Abstract
Leber hereditary optic neuropathy (LHON) is a genetic disorder primarily due to mutations of mitochondrial DNA (mtDNA). Environmental factors are thought to precipitate the visual failure and explain the marked incomplete penetrance of LHON, but previous small studies have failed to confirm this to be the case. LHON has no treatment, so identifying environmental triggers is the key to disease prevention, whilst potentially revealing new mechanisms amenable to therapeutic manipulation. To address this issue, we conducted a large, multicentre epidemiological study of 196 affected and 206 unaffected carriers from 125 LHON pedigrees known to harbour one of the three primary pathogenic mtDNA mutations: m.3460G>A, m.11778G>A and m.14484T>C. A comprehensive history of exposure to smoking, alcohol and other putative environmental insults was collected using a structured questionnaire. We identified a strong and consistent association between visual loss and smoking, independent of gender and alcohol intake, leading to a clinical penetrance of 93% in men who smoked. There was a trend towards increased visual failure with alcohol, but only with a heavy intake. Based on these findings, asymptomatic carriers of a LHON mtDNA mutation should be strongly advised not to smoke and to moderate their alcohol intake.
Keywords: Leber hereditary optic neuropathy, mitochondrial DNA, alcohol, tobacco, epigenetics
Introduction
Leber hereditary optic neuropathy (LHON, MIM 535000) is a mitochondrial genetic disease that preferentially affects young adults in their second and third decades of life, with over 95% of cases arising due to one of three point mutations in the mitochondrial genome: m.3460G>A, m.11778G>A and m.14484T>C (Harding et al., 1995; Mackey et al., 1996; Man et al., 2002; Newman and Biousse, 2004; Yu-Wai-Man et al., 2009). LHON is a common cause of inherited visual failure, with a minimum prevalence of 1 in 30 000 in Northern Europe, and with an estimated mutation carrier rate of ∼1 in 350 (Man et al., 2003; Spruijt et al., 2006; Elliott et al., 2008). Clinically, LHON is characterized by bilateral sub-acute loss of central vision as a result of focal degeneration of the retinal ganglion cell layer within the papillomacular bundle (Yu-Wai-Man et al., 2009). The visual prognosis is poor and the majority of patients remain severely visually impaired secondary to the marked reduction in visual acuity and the dense scotoma in their visual fields.
LHON shows marked incomplete penetrance with only ∼50% of male and ∼10% of female carriers developing the optic neuropathy in their lifetime (Seedorff, 1985; Brown and Wallace, 1994; Nikoskelainen, 1994). The secondary factors modulating the mitochondrial DNA (mtDNA) LHON mutations still remain largely undefined, although the gender bias has been linked to the synergistic influence of visual-loss susceptibility loci on the X-chromosome (Hudson et al., 2005; Shankar et al., 2008). Genetic factors, however, cannot provide a complete explanation for the reduced penetrance. Five pairs of monozygotic twins with a primary LHON mutation have been identified in the literature (Nikoskelainen et al., 1987; Newman et al., 1991; Johns et al., 1993; Harding et al., 1995; Biousse et al., 1997; Lam, 1998) and among two pairs, one sibling has remained visually unaffected on long-term follow-up (Johns et al., 1993; Biousse et al., 1997). Whilst it is possible that the unaffected sibling will lose vision in later life, this discordance supports the role of environmental factors in triggering visual loss among at-risk carriers. LHON is therefore likely to be a complex multifactorial disease, with environmental triggers operating at the individual level contributing to the observed intra- and inter-familial variability in penetrance.
A limited number of relatively small case–control studies have attempted to address this important issue, some of which suggest an increased risk of visual loss among LHON carriers with high alcohol and tobacco consumption (Cullom et al., 1993; Golnik and Shaible, 1994; Riordan-Eva et al., 1995; Chalmers and Harding, 1996; Tsao et al., 1999; Sadun et al., 2003). However, the largest study to date involving affected and unaffected siblings from 80 LHON sibships found no relationship between smoking or alcohol and the likelihood of visual failure (Kerrison et al., 2000). There are also anecdotal reports of trauma, nutritional deprivation, metabolic disturbance, exposure to industrial toxins, anti-retroviral drugs, psychological stress or acute illness precipitating the onset of blindness in LHON, but the strength of the causal relationship is difficult to establish (DuBios and Feldon, 1992; Johns et al., 1993; Hwang and Park, 1996; Mackey et al., 2003; Sadun et al., 2003; Sanchez et al., 2006; Carelli et al., 2007).
There is currently no proven treatment in LHON that will either prevent disease conversion or improve visual prognosis following the onset of optic neuropathy. The identification of potentially modifiable risk factors would therefore contribute significantly to counselling these families, and reveal potential disease mechanisms. To address this issue we carried out the largest multi-centre study of potential environmental triggers in 402 LHON mtDNA mutation carriers.
Subjects and Methods
Subjects
A structured questionnaire was conducted on 196 affected and 206 unaffected carriers (n = 402) from 125 genealogically distinct LHON pedigrees in three centres: Newcastle, UK (n = 47); Rotterdam, the Netherlands (n = 46); and Munich, Germany (n = 32). All LHON carriers were homoplasmic for one of the three primary mtDNA mutations: m.3460G>A (n = 71), m.11778G>A (n = 270) and m.14484T>C (n = 61), confirmed by mtDNA sequencing, restriction fragment length polymorphism analysis or primer extension assay. With the exception of one Asian individual, all participants were of white Caucasian origin. As well as having ethical approval, this study complied with the Declaration of Helsinki.
Data collection
Participants were interviewed via telephone by three investigators (MAK, AK and ML) using a standardized questionnaire that has been adapted from previous studies (Tsao et al., 1999; Kerrison et al., 2000) (Supplementary data). The dataset collected included basic demographic details, affected status, age of onset (time at which first symptoms were noted), time course and progression of visual loss, and a detailed account of exposure to possible environmental triggers to allow a quantitative analysis where applicable. The lifestyle factors specifically queried were (i) smoking, (ii) alcohol, (iii) trauma, (iv) recreational drugs, (v) occupational and industrial toxin exposure, (vi) diet, (vii) physical exercise, (viii) medication history including vitamins and herbal remedies and (ix) any relevant medical comorbidity. Consumption of alcoholic beverages (beer, wine, liquor and alcopops) among subjects was combined and converted to standardized units of alcohol as outlined by the UK Office for National Statistics (Office for National Statistics, 2007). Cumulative consumption of alcohol was measured as ‘drink years’, which was calculated by the units of alcohol per week multiplied by the number of years of drinking, taking into account the variations in levels of consumption as identified through the questionnaire. Maximum intensity of alcohol consumption was quantified as the maximum units of alcohol consumed in a single week. In line with a previous study (Kerrison et al., 2000), cumulative consumption and maximum intensity were analysed as both continuous variables and categorical variables by creating three subject groups: (i) non-drinkers, (ii) those drinking <75th percentile (light drinking) and (iii) those drinking ≥75th percentile (heavy drinking), using the level of alcohol consumption among unaffected LHON carriers to define the reference percentile ranges. Affected LHON carriers who only began drinking after losing vision had an alcohol exposure of zero and for those who started drinking beforehand, only their consumption (cumulative and maximum intensity) up to the onset of visual loss was considered for the purposes of statistical analysis. A similar analysis protocol was applied to smoking, with cumulative smoking expressed in terms of ‘pack years’, calculated by multiplying the number of packs of cigarettes smoked per day by the number of years of smoking, and maximum intensity of smoking quantified in terms of the maximum number of cigarettes consumed in a single day.
Statistical analysis
Statistical analyses were performed using SPSS™ v.15 statistical software (Chicago, IL, USA). Survival analysis was carried out by constructing Kaplan–Meier survival curves plotted against age, gender and the specific LHON mutation and including censored cases i.e. unaffected individuals who, at the time of interview, had not lost vision. Binary logistic regression was used to determine which variables in our dataset influenced the risk of visual loss among LHON carriers. This form of analysis assumes that the logarithm of the odds ratio is a linear function of the predictor variables included in the model:
where P is the probability of a LHON carrier converting to affected disease status; X1, X2 … Xn represent the chosen predictor variables; and B0, B1, … Bn are coefficients reflecting the nature of each predictor (Bland, 2000). Visual failure was the dependent response variable used in the model, with the following as independent variables: (i) age, (ii) gender, (iii) LHON mutation (m.11778G>A, m.3460G>A, m.14484T>C), (iv) cumulative consumption of tobacco and alcohol and (v) maximum intensity of tobacco and alcohol consumption. Categorical smoking and alcohol data incorporated into the model was based on the 75th percentiles for the unaffected LHON carrier group.
Results
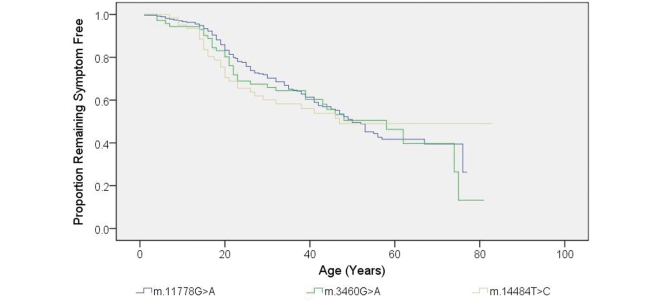
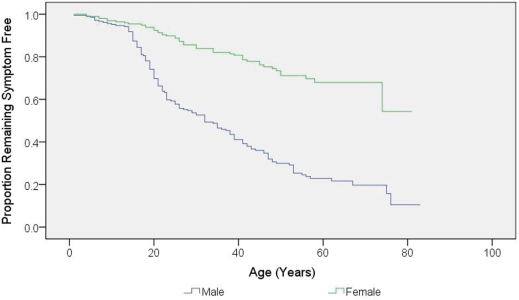
The mean age at onset of visual loss in the affected individuals was 27.9 years (SD = 14.9), with a mean disease duration of 15.5 years (SD = 15.4) (Table 1). In a minority of affected carriers, visual failure occurred before the age of 10 years (n = 15, 7.7%) and after the age of 50 years (n = 18, 9.2%). There was no difference in the age of onset of visual loss among the three primary LHON mutations (Kaplan–Meier, log rank P = 0.946) (Fig. 1). Among the affected group, 74.5% were male, with a male to female ratio of 2.9:1 and male gender was a significant risk factor for visual loss [Odds ratio (OR) = 7.11, 95% confidence interval (CI) = 4.58–11.03, P < 0.001] (Fig. 2).
Table 1.
Characteristics of the study population
| Affected | Unaffected | |
|---|---|---|
| Number of individuals | ||
| m.11778G>A (%) | 132 (67.3) | 138 (67.0) |
| m.3460G>A (%) | 35 (17.9) | 36 (17.5) |
| m.14484T>C (%) | 29 (14.8) | 32 (15.5) |
| Total | 196 | 206 |
| Sex, N (%) | ||
| Male | 146 (74.5) | 60 (29.1) |
| Female | 50 (25.5) | 146 (70.9) |
| Male: female ratio | 2.9 | 0.4 |
| Age at time of study (years) | ||
| Mean (SD) | 43.3 (16.9) | 47.8 (14.9) |
| Range | 13–82 | 14–83 |
| Numbers of tobacco smokers (%) | ||
| Male | 107 (73.3) | 33 (55.0) |
| Female | 27 (54.0) | 75 (51.4) |
| Whole group | 134 (68.4) | 108 (52.4) |
| Numbers of alcohol drinkers (%) | ||
| Male | 139 (95.2) | 57 (95.0) |
| Female | 46 (92.0) | 131 (89.7) |
| Whole group | 185 (94.4) | 188 (91.3) |
Figure 1.
Kaplan–Meier curve showing disease onset with regard to the three primary LHON mutations (P = 0.946 by log-rank test). Age of onset (years) was defined as the age when the patients first noticed their visual symptoms.
Figure 2.
Kaplan–Meier curve of disease onset for male and female LHON carriers (P < 0.001 by log-rank test).
Approximately two-thirds of affected individuals were smokers, compared with approximately half of the unaffected LHON carriers (Tables 1 and 2). There was no significant difference in mean cumulative tobacco consumption between affected and unaffected LHON carriers (6.57 pack years, SD = 12.37 versus 7.25 pack years, SD = 11.30, P = 0.57), but mean maximum intensity of smoking was significantly higher among affected individuals (16.66 cigarettes per day, SD = 19.62 versus 11.55 cigarettes per day, SD = 14.97, P < 0.001).
Table 2.
Levels of tobacco consumption among LHON carriers and ORs for vision loss
| Affected (%) | Unaffected (%) | Mean (SD) | ORsa (95% CI) | P-value | |
|---|---|---|---|---|---|
| Cumulative smoking consumption (Pack years) | |||||
| None | 62 (31.6) | 98 (47.6) | – | – | – |
| Light smoking | 114 (58.2) | 81 (39.3) | 5.82 (5.45) | 2.23 (1.45–3.41) | <0.001c |
| Heavy smoking | 20 (10.2) | 27 (13.1) | 32.47 (14.63) | 1.17 (0.61–2.27) | 0.735 |
| Whole groupb | 134 (68.4) | 108 (52.4) | 11.00 (13.28) | 1.96 (1.31–2.95) | 0.001c |
| Maximum smoking consumption | |||||
| None | 62 (31.6) | 98 (47.6) | – | – | – |
| Light smoking | 78 (39.8) | 80 (38.8) | 12.50 (8.82) | 1.54 (0.99–2.41) | 0.071 |
| Heavy smoking | 56 (28.6) | 28 (13.6) | 42.32 (12.84) | 3.16 (1.82–5.50) | <0.001c |
| Whole groupb | 132 (68.4) | 108 (52.4) | 22.85 (17.60) | 1.96 (1.31–2.95) | 0.001c |
The light and heavy smoking subgroups were based on whether the subject was below or above the 75th percentile values derived from the level of smoking consumption among unaffected LHON carriers.
a Comparison to unaffected LHON carriers who were non-smokers.
b Whole group includes both light and heavy tobacco consumers
c Significant at P < 0.05 level.
The majority of study subjects drank alcohol (94.4% of the affected individuals and 91.2% of the unaffected individuals, Tables 1 and 3). Mean cumulative alcohol consumption among affected carriers (41.95 drink years, SD = 68.34) was not significantly different to unaffected carriers (43.26 drink years, SD = 73.39, P = 0.853). However, the mean maximum intensity of alcohol consumption for the affected group was significantly higher than for the unaffected group (54.43 U/week, SD = 104.21 versus 23.78 U/week, SD = 36.88, P < 0.001).
Table 3.
Levels of alcohol consumption among LHON carriers and ORs for vision loss
| Affected (%) | Unaffected (%) | Mean (SD) | ORa (95% CI) | P-value | |
|---|---|---|---|---|---|
| Cumulative alcohol consumption (Drink years) | |||||
| None | 11 (5.6) | 18 (8.8) | – | – | – |
| Light drinking | 137 (69.9) | 141 (68.4) | 16.10 (16.31) | 1.59 (0.72–3.49) | 0.329 |
| Heavy drinking | 48 (24.5) | 47 (22.8) | 132.02 (99.93) | 1.67 (0.71–3.91) | 0.290 |
| Whole groupb | 185 (94.4) | 188 (91.3) | 45.62 (72.66) | 1.61 (0.74–3.50) | 0.252 |
| Maximum alcohol consumption (Units of alcohol per week) | |||||
| None | 11 (5.6) | 18 (8.8) | – | – | – |
| Light drinking | 85 (43.4) | 138 (67.0) | 8.32 (8.98) | 1.01 (0.45–2.24) | 1.000 |
| Heavy drinking | 100 (51.0) | 50 (24.3) | 90.95 (110.47) | 3.27 (1.44–7.46) | 0.006c |
| Whole groupb | 185 (94.4) | 188 (91.3) | 41.55 (81.13) | 1.61 (0.74–3.50) | 0.252 |
The light and heavy smoking subgroups were based on whether the subject was below or above the 75th percentile values derived from the level of alcohol consumption among unaffected LHON carriers.
a Comparison to unaffected LHON carriers who were non-drinkers.
b Whole group includes both light and heavy alcohol consumers
c Significant at P < 0.05 level.
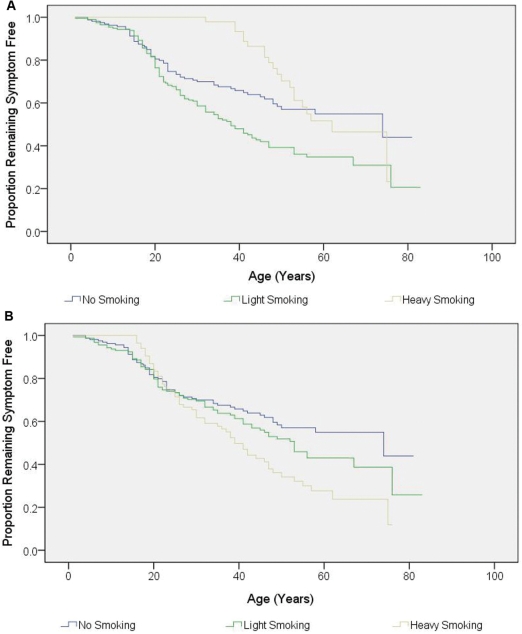
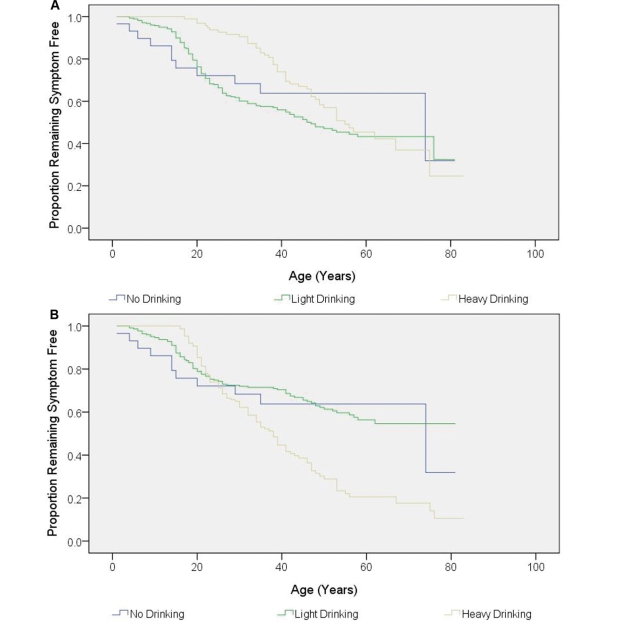
Kaplan–Meier survival analysis revealed significant relationships with (i) cumulative smoking, (ii) maximum intensity smoking and (iii) maximum intensity drinking, but not cumulative drinking (Figs 3 and 4). These initial results suggested that both smoking and alcohol consumption increased the risk of visual failure in LHON pedigrees. However, male carriers drank and smoked more than female carriers (maximum smoking: P = 0.001; cumulative smoking P = 0.096; maximum alcohol: P < 0.001; cumulative alcohol: P < 0.001), raising the possibility that the apparent association of visual loss with alcohol and smoking could actually be secondary to the gender bias in LHON i.e. the greater number of affected males. To address this issue, binary logistic regression was performed which allows a more stringent statistical analysis by simultaneously analysing all the variables that could influence the risk of a LHON carrier converting, therefore minimizing the chance of detecting a spurious statistical association.
Figure 3.
Kaplan–Meier curve showing the disease onset according to tobacco consumption. (A) Cumulative smoking (P < 0.001 by log-rank test). (B) Maximum intensity smoking (P = 0.011 by log-rank test).
Figure 4.
Kaplan–Meier curve showing the disease onset according to alcohol consumption. (A) Cumulative drinking (P = 0.108 by log-rank test). (B) Maximum intensity drinking (P < 0.001 by log-rank test).
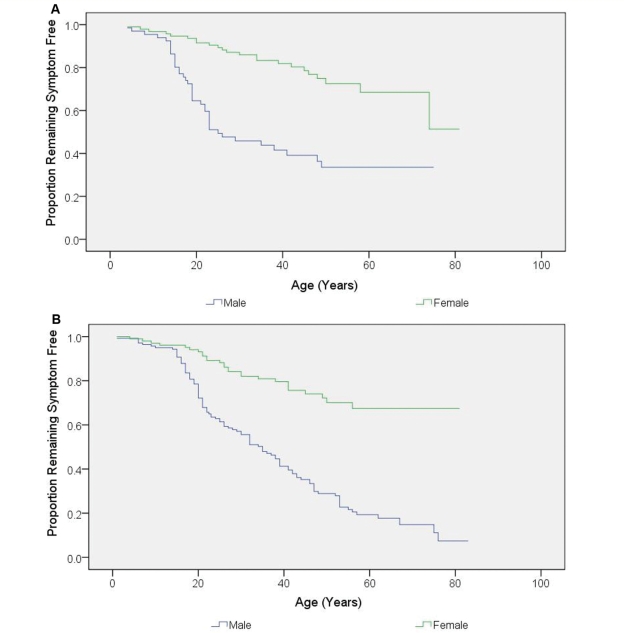
With smoking and alcohol consumption treated as categorical variables in the logistic regression model, both light (OR = 1.93; 95% CI = 1.08–3.46; P = 0.027) and heavy (OR = 3.26; 95% CI = 1.31–8.07; P = 0.011) cumulative smoking were independent risk factors for visual failure compared with the non-smoking group, but for maximum smoking intensity, only heavy smoking (OR = 2.80; 95% CI = 1.31–5.99; P = 0.008) showed a significant association (Table 4). When analysed as continuous variables, both cumulative (OR = 1.04; 95% CI = 1.01–1.06; P = 0.004) and maximum intensity (OR = 1.02; 95% CI = 1.00–1.04; P = 0.021) of smoking were significantly associated with increased risk of visual loss (Table 5). Although there was a trend towards a greater likelihood of conversion among LHON carriers who were heavy drinkers (≥75th percentile), both cumulative and maximum intensity of alcohol consumption did not reach statistical significance either as categorical or continuous variables (Tables 4 and 5). There was also no significant statistical interaction between smoking, alcohol and gender intake when considered as either continuous or categorical variables. Further evidence that gender and smoking are independent risk factors for visual failure in LHON was apparent from Kaplan–Meier survival analysis of male and female smokers and non-smokers. Clinical penetrance was greater in male non-smokers than female non-smokers (Fig. 5A, P < 0.001). However, the striking effect of smoking in males was associated with a 93% life-time penetrance of visual failure, compared with only 66% in non-smoking males and 33% in smoking females (Fig. 5B, P < 0.001).
Table 4.
Binary logistic regression models for the prediction of vision loss: categorical consumption levels of tobacco and alcohol
| Model 1 (cumulative) |
Model 2 (maximum) |
|||
|---|---|---|---|---|
| Predictor variables | ORs (95% CI) | P-value | ORs (95% CI) | P-value |
| Light smokinga | 1.93 (1.08–3.46) | 0.027c | 1.63 (0.88–3.02) | 0.118 |
| Heavy smokinga | 3.26 (1.31–8.07) | 0.011c | 2.80 (1.31–5.99) | 0.008c |
| Light drinkingb | 1.52 (0.50–4.64) | 0.459 | 1.32 (0.44–3.98) | 0.625 |
| Heavy drinkingb | 2.75 (0.76–10.02) | 0.125 | 2.31 (0.73–7.30) | 0.154 |
a Comparison with non-smokers.
b Comparison with non-drinkers
c Significant at P < 0.05 level. Both models also incorporated the following variables: age, gender and LHON mutation.
Table 5.
Binary logistic regression models for the prediction of vision loss: continuous consumption levels of tobacco and alcohol
| Model 3 (cumulative) |
Model 4 (maximum) |
|||
|---|---|---|---|---|
| Predictor variables | ORs (95% CI) | P-value | ORs (95% CI) | P-value |
| Smoking | 1.04 (1.01–1.06) | 0.004a | 1.02 (1.00–1.04) | 0.021a |
| Alcohol | 1.00 (1.00–1.01) | 0.093 | 1.00 (1.00–1.01) | 0.176 |
a Significant at P < 0.05 level. Both models also incorporated the following variables: age, gender and LHON mutation.
Figure 5.
Kaplan–Meier curve showing the disease onset according to gender and smoking status. (A) Non-smoking individuals (P < 0.001 by log-rank test). (B) Smoking individuals (P < 0.001 by log-rank test).
The most commonly reported triggers for visual loss were psychological stress (13.3%, n = 26) and physical trauma (5.6%, n = 11), with other subjects reporting a concurrent physical illness (4.6%, n = 9), poor nutrition (0.5%, n = 1) and chemotherapy (0.5%, n = 1) as significant personal events prior to the onset of symptoms. The nature of the physical trauma included head injury (n = 7), road traffic accident (n = 3) and limb injury (n = 1). A proportion of affected LHON carriers (33.7%, n = 66) also reported occupational or accidental toxin exposure in the immediate period preceding visual loss, which included exhaust fumes (n = 16), dry cleaning solvents (n = 13), asbestos (n = 5), lead (n = 4), scrap metals (n = 4) and fibreglass (n = 2), at high levels not encountered in a normal environment. There was no significant difference between affected and unaffected LHON carriers in their diet, level of physical exercise, recreational drugs, medication history or medical comorbidity.
Discussion
The evidence supporting the role of environmental risk factors in precipitating the optic neuropathy in LHON has so far been largely based on anecdotal reports (DuBios and Feldon, 1992; Golnik and Shaible, 1994; Rizzo, 1995; Hwang and Park, 1996; Mackey et al., 2003; Sanchez et al., 2006; Carelli et al., 2007) and small case series (Newman et al., 1991; Cullom et al., 1993; Riordan-Eva et al., 1995). Larger studies specifically investigating the role of tobacco and alcohol have produced conflicting results (Chalmers and Harding, 1996; Tsao et al., 1999; Kerrison et al., 2000; Sadun et al., 2003) (Table 6). Two separate studies of large m.11778G>A LHON pedigrees did suggest an important role for smoking in disease conversion (Tsao et al., 1999; Sadun et al., 2003), but crucially, only one study (Kerrison et al., 2000) considered exposure levels before the onset of visual loss. By carrying out the largest study to date, and controlling for the major factors known to influence disease penetrance, we have provided strong evidence that smoking is associated with an increased risk of visual failure among LHON carriers. For both cumulative and maximum intensity levels, heavy smokers were also more likely to be affected than light smokers, providing further support for a biologically plausible dose–response relationship.
Table 6.
Summary of previous studies assessing the role of smoking and alcohol in LHON
| References | Study group | Main findings | Limitations |
|---|---|---|---|
| Chalmers and Harding, 1996 | 50 unrelated, matched controls. | Maximum alcohol and tobacco consumption were analysed at the time of onset of visual symptoms. | Small sample size. |
| 50 affected LHON carriers with one of the three primary LHON mutations: m.11778G>A, n = 35 m.3460G>A, n = 8 m.14484T>C, n = 7. | Alcohol, but not tobacco consumption, was significantly higher among affected patients compared with controls. | Cumulative consumption and variation over the years preceding visual loss was not recorded. | |
| Tsao et al., 1999 | A North American pedigree of 65 family members harbouring the m.11778G>A mutation | Tobacco consumption was compared between affected and unaffected family members at the two age cut-offs of 25 and 35 years. | Small sample size. |
| All affected carriers were smokers compared with only 25–42% of unaffected carriers. | Cumulative consumption and variation over the years preceding visual loss was not recorded. | ||
| Kerrison et al., 2000 | 103 affected and 158 unaffected from 80 LHON pedigrees: m.11778G>A, n = 63 m.3460G>A, n = 7 m.14484T>C, n = 10 | Maximum intensity and cumulative consumption of tobacco or alcohol were not associated with an increased risk of visual loss. | Possibility of ascertainment bias since only 56% of postal questionnaires were returned |
| Sadun et al., 2003 | A large Brazilian pedigree of 265 family members harbouring the m.11778G>A mutation. | Disease penetrance was significantly higher among smokers but not drinkers. | Actual consumption levels were not quantified, only whether an individual drank or smoked. |
Our LHON cohort is comparable with other published epidemiological case series, with a predominance of the m.11778G>A mutation, most patients becoming affected in their twenties, and males having a higher risk of visual loss than female carriers. The gender bias was slightly lower than the figures reported in previous LHON case series, where males were on average five times more likely to be affected than female carriers (Man et al., 2002; Yu-Wai-Man, 2009). Although this discrepancy can be accounted for by the recruitment of a proportionally larger number of affected female carriers, this ascertainment bias is unlikely to affect the general applicability of our findings.
Initial analysis of our dataset indicated an increased risk of disease conversion among LHON carriers who were heavy drinkers. However, after controlling for possible confounding variables such as age, gender and the primary LHON mutations using a logistic regression model, both cumulative and maximum intensity of alcohol failed to reach statistical significance. The trend towards a significant relationship between visual loss and heavy alcohol intake suggests a weaker causal relationship between levels of alcohol consumption and visual failure, but this cannot be substantiated at present. Although we aimed to investigate the role of other environmental triggers in LHON, besides smoking and alcohol, and about a third of our patients reported possible insults prior to the onset of visual loss, the retrospective nature of our study made it difficult to draw any firm conclusions regarding their actual relevance.
What pathophysiological mechanisms could explain the increased susceptibility to optic neuropathy among smoking LHON carriers? Oxidative phosphorylation (OXPHOS) produces most of the adenosine triphosphate (ATP) requirements and this process is achieved by a chain of five respiratory complexes (I–V) along the inner mitochondrial membrane. All three primary LHON mutations result in amino acid substitutions within subunits that assemble into Complex I and depending on the experimental assays used, a mild to moderate reduction in respiratory chain activity has been identified (Yu-Wai-Man et al., 2009). Cigarette smoking could potentiate this energy deficit by either compromising complex I activity directly (Smith et al., 1993, 1994), or limiting OXPHOS through elevated carboxyhaemoglobin levels and a reduction in arterial oxygen content (Gvozdjak et al., 1987; van Jaarsveld et al., 1992). In a study, cytochrome c oxidase (COX, complex IV) activity in rats was reduced by 25% after 30 min exposure to cigarette smoke, and prolonged exposure resulted in further decreases in COX activity (Gvozdjak et al., 1987). This led to an increase in reactive oxygen species, which have been implicated as potent triggers of apoptosis and retinal ganglion cell loss in LHON (Yu-Wai-Man et al., 2009). There is also a suggestion that secondary mtDNA abnormalities are more prevalent among smokers, with an increased rate of somatic mtDNA mutations and a compensatory increase in mtDNA copy number (Tan et al., 2008). These findings are consistent with a deleterious effect of smoking on mitochondrial biogenesis, which potentially could further exacerbate the pre-existing LHON-induced complex I defect in retinal ganglion cells, precipitating the onset of optic nerve dysfunction.
We identified 15 individuals who reported visual loss before the age of 10 years, none of whom had consumed tobacco or alcohol prior to visual loss, and there was not an increased rate of smoking among close family members compared with the rest of the affected group. This is in keeping with the prevailing view that LHON is a multifactorial disease, and the visual failure is likely due to a complex interaction between genetic and environmental factors, with smoking as only one of the susceptibility factors. Although we applied a stringent logistic regression model to reduce the risk of finding a spurious association, the nature of our study did not allow us to control for the influence of mtDNA haplogroups (Hudson et al., 2007) and the presence or absence of the high-risk haplotype at Xp21 which has been shown to increase the risk of visual failure ∼35-fold for the m.11778G>A and m.14484T>C mutations but not for m.3460G>A (Hudson et al., 2005). However, our results clearly indicate that future association studies of possible nuclear modifier genes in LHON should ideally control for the level of smoking and alcohol consumption in any analyses performed. Being a retrospective study based upon telephone interviews, our study was also potentially subject to recall bias, with patients reporting information about their alcohol and smoking history over a time period sometimes spanning several decades.
Our large multi-centre study of 125 LHON pedigrees has revealed an important and consistent role for smoking in increasing disease penetrance among carriers of the three primary mtDNA mutations. Perhaps the most striking finding is that 93% of men who smoke develop visual failure if they harbour the LHON mtDNA mutation. The effect of smoking is also apparent in women, albeit with a reduced overall clinical penetrance due to the gender bias. These findings have important practical implications for genetic counselling and LHON carriers should be strongly advised not to smoke. Although the association between visual failure and heavy alcohol consumption was not statistically significant in the logistic regression analysis, it would also seem sensible for LHON mutation carriers to moderate their alcohol intake and avoid binge drinking episodes.
Supplementary material
Supplementary material is available at Brain online.
Funding
Funding to pay the Open Access publication charges for this article was provided by The Wellcome Trust.
Acknowledgements
PFC is a Wellcome Trust Senior Fellow in Clinical Science who also receives funding from the Medical Research Council (UK), the UK Parkinson's Disease Society and the UK NIHR Biomedical Research Centre for Ageing and Age-related disease award to the Newcastle upon Tyne Foundation Hospitals NHS Trust. PYWM is an MRC Research Fellow. This research was presented at the 2009 American Academy of Neurology Annual Meeting with support from a Brain Travel Grant to MAK. We would like to thank the Dutch patients’ association ‘De LOA-contactgroep’ (Eindhoven) for their contribution and all the LHON families who have kindly taken part in this survey.
References
- Biousse V, Browne MD, Newman NJ, Allen JC, Rosenfeld J, Meola G, et al. De novo 14484 mitochondrial DNA mutation in monozygotic twins discordant for Leber's hereditary optic neuropathy. Neurology. 1997;49:1136–8. doi: 10.1212/wnl.49.4.1136. [DOI] [PubMed] [Google Scholar]
- Bland M. An Introduction to medical statistics. 3rd. Oxford: Oxford University Press; 2000. [Google Scholar]
- Brown MD, Wallace DC. Spectrum of mitochondrial-DNA mutations in Lebers hereditary optic neuropathy. Clin Neurosci. 1994;2:138–45. [Google Scholar]
- Carelli V, Franceschini F, Venturi S, Barboni P, Savini G, Barbieri G, et al. Grand rounds: Could occupational exposure to n-hexane and other solvents precipitate visual failure in Leber hereditary optic neuropathy? Environ Health Perspect. 2007;115:113–5. doi: 10.1289/ehp.9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers RM, Harding AE. A case-control study of Leber's hereditary optic neuropathy. Brain. 1996;119:1481–6. doi: 10.1093/brain/119.5.1481. [DOI] [PubMed] [Google Scholar]
- Cullom ME, Heher KL, Miller NR, Savino PJ, Johns DR. Leber's hereditary optic neuropathy masquerading as tobacco-alcohol amblyopia. Arch Ophthalmol. 1993;111:1482–5. doi: 10.1001/archopht.1993.01090110048021. [DOI] [PubMed] [Google Scholar]
- DuBois LG, Feldon SE. Evidence for a metabolic trigger for Leber's hereditary optic neuropathy. A case report. J Clin Neuro-Ophthalmol. 1992;12:15–16. [PubMed] [Google Scholar]
- Elliott HR, Samuels DC, Eden JA, Relton CL, Chinnery PF. Pathogenic mitochondrial DNA mutations are common in the general population. Am J Hum Genet. 2008;83:254–60. doi: 10.1016/j.ajhg.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golnik KC, Schaible ER. Folate-responsive optic neuropathy. J Neuroophthalmol. 1994;14:163–9. [PubMed] [Google Scholar]
- Gvozdjak J, Gvozdjakova A, Kucharska J, Bada V. The effect of smoking on myocardial metabolism. Czech Med. 1987;10:47–53. [PubMed] [Google Scholar]
- Harding AE, Sweeney MG, Govan GG, Riordan-Eva P. Pedigree analysis in Leber hereditary optic neuropathy families with a pathogenic mtDNA mutation. Am J Hum Genet. 1995;57:77–86. [PMC free article] [PubMed] [Google Scholar]
- Hudson G, Carelli V, Spruijt L, Gerards M, Mowbray C, Achilli A, et al. Clinical expression of Leber hereditary optic neuropathy is affected by the mitochondrial DNA-haplogroup background. Am J Hum Genet. 2007;81:228–33. doi: 10.1086/519394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson G, Keers S, Yu-Wai-Man P, Griffiths P, Huponen K, Savontaus ML, et al. Identification of an X-chromosomal locus and haplotype modulating the phenotype of a mitochondrial DNA disorder. Am J Hum Genet. 2005;77:1086–91. doi: 10.1086/498176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JM, Park HW. Carbon monoxide poisoning as an epigenetic factor for Leber's hereditary optic neuropathy. Korean J Ophthalmol. 1996;10:122–3. doi: 10.3341/kjo.1996.10.2.122. [DOI] [PubMed] [Google Scholar]
- Johns DR, Smith KH, Miller NR, Sulewski ME, Bias WB. Identical twins who are discordant for Leber's hereditary optic neuropathy. Arch Ophthalmol. 1993;111:1491–4. doi: 10.1001/archopht.1993.01090110057023. [DOI] [PubMed] [Google Scholar]
- Kerrison JB, Miller NR, Hsu F, Beaty TH, Maumenee IH, Smith KH, et al. A case-control study of tobacco and alcohol consumption in Leber's hereditary optic neuropathy. Am J Ophthalmol. 2000;130:803–12. doi: 10.1016/s0002-9394(00)00603-6. [DOI] [PubMed] [Google Scholar]
- Lam BL. Identical twins no longer discordant for Leber's hereditary optic neuropathy. Arch Ophthalmol. 1998;116:956–7. [PubMed] [Google Scholar]
- Mackey DA, Fingert JH, Luzhansky JZ, McCluckey PJ, Howell N, Hall AJ, et al. Leber's hereditary optic neuropathy triggered by antiretroviral therapy for human immunodeficiency virus. Eye. 2003;17:312–7. doi: 10.1038/sj.eye.6700362. [DOI] [PubMed] [Google Scholar]
- Mackey DA, Oostra RJ, Rosenberg T, Nikoskelainen E, Bronte-Stewart J, Poulton J, et al. Primary pathogenic mtDNA mutations in multigeneration pedigrees with Leber hereditary optic neuropathy. Am J Hum Genet. 1996;59:481–5. [PMC free article] [PubMed] [Google Scholar]
- Man PY, Griffiths PG, Brown DT, Howell N, Turnbull DM, Chinnery PF. The epidemiology of Leber hereditary optic neuropathy in the North East of England. Am J Hum Genet. 2003;72:333–9. doi: 10.1086/346066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man PY, Turnbull DM, Chinnery PF. Leber hereditary optic neuropathy. J Med Genet. 2002;39:162–9. doi: 10.1136/jmg.39.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman NJ, Biousse V. Hereditary optic neuropathies. Eye. 2004;18:1144–60. doi: 10.1038/sj.eye.6701591. [DOI] [PubMed] [Google Scholar]
- Newman NJ, Lott MT, Wallace DC. The clinical characteristics of pedigrees of Leber's hereditary optic neuropathy with the 11778 mutation. Am J Ophthalmol. 1991;111:750–62. doi: 10.1016/s0002-9394(14)76784-4. [DOI] [PubMed] [Google Scholar]
- Nikoskelainen EK. Clinical picture of LHON. Clin Neurosci. 1994;2:115–20. [Google Scholar]
- Nikoskelainen EK, Savontaus ML, Wanne OP, Katila MJ, Nummelin KU. Leber's hereditary optic neuropathy a maternally inherited disease. A genealogic study in four pedigrees. Arch Ophthalmol. 1987;105:665–71. doi: 10.1001/archopht.1987.01060050083043. [DOI] [PubMed] [Google Scholar]
- Office for National Statistics. National Statistics Methodological Series No. 37: estimating alcohol consumption from survey data: updated method of converting volumes to units. Newport, Wales: Office for National Statistics; 2007. [Google Scholar]
- Riordan-Eva P, Sanders MD, Govan GG, Sweeney MG, Da Costa J, Harding AE. Clinical features of Leber's hereditary optic neuropathy defined by the presence of a pathogenic mitochondrial DNA mutation. Brain. 1995;118:319–37. doi: 10.1093/brain/118.2.319. [DOI] [PubMed] [Google Scholar]
- Rizzo JF., III Adenosine triphosphate deficiency: a genre of optic neuropathy. Neurology. 1995;45:11–16. doi: 10.1212/wnl.45.1.11. [DOI] [PubMed] [Google Scholar]
- Sadun AA, Carelli V, Salomao SR, Berezovsky A, Quiros PA, Sadun F, et al. Extensive investigation of a large Brazilian pedigree of 11778/haplogroup J Leber hereditary optic neuropathy. Am J Ophthalmol. 2003;136:231–8. doi: 10.1016/s0002-9394(03)00099-0. [DOI] [PubMed] [Google Scholar]
- Sanchez RN, Smith AJ, Carelli V, Sadun AA, Keltner JL. Leber hereditary optic neuropathy possibly triggered by exposure to tire fire. J Neuroophthalmol. 2006;26:268–72. doi: 10.1097/01.wno.0000249320.27110.ab. [DOI] [PubMed] [Google Scholar]
- Seedorff T. The inheritance of Leber's disease. A genealogical follow-up study. Acta Ophthalmol. 1985;63:135–45. doi: 10.1111/j.1755-3768.1985.tb01526.x. [DOI] [PubMed] [Google Scholar]
- Shankar SP, Fingert JH, Carelli V, Valentino ML, King TM, Daiger SP, et al. Evidence for a novel x-linked modifier locus for leber hereditary optic neuropathy. Ophthalmic Genet. 2008;29:17–24. doi: 10.1080/13816810701867607. [DOI] [PubMed] [Google Scholar]
- Smith PR, Cooper JM, Govan GG, Harding AE, Schapira AH. Smoking and mitochondrial function: a model for environmental toxins. Q J Med. 1993;86:657–60. doi: 10.1093/qjmed/86.10.657. [DOI] [PubMed] [Google Scholar]
- Smith PR, Cooper JM, Govan GG, Harding AE, Schapira AH. Platelet mitochondrial function in Leber's hereditary optic neuropathy. J Neurol Sci. 1994;122:80–3. doi: 10.1016/0022-510x(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Spruijt L, Kolbach DN, de Coo RF, Plomp AS, Bauer NJ, Smeets HJ, et al. Influence of mutation type on clinical expression of Leber hereditary optic neuropathy. Am J Ophthalmol. 2006;141:676–82. doi: 10.1016/j.ajo.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Tan D, Goerlitz DS, Dumitrescu RG, Han D, Seillier-Moiseiwitsch F, Spernak SM, et al. Associations between cigarette smoking and mitochondrial DNA abnormalities in buccal cells. Carcinogenesis. 2008;29:1170–7. doi: 10.1093/carcin/bgn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao K, Aitken PA, Johns DR. Smoking as an aetiological factor in a pedigree with Leber's hereditary optic neuropathy. Br J Ophthalmol. 1999;83:577–81. doi: 10.1136/bjo.83.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Jaarsveld H, Kuyl JM, Alberts DW. Exposure of rats to low concentrations of cigarette smoke increases myocardial sensitivity to ischaemia/reperfusion. Basic Res Cardiol. 1992;87:393–9. doi: 10.1007/BF00796524. [DOI] [PubMed] [Google Scholar]
- Yu-Wai-Man P, Griffiths PG, Hudson G, Chinnery PF. Inherited mitochondrial optic neuropathies. J Med Genet. 2009;46:145–58. doi: 10.1136/jmg.2007.054270. [DOI] [PMC free article] [PubMed] [Google Scholar]