indication of dopamine pathology in RLS is revealed in this autopsy study. The results suggest cellular regulation of dopamine production that closely matches the data from cellular and animal iron insufficiency models. The results are consistent with the hypothesis that a primary iron insufficiency produces a dopaminergic abnormality characterized as an overly activated dopaminergic system as part of the RLS pathology.
Keywords: PC12 cells, iron deficiency, movement disorders, sleep disorders, basal ganglia
Introduction
Despite the common assumption that dopaminergic pathophysiology causes the sensorimotor disorder restless leg syndrome (RLS), the very limited data supporting this view seem neither conclusive nor informative about the nature of this dopamine abnormality. The dramatic treatment response to dopamine agonists and levodopa (Early, 2003; Hening et al., 2004) and the limited data suggesting adverse reactions to dopamine antagonists (Winkelmann et al., 2001) have provided the basis for the hypothesis of dopamine pathology. Most attempts to document any dopamine pathology in RLS to date have been unconvincing. Brain imaging studies (Trenkwalder et al., 1999; Turjanski et al., 1999; Eisensehr et al., 2001; Michaud et al., 2002; Tribl et al., 2002; Cervenka et al., 2006) have failed to provide a consistent pattern indicating a dopamine deficit. Two autopsy studies found no evidence for loss of neuromelanin cells in RLS (Erikson et al., 2001; Wang et al., 2004). Three CSF studies (Earley et al., 2001, 2006b; Stiasny-Kolster et al., 2004) have reported no dopamine-related abnormalities, although one recent report presented two CSF studies both showing a surprisingly greater CSF 3-O-methyldopa (3OMD) for RLS patients that correlated with greater HVA indicating possible increased dopamine production (Allen et al., 2008). Since the assumption had been that there was decreased dopaminergic activity this finding has been somewhat difficult to interpret.
Unlike the lack of direct evidence for a dopamine pathology, all studies of CNS iron have consistently shown iron insufficiency in RLS (Early, 2000, 2005, 2006c; Allen et al., 2001; Mizuno et al., 2005; Schmidauer et al., 2005; Clardy et al., 2006). Autopsy analysis revealed that the immunostaining for iron management proteins was altered in the substantia nigra of RLS brains and the profile of proteins responsible for iron management in the neuromelanin cells indicated iron deficiency (Connor et al., 2004). There is significant animal literature that indicates a close relationship between brain iron status and the dopaminergic system (Beard et al., 1994; Nelson et al., 1997; Erikson et al., 2000, 2001; Jones et al., 2002; Beard, 2003; Beard and Connor, 2003; Beard et al., 2003; Allen, 2004; Earley et al., 2004, 2006c). If the iron-deficiency model of RLS is correct then brain dopaminergic abnormalities induced by iron deficiency in animals should be at least partly observed in RLS patients. In particular, the striatal decrease in D2 receptors observed in the iron-deprived rat model would be expected to occur in the brain tissue from RLS patients. Conversely, dopamine abnormalities seen in RLS brains would be expected to be seen both in vivo in iron-deprived animals and in vitro in iron-chelated cells. Thus, to the extent the iron insufficiency is central to RLS we expect that the dynamic interaction of discovery from the bench to the clinical setting back to the bench should inform about the nature of the dopamine pathology in RLS.
In this research, we used a translational approach comparing autopsy tissue with both in vivo animal and in vitro cell studies. We first tested a primary hypothesis that the RLS putamen of RLS patients compared with controls would show the same D2 decreases seen in the iron-deprived animal studies. We also explored for other significant dopaminergic differences between RLS and control substantia nigra and putamen tissue. We then sought to determine whether or not these autopsy differences also occurred with in vitro cellular iron chelation and in vivo iron deprived rats.
Methods
Patient characteristics
Substantia nigra and putamen were obtained at autopsy from the brains of eight patients selected from a collection maintained by the RLS foundation. The clinical RLS diagnosis was confirmed by an RLS expert board certified in Sleep Medicine who reviewed a detailed RLS questionnaire previously completed by the RLS individual at 0.08–2.4 (average 0.79) years before death. This questionnaire provided RLS clinical information including the critical diagnostic symptoms, RLS characteristics, family history and medication treatments (Table 1). The accepted patients met the diagnostic criteria established by the NIH consensus workshop on RLS (Allen et al., 2003), had no evidence for any contributing secondary causes of RLS, and expressed the early-onset phenotype of RLS (symptoms starting at or before age 45) (Allen and Earley, 2000). These criteria were selected to reduce variance of disease expression noting evidence for different pathologies related to age-of-onset phenotype (Earley et al., 2005; Clardy et al., 2006).
Table 1.
RLS patients’ clinical characteristics
| Patient No. | Gender | Age at death | RLS onset age | RLS Family history | Usual daily time RLS starts | JHRLSS | IRLS | Rx |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 77 | 6 | No | 19:00 | 2 | 32 | Vicodina |
| 2 | F | 68 | 12 | Yes | 09:00 | 4 | 28 | Carbidopa/levodopa |
| 3 | F | 76 | 10 | Yes | 20:00 | 2 | 27 | Neurontin, Xanaxb |
| 4 | F | 84 | 42 | Yes | 09:00 | 4 | 26 | Bromocriptine, Propoxyphene |
| 5 | F | 53 | 21 | No | 22:00 | 1 | 38 | Carbidopa/levodopa, Neurotin |
| 6 | F | 77 | 43 | YES | 23:00 | 1 | 36 | Pramipexolie, Tylenol with codeine PRN |
| 7 | F | 86 | 44 | No | 13:00 | 3 | 28 | Permax |
| 8 | F | 83 | 5 | Yes | 14:00 | 3 | 32 | Duragesic patch, Neurontina |
| Average ± SD | — | 75.5 ± 10.7 | 22.9 ± 17.4 | — | 16.1 ± 5.6 | 2.5 ± 1.2 | 30.9 ± 4.4 | – |
a No dopaminergic treatment.
b brief prior exposure to carbidopa/levodopa.
All patients reported daily RLS symptoms and RLS disturbance of their sleep. They reported off-treatment RLS severity on the international restless syndrome study group severity scale (IRLS) ranging from 26 to 38 (average 30.9) on this 40-point scale, indicating severe to very severe RLS. All reported a gradual worsening of RLS with symptoms taking 3–56 (average 20) years to become daily. Five of the eight reported a family history of RLS. Five were treated with levodopa or dopamine agonists, while the other three were on non-dopaminergic treatment and reported no significant dopaminergic treatment. The range of age at time of death of the RLS patients was 53–86 (average 75.5) years and all were female (Table 1). Of the eight RLS brains used in this study, seven of them had previously been shown to have altered iron management proteins in a separate histopathology study (Connor et al., 2004); the iron protein profile was not determined in the other sample.
The average ± SD post-mortem interval was 17.8 ± 7.6 h in the RLS group. One control group consisted of the substantia nigra from eight females. Their average ± SD age was 72.2 ± 9.7 and post-mortem interval was 18.6 ± 9.0. The second control group consisted of the putamen from seven females. Their average ± SD age was 71.4 ± 15.7 and post-mortem interval was 19.0 ± 6.3. There were no significant differences between these groups in the ages at death or the post-mortem intervals. All control subjects lacked any significant history of neurological disease at the time of death. None of these brains had been used in our previous studies. They were obtained from either the Brain Bank at Johns Hopkins University or the Harvard Brain Bank.
Pheochromocytoma cell cultures
Pheochromocytoma cells (PC12) were chosen for this study because they are an established model to mimic catecholaminergic cells in culture (Greene and Tischler, 1976). The cells were obtained commercially (ATCC, Manassas, VA, USA) and were grown in T-25 collagen IV coated flasks (BD Biosciences, Bedford, MA, USA) to 75% confluence in RPMI 1,640 medium supplemented with 10% equine serum, 5% fetal bovine serum, 100 μg/ml streptomycin and 100 U/ml of penicillin at 37°C in a water-saturated atmosphere containing 7% CO2. For iron chelation experiments, PC12 cells were washed twice with ice-cold phosphate buffered saline (PBS) and incubated with deferoxamine mesylate (DFO, dissolved in media, Sigma, St Louis, MO, USA) at final concentrations of 50, 100 and 200 μM for 24 h time intervals. All cells were collected by centrifugation and solubilized in 300 μl of ice-cold RIPA buffer (25 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1% IGEPAL CA-630, 0.5% sodium deoxycholate and 0.1% SDS) supplemented with protease inhibitors (500 μM phenylmethylsulfonyl fluoride and 2 μg/ml leupeptin/aprotinin/pepstatin A) for 60 min at 4°C with constant shaking. Solubilized extracts were centrifuged (16 000 g, 10 min, 4°C) and protein content of supernatants was assessed using the BCA protein assay (Pierce, Rockford, IL, USA) with bovine serum albumin as the standard.
Rats
The experiment was approved by the Institutional Animal Care and Utilization Committees of the University of Michigan and the Pennsylvania State University. A total of 15 Sprague–Dawley adult rats were used in this study. The control rats were maintained on a standard iron-sufficient diet (198 mg Fe/kg, Teklad, Madison, WI, USA) throughout pregnancy and post-partum. There were two experimental groups (five rats per group), designated as Groups I or II). The rats in each experimental group were born to dams that were maintained on a low-iron diet; 3–5 mg Fe/kg (Formula TD 80396, Harlan Teklad, Madison WI) from gestation Day 5 to postnatal Day 7, then 6–10 mg Fe/kg (Formula TD 01094 Harlan Teklad) from postnatal Day 7 to Day 21. Thereafter, experimental Group I pups continued the 6–10 mg Fe/kg diet for 65 days. Group II offspring were weaned onto an iron sufficient diet (40–50 mg Fe/kg, Formula TD 89300, Harlan Teklad) and continued until assessment at Day 65. Rats were deeply anesthetized at termination of the study (postnatal Day 65) with sodium pentobarbital (60 mg/kg i.p.) and perfused through the ascending aorta with 0.1 M PBS. The ventral midbrain was dissected and stored for analysis. Samples from animals were stored at −80°C.
Protein isolation and immunoblot analysis
The substantia nigra and putamen from humans or the ventral midbrain from each rat were collected and homogenized in 10 volumes of cold lysis buffer. Immunoblots were carried out using the methods described in our previous publication (Connor et al., 2004; Earley et al., 2005). Briefly, the homogenates were centrifuged at 14 000 r.p.m. for 20 min at 4°C, and proteins in the supernatant were collected. Protein concentrations were determined by Bradford assay. Immunoblot (slot blot) analysis was performed to obtain the dopaminergic profiles in the human tissue, PC12 cells or rat brains. Equivalent samples (total protein content = 0.2 μg) were loaded (500 μl aliquots), in triplicate, into a Minifold II slot-blot (immunoblot) systems (Schleicher and Schuell, Keene, NH, USA) containing a nitrocellulose membrane (Schleicher and Schuell, pore size = 0.45 μm). The membrane was incubated overnight at 4°C with antibodies (dilution 1:500 in PBS) directed against TH (Pel Freez), phosphorylated TH (Ser 40, Zymed, San Francisco, CA), dopamine (Chemicon, Billerica, MA), VMAT2 (Chemicon), dopamine receptor 1 (D1, Sigma), dopamine receptor 2 (D2, Chemicon) and dopamine transporter (DAT, Chemicon). The membrane was developed for visualization of the reaction product using an enhanced chemiluminescence system (KPL, Gaithersburg, MD, USA), and the blots were exposed to film. The blot was analysed to determine relative concentrations of standards and samples using a laser densitometer (100A, Molecular Dynamics, Sunnyvale, CA, USA) coupled to a computer (software = Quantity One, Biorad). The average optical density for the triplicate aliquots for each sample was calculated.
Statistics
The Mann–Whitney U-test was used when two groups were being compared. Statistical significance was set at P < 0.05. We tested our primary hypothesis that D2R would be decreased in the RLS putamen, compared with controls. As an exploratory analysis, we then evaluated six other potential dopaminergic abnormalities using a Bonferroni correction for six independent multiple comparisons (setting α = 0.0083) for the putamen and evaluated the same measures plus the D2R for the substantia nigra (setting α = 0.007 to adjust for the multiple comparisons). In the exploratory analyses, we noted some marked outlier data. We therefore used the Dixon test for outliers 16 and if one outlier was identified made the exploratory analyses with the outlier removed. We further evaluated the Pearson's correlation coefficient for the relation between RLS severity and any dopaminergic abnormality in the RLS tissue. The iron-deprived animal and cell model data were analysed using a repeated measure ANOVA and the Tukey/Kramer post hoc test with α = 0.05.
Results
Human putamen and substantia nigra
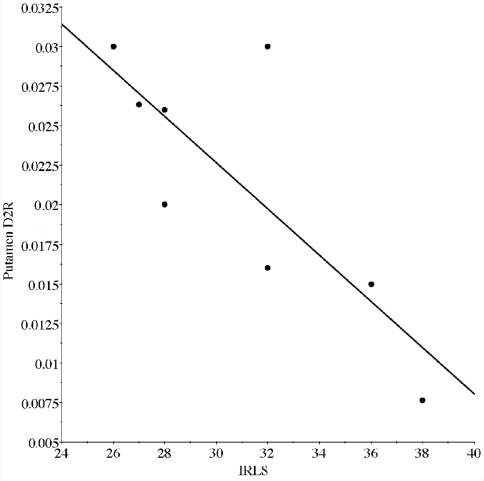
Evaluation of the primary hypothesis found D2 was significantly less (Fig. 1B) in homogenates from the putamen (U = 9, P = 0.028). Since there may be some concern about the long-term effects of dopaminergic treatment for RLS on these dopamine-related measurements, all the figures indicate whether or not the patients had dopaminergic treatment for their RLS. Because our primary hypothesized difference was a decrease in D2 expression in RLS, we also examined the relationship of D2 expression to RLS severity. Severity was measured by the IRLS score obtained from the RLS patients at the time they completed the diagnostic questionnaire required for subscribing to the brain bank. The D2 correlated significantly with the IRLS score (r = 0.80, P = 0.018) (Fig. 2).
Figure 1.
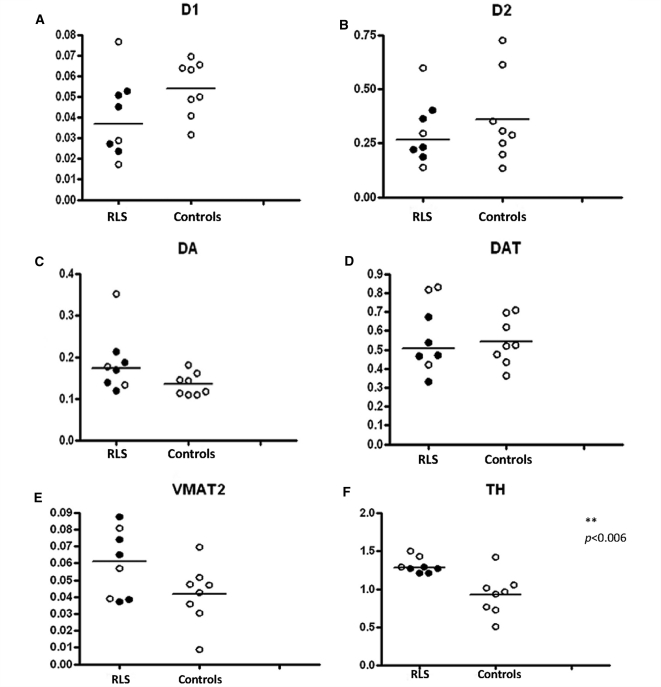
Dopaminergic profile in the putamen. The expression of D1 (A) D2 (B), dopamine; DA (C), DAT (D), VMAT2 (E), TH (F), pTH (G) and the pTH/TH ratio (H), in the putamen of control (right column in each graph) and RLS patients (left column in each graph). The data are plotted as individual values and the median is indicated by the bar in each graph. Those RLS patients who received dopaminergic agents as part of the treatment regimen are indicated by the closed circles and those who were not treated with dopaminergic agents are shown as open circles. Statistical significance was determined using the Mann–Whitney U-test.
Figure 2.
Correlational analysis of putamenal D2 receptors and IRLS score. There was a strong inverse correlation (r = 0.80, P = 0.018) between the amount of D2 expression measured in the autopsy samples in the putamen and the patient's score on the IRLS scale of RLS severity. The higher the score on the rating scale indicating the more severe the symptoms.
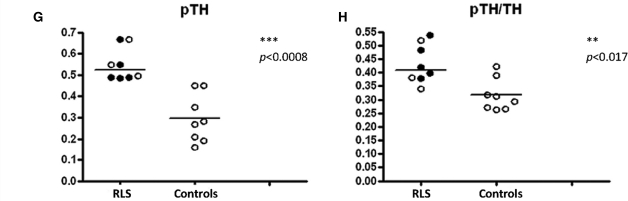
We examined six other proteins in the autopsy samples, characterizing the dopamine profile in the putamen. The control group included one outlier tyrosine hydroxylase (TH) value that for the Dixon test gave a Q = 0.84 exceeding the criterion for this sample size of 0.76. This outlier value exceeded the next largest value by 17 SDs of all the data besides this one. It was identified as an outlier for TH, phosphorylated tyrosine hydroxylase (pTH) and the ratio of the two and was removed from the analyses for these three measurements only. There was significantly greater expression in pTH for the putamen (U = 3, P = 0.0067), but not in TH for RLS compared with controls (U = 4, p = 0.0098—critical P-value with Bonferroni correction is 0.0083) (Fig. 1F and G). There were no other statistically significant differences between RLS and controls for dopaminergic measures from the putamen (Fig. 1).
We examined all seven dopaminergic proteins in the substantia nigra for differences between RLS and controls. There were no outliers identified in these data. The RLS tissue showed significantly higher values for both TH (U = 6, P = 0.006) and pTH (U = 0, P = 0.0008). The ratio of pTH/TH was elevated but not significantly (U = 8, P = 0.017, critical P-value with Bonferroni correction is 0.007). There were no other significant differences between RLS and control groups (Fig. 3).
Figure 3.
Dopaminergic profile in the substantia nigra. The expression of D1 (A), D2 (B), dopamine; DA (C), DAT (D), VMAT (E), TH (F), pTH (G), and the pTH/TH ratio (H), in the substantia nigra of control (right column in each graph) and RLS patients (left column in each graph). The data are plotted as individual values and the median is indicated by the bar in each graph. Those RLS patients who received dopaminergic agents as part of the treatment regimen are indicated by the closed circles and those who were not treated with dopaminergic agents are shown as open circles. Statistical significance was determined using the Mann–Whitney U-test.
Graphical analyses showed no differences for the three RLS patients not treated, compared with the five treated with dopaminergic agents for any of the measures showing significant differences between RLS and control groups (Figs 1 and 3).
Iron deficient rat brains and PC12 cells
The finding of markedly elevated TH and pTH in the nigra and to a somewhat less extent in the putamen of RLS brains surprised us. This had not been anticipated as a consequence of iron deficiency and accordingly had not been previously examined in either in vivo or in vitro iron deficiency conditions. As noted before, there is significant literature on dopaminergic parameters and iron status, but before seeing these data there had been no consideration of the possibility that iron deficiency itself would increase TH and pTH. We therefore, measured TH levels in a rat model of iron deficiency and a cell line treated with the iron chelator, deferroxamine, following methods previously reported (Wang et al., 2004).
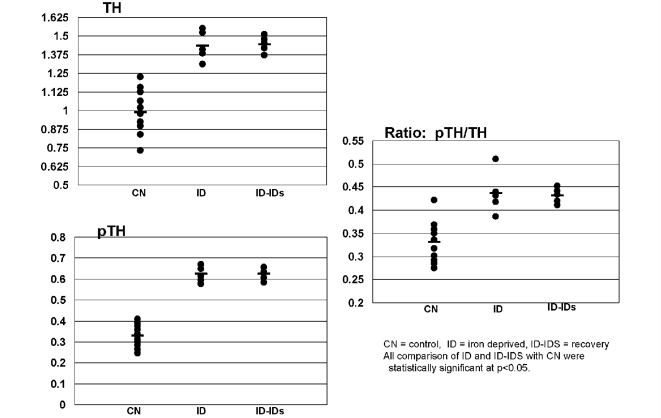
Rat pups that were iron deficient (ID) during development had significantly higher levels of both TH and pTH, as well as pTH/TH ratio, compared with the control group which were fed a normal diet (Fig. 4A–C). Weaning a group of rats onto an iron sufficient diet until 65 days of age did not reverse the effect of the developmental iron deficiency on either the TH, pTH levels or pTH/TH ratio (Fig. 4A–C).
Figure 4.
TH and pTH. Immunoblot analysis for homogenates of rat ventral tegmentum (65-day-old rats). There is a significant increase in relative amounts of TH in rats that were exposed to a low-iron diet from gestational Day 5 through weaning at postnatal Day 21 and continued until 65 days (ID) compared with an age-matched control group (CN). Introducing an iron sufficient diet at the time of weaning did not result in a return to normal levels of TH by 65 days of age (ID–IDs). The levels of pTH and the ratio of pTH/TH followed the same patterns as described for TH. The differences for TH, pTH and the ratio from control are all significant (P < 0.001). The data are presented as X ± SE.
To determine the impact of iron deficiency on TH more directly, a cell culture model was used. PC12 cells were exposed to increasing concentrations of the iron chelator DFO and the relative amount of TH and pTH expression were determined for each group. Tyrosine hydroxylase expression is relatively stable in the presence of increasing concentrations of DFO. A 10% increase in TH (P < 0.05) was only seen at the highest concentration of DFO (Fig. 5A). On the other hand, the levels of pTH showed an upward trend with increasing concentrations of DFO and reached statistical significance at 200 μM (30% P < 0.001) (Fig. 5B). The pTH and TH ratios were also increased at 200 μM (P < 0.01, Fig. 5C). The concentrations of iron chelator used in this study have been previously shown to induce a cellular iron deficiency as indicated by an increase in the Tf receptor (Wang et al., 2004).
Figure 5.
The effect of iron chelation on TH, pTH concentrations and pTH/TH ratios (pTH/TH) in PC12 cells. The concentrations of the iron chelator (DFO) are shown. Exposing cells to an iron chelator is associated with a trend of increasing amounts of TH, pTH and the pTH/TH ratio. The data are presented as X ± SE. Asterisk refers to significantly different compared with control.
Discussion
We confirmed our primary hypothesis that the D2 receptor would be decreased in the putamen of RLS brains and found the degree of decrease was significantly greater for more severe RLS. These results are consistent with our a priori primary hypothesis based on prior animal studies of effects of iron deficiency (Erikson et al., 2001). We did not expect, and did not see, a similar decrease in the nigra, nor has any such nigral decrease been reported for the iron-deprived animals. It is the receptor density in the terminal fields that are likely to be the most biologically and clinically relevant.
We also report two new discoveries: first, the surprisingly large increase of pTH in the putamen and both TH and pTH in the substantia nigra in RLS, compared with controls produced essentially non-overlapping data. The nigral TH was, on average, 40% higher and the pTH was 86% higher for RLS compared with control brains. Second, that ID rats and iron chelation of PC12 cells both show increased TH activation overall, similar to that seen in the autopsy tissue of RLS patients. The particularly striking aspect of these two new results lies not with the degree of exact concordance but rather the surprising discovery that iron deficiency and RLS both appear to produce states that would be essentially associated with increased presynaptic dopaminergic activity.
The indication in the analysis of post-mortem tissue that there is an increase in dopamine activity is very consistent with the recent CSF studies showing increased 3OMD in RLS patients off dopamine treatment that correlates well with increased HVA (Allen et al., 2008). Both the CSF data and these autopsy results indicate that more severe RLS produces increased dopamine production and turnover and, as seen in Figs 1 and 3, this occurs even for patients not previously treated with dopamine agents.
One potential confounding factor that might contribute to these findings is prior drug treatment for RLS. We have fairly recent drug treatment information on all of these subjects and at least three of them were not on dopaminergic treatment for RLS. We have limited medical records at the time of death and while we cannot rule out added use of dopamine medications shortly before death, there is no indication of that for these three patients. The data in Figs 1 and 3 do not show any overall indication of differences related to prior dopaminergic treatment. Particularly striking is the lack of overlap between RLS and controls for TH in the nigra as shown in Fig. 3A, clearly indicating that even those subjects not on dopaminergic treatment have increased TH. It may seem somewhat surprising that these results do not show some indication of difference related to dopaminergic treatment. Previous PET (Turjanski et al., 1999) and SPECT (Eisensehr et al., 2001) studies, however, also found no differences between dopamine treated and untreated RLS patients, some of whom were drug naïve. Moreover, chronic use of even high l-dopa doses does not alter levels of dopamine receptors in the striatum of normal monkeys (Zeng et al., 2001). Chronic dopamine treatment would also be expected, if anything, to lead to reduced—not increased—TH. The magnitude, consistency and direction of change for the differences between RLS and controls make it difficult to dismiss these findings as effects of chronic dopaminergic treatment. Rather they seem likely to reflect effects of the underlying disease process.
Perhaps the strongest indication that these post-mortem results reflect RLS pathology comes from the remarkable convergence with the biological results of both in vivo and in vitro iron deprivation models. An unexpected finding in the RLS tissue was the marked increase in pTH in both the putamen and substantia nigra and TH in the substantia nigra in RLS compared with control that was statistically significant even after the Bonferroni correction. Given the limited data on TH in iron-deficiency models, we examined both a developmental ID rat model and a cell culture model in which iron status can be directly manipulated. In both of these models, TH and pTH levels were increased by iron deficiency. Thus, the increase in TH and pTH in RLS is again consistent with the concept that the RLS brain is ID. It may be noteworthy that iron supplementation for 35 days post-weaning was not capable of reversing the early effects of iron deficiency on TH or pTH; suggesting that the feedback system may have been remodeled during development. The relevance of this observation to RLS remains to be determined.
Only one of the findings in this post-mortem study fails to match those from the iron deprived rat. There was no decrease in striatal DAT in RLS, in contrast to the decreased striatal DAT function reported in iron deprived rats (Erikson et al., 2000). This may reflect differences in degree of iron deficiency, species differences in this aspect of iron deficiency effects or perhaps differences in total expression evaluated in this post-mortem study versus functional expression on the cell surface evaluated in the animal studies. The post-mortem analysis is consistent with SPECT imaging data in RLS patients (Eisensehr et al., 2001; Michaud et al., 2002; Tribl et al., 2002; Mrowka et al., 2005) that also failed to show any indication of decreased DAT. Although our studies focused on the nigrostriatal system, it is sometimes argued that other dopaminergic systems, particularly the A11 should be considered as more likely to be involved in producing RLS symptoms. While this is a valuable theoretical argument that should be considered, it is somewhat irrelevant here. The question in this study was the nature of the dopamine pathology in RLS and its similarity to iron deficiency effects. RLS does not show any indication of being a neurodegenerative disease. Histopathological analyses report no signs of neurodegeneration nor cell loss in either the substantia nigra (Connor et al., 2003; Pittock et al., 2004) or in A11 (Earley et al., in press). The critical issue then becomes specifying abnormal functioning in an intact dopamine system and comparing that with expected results from the in vivo and in vitro iron studies. The question for the dopamine pathology, therefore, becomes more ‘what’ than ‘where’. The nigrostriatal system allows this type of analysis. While the post-mortem analysis and iron-deficiency models show the same decrease in striatal D2, the striatal imaging studies in RLS patients have not shown similarly consistent results. A ‘mild, but significant’ decrease in D2 binding has been reported in the putamen in RLS in one study using PET analysis (Turjanski et al., 1999) and a similar finding was reported in a study using SPECT (Michaud et al., 2002). Other imaging studies comparing RLS to controls, however, have either found no significant differences (Trenkwalder et al., 1999; Eisensehr et al., 2001; Tribl et al., 2002) or, in one study, found a significant increase in D2 binding (Cervenka et al., 2006). It is important to note that these PET measures reflect competitive uptake of the ligand and are therefore altered both by the receptor status on the membrane and the amount of intra-synaptic dopamine, whereas the post-mortem analysis is a direct measurement of total D2 receptors. The complicated interaction with endogenous ligand may account for some of the differences in the imaging studies. The 30% decrease in D2 receptors seen in this post-mortem analysis came from patients who all had very severe RLS, whereas the one imaging study that found an increase in D2 receptors was performed on mostly patients with very mild RLS, some of who had RLS severity scores below the usual cut-off to be considered candidates for medication treatment trials (Dixon, 1995). The patients in this post-mortem analysis were like the fairly severely affected patients whose PET studies indicated decreased D2R binding consistent with our findings (Turjanski et al., 1999). Moreover the data in Fig. 2 indicate that the abnormal reduction in D2R occurs only for patients with severe to very severe RLS, as indicated by IRLS scores of 28 or higher. These data suggest that even moderately severe RLS—with IRLS scores of 20–25—would likely have fairly normal D2R densities, but not so the very severe RLS.
The results of this study reveal that the dopaminergic profile in the putamen and substantia nigra of individuals with RLS have a number of significant differences from controls. The results provide the best direct biological evidence documenting both the existence and nature of dopamine pathology in moderate to severe chronic RLS. This supports the clinical evidence that alterations in the dopaminergic system may underlie the symptoms associated with RLS. The striking parallel with the iron studies also provide further support for the importance of iron status in RLS pathology.
Funding
This work was supported by National Institutes of Health, (grant number 1 P01 AG021190 to C.J.E.) awarded to the group studying pathophysiology of RLS; (NIH, 2R24 MH/NS068855 to RLS Foundation, to Harvard Brain Bank); National Institutes of Health (grants NS042857, NS35088); National Institutes of Health Program project grant (NICHD 39386 to Betsy Lozoff) awarded to the group studying Brain and Behaviour in Early Iron Deficiency (JRC, BTF co-investigators).
Acknowledgements
The authors are grateful to the RLS Foundation for access to the RLS Brain Donation Center, to the Harvard Brain Bank for access both to the RLS and control tissue and Juan Troncoso for access to control tissue from the Johns Hopkins Brain Bank. The authors are grateful to Michelle Lauzon for her assistance in preparing the manuscript.
Glossary
Abbreviations
- DAT
dopamine transporter
- DFO
deferoxamine mesylate
- ID
iron deficient
- PC12
pheochromocytoma cell
- pTH
phosphorylated tyrosine hydroxylase
- RLS
restless leg syndrome
- TH
tyrosine hydroxylase
References
- Allen R. Dopamine and iron in the pathophysiology of restless legs syndrome (RLS) Sleep Med. 2004;5:385–91. doi: 10.1016/j.sleep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Allen RP, Barker PB, Wehrl F, Song HK, Earley CJ. MRI measurement of brain iron in patients with restless legs syndrome. Neurology. 2001;56:263–5. doi: 10.1212/wnl.56.2.263. [DOI] [PubMed] [Google Scholar]
- Allen RP, Connor JR, Hyland K, Earley CJ. Abnormally increased CSF 3-Ortho-methyldopa (3-OMD) in untreated restless legs syndrome (RLS) patients indicates more severe disease and possibly abnormally increased dopamine synthesis. Sleep Med. 2008;10:123–8. doi: 10.1016/j.sleep.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RP, Earley CJ. Defining the phenotype of the restless legs syndrome (RLS) using age-of-symptom-onset. Sleep Med. 2000;1:11–9. doi: 10.1016/s1389-9457(99)00012-x. [DOI] [PubMed] [Google Scholar]
- Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- Beard J. Iron deficiency alters brain development and functioning. J Nutr. 2003;133(5 Suppl. 1):1468S–72S. doi: 10.1093/jn/133.5.1468S. [DOI] [PubMed] [Google Scholar]
- Beard JL, Chen Q, Connor J, Jones BC. Altered monamine metabolism in caudate-putamen of iron-deficient rats. Pharmacol Biochem Behav. 1994;48:621–4. doi: 10.1016/0091-3057(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr. 2003;2:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- Beard J, Erikson KM, Jones BC. Neonatal iron deficiency results in irreversible changes in dopamine function in rats. J Nutr. 2003;133:1174–9. doi: 10.1093/jn/133.4.1174. [DOI] [PubMed] [Google Scholar]
- Cervenka S, Palhagen SE, Comley RA, Panagiotidis Z, Cselenyi JC, Matthews RY, et al. Support for dopaminergic hypoactivity in restless legs syndrome: a PET study on D2-receptor binding. Brain. 2006;129:2017–28. doi: 10.1093/brain/awl163. [DOI] [PubMed] [Google Scholar]
- Clardy SL, Wang X, Boyer PJ, Earley C, Allen R, Connor J. Is ferroportin–hepcidin signaling altered in restless legs syndrome? J Neurol Sci. 2006;247:173–9. doi: 10.1016/j.jns.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Connor JR, Boyer PJ, Menzies SL, Dellinger B, Allen RP, Earley CJ. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology. 2003;61:304–9. doi: 10.1212/01.wnl.0000078887.16593.12. [DOI] [PubMed] [Google Scholar]
- Connor JR, Wang XS, Patton SM, Menzies SL, Troncoso JC, Earley CJ, et al. Decreased transferrin receptor expression by neuromelanin cells in restless legs syndrome. Neurology. 2004;62:1563–7. doi: 10.1212/01.wnl.0000123251.60485.ac. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. Analysis of extreme values. Ann Math Stat. 1951;21:488–506. [Google Scholar]
- Earley CJ. Clinical practice. Restless legs syndrome. N Engl J Med. 2003;348:2103–9. doi: 10.1056/NEJMcp021288. [DOI] [PubMed] [Google Scholar]
- Earley C, Allen RP, Connor JR, Ferrucci L, Troncoso J. The dopaminergic neurons of the A11 system in RLS autopsy brains appear normal. Sleep Med. doi: 10.1016/j.sleep.2009.01.006. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley CJ, Barker PB, Horská A, Allen RP. MRI-determined regional brain iron concentrations in early and late-onset restless legs syndrome. Sleep Med. 2006a;7:458–61. doi: 10.1016/j.sleep.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Earley CJ, Connor JR, Beard JL, Clardy SL, Allen RP. Ferritin levels in the cerebrospinal fluid and restless legs syndrome: effects of different clinical phenotypes. Sleep. 2005;28:1069–75. doi: 10.1093/sleep/28.9.1069. [DOI] [PubMed] [Google Scholar]
- Earley CJ, Connor JR, Beard JL, Malecki EA, Epstein DK, Allen RP. Abnormalities in CSF concentrations of ferritin and transferrin in restless legs syndrome. Neurology. 2000;54:1698–700. doi: 10.1212/wnl.54.8.1698. [DOI] [PubMed] [Google Scholar]
- Earley CJ, Heckler D, Horská A, Barker PB, Allen RP. The treatment of restless legs syndrome with intravenous iron dextran. Sleep Med. 2004;5:231–5. doi: 10.1016/j.sleep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Earley CJ, Hyland K, Allen RP. CSF dopamine, serotonin, and biopterin metabolites in patients with restless legs syndrome. Mov Disord. 2001;16:144–9. doi: 10.1002/1531-8257(200101)16:1<144::aid-mds1009>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Earley CJ, Hyland K, Allen RP. Circadian changes in CSF dopaminergic measures in restless legs syndrome. Sleep Med. 2006b;7:263–8. doi: 10.1016/j.sleep.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Eisensehr I, Wetter TC, Linke R, Noachtar S, Lindeiner Hv, Gildehaus FJ, et al. Normal IPT and IBZM SPECT in drug-naive and levodopa-treated idiopathic restless legs syndrome. Neurology. 2001;57:1307–9. doi: 10.1212/wnl.57.7.1307. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Jones BC, Beard JL. Iron deficiency alters dopamine transporter functioning in rat striatum. J Nutr. 2000;130:2831–7. doi: 10.1093/jn/130.11.2831. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Jones BC, Hess EJ, Zhang Q, Beard JL. Iron deficiency decreases dopamine D1 and D2 receptors in rat brain. Pharmacol Biochem Behav. 2001;69:409–18. doi: 10.1016/s0091-3057(01)00563-9. [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–8. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hening WA, Allen RP, Earley CJ, Picchietti DL, Silber MH. An update on the dopaminergic treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 2004;27:560–83. doi: 10.1093/sleep/27.3.560. [DOI] [PubMed] [Google Scholar]
- Jones BC, Wheeler DS, Beard JL, Grigson PS. Iron deficiency in rats decreases acquisition of and suppresses responding for cocaine. Pharmacol Biochem Behav. 2002;73:813–9. doi: 10.1016/s0091-3057(02)00906-1. [DOI] [PubMed] [Google Scholar]
- Michaud M, Soucy JP, Chabli A, Lavigne G, Montplaisir J. SPECT imaging of striatal pre- and postsynaptic dopaminergic status in restless legs syndrome with periodic leg movements in sleep. J Neurol. 2002;249:164–70. doi: 10.1007/pl00007859. [DOI] [PubMed] [Google Scholar]
- Mizuno S, Mihara T, Miyaoka T, Inagaki T, Horiguchi J. CSF iron, ferritin and transferrin levels in restless legs syndrome. J Sleep Res. 2005;14:43–7. doi: 10.1111/j.1365-2869.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- Mrowka M, Jobges M, Berding G, Schimke N, Shing M, Odin P. Computerized movement analysis and beta-CIT-SPECT in patients with restless legs syndrome. J Neural Transm. 2005;112:693–701. doi: 10.1007/s00702-004-0217-9. [DOI] [PubMed] [Google Scholar]
- Nelson C, Erikson K, Pinero DJ, Beard JL. In vivo dopamine metabolism is altered in iron-deficient anemic rats. J Nutr. 1997;127:2282–8. doi: 10.1093/jn/127.12.2282. [DOI] [PubMed] [Google Scholar]
- Pittock SJ, Parrett T, Adler CH, Parisi JE, Dickson DW, Ahlskog JE. Neuropathology of primary restless leg syndrome: absence of specific tau- and alpha-synuclein pathology. Mov Disord. 2004;19:695–9. doi: 10.1002/mds.20042. [DOI] [PubMed] [Google Scholar]
- Schmidauer C, Sojer M, Seppi K, Stockner H, Högl B, Biedermann B, et al. Transcranial ultrasound shows nigral hypoechogenicity in restless legs syndrome. Ann Neurol. 2005;58:630–4. doi: 10.1002/ana.20572. [DOI] [PubMed] [Google Scholar]
- Stiasny-Kolster K, Moller JC, Zschocke J, Bandmann O, Cassel W, Oertel WH, et al. Normal dopaminergic and serotonergic metabolites in cerebrospinal fluid and blood of restless legs syndrome patients. Mov Disord. 2004;19:192–6. doi: 10.1002/mds.10631. [DOI] [PubMed] [Google Scholar]
- Trenkwalder C, Walters AS, Hening WA, Chokroverty S, Antonini A, Dhawan V, et al. Positron emission tomographic studies in restless legs syndrome. Mov Disord. 1999;14:141–5. doi: 10.1002/1531-8257(199901)14:1<141::aid-mds1024>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Tribl GG, Asenbaum S, Klosch G, Mayer K, Bonelli RM, Auff E, et al. Normal IPT and IBZM SPECT in drug naive and levodopa-treated idiopathic restless legs syndrome (letter to editor) Neurology. 2002;59:649–50. doi: 10.1212/wnl.59.4.649. [DOI] [PubMed] [Google Scholar]
- Turjanski N, Lees AJ, Brooks DJ. Striatal dopaminergic function in restless legs syndrome: 18F-dopa and 11C-raclopride PET studies. Neurology. 1999;52:932–7. doi: 10.1212/wnl.52.5.932. [DOI] [PubMed] [Google Scholar]
- Wang X, Wiesinger J, Beard J, Felt B, Menzies S, Earley C, et al. Thy1 expression in the brain is affected by iron and is decreased in Restless Legs Syndrome. J Neurol Sci. 2004;220:59–66. doi: 10.1016/j.jns.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Winkelmann J, Schadrack J, Wetter TC, Zieglgansberger W, Trenkwalder C. Opioid and dopamine antagonist drug challenges in untreated restless legs syndrome patients. Sleep Med. 2001;2:57–61. doi: 10.1016/s1389-9457(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Zeng BY, Pearce RK, MacKenzie GM, Jenner P. Chronic high dose L-dopa treatment does not alter the levels of dopamine D-1, D-2 or D-3 receptor in the striatum of normal monkeys: an autoradiographic study. J Neural Transm. 2001;108:925–41. doi: 10.1007/s007020170013. [DOI] [PubMed] [Google Scholar]