Abstract
G protein-coupled receptor (GPR)-39 is a seven-transmembrane receptor expressed mainly in endocrine and metabolic tissues that acts as a Zn++ sensor signaling mainly through the Gq and G12/13 pathways. The expression of GPR39 is regulated by hepatocyte nuclear factor (HNF)-1α and HNF-4α, and in the present study, we addressed the importance of GPR39 for glucose homeostasis and pancreatic islets function. The expression and localization of GPR39 were characterized in the endocrine pancreas and pancreatic cell lines. Gpr39(−/−) mice were studied in vivo, especially in respect of glucose tolerance and insulin sensitivity, and in vitro in respect of islet architecture, gene expression, and insulin secretion. Gpr39 was down-regulated on differentiation of the pluripotent pancreatic cell line AR42J cells toward the exocrine phenotype but was along with Pdx-1 strongly up-regulated on differentiation toward the endocrine phenotype. Immunohistochemistry demonstrated that GRP39 is localized selectively in the insulin-storing cells of the pancreatic islets as well as in the duct cells of the exocrine pancreas. Gpr39(−/−) mice displayed normal insulin sensitivity but moderately impaired glucose tolerance both during oral and iv glucose tolerance tests, and Gpr39(−/−) mice had decreased plasma insulin response to oral glucose. Islet architecture was normal in the Gpr39 null mice, but expression of Pdx-1 and Hnf-1α was reduced. Isolated, perifused islets from Gpr39 null mice secreted less insulin in response to glucose stimulation than islets from wild-type littermates. It is concluded that GPR39 is involved in the control of endocrine pancreatic function, and it is suggested that this receptor could be a novel potential target for the treatment of diabetes.
Deletion of GPR39–expressed in β-cells and ducts of the pancreas–leads to glucose intolerance, decreased Pdx-1 expression, and impaired glucose-stimulated insulin secretion.
G protein-coupled receptor (GPR)-39 is a member of the ghrelin subfamily of seven-transmembrane (7TM) segments (1) of which other members have been shown to be important regulators of food intake, energy metabolism, and regulators specifically of the glucose homeostasis (2,3,4). Originally, GPR39 was reported to be ubiquitously expressed in the body including multiple areas of the central nervous system (1). However, the full-length biologically active form of the GPR39, called GPR39-1a, is found exclusively in peripheral tissues (5). Importantly, the expression of GPR39 is confined to endocrine, metabolic organs such as the pancreas, the liver, the gastrointestinal tract, and white adipose tissue (5).
GPR39 signals mainly through the Gαq and Gα12/13 pathways leading to, for example, inositol phosphate accumulation and activation of downstream pathways such as the serum response element and calcineurin/nuclear factor of activated T cells (NFAT) transcriptional pathways (6) (Fig. 1A). A peptide fragment from the ghrelin precursor, called obestatin, was suggested a few years ago to be a ligand for GPR39 and having the opposite function of ghrelin (7). However, neither the function of obestatin nor its interaction with GPR39 could be confirmed by independent groups, although this point is still debated (8,9,10,11). Importantly, at an early stage, we reported that GPR39 is activated by zinc ions and suggested that the receptor could act as a sensor of presumably physiological concentrations of Zn2+, an observation that has been confirmed by other groups (6,8,9,12). In the context of the endocrine pancreas, it is particularly interesting that GPR39 acts as a zinc sensor because Zn2+ released form the secretory granules of the β-cells is believed to function as a paracrine regulator of islet function (13,14).
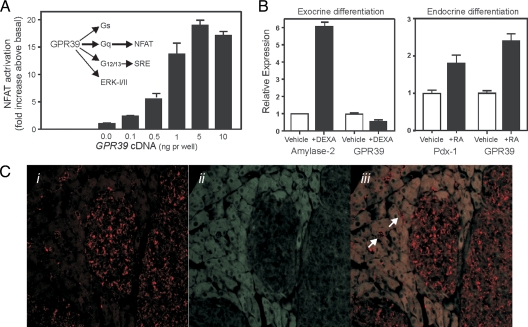
Figure 1.
The GPR39 receptor signaling pathways and GPR39 expression in the endocrine pancreas. A, Simplified scheme of the GPR39 signal transduction pathways with emphasis on the Gαq and Gα12/13 pathways including calcineurin/NFAT, serum response element, and the MAPK ERK1/2 (45). The bar diagram shows the signaling of GPR39 through the NFAT pathway as determined by a transcriptional activity reporter assay in HEK 293 cells transiently transfected with increasing amount of GPR39 cDNA to increase the cell surface expression of the constitutive active receptor; mean ± sem (n = 5, made in triplicates). B, GPR39 expression in pancreatic acinar-derived AR42J cells differentiated toward exocrine phenotype by administration of 100 nm of dexamethasone (DEXA) using expression of amylase 2 as control (left panel) and differentiated toward endocrine phenotype by administration of 1 μm trans-retinoic acid (RA) using expression pdx-1 as control (to the right). C, Immunohistochemical localization of GPR39 in mouse pancreas using a rabbit antiserum raised against the C-terminal tail of the full-length GPR39-1a receptor visualized with biotinylated donkey antirabbit serum plus streptavidin-horseradish peroxidase and tyramide signal amplification-cy3 (panels i and iii, red). In panel ii and iii, the gray autofluorescence of especially the acinar cells shows the outline of the cells and the dark endocrine islet tissue (using the 405 laser with 80/20 neutral density filter). GPR39 is found in the endocrine isles and small ducts in the exocrine tissue, two of which are indicated by white arrows in panel iii. GPR39 is also found in the larger ducts (data not shown). Selective staining of GPR39 transfected vs. mock-transfected HEK-293 cells with this antiserum is shown in supplemental Fig. 1.
The physiological function of GPR39 has previously been investigated by use of genetic knockout models in which, especially the effect on food intake and gastrointestinal function, were addressed (15,16). Moechars et al. (15) found enhanced gastric fluid secretion, enhanced gastric emptying, and increased gastrointestinal transit time in the Gpr39-deficient mice compared with wild-type mice, whereas body weight, food intake, and glucose and insulin levels were unaffected in mice with disrupted GPR39 gene up to 16 wk of age. Similar conclusions were made by Tremblay et al. (16), who studied a number of metabolic parameters without observing significant differences between wild-type and Gpr39-deficient mice.
Recently we characterized the promoter region of the human GPR39 gene and presented functional data supporting the notion that this gene is regulated by both hepatocyte nuclear factor (HNF)-1α and HNF-4α (5), in what Odom et al. (17) had described as a feed-forward multicomponent loop manner. Only relatively few genes behave like this and GPR39 was one of only two of the approximately 400 known 7TM receptors found to be regulated by HNF-1α and HNF-4α in this way (17). HNF-1α and HNF-4α are both so-called maturity-onset diabetes of the young (MODY) genes that are associated with the development of MODY (18). In the present study, we report that Gpr39 specifically follows the endocrine pancreatic differentiation and is selectively expressed in the β-cells of the pancreatic islets. Moreover, deletion of Gpr39 in mice is found to be associated with impaired glucose tolerance and altered pancreatic β-cell gene expression and function including decreased glucose-induced insulin secretion from isolated perifused islets.
Materials and Methods
Immunohistochemistry
Rabbit antiserum against GPR39-1a was generated through immunization with 50 μg keyhole limpet hemocyanin-mouse (m) Gpr39-1a (aa428-446, C-PLSPESPQ-TGSETKPAGST corresponding to the C terminal tail region) biweekly initially with Freund’s complete adjuvant and the next three times with Freund’s incomplete adjuvant followed by monthly immunizations with 10 μg keyhole limpet hemocyanin-mGpr39-1a with Freund’s incomplete adjuvant. All animal experiments were performed according to national guidelines and approved by the national ethics committee.
Tissue
Pancreatic tissue from mice (NMRI) was fixed overnight in 4% fresh paraformaldehyde at +5 C, cryoprotected overnight in 30% sucrose in PBS, and embedded in Tissue-Tek (Sakura, Værløse, Denmark). Eight-micrometer sections were cut on a cryostat and stored at −80 C until use.
Immunohistochemistry
Immunohistochemistry was carried out as described in detail (19); briefly, cryosections were thawed and rinsed in PBS, microwaved in citrate buffer [0.01 m citric acid (pH 6.0)] for 4 min at 600 W in 200 ml buffer followed by 15 min at 250 W and finally left to cool for 20 min and incubated with 1% H2O2 in PBS for 30 min and subsequently blocked with 0.5% Tris-HCl NaCl Blocking Reagent (PerkinElmer, Norwalk, CT). Sections were incubated with primary anti-mGpr39-1a antiserum (1:7000) overnight at room temperature. The following day sections were rinsed in PBS, and biotinylated donkey antirabbit antibody (1:300, Jackson ImmunoResearch, West Grove, PA) was added and incubated for 45 min. Sections were then incubated with streptavidin-horseradish peroxidase, washed in PBS, tyramide signal amplification (TSA-cy3; PerkinElmer) added, and sections washed with PBS. On top of this, new primary antibodies were added goat anti-pancreatic duodenal homeobox-1 (PDX1; 1:5000; Chris Wright Laboratory, Vanderbilt University, Nashville, TN), mouse antiglucagon (1:100, Glu001; New England Biolabs, Ipswich, MA), mouse anti-insulin (1:100, HUI18; Biolabs), mouse antisomatostatin (1:100, Som-018; Biolabs), guinea pig antipancreatic polypeptide (PP; 1:400; Linco, St. Charles, MO). 4′,6-Diamidino-2-phenylindole (0.2ug/ml) was used to outline nuclei. Images were taken on a LSM510 laser-scanning confocal microscope (Zeiss, Birkerød, Denmark). The anti-mGPR39-1a antiserum stained HEK-293 cells transfected with mGPR39-1a as opposed to mock transfected cells (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org).
Gpr39(−/−) mice
Gpr39 knockout mice were generated by Deltagen, Inc. through homologous recombination targeting of the first exon of Gpr39, exchanging the nucleotides from position 278 to 647 of the open reading frame with a neomycin-containing cassette. The chimeric males were mated with C57BL/6 females and backcrossed for five generations into C57BL/6 mice. The mice used in this study were obtained from heterozygous breeding resulting in wild-type, homozygous, or heterozygous littermates, and no further backcrossing was performed. Genotypes were verified through PCR using primers specific for the wild-type (G1 and G3) and one primer specific for the insert (G2) sequences shown in supplemental Table 1. The mice were housed in a normal 12-h light, 12-h dark cycle and had free access to food and water. The animal studies were conducted in accordance with institutional guidelines and approved by the Animal Experiments Inspectorate in Denmark.
In vivo mice studies
Oral glucose tolerance tests (OGTT) were carried out in mice at the age of 11–12 wk. Mice were fasted for 16–18 h with free access to water. Glucose (1.5 g/kg body weight) was administered by oral gavage. Concentration of blood glucose was measured, using a glucometer (Ascensia Elite XL diabetes care system; Bayer HealthCare, Berkley, CA). At time point −30 and 15 min, blood was collected from the orbital sinus for insulin measurement. In between each glucose measurement, the mice were contained individually in small boxes. For iv glucose tolerance tests (IVGTTs), mice of 17–18 wk of age were fasted for 16–18 h with free access to water. Glucose (1 g/kg body weight) was injected iv in a tail vein. For insulin tolerance test (ITT), mice of 15–16 wk of age were used. Food was removed for 2 h before the test. Mice were challenged with insulin ip (Actrapid; Novo Nordisk, Copenhagen, Denmark) at a dose of 0.75 U/kg. The average basal blood glucose after 2 h fasting was for Gpr39(+/+) 6.8 ± 0.4 mm and for Gpr39(−/−) 6.8 ± 0.3 mm measured in blood from tail punctures. Tissues were collected in mice at the age of 21–22 wk. After 16–18 h, fasting blood was sampled from the orbital sinus under anesthesia. Plasma was separated and stored at −20 C until further use. Tissues for quantitative PCR (QPCR) were dissected and frozen in liquid nitrogen and stored at −80 C until further use. Tissue for immunohistochemistry were fixed overnight in Stefanini’s solution; rinsed in Tyrode’s solution; and indirect immunofluorescence for insulin, glucagon, somatostatin, and PP performed as previously described (20). The islet area was measured by MetaMorph imaging software Molecular Device, Downington, PA.
Real-time QPCR
Real-time QPCR was performed using the Mx3000P (Stratagene, La Jolla, CA) as previously described (5). The SYBR Premix Ex Taq (Takara, Shiga, Japan) was used for standard SYBR green-based QPCR and for evaluation of the primers. Premix Ex Taq (Takara) and dual-labeled LNA containing fluorogenic probes (Sigma Genosys, Haverhill, UK) were used for TaqMan assays, in which a target gene and a reference gene were assayed in a duplex reaction. All primers and probes are listed in supplemental Table 1. The data were normalized by setting the maximum expression value to 1, thus showing the relative expression of the gene. As a reference gene, tyrosine 3-monooxygenase (YWHAZ) was used for the mouse studies of pancreatic tissue and β-actin was used for the tissue expression studies. RNA from tissue was extracted using the SV total RNA isolation system (Promega, Madison, WI) followed by cDNA synthesis using the ImProm-II Reverse Transcriptase (Promega).
Insulin content and secretion from islets in culture
Islets from wild-type or Gpr39 knockout mice were isolated by collagenase digestion technique as described previously (21). Islets were precultured for 2–5 d in RPMI 1640 medium containing 11 mm glucose and 10% newborn calf serum and supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin (all from Invitrogen/Life Technologies, Inc., Carlsbad, CA). For insulin secretion, the islets were stimulated with 11 mm glucose for 24–48 h. For total islet insulin content determination, islets were lysed on ice for 30 min in lysis buffer. Insulin in media or islet lysates was measured by competitive ELISA as previously described (22).
Perifusion of islets
Islets from equal numbers of male and female mice were prepared by collagenase digestion and kept in tissue culture for 24 h in RPMI 1640 medium containing 10% newborn calf serum as previously described (23). Insulin release from islets was determined by perifusion in a noncirculating system with beads of 0.25 ml Bio-Gel P2 (Bio-Rad Laboratories, Rockville Center, NY) as a supporting medium. Twenty-five islets per chamber were perifused at 37 C at a flow rate of 0.26 ml/min in Krebs-Ringer medium supplemented with 20 mm HEPES, 5 mm NaHCO3, 2 mg/ml BSA, and 3.3 mm glucose. Islets were initially perifused to obtain a basal release rate and then challenged with 11 and 16.7 mm glucose or 16.7 mm glucose and 0.1 μm glucagon-like peptide-1-(7-36)-amide (GLP-1). The effluent medium was collected for periods of 5 or 10 min. Insulin was determined by RIA (23).
Signaling through NFAT-induced transcription activity
HEK-293 cells were grown in DMEM 31966 supplemented with 10% fetal calf serum, 2 mm glutamine, and 0.01 mg/ml gentamicin. Cells were transfected with Lipofectamine 2000 (Life Technologies). HEK-293 cells (30,000 cells/well) seeded in 96-well plates were transiently transfected with NFAT-Luc (PathDetect NFAT cis-reporting system; Stratagene) with the indicated amounts of GPR39 cDNA, and the signaling was measured according to the protocol provided by the manufacturer.
Expression pattern in the AR42J rat acinar cell line
The cells were grown in DMEM containing GlutaMAX supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. For transdifferentiation into an exocrine- and endocrine-like cell type, AR42J cells were grown at a cell density of 20,000 cells/cm2 in the growth media plus 100 nm dexamethasone (Sigma, St. Louis, MO) or 1 μm all trans-retinoic acid (Sigma). The cells were incubated for 72 h.
Determination of metabolites and hormones
Plasma level of nonesterified free fatty acid (FFA) and triglycerides (TGs) were determined by kits from Wako (Richmond, VA) and Sigma, respectively. RIAs were used for determination of plasma level of insulin (Linco) and glucagon (24). Glucose concentration was determined in whole blood by a calibrated and standardized glucometer (Ascensia Elite XL diabetes care system; Bayer HealthCare).
The protein level of PDX-1
PDX-1was determined by Western blot of lysates of isolated islets. Total-cell lysate from 200 islets in each determination was separated on SDS-PAGE and transferred to polyvinylidine fluoride membranes. Primary antibody against total PDX-1 and secondary antibody were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Statistical analysis
Data from OGTT, IVGTT, and ITT tests were analyzed by two-way ANOVA followed by post hoc individual comparisons. In the figure captions, it is indicated where a Mann-Whitney U test is used.
Results
GPR39 expression in endocrine pancreatic cells
Gpr39 is expressed in the pluripotent pancreatic acinar cell line AR42J (Fig. 1B). When exposed to dexamethasone, which induces the expression of α-amylase as an indicator of differentiation toward an exocrine phenotype, the expression of Gpr39 was decreased (Fig. 1B, left panel). In contrast, when exposed to retinoic acid, which strongly induced the expression of the Pdx-1, i.e. the homeobox, the key regulator gene for islet development and differentiation (25), Gpr39 expression was increased in the AR42J cells (Fig. 1B, right panel).
Previously Gpr39 was by QPCR analysis shown to be expressed in the pancreas (5) and β-galactosidase under the control of the Gpr39 promoter has been located in the pancreatic islets but not in specific endocrine cells (15). QPCR analysis demonstrated that Gpr39 is expressed in isolated pancreatic islets and that the receptor occurs also in cell lines derived from pancreatic islet cells (supplemental Fig. 2).
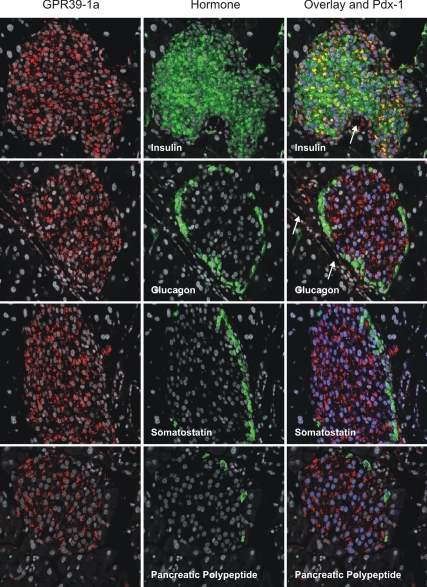
By use of an antiserum raised against a peptide corresponding to part of the C-terminal tail of GPR39-1a, which is not found in GPR39-1b, the full-length GPR39-1a receptor was in the mouse pancreas localized to the endocrine islets and the duct epithelium (Fig. 1C). By double and triple staining, GPR39-1a was in the mouse pancreatic islets found exclusively in the insulin storing β-cells, which also stained positively for Pdx-1, as opposed to the glucagon, somatostatin, and pancreatic polypeptide cells (Fig. 2). As indicated in Fig. 2 (by arrows in the top right panels), cells lining the ducts stained positively for GPR39-1a but not insulin or glucagon.
Figure 2.
Immunohistochemical colocalization of GPR39 with pancreatic islets hormones and PDX-1. Left column of panels, Pancreatic sections from WT mice stained (red) with the GPR39-1a selective rabbit anti-mGPR39 serum (see Materials and Methods for details). Middle column of panels, Same sections stained (green) with antisera selective for the indicated pancreatic hormones: insulin, glucagon, somatostatin, and PP, respectively. Right column of panels, Triple staining of the pancreatic sections for GPR39-1a (red), pancreatic hormones (green), and PDX-1 (nuclear staining in blue). Colocalization of GPR39-1a and insulin appears as yellow. White arrows point to ducts lined by GPR39-1a-positive cells not staining for insulin or other islet hormones.
In vivo characterization of the metabolic phenotype of Gpr39-deficient mice
To study the contribution of the GPR39 receptor to the control of glucose metabolism, we turned to Gpr39-deficient mice in which a major part of the first exon of Gpr39 was replaced by a construct coding for the neomycin resistance gene. Approximately 50% loss of expression of Gpr39 was observed in Gpr39(+/−) mice, and total loss of expression was found in Gpr39(−/−) mice as determined by QPCR analysis of pancreatic tissue (supplemental Fig. 3). The Gpr39(−/−) mice did not display any overt, general phenotype but did have a slightly higher body weight than the wild-type (WT) mice as previously reported (Table 1) (15,16). We did not observe any significant difference between Gpr39(−/−) and WT littermates in, for example, plasma FFAs and TGs (Table 1). Fasting blood glucose was somewhat lower, 5.6 ± 0.3 mm (n = 25) in the Gpr39(−/−) mice compared with 6.6 ± 0.4 mm (n = 24) in the WT littermates; however, this difference was not significant (P = 0.057), whereas plasma glucagon was significantly decreased (130 ± 3 vs. 117 ± 5, P = 0.04) (Table 1).
Table 1.
General phenotype of GPR39-deficient mice
|
Gpr39(+/+)
|
Gpr39(+/−)
|
Gpr39(−/−)
|
||||
|---|---|---|---|---|---|---|
| Mean ± sem | n | Mean ± sem | n | Mean ± sem | n | |
| Body weight (g) | 24.5 ± 1.3 | 10 | 26.3 ± 1.1 | 10 | 26,7 ± 1,3 | 11 |
| FFAs (mg/dl) | 27 ± 5 | 11 | 23 ± 4 | 11 | 29 ± 5 | 9 |
| TGs (mg/dl) | 56 ± 8 | 11 | 58 ± 12 | 11 | 69 ± 14 | 9 |
| Glucose (mm) | 6.6 ± 0.4 | 25 | 6.5 ± 0.3 | 24 | 5.6 ± 0.3 | 24 |
| Insulin (ng/ml) | 0.46 ± 0.05 | 18 | 0.49 ± 0.07 | 18 | 0.45 ± 0.05 | 14 |
| Glucagon (pm) | 130 ± 3 | 11 | 132 ± 4 | 11 | 117 ± 5a | 9 |
Body weight and plasma measurements of nonesterified FFAs, TGs, glucose, insulin, and glugacon in 16- to 18-h fasted male and female mice at 21–22 wk of age.
Significantly lower value in knockout group compared with WT.
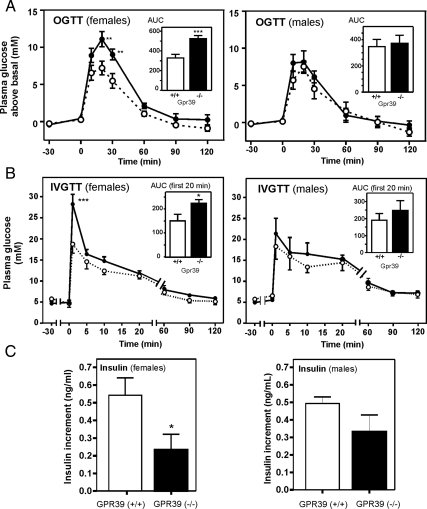
When Gpr39(−/−) mice were subjected to OGTTs, the females turned out to be moderately glucose intolerant (Fig. 3A), with the area under the curve being 524 ± 33 in Gpr39(−/−) mice compared with 328 ± 37 in the WT littermates (P < 0.001). The heterozygous Gpr39(+/−) mice were indistinguishable from the WT mice during OGTT (supplemental Fig. 4).
Figure 3.
In vivo metabolic phenotype of Gpr39-deficient mice. A, OGTTs performed in female [Gpr39(+/+), n = 12, and Gpr39(−/−), n = 10] and male mice [Gpr39(+/+), n = 5 and Gpr39(−/−), n = 5], respectively, at 11–12 wk of age; glucose was measured in tail blood. For female mice significantly higher blood glucose was observed in Gpr39(−/−) (in black) compared with WT (in white) controls at time point 20 and 30 min after glucose administration with P = 0.009 and 0.011, respectively. Area under the curve (AUC) is shown as an insert (***, P = 0.001). B, IVGTTs performed in female [Gpr39(+/+), n = 6, and Gpr39(−/−), n = 4] and male mice [Gpr39(+/+), n = 5, and Gpr39(−/−), n = 5], respectively, at 17–18 wk of age; glucose was measured in venous tail blood. Area under the curve for the first 20 min is shown as an insert (*, P = 0.03; ***, P = 0.0003). C, Increment in plasma insulin during the first 15 min after an oral glucose load (∼OGTT) for female (left panel) and male (right panel) Gpr39(+/+) mice (black column, n = 4–6) and in Gpr39(−/−) animals (white column, n = 5–6). *, P = 0.04.
During iv glucose challenge, the female Gpr39(−/−) mice also displayed significantly elevated blood glucose compared with their WT littermates at the first time point and a significantly increased area under the curve (Fig. 3B). In males this phenotype was much weaker because only a nonsignificant trend toward impaired glucose tolerance was observed both in OGTT and IVGTT (Fig. 3, A and B, right panels).
Importantly, the insulin response measured in serum 15 min after an oral glucose load was lower in the Gpr39-deficient animals compared with the WT mice (Fig. 3C). This was observed in both female and male animals, but the difference was significant only in the females.
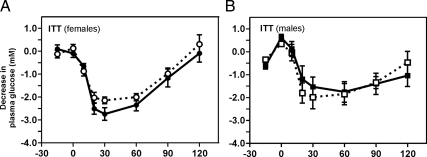
No sign of insulin insensitivity was observed because comparable blood glucose curves with similar degrees of hypoglycemia were observed during ITTs in the homozygous Gpr39(−/−) and WT mice in both genders (Fig. 4).
Figure 4.
Insulin sensitivity of GPR39(−/−) mice. A, Female mice; B, male mice. ITT was performed with 0.75 U/kg insulin administered ip to 15- to 16-wk-old mice to induce moderate hypoglycemia.
Thus, female Gpr39-deficient mice are moderately glucose intolerant and have a lower insulin response to glucose pointing to an islet dysfunction.
Endocrine pancreas function in Gpr39-deficient mice
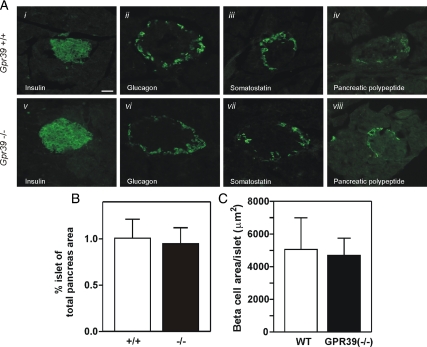
General histological and immunohistochemical analysis of pancreatic tissue from Gpr39(−/−) and WT mice showed similar islet architecture with comparable composition of endocrine cells (Fig. 5A). This was also reflected in a similar overall area and islet size (Fig. 5B) and similar number of β-cells per islet (Fig. 5C).
Figure 5.
Islet morphology and gene expression in GPR39-deficient mice. A, Immunohistochemistry of representative islets from WT mice (upper panels) and Gpr39(−/−) mice (lower panels). Indirect immunofluorescence for insulin (i and v), glucagons (ii and vi), somatostatin (iii and vii), and PP (iv and viii) was performed on fixed pancreas tissue. B, The islet cell area as determined in hematoxylin and eosin-stained pancreatic slides was 1.0 ± 0.2% in Gpr39(+/+) and 0.94 ± 0.2% in Gpr39(−/−). C, Quantification of the β-cell number per islets performed by histomorphometric analysis.
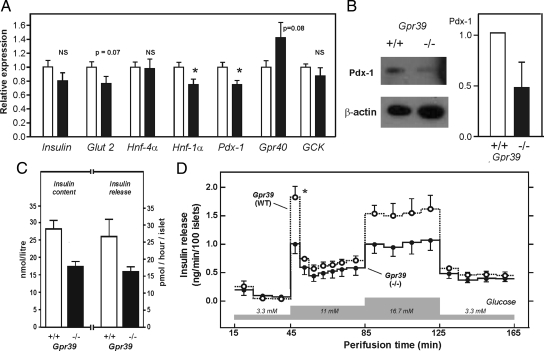
However, the expression of the genes for Pdx-1 and Hnf-1α was significantly decreased in the Gpr39(−/−) mice as determined by QPCR analysis (Fig. 6A). The expression of insulin and the glucose transporter, Glut2, was also lower in the Gpr39-deficient mice; however, the difference did not reach statistical significance (P = 0.07 for Glut2). In contrast, the expression of Hnf-4α was unchanged, and the expression of the Gpr40 receptor gene even tended to be increased (P = 0.08) in the Gpr39-deficient mice (Fig. 6A). Importantly, the decrease in Pdx-1 expression in Gpr39(−/−) mice was confirmed at the protein level by Western blot analysis of isolated islet proteins (Fig. 6B).
Figure 6.
Gene expression and secretory function of isolated islets in GPR39-deficient mice. A, Relative expression of various islet-specific genes measured by QPCR using tyrosine 3-monooxygenase (YWHAZ) as reference gene in WT mice (white bars) and Gpr39(−/−) mice (black bars) (n = 12). *, P < 0.05. GCK, Glucokinase. B, Western blot determination of PDX-1 and β-actin protein expression in freshly isolated islets from WT and Gpr39(−/−) mice. A representative blot of three independent experiments is shown to the left and to the right is shown the densitometric quantification of three independent Western blot (each consists of tissue from one or two mice) in which the expression in WT mice is normalized to 1. Equal numbers of female and males were included in these experiments and no differences were observed. C, Insulin content of islets cultured for 5 d (n = 2) (two additional experiments performed under different culture conditions are shown in supplemental Table 2) and insulin release after 24–48 h of incubation with 11 mm glucose (n = 3). D, Perifusion of islets isolated from WT mice (open circles and dashed line) and Gpr39(−/−) mice (filled circles and solid line) exposed to step-wise increasing concentrations of glucose. Data are mean ± sem (n = 4). *, P = 0.017. NS, Not significant.
The insulin content of isolated islets from Gpr39(−/−) mice was lower than that of islets isolated from WT littermates as shown in Fig. 6C for islets after 5 d of culture (supplemental Table 2). A similar difference was also observed in the accumulated insulin release determined in the culture medium from islets after 24–48 h in culture (Fig. 6C). However, to characterize the glucose-induced insulin secretion in more detail we used an isolated perifused islets model. Here a step-wise glucose challenge from 3.3 to 11.0 mm demonstrated that especially the first phase of the glucose-induced insulin secretion was impaired in islets isolated from 12-wk-old Gpr39(−/−) mice compared with islets isolated from WT littermates despite a similar basal insulin secretion (Fig. 6D). The impaired insulin release was also apparent during subsequent further increase in glucose to 16.7 mm. This impairment in acute insulin secretion was also observed in islets isolated from 20-wk-old animals (supplemental Fig. 5). It should be noted that normal glucose-induced insulin secretion is observed in islets from mice deficient in other 7TM receptors such as the GLP-1 receptor (26,27). The insulin response to GLP-1 was not impaired in the perifused islet from Gpr39(−/−) mice (supplemental Fig. 6).
In conclusion, Gpr39-deficient animals display decreased expression of the Pdx-1 and Hnf-1α genes involved in islet development and β-cell function, which may be involved in the observed impairment in glucose stimulated insulin secretion. In man, loss-of-function mutations of PDX-1 and HNF-1α are linked to maturity onset of diabetes, i.e. MODY4 and MODY3, respectively (28,29).
Discussion
In the present study, we found that the GPR39 receptor in the pancreas is expressed in duct epithelium and the β-cells of the islets and that this receptor is required for normal glucose tolerance in mice, which conceivably is related to a dependency of the pancreatic islets on GPR39 signaling for normal expression of, for example, PDX-1, and normal, glucose-stimulated insulin secretion from the islets. Thus, GPR39, which signals through for example the Gq and NFAT pathways (6), appears to be an important factor in the control of endocrine pancreatic function and glucose metabolism.
The phenotype of Gpr39-deficient mice
The overall architecture and distribution of endocrine cells within the pancreatic islets is normal in the Gpr39(−/−) animals. However, expression of the key or master regulator of islet development and differentiation, the homeobox gene Pdx-1, is slightly down-regulated at both the mRNA and protein level in the Gpr39(−/−) mice. This down-regulation is associated with a decreased expression of, most notably, the transcription factor HNF-1α. Importantly, pancreatic islets isolated from unchallenged Gpr39(−/−) mice display an impaired, especially first-phase, glucose-induced insulin secretion in perifusion experiments (Fig. 6). Consequently, the Gpr39(−/−) mice have an impaired plasma insulin response to oral glucose, and as revealed by both OGTT and IVGTT, they have a tendency toward glucose intolerance but apparently have normal insulin sensitivity. In addition, the fasting plasma level of glucagon was significantly decreased in the Gpr39(−/−) mice.
Like several other knockout animals in which related endocrine, metabolic systems were targeted, such as Hnf-4α (30) and the ghrelin receptor (31), rather evident gender differences are observed also in the Gpr39(−/−) mice in which the glucose intolerance is observed only in the female animals. Importantly, in an accompanying paper, Tremblay et al. (46) demonstrated that by age or especially when exposed to a high-fat combined with a high-sucrose diet also, the male Gpr39(−/−) mice become more glucose intolerant and display significantly decrease glucose-induced insulin secretion than WT mice.
In several ways, the phenotype of the Gpr39(−/−) mice is more similar to the phenotype observed in mice with β-cell-specific deletion of, for example, the MODY-1 gene, HNF-4α, than mice deficient in other cell surface receptors such as the GLP-1, GPR40, or GPR119 receptors (26,32,33). As in the HNF-4α null mice, we observed slightly impaired glucose tolerance and especially impaired first-phase, glucose-induced insulin secretion but nevertheless a trend toward low fasting blood glucose combined with normal insulin sensitivity and normal islet architecture (30). Mutations in the HNF-4α gene in man are associated with increased birth weight and hyperinsulinemic hypoglycemia (34). Importantly, later in life such mutations lead to a typical MODY phenotype with decreased insulin secretion and diabetes. Despite many similarities between the Hnf-4α and Gpr39 knockout phenotypes, we did not observe any change in Hnf-4α expression in our study, which could indicate that Hnf-4α acts as an upstream regulator of Gpr39, as also previously reported (5,17). In contrast to the Hnf-4α null phenotype, we found decreased expression of Pdx-1 in the Gpr39(−/−) mice (Fig. 6). Similarly, Tremblay et al. (46) in the accompanying paper find that Pdx-1 expression is decreased on selective small interfering RNA reduction of Gpr39 expression in the β-cell line NIT-1. One of the major roles of Pdx-1 in the adult animal is to control glucose-induced insulin secretion (35) and the maintenance of islet mass, architecture, and plasticity involving β-cell neogenesis, differentiation, and apoptosis (35,36). Thus, Pdx-1 deficiency, as observed in Pdx1(+/−) mice, results in increased susceptibility to apoptosis of the islets and a lack of ability of the islets to adapt to the increased demand, i.e. by age (36). In man, mutations in PDX-1 leads to MODY4 (28).
Recently it was realized that the NFAT signal-transduction pathway is crucial in controlling the expression of hallmark β-cell genes including Pdx-1 as well as cell-cycle regulators and consequently is involved in the control of β-cell proliferation and adaptive islet responses in vivo (37). As shown in Fig. 1A, the GPR39 receptor is a highly efficient activator of NFAT signaling, which consequently could be a connecting signaling pathway between GPR39, β-cell gene expression, and the observed effects on β-cell function. Interestingly, GPR39 was recently rediscovered by Methner and coworkers (38), who cloned GPR39 as one of two 7TM receptors being overexpressed in a naturally occurring, apoptosis-resistant cell line. Importantly, transfection of GPR39, but not the other receptor, into another host cell line protected against oxidative and endoplasmic reticulum stress (38). Thus, it is possible that the mechanism by which GPR39 affects islet cell function is through protection against cell death and/or control of proliferation and adaptive responses. In the accompanying paper Tremblay et al. (46) found that Gpr39-deficient mice do not display the normal pancreatic islet hyperplasia in response to diet-induced obesity.
Zn2+, islet function, and GPR39
Zn2+ is stored and released together with insulin from the β-cells, in which a zinc transporter, ZnT-8 or SLC30A8, is responsible for the transport of zinc ions from the cytoplasm into the secretory granules (39,40). A nonsynonymous polymorphism (R325W) in SLC30A8 was reported by Sladek et al. (40) in a genome-wide association study identified as a highly significant risk locus for type 2 diabetes, which has been confirmed in several similar studies. Importantly, as demonstrated by Wollheim and coworkers (13,14), Zn2+ appears to act as a β-cell secretory product in its own right, modulating islet cell function locally. Zn2+ also has a general antiapoptotic effect (41), and importantly, treatment with Zn2+ has been shown to specifically protect against streptozotocin-induced diabetes (42,43) and protect against the spontaneous development of diabetes in NOD mice and biobreeding rats (43,44). In view of the results of the present study and the demonstration that GPR39 appears to protect against oxidative and endoplasmic reticulum stress (38), we suggest that GPR39, which is activated by what is believed to be physiological concentrations of Zn2+ (6,8,9), could be involved in these islet protective effects of Zn2+.
Thus, GPR39 is the second gene, aside from SLC30A8/ZnT-8, that is involved in zinc biology and appears to be associated with impaired pancreatic islet cell function and impaired glucose-induced insulin release. Importantly, the fact that GPR39 is a 7TM receptor, which is known to be highly drugable proteins, directly makes it an interesting target for the development of agonists of potential use in the treatment or prevention of diabetes and related metabolic diseases.
Supplementary Material
Acknowledgments
Mette Simons, Elisabeth Ringvard, and Bente Friis are thanked for expert technical assistance. Kenneth Polonsky and Klaus H. Kaestner are thanked for critical, constructive reading of an early version of the manuscript.
Footnotes
This work was supported by grants from The Danish and The Swedish Medical Research Council, The NovoNordisk Foundation, The Lundbeck Foundation, The Alfred Benzon Foundation, The Danish Diabetes Association, The Sixth Framework Program Grant GPCR LSHB-CT-2003-503337 (to B.H. and T.W.S.) from the European Union, and Grant DK-072473) from the National Institutes of Health (to O.D.M.). K.L.E. was the recipient of a stipend from the Health Science Faculty of University of Copenhagen.
Major parts of the present study were presented at the Keystone meeting on β-cell function, April 6–11, 2008.
Disclosure Summary: The authors have nothing to declare.
First Published Online February 12, 2009
Abbreviations: FFA, Free fatty acid; GLP-1, glucagon-like peptide-1-(7-36)-amide; GPR, G protein-coupled receptor; HNF, hepatocyte nuclear factor; ITT, insulin tolerance test; IVGTT, iv glucose tolerance test; m, mouse; MODY, maturity-onset diabetes of the young; NFAT, nuclear factor of activated T cells; OGTT, oral glucose tolerance test; PDX-1, pancreatic duodenal homeobox-1; PP, pancreatic polypeptide; QPCR, quantitative PCR; TG, triglyceride; 7TM, seven-transmembrane; WT, wild type.
References
- McKee KK, Tan CP, Palyha OC, Liu J, Feighner SD, Hreniuk DL, Smith RG, Howard AD, Van der Ploeg LH 1997 Cloning and characterization of two human G protein-coupled receptor genes (GPR38 and GPR39) related to the growth hormone secretagogue and neurotensin receptors. Genomics 46:426–434 [DOI] [PubMed] [Google Scholar]
- Tschöp M, Smiley DL, Heiman ML 2000 Ghrelin induces adiposity in rodents. Nature 407:908–913 [DOI] [PubMed] [Google Scholar]
- Theander-Carrillo C, Wiedmer P, Cettour-Rose P, Nogueiras R, Perez-Tilve D, Pfluger P, Castaneda TR, Muzzin P, Schürmann A, Szanto I, Tschöp MH, Rohner-Jeanrenaud F 2006 Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest 116:1983–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Asnicar M, Saha PK, Chan L, Smith RG 2006 Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab 3:379–386 [DOI] [PubMed] [Google Scholar]
- Egerod KL, Holst B, Petersen PS, Hansen JB, Mulder J, Hökfelt T, Schwartz TW 2007 GPR39 splice variants versus antisense gene LYPD1: expression and regulation in gastrointestinal tract, endocrine pancreas, liver, and white adipose tissue. Mol Endocrinol 21:1685–1698 [DOI] [PubMed] [Google Scholar]
- Holst B, Holliday ND, Bach A, Elling CE, Cox HM, Schwartz TW 2004 Common structural basis for constitutive activity of the ghrelin receptor family. J Biol Chem 279:53806–53817 [DOI] [PubMed] [Google Scholar]
- Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, Hsueh AJ 2005 Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science 310:996–999 [DOI] [PubMed] [Google Scholar]
- Holst B, Egerod KL, Schild E, Vickers SP, Cheetham S, Gerlach LO, Storjohann L, Stidsen CE, Jones R, Beck-Sickinger AG, Schwartz TW 2007 GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology 148:13–20 [DOI] [PubMed] [Google Scholar]
- Lauwers E, Landuyt B, Arckens L, Schoofs L, Luyten W 2006 Obestatin does not activate orphan G protein-coupled receptor GPR39. Biochem Biophys Res Commun 351:21–25 [DOI] [PubMed] [Google Scholar]
- Chartrel N, Alvear-Perez R, Leprince J, Iturrioz X, Reaux-Le GA, Audinot V, Chomarat P, Coge F, Nosjean O, Rodriguez M, Galizzi JP, Boutin JA, Vaudry H, Llorens-Cortes C 2007 Comment on “Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake.” Science 315:766 [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Pfluger P, Tovar S, Arnold M, Mitchell S, Morris A, Perez-Tilve D, Vázquez MJ, Wiedmer P, Castañeda TR, DiMarchi R, Tschöp M, Schurmann A, Joost HG, Williams LM, Langhans W, Diéguez C 2007 Effects of obestatin on energy balance and growth hormone secretion in rodents. Endocrinology 148:21–26 [DOI] [PubMed] [Google Scholar]
- Yasuda S, Miyazaki T, Munechika K, Yamashita M, Ikeda Y, Kamizono A 2007 Isolation of Zn2+ as an endogenous agonist of GPR39 from fetal bovine serum. J Recept Signal Transduct Res 27:235–246 [DOI] [PubMed] [Google Scholar]
- Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB 2005 β-Cell secretory products activate α-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 54:1808–1815 [DOI] [PubMed] [Google Scholar]
- Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB 2003 Islet β-cell secretion determines glucagon release from neighbouring α-cells. Nat Cell Biol 5:330–335 [DOI] [PubMed] [Google Scholar]
- Moechars D, Depoortere I, Moreaux B, de Smet B, Goris I, Hoskens L, Daneels G, Kass S, Ver Donck L, Peeters T, Coulie B 2006 Altered gastrointestinal and metabolic function in the GPR39-obestatin receptor-knockout mouse. Gastroenterology 131:1131–1141 [DOI] [PubMed] [Google Scholar]
- Tremblay F, Perreault M, Klaman LD, Tobin JF, Smith E, Gimeno RE 2007 Normal food intake and body weight in mice lacking the G protein-coupled receptor GPR39. Endocrinology 148:501–506 [DOI] [PubMed] [Google Scholar]
- Odom DT, Zizlsperger N, Gordon DB, Bell GW, Rinaldi NJ, Murray HL, Volkert TL, Schreiber J, Rolfe PA, Gifford DK, Fraenkel E, Bell GI, Young RA 2004 Control of pancreas and liver gene expression by HNF transcription factors. Science 303:1378–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stride A, Hattersley AT 2002 Different genes, different diabetes: lessons from maturity-onset diabetes of the young. Ann Med 34:207–216 [PubMed] [Google Scholar]
- Pedersen IL, Klinck R, Hecksher-Sorensen J, Zahn S, Madsen OD, Serup P, Jorgensen MC 2006 Generation and characterization of monoclonal antibodies against the transcription factor Nkx6.1. J Histochem Cytochem 54:567–574 [DOI] [PubMed] [Google Scholar]
- Wierup N, Yang S, McEvilly RJ, Mulder H, Sundler F 2004 Ghrelin is expressed in a novel endocrine cell type in developing rat islets and inhibits insulin secretion from INS-1 (832/13) cells. J Histochem Cytochem 52:301–310 [DOI] [PubMed] [Google Scholar]
- Brunstedt J, Nielsen JH, Lernmark Å, Hagedorn Study Group 1984 In: Larner J, Pohl SL, eds. Methods in diabetes research. New York: John Wiley &Sons, Inc.; 254–288 [Google Scholar]
- Nielsen K, Karlsen AE, Deckert M, Madsen OD, Serup P, Mandrup-Poulsen T, Nerup J 1999 β-Cell maturation leads to in vitro sensitivity to cytotoxins. Diabetes 48:2324–2332 [DOI] [PubMed] [Google Scholar]
- Thams P, Capito K 2001 Differential mechanisms of glucose and palmitate in augmentation of insulin secretion in mouse pancreatic islets. Diabetologia 44:738–746 [DOI] [PubMed] [Google Scholar]
- Thulesen J, Orskov C, Holst JJ, Poulsen SS 1997 Short-term insulin treatment prevents the diabetogenic action of streptozotocin in rats. Endocrinology 138:62–68 [DOI] [PubMed] [Google Scholar]
- Tulachan SS, Doi R, Kawaguchi Y, Tsuji S, Nakajima S, Masui T, Koizumi M, Toyoda E, Mori T, Ito D, Kami K, Fujimoto K, Imamura M 2003 All-trans retinoic acid induces differentiation of ducts and endocrine cells by mesenchymal/epithelial interactions in embryonic pancreas. Diabetes 52:76–84 [DOI] [PubMed] [Google Scholar]
- Flamez D, Van Breusegem A, Scrocchi LA, Quartier E, Pipeleers D, Drucker DJ, Schuit F 1998 Mouse pancreatic β-cells exhibit preserved glucose competence after disruption of the glucagon-like peptide-1 receptor gene. Diabetes 47:646–652 [DOI] [PubMed] [Google Scholar]
- Scrocchi LA, Brown TJ, MaClusky N, Brubaker PL, Auerbach AB, Joyner AL, Drucker DJ 1996 Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med 2:1254–1258 [DOI] [PubMed] [Google Scholar]
- Stoffers DA, Ferrer J, Clarke WL, Habener JF 1997 Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet 17:138–139 [DOI] [PubMed] [Google Scholar]
- Yamagata K, Oda N, Kaisaki PJ, Menzel S, Furuta H, Vaxillaire M, Southam L, Cox RD, Lathrop GM, Boriraj VV, Chen X, Cox NJ, Oda Y, Yano H, Le Beau MM, Yamada S, Nishigori H, Takeda J, Fajans SS, Hattersley AT, Iwasaki N, Hansen T, Pedersen O, Polonsky KS, Bell GI 1996 Mutations in the hepatocyte nuclear factor-1α gene in maturity-onset diabetes of the young (MODY3). Nature 384:455–458 [DOI] [PubMed] [Google Scholar]
- Gupta RK, Vatamaniuk MZ, Lee CS, Flaschen RC, Fulmer JT, Matschinsky FM, Duncan SA, Kaestner KH 2005 The MODY1 gene HNF-4α regulates selected genes involved in insulin secretion. J Clin Invest 115:1006–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK 2005 Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest 115:3564–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steneberg P, Rubins N, Bartoov-Shifman R, Walker MD, Edlund H 2005 The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab 1:245–258 [DOI] [PubMed] [Google Scholar]
- Chu ZL, Jones RM, He H, Carroll C, Gutierrez V, Lucman A, Moloney M, Gao H, Mondala H, Bagnol D, Unett D, Liang Y, Demarest K, Semple G, Behan DP, Leonard J 2007 A role for β-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology 148:2601–2609 [DOI] [PubMed] [Google Scholar]
- Pearson ER, Boj SF, Steele AM, Barrett T, Stals K, Shield JP, Ellard S, Ferrer J, Hattersley AT 2007 Macrosomia and hyperinsulinaemic hypoglycaemia in patients with heterozygous mutations in the HNF4A gene. PLoS Med 4:e118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habener JF, Kemp DM, Thomas MK 2005 Minireview: transcriptional regulation in pancreatic development. Endocrinology 146:1025–1034 [DOI] [PubMed] [Google Scholar]
- Johnson JD, Ahmed NT, Luciani DS, Han Z, Tran H, Fujita J, Misler S, Edlund H, Polonsky KS 2003 Increased islet apoptosis in Pdx1+/− mice. J Clin Invest 111:1147–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heit JJ, Apelqvist AA, Gu X, Winslow MM, Neilson JR, Crabtree GR, Kim SK 2006 Calcineurin/NFAT signalling regulates pancreatic β-cell growth and function. Nature 443:345–349 [DOI] [PubMed] [Google Scholar]
- Dittmer S, Sahin M, Pantlen A, Saxena A, Toutzaris D, Pina AL, Geerts A, Golz S, Methner A 2008 The constitutively active orphan G-protein coupled receptor GPR39 protects from cell death by increasing secretion of pigment-epithelial derived growth factor PEDF. J Biol Chem 283:7074–7081 [DOI] [PubMed] [Google Scholar]
- Chimienti F, Devergnas S, Pattou F, Schuit F, Garcia-Cuenca R, Vandewalle B, Kerr-Conte J, Van Lommel L, Grunwald D, Favier A, Seve M 2006 In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J Cell Sci 119:4199–4206 [DOI] [PubMed] [Google Scholar]
- Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P 2007 A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445:881–885 [DOI] [PubMed] [Google Scholar]
- Beyersmann D, Haase H 2001 Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals 14:331–341 [DOI] [PubMed] [Google Scholar]
- Ohly P, Dohle C, Abel J, Seissler J, Gleichmann H 2000 Zinc sulphate induces metallothionein in pancreatic islets of mice and protects against diabetes induced by multiple low doses of streptozotocin. Diabetologia 43:1020–1030 [DOI] [PubMed] [Google Scholar]
- Schott-Ohly P, Lgssiar A, Partke HJ, Hassan M, Friesen N, Gleichmann H 2004 Prevention of spontaneous and experimentally induced diabetes in mice with zinc sulfate-enriched drinking water is associated with activation and reduction of NF-κB and AP-1 in islets, respectively. Exp Biol Med (Maywood) 229:1177–1185 [DOI] [PubMed] [Google Scholar]
- Tobia MH, Zdanowicz MM, Wingertzahn MA, McHeffey-Atkinson B, Slonim AE, Wapnir RA 1998 The role of dietary zinc in modifying the onset and severity of spontaneous diabetes in the BB Wistar rat. Mol Genet Metab 63:205–213 [DOI] [PubMed] [Google Scholar]
- Schwartz TW, Holst B 2002 Molecular structure and function of 7TM/G-protein coupled receptors. In: Forman JC, Johansen T, eds. Textbook of receptor pharmacology. Boca Raton, FL: CRC Press; 65–84 [Google Scholar]
- Tremblay F, Richard AM, Will S, Syed J, Stedman N, Perrault M, Gimeno RE 2009 Disruption of G protein-coupled receptor 39 impairs insulin secretion in vivo. Endocrinology 150:2586–2595 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.