Abstract
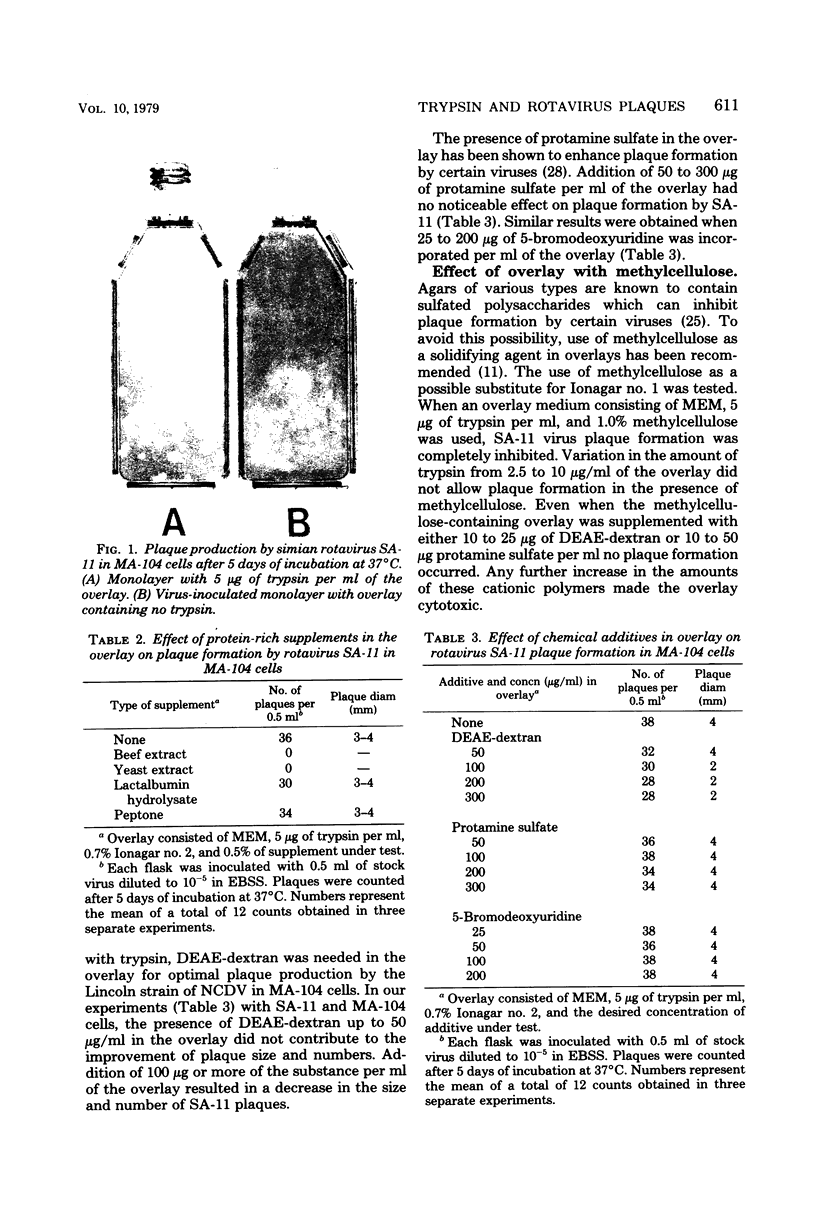
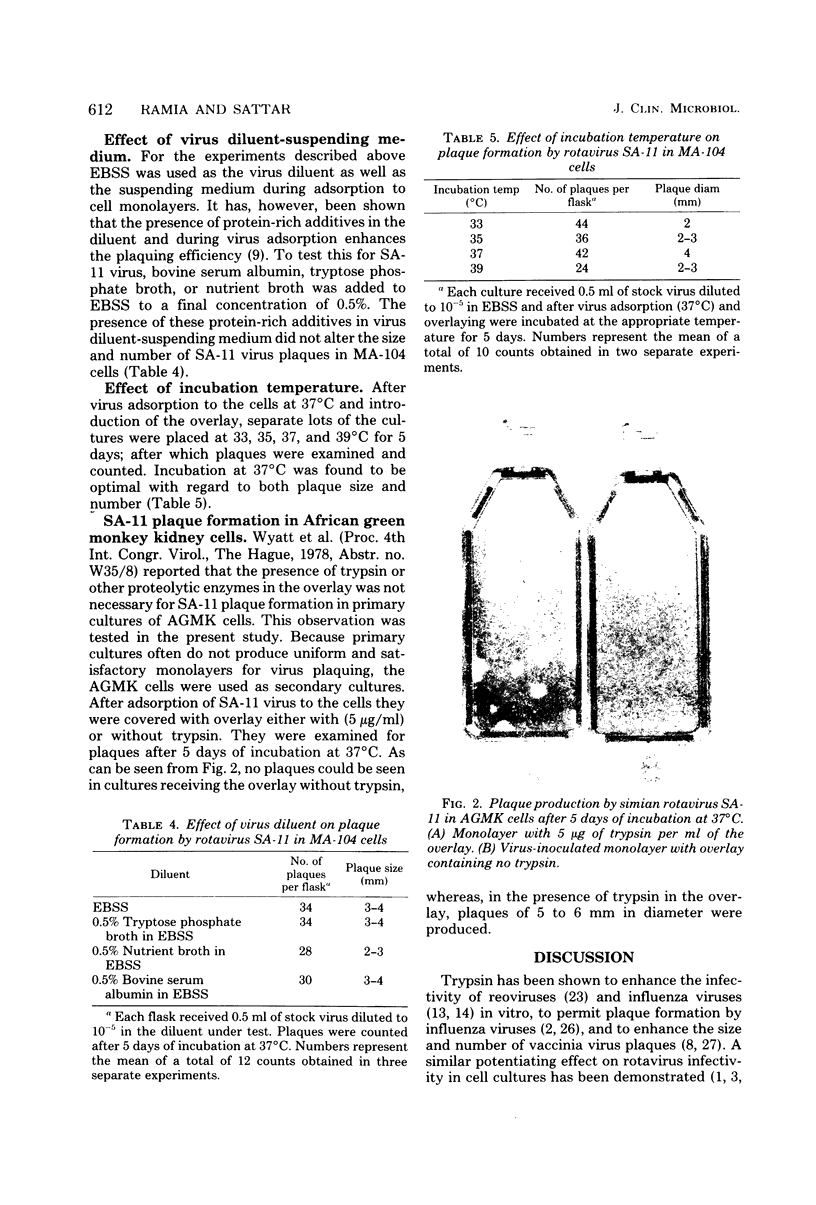
Incorporation of 5 micrograms of trpsin per ml of the overlay (Eagle minimal essential medium-0.7% Ionagar no. 2) was found to be necessary for plaque formation by simian rotavirus SA-11. Plaques of 3 to 4 mm in diameter were produced in MA-104 cells after 5 days of incubation at 37 degrees C. Plaque size was even larger (5 to 6 mm) in monolayers of African green monkey kidney cells. Addition of diethyl-aminotheyl-dextran, protamine sulfate, or 5-bromodeoxyuridine to the trypsin-containing overlay did not improve plaque formation by the virus. Incorporation of beef extract or yeast extract to a final concentration of 0.5% in the trypsin-containing overlay inhibited plaque formation. On the other hand, the presence of lactalbumin hydrolysate or peptone at a similar concentration in the overlay did not inhibit plaque formation. When methylcellulose was used instead of the agar as the solidifying agent in the overlay, no plaques were seen. SA-11 is a useful model for the study of human rotaviruses, and this relatively simple plaque assay system should further enhance its usefulness in this regard.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida J. D., Hall T., Banatvala J. E., Totterdell B. M., Chrystie I. L. The effect of trypsin on the growth of rotavirus. J Gen Virol. 1978 Jul;40(1):213–218. doi: 10.1099/0022-1317-40-1-213. [DOI] [PubMed] [Google Scholar]
- Appleyard G., Maber H. B. Plaque formation by influenza viruses in the presence of trypsin. J Gen Virol. 1974 Dec;25(3):351–357. doi: 10.1099/0022-1317-25-3-351. [DOI] [PubMed] [Google Scholar]
- Babiuk L. A., Mohammed K., Spence L., Fauvel M., Petro R. Rotavirus isolation and cultivation in the presence of trypsin. J Clin Microbiol. 1977 Dec;6(6):610–617. doi: 10.1128/jcm.6.6.610-617.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. M., Barnett B. B., Spendlove R. S. Production of high-titer bovine rotavirus with trypsin. J Clin Microbiol. 1979 Mar;9(3):413–417. doi: 10.1128/jcm.9.3.413-417.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah S. R., Goyal S. M., Gerba C. P., Conklin R. H., Smith E. M. Comparison between adsorption of poliovirus and rotavirus by aluminum hydroxide and activated sludge flocs. Appl Environ Microbiol. 1978 Feb;35(2):360–363. doi: 10.1128/aem.35.2.360-363.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flewett T. H., Woode G. N. The rotaviruses. Arch Virol. 1978;57(1):1–23. doi: 10.1007/BF01315633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford G. E., Klapper D. G. Enhancement of vaccinia virus plaque formation by trypsin. Proc Soc Exp Biol Med. 1967 Nov;126(2):515–517. doi: 10.3181/00379727-126-32492. [DOI] [PubMed] [Google Scholar]
- HOLMES A. W., GILSON J., DEINHARDT F. INHIBITION OF INTERFERON PRODUCTION BY 5-IODO-2-DEOXYURIDINE. Virology. 1964 Oct;24:229–232. doi: 10.1016/0042-6822(64)90111-4. [DOI] [PubMed] [Google Scholar]
- HOTCHIN J. E. Use of methyl cellulose gel as a substitute for agar in tissue-culture overlays. Nature. 1955 Feb 19;175(4451):352–352. doi: 10.1038/175352a0. [DOI] [PubMed] [Google Scholar]
- Hamblet F. E., Hill W. F., Akin E. W. Effect of plaque assay diluent upon enumeration of poliovirus type 1. Appl Microbiol. 1967 Jan;15(1):208–208. doi: 10.1128/am.15.1.208-.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian A. Z., Cline W. L., Kim H. W., Kalica A. R., Wyatt R. G., Vankirk D. H., Chanock R. M., James H. D., Jr, Vaughn A. L. Antigenic relationships among five reovirus-like (RVL) agents by complement fixation (CF) and development of new substitute CF antigens for the human RVL agent of infantile gastroenteritis. Proc Soc Exp Biol Med. 1976 Sep;152(4):535–539. doi: 10.3181/00379727-152-39434. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Orlich M., Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975 Dec;68(2):426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Choppin P. W. Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology. 1975 Dec;68(2):440–454. doi: 10.1016/0042-6822(75)90285-8. [DOI] [PubMed] [Google Scholar]
- Lycke E., Blomberg J., Berg G., Eriksson A., Madsen L. Epidemic acute diarrhoea in adults associated with infantile gastroenteritis virus. Lancet. 1978 Nov 11;2(8098):1056–1057. doi: 10.1016/s0140-6736(78)92389-9. [DOI] [PubMed] [Google Scholar]
- Malherbe H. H., Strickland-Cholmley M. Simian virus SA11 and the related O agent. Arch Gesamte Virusforsch. 1967;22(1):235–245. doi: 10.1007/BF01240518. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Inouye S., Kono R. Plaque assay of neonatal calf diarrhea virus and the neutralizing antibody in human sera. J Clin Microbiol. 1977 Jan;5(1):1–4. doi: 10.1128/jcm.5.1.1-4.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty M. S. Rotaviruses. J Gen Virol. 1978 Jul;40(1):1–18. doi: 10.1099/0022-1317-40-1-1. [DOI] [PubMed] [Google Scholar]
- Mebus C. A., Kono M., Underdahl N. R., Twiehaus M. J. Cell culture propagation of neonatal calf diarrhea (scours) virus. Can Vet J. 1971 Mar;12(3):69–72. [PMC free article] [PubMed] [Google Scholar]
- PRESTON N. W., MORRELL A. Reproducible results with the Gram stain. J Pathol Bacteriol. 1962 Jul;84:241–243. doi: 10.1002/path.1700840131. [DOI] [PubMed] [Google Scholar]
- Paul N. R., Iwakata S., Rhodes A. J., Labzoffsky N. A. Enhancing effect of halogenated pyrimidines (BUdR and IUdR) on the growth of Rubella virus in BHK-21 cells. Arch Gesamte Virusforsch. 1974;44(2):144–146. doi: 10.1007/BF01250223. [DOI] [PubMed] [Google Scholar]
- Schoub B. D., Lecatsas G., Prozesky O. W. Antigenic relationship between human and simian rotaviruses. J Med Microbiol. 1977 Feb;10(1):1–6. doi: 10.1099/00222615-10-1-1. [DOI] [PubMed] [Google Scholar]
- Spendlove R. S., McClain M. E., Lennette E. H. Enhancement of reovirus infectivity by extracellular removal or alteration of the virus capsid by proteolytic enzymes. J Gen Virol. 1970 Aug;8(2):83–94. doi: 10.1099/0022-1317-8-2-83. [DOI] [PubMed] [Google Scholar]
- St Jeor S., Rapp F. Cytomegalovirus: conversion of nonpermissive cells to a permissive state for virus replication. Science. 1973 Sep 14;181(4104):1060–1061. doi: 10.1126/science.181.4104.1060. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K. Plaque mutants of animal viruses. Prog Med Virol. 1966;8:314–348. [PubMed] [Google Scholar]
- Tobita K., Kilbourne E. D. Genetic recombination for antigenic markers of antigenically different strains of influenza B virus. J Virol. 1974 Feb;13(2):347–352. doi: 10.1128/jvi.13.2.347-352.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Mechanism of enhancement of virus plaques by cationic polymers. J Virol. 1968 Apr;2(4):267–274. doi: 10.1128/jvi.2.4.267-274.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]





