Abstract
Increases in rates of punished behavior by the administration of anxiolytic drugs (called antipunishment effects) are well established in animals but not humans. The present study examined antipunishment effects of ethanol in humans using a choice procedure. The behavior of 5 participants was placed under six concurrent variable-interval schedules of monetary reinforcement. In three of the six concurrent schedules, punishment, in the form of monetary loss, was superimposed on one alternative. Data were analyzed according to the generalized matching equation which distinguishes between bias (allocation of behavior beyond what matching to relative reinforcer densities would predict) and sensitivity to reinforcement (how well behavior tracks relative reinforcer densities). In addition, participants completed a pencil-tapping test. Under placebo punishment conditions, all participants demonstrated low response rates and a bias against the alternative associated with punishment, despite a resultant loss of available reinforcers. Bias against the punished alternative was dose-dependently reduced in participants shown to be most sensitive to ethanol (0.6, 1.2, and 1.8 g/kg) in measures of overall responding and on the pencil-tapping test. No ethanol-induced change in bias was noted when punishment was not imposed. Sensitivity to reinforcement also decreased for participants shown to be sensitive to ethanol. In addition to extending antipunishment effects to humans, these results also show that antipunishment effects can be quantified via the matching equation.
Keywords: antipunishment, choice, concurrent schedules, ethanol, generalized matching equation, humans, matching, punishment
Anxiolytic drugs can be characterized by their ability to increase rates of responding suppressed by punishment, and a vast literature documents these effects with a variety of anxiolytic compounds in nonhuman species (see Commissaris, 1993, Pollard & Howard, 1990, and Rasmussen, 2006, for reviews.) These reports also demonstrate the specificity of antipunishment effects to mostly benzodiazepines and barbiturates. Stimulants, opiates and other compounds do not reliably increase behavior that has been suppressed by punishment. Because most punishing stimuli induce pain, the role of analgesia in antipunishment effects has been studied. Results of several studies have indicated that analgesia is unlikely to play a role since antipunishment effects have not been noted with potent analgesics like morphine (e.g., McCloskey, Paul, & Commissaris, 1987; McMillan & Leander, 1975). Moreover, antipunishment effects to anxiolytics are observed with several different types of punishers, including electric shock, pressurized air (Spealman, 1979), and timeout from reinforcement (van Haaren & Anderson, 1997). The phenomenon is most consistently observed with drugs that act on GABAergic systems, though it has also been noted with serotonergic anxiolytics (McCloskey, Paul, & Commissaris, 1987; van Haaren & Anderson, 1997), but with less consistency (Sanger, 1990).
Several studies document antipunishment effects with ethanol, another GABAergic compound, in nonhuman species (Barrett, Brady, & Witkin, 1985; Glowa & Barrett, 1976; Koob, Braestrup & Britton, 1986; Vogel, 1980). In a study by Vogel (1980), for example, licking of a water tube by rats was suppressed when each twentieth lick produced shock (FR 20). Ethanol (0.5–2 g/kg) increased punished licking in a manner that resembled the increases observed with chlordiazepoxide, a drug that has well established antipunishment effects. Licking the tube when punishment was not scheduled was not increased by these same doses, so rate increases were specific to the presence of a punishment contingency. Antipunishment effects with ethanol have also been reported with rats (Barrett et al., 1985) and squirrel monkeys (Glowa & Barrett, 1976) in a variety of punishment procedures.
There is indirect evidence that antipunishment effects are relevant to the use of anxiolytics in human clinical psychopharmacology. As noted earlier, the effect is obtained with drugs that are used clinically for managing anxiety. Doses of these drugs that produce anxiolytic effects in animals are closely correlated with doses that are clinically efficacious with humans (Cook & Davidson, 1973; Kleven & Koek, 1999). The evidence for generalization of antipunishment effects from animals to humans is indirect, but intriguing. Socially inappropriate behavior, such as aggression (e.g., Cherek, Steinberg, & Manno, 1985; Cherek, Steinberg, & Vines, 1984; Dougherty, Cherek, & Bennett, 1996) or observing sexually explicit pictures (Kallmen & Gustafson, 1998) is increased by alcohol in humans. Under nondrug conditions, aggression or viewing sexually explicit pictures occurred at a low rate, and it may be the case that these behaviors were suppressed by punishers from, for example, social or cultural sources prior to the experiment. The direct demonstration of antipunishment effects, however, requires the suppression of baseline behavior by a response-contingent punisher, and the subsequent increase in punished behavior produced by the drug. The present study was designed to study ethanol using this approach.
Punishment has been studied and quantified in humans by applying the generalized matching equation, a model of choice, to behavior under concurrent schedules of reinforcement. Under concurrent schedules, two response alternatives are available simultaneously and the two responses are often maintained under separate variable-interval schedules. Allocation of responses or time to the two alternatives approximately matches the relative rates of reinforcement that they produce (Herrnstein, 1961; Davison & McCarthy, 1988). This relationship also has been described quantitatively using the generalized matching equation (Baum, 1974), a power-law formulation that partitions two sources of deviation from matching: bias and sensitivity.
The generalized matching equation is often expressed as:
| 1 |
where the ratio of behavioral responses allocated to two reinforcer alternatives (Ba and Bb) is related to the ratio of reinforcers earned by the two alternative responses (Ra and Rb). The two free parameters, log k and c, describe bias and sensitivity to reinforcement, respectively. Bias (log k) appears as a preference for one alternative and may come from characteristics of the experimental setting, such as difficulty operating one response device (Davison & McCarthy, 1988) or, here, punishment of responding on one alternative. A log k > 0 means behavior is biased toward the numerator (alternative a in equation 1); log k < 0 means that behavior is biased toward the denominator (alternative b). Sensitivity to reinforcement refers to the manner in which the ratio of responding on the two alternatives tracks the reinforcer ratio delivered by them. A value of c = 1, which is not commonly seen, is matching, and the response ratios equal reinforcer ratios. If c < 1, then response ratios are less sensitive to reinforcer ratios, which is called undermatching and is a common finding in animals (Baum, 1974) and humans (Kollins, Newland, & Critchfield, 1997; Pierce & Epling, 1983). A value of c > 1, called overmatching, indicates that changes in response ratios are highly sensitive to changes in reinforcer ratios. Overmatching occurs, for example, when there is a high cost for switching alternatives or if changing from one alternative to another is punished (Todorov, 1971).
A schedule of punishment superimposed on one response alternative of a concurrent schedule shifts the distribution of behavior toward the alternative associated with no punishment even when there is a net loss of reinforcement. This shift has been reported with animals (Deluty & Church, 1978; de Villiers, 1980; Farley & Fantino, 1978; Wojnicki & Barrett, 1993) and humans (e.g., Bradshaw, Szabadi, & Bevan, 1979; Carlton, Siegel, Murphee, & Cook, 1981; Critchfield, Paletz, & MacAleese, 2003; Katz, 1973; Rasmussen & Newland, 2008). A global shift in preference is captured by the bias parameter (log k) in the generalized matching equation (Carlton et al., 1981; Rasmussen & Newland, 2008). In some cases, punishment also lowers sensitivity to reinforcement. For example, a study by Rasmussen and Newland (2008) reported on punishment effects with humans in a concurrent schedule arrangement with monetary gain as a reinforcer and monetary loss as a punisher. When no punisher was present, both matching and undermatching occurred and no bias was demonstrated toward either alternative. Under the punishment condition, all participants exhibited a bias (log k range −0.20 to −0.76) toward the alternative associated with no punishment, and this was true whether obtained reinforcers (total reinforcers earned on each alternative), or net reinforcers (reinforcers on each alternative minus those lost due to punishment on an alternative) were used in the analysis. Moreover, all participants showed greater undermatching, as indicated by shallower slopes, under punishment conditions than under no-punishment conditions, suggesting punishment also reduced sensitivity to reinforcement densities of the two alternatives.
The present study examined the effects of ethanol on punished responding in human participants in order to assess further the extension of antipunishment effects to the behavior of human participants. Behavior was maintained under concurrent schedules of reinforcement with a conjoint schedule of punishment superimposed on one response alternative. Ethanol's antipunishment effects were examined using the generalized matching equation. A detailed description of behavior under nondrug conditions with the same participants as the present study and the ability of the matching equation to characterize punished responding has been described previously (Rasmussen & Newland, 2008). In that study, punishment created a bias toward the alternative associated with no punishment. In the present study, however, we extended the earlier findings by examining the degree to which ethanol increases rates of punished responding and diminishes bias toward the alternative associated with no punishment. Based on previous studies (e.g., Carlton et al., 1981; Rasmussen & Newland, 2008), it was hypothesized that bias of 0 would appear under the no-punishment condition, and bias would be less than 0 (toward the unpunished component) in the punishment condition during placebo conditions. It was further hypothesized that ethanol would, in a dose-related fashion, shift bias toward 0 and increase response rate under punishment.
METHOD
Participants
Five male college students, at least 21 years of age and weighing between 74–83 kg (165–185 pounds) participated. Participants were screened to ensure that there was no history of problem drinking patterns or other medical problems by completing the Rutgers Ethanol Problem Inventory and a modified version of the Daily Drinking Questionnaire (Collins, Parks, & Marlatt, 1985), which includes questions about medical history. Male participants were used so gender differences in alcohol metabolism did not introduce variability. Over the course of the subjects' participation in the study, they were asked to refrain from ethanol consumption outside of the experiment, such that tolerance to ethanol would be minimized. All experiments were approved by the Auburn University Institutional Review Board.
Materials
A DOS-based computer was used to present visual images, transduce responses from the participants, present stimuli, and record data. All code was written in VisualBasic®. Participants were placed individually in small, separate, office-sized rooms containing a desk, chair, computer monitor, mouse, and mouse pad. A Breathalyzer was used to monitor breath-alcohol concentration (BAC). BAC is a reliable, valid, and noninvasive predictor of blood-ethanol concentration (Jones & Andersson, 1996).
Procedure
Procedures were similar to those reported in Rasmussen and Newland (2008), and the same 5 participants from that report were used in the present study. A participant was escorted into a 400-square-foot room and seated at a desk with a computer monitor. On the first day of the study, before participation began, each participant completed a consent form and was given a set of instructions to read before beginning the computer task. After the participant mouse-clicked a START icon on the monitor, an 8-min concurrent schedule session began. In the 8-min session, the monitor screen was split vertically into halves and a small colored box was located in the middle of each half of the screen. For each participant, the left box (alternative A) and right box (alternative B) were different colors. A mouse-click on either box started both boxes moving in a random pattern at a constant rate throughout each half of the screen. Participants could mouse-click (the response of interest) on one of the two moving colored box icons, but could not click on both boxes simultaneously. Each box was associated with one component of a VI schedule of reinforcement in which a reinforcer (a flashing “+4¢” icon that appeared on the screen for 2 s, representing that 4¢ had been earned) was delivered on each alternative after the first response after a varying amount of time elapsed (described below). Participants could switch alternatives throughout the session; a 2-s changeover delay during which points could not be delivered or subtracted was imposed after each switch.
No-punishment condition
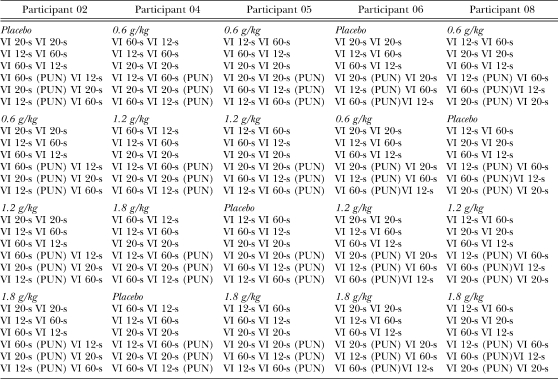
Each participant's mouse-clicking was reinforced under a concurrent VIA VIB (conc VIA VIB) schedule (A represented the value on the left alternative, or alternative A; B represented the value on the right alternative, or alternative B). The following schedules were used: conc VI 12-s VI 60-s, conc VI 20-s VI 20-s, and conc VI 60-s VI 12-s. For example, under conc VI 12-s VI 60-s, the first response on the alternative A was reinforced after the lapse of an average of 12 s; for alternative B, the first response after the lapse of an average of 60 s on an independent, but continuously running, clock produced a reinforcer. The VI clock reset at the onset of the 2-s reinforcer interval. The VI schedules were arranged using Fleshler and Hoffman's (1962) constant probability distributions. There were no counters available to the participant that tallied earnings or losses. Programmed reinforcement ratios for these schedules were 5∶1, 1∶1, and 1∶5, respectively and the specific values used resulted in the same overall programmed reinforcement rate for all conditions. Each concurrent schedule was in effect until stability occurred. Stability was defined as three consecutive sessions in which response allocation (percent of responses on the left alternative) differed by no more than 5% of the mean of the last three sessions, with no trends apparent. Table 1 summarizes the order of conditions that each participant experienced. The order of schedules was counterbalanced across participants.
Table 1.
Order of VIA VIB schedules for each participant. Reinstatement of no-punishment conditions is omitted for brevity.
| Participant 02 | Participant 04 | Participant 05 | Participant 06 | Participant 08 |
| Placebo | 0.6 g/kg | 0.6 g/kg | Placebo | 0.6 g/kg |
| VI 20-s VI 20-s | VI 60-s VI 12-s | VI 12-s VI 60-s | VI 20-s VI 20-s | VI 12-s VI 60-s |
| VI 12-s VI 60-s | VI 12-s VI 60-s | VI 60-s VI 12-s | VI 12-s VI 60-s | VI 20-s VI 20-s |
| VI 60-s VI 12-s | VI 20-s VI 20-s | VI 20-s VI 20-s | VI 60-s VI 12-s | VI 60-s VI 12-s |
| VI 60-s (PUN) VI 12-s | VI 12-s VI 60-s (PUN) | VI 20-s VI 20-s (PUN) | VI 20-s (PUN) VI 20-s | VI 12-s (PUN) VI 60-s |
| VI 20-s (PUN) VI 20-s | VI 20-s VI 20-s (PUN) | VI 60-s VI 12-s (PUN) | VI 12-s (PUN) VI 60-s | VI 60-s (PUN)VI 12-s |
| VI 12-s (PUN) VI 60-s | VI 60-s VI 12-s (PUN) | VI 12-s VI 60-s (PUN) | VI 60-s (PUN)VI 12-s | VI 20-s (PUN) VI 20-s |
| 0.6 g/kg | 1.2 g/kg | 1.2 g/kg | 0.6 g/kg | Placebo |
| VI 20-s VI 20-s | VI 60-s VI 12-s | VI 12-s VI 60-s | VI 20-s VI 20-s | VI 12-s VI 60-s |
| VI 12-s VI 60-s | VI 12-s VI 60-s | VI 60-s VI 12-s | VI 12-s VI 60-s | VI 20-s VI 20-s |
| VI 60-s VI 12-s | VI 20-s VI 20-s | VI 20-s VI 20-s | VI 60-s VI 12-s | VI 60-s VI 12-s |
| VI 60-s (PUN) VI 12-s | VI 12-s VI 60-s (PUN) | VI 20-s VI 20-s (PUN) | VI 20-s (PUN) VI 20-s | VI 12-s (PUN) VI 60-s |
| VI 20-s (PUN) VI 20-s | VI 20-s VI 20-s (PUN) | VI 60-s VI 12-s (PUN) | VI 12-s (PUN) VI 60-s | VI 60-s (PUN)VI 12-s |
| VI 12-s (PUN) VI 60-s | VI 60-s VI 12-s (PUN) | VI 12-s VI 60-s (PUN) | VI 60-s (PUN)VI 12-s | VI 20-s (PUN) VI 20-s |
| 1.2 g/kg | 1.8 g/kg | Placebo | 1.2 g/kg | 1.2 g/kg |
| VI 20-s VI 20-s | VI 60-s VI 12-s | VI 12-s VI 60-s | VI 20-s VI 20-s | VI 12-s VI 60-s |
| VI 12-s VI 60-s | VI 12-s VI 60-s | VI 60-s VI 12-s | VI 12-s VI 60-s | VI 20-s VI 20-s |
| VI 60-s VI 12-s | VI 20-s VI 20-s | VI 20-s VI 20-s | VI 60-s VI 12-s | VI 60-s VI 12-s |
| VI 60-s (PUN) VI 12-s | VI 12-s VI 60-s (PUN) | VI 20-s VI 20-s (PUN) | VI 20-s (PUN) VI 20-s | VI 12-s (PUN) VI 60-s |
| VI 20-s (PUN) VI 20-s | VI 20-s VI 20-s (PUN) | VI 60-s VI 12-s (PUN) | VI 12-s (PUN) VI 60-s | VI 60-s (PUN)VI 12-s |
| VI 12-s (PUN) VI 60-s | VI 60-s VI 12-s (PUN) | VI 12-s VI 60-s (PUN) | VI 60-s (PUN)VI 12-s | VI 20-s (PUN) VI 20-s |
| 1.8 g/kg | Placebo | 1.8 g/kg | 1.8 g/kg | 1.8 g/kg |
| VI 20-s VI 20-s | VI 60-s VI 12-s | VI 12-s VI 60-s | VI 20-s VI 20-s | VI 12-s VI 60-s |
| VI 12-s VI 60-s | VI 12-s VI 60-s | VI 60-s VI 12-s | VI 12-s VI 60-s | VI 20-s VI 20-s |
| VI 60-s VI 12-s | VI 20-s VI 20-s | VI 20-s VI 20-s | VI 60-s VI 12-s | VI 60-s VI 12-s |
| VI 60-s (PUN) VI 12-s | VI 12-s VI 60-s (PUN) | VI 20-s VI 20-s (PUN) | VI 20-s (PUN) VI 20-s | VI 12-s (PUN) VI 60-s |
| VI 20-s (PUN) VI 20-s | VI 20-s VI 20-s (PUN) | VI 60-s VI 12-s (PUN) | VI 12-s (PUN) VI 60-s | VI 60-s (PUN)VI 12-s |
| VI 12-s (PUN) VI 60-s | VI 60-s VI 12-s (PUN) | VI 12-s VI 60-s (PUN) | VI 60-s (PUN)VI 12-s | VI 20-s (PUN) VI 20-s |
In the current study, participants performed five to seven 8-min sessions within an approximate 1-hr block of sessions. (Session blocks sometimes ran up to 65 min, depending on whether a participant needed a short break between sessions at some point during the session block to, for example, use the restroom.) One session block (or visit to the laboratory) was conducted per day, and at least 2 days separated each session block. Therefore a participant could make up to four visits a week for a total of four session blocks per week. If stability under one concurrent schedule was reached before the end of a session block (e.g., stability under conc VI 12-s VI 60-s was reached within three sessions), the next concurrent schedule was placed in effect in the next session and remained in effect until stability occurred (e.g., conc VI 20-s VI 20-s would then be implemented). If stability within a concurrent schedule did not occur by the end of a session block, the same schedule was implemented again at the beginning of the next session block.
Punishment condition
After behavior stabilized under the three concurrent schedules of the no-punishment condition, a VI schedule of punishment (a flashing “−4¢” that appeared on the screen for 2 s and represented the loss of 4 cents) was superimposed on one alternative of the concurrent schedule, forming a conjoint schedule for that response alternative. The average interval for the punishment VI was programmed to be 25% greater than the average interval in the schedule of reinforcement on which it was superimposed. For example, if a VI 20-s schedule of reinforcement was programmed for alternative A responses, then the punishment schedule, also on the alternative A responses, would be VI 25-s schedule. For each participant, the punishment schedule was always on the same response alternative (A or B). As in the earlier phase, each of the three concurrent schedules with punishment was in effect until stable responding was obtained. After stability was obtained with punishment, one of the three no-punishment concurrent schedules was placed in effect again until stability was obtained, such that each concurrent schedule with punishment alternated with a no-punishment concurrent schedule. This was done to reestablish matching and to ensure that punishment did not affect subsequent matching. The reestablished conditions yielded data that were nearly identical to the initial matching sessions, suggesting punishment did not affect subsequent matching (see data presented in Rasmussen & Newland, 2008, for details).
Drug conditions
A complete series of conditions, including concurrent schedules with punishment and no-punishment, were conducted before the present study commenced. The results of this baseline phase are discussed in detail in Rasmussen and Newland (2008) and will not be repeated here. The placebo and drug conditions for the present study began after the baseline phase was completed.
Three volumes of vodka (40% ethyl alcohol) were mixed in orange juice so that the total solution administered was 16 oz, yielding ethanol doses of 0.6, 1.2 and 1.8 g/kg body weight. Doses were administered to all participants in increasing order. These doses yield approximately 0.64, 1.28, and 1.95 oz of absolute ethanol, respectively, for a 170 pound male, and produced respective mean BACs of approximately 0.02 (SD = 0.003), 0.04 (SD = 0.01), and 0.07 (SD = 0.01) for our participants. Peak BACs did not exceed 0.09 under any condition. A placebo condition was implemented in which 1 mL of ethanol was floated on top of the mixer and rubbed on the sides of the drinking glass. The placement of the placebo condition within the dose series was counterbalanced across participants (see Table 1). Doses (including placebo), and therefore session blocks, were separated by at least 2 days.
Participants were asked to refrain from eating or drinking for 2 hr prior to the experiment. When a participant arrived, he was given the drink with ethanol as described above, 1 hr before the session commenced. The drink was given in thirds that were spaced apart by 5 min to control for rate of consumption. BAC data were collected 1 hr after the first third was administered. During this 1-hr waiting period, the participant was allowed to engage in quiet activities in the waiting area, such as reading a magazine or studying.
To measure other effects of ethanol (e.g., motor effects), a pencil-tapping task was used. Tapping has been used as a measure of motor function, and has assessed motor dysfunction in degenerative motor disorders, such as Parkinson's and Huntington's diseases (e.g., Mitchell et al., 2008; Nagasaki, Itoh, Maruyama, & Hashizume, 1988; Ziv et al., 1999), in aged populations (e.g., Cousins, Corrow, Finn, & Salamone, 1998; Nagasaki et al., 1988), and in alcohol-dependent populations (e.g., Parks et al., 2003). Tapping has been used also in assessing the acute effects of CNS depressants, such as ethanol and diazepam (e.g., Lindenschmist, Brown, Cerimele, Walle, & Forney, 1983; Palva, 1985). In this task, participants were asked to tap a pencil against paper as quickly as possible for 60 s, and were told that each pencil tap would result in 2 cents added to their total earnings at the end of the experiment. One pencil-tap test was given to each participant under each dose of ethanol, and each test was given immediately before a session block began.
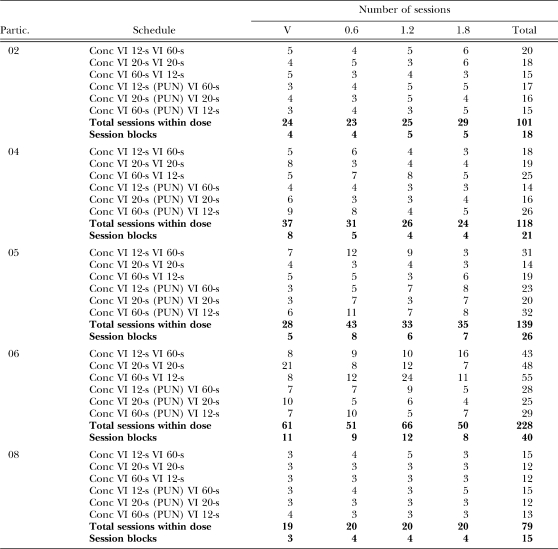
The participant then was placed in the experimental conditions for a 1-hr session block; only one session block was conducted per day. During this time, 8-min sessions of a particular concurrent schedule were conducted until responding stabilized. Since it took more than one session block to run all six concurrent schedules until stability was observed under each dose (stability often required more than three sessions—see Table 2), multiple session blocks were conducted under each dose of ethanol, usually four to five (though some participants completed a higher number, e.g., Participant 06), so participants experienced between 15 to 26 session blocks across the experiment, with the exception of Participant 06, who completed 40 session blocks. Under a particular dose of ethanol, if a participant completed six of the concurrent schedules across several session blocks, but reached stability before the hour was up, the session block ended early, i.e., he was not given the next dose of ethanol. On the next visit, he was given the next dose and the concurrent schedule sequence continued. Table 2 shows the number of sessions and session blocks completed for each participant under each schedule and dose of ethanol (Note: Reestablishment data are not included in this table, but typically increased the total session blocks by 2–4 per individual per drug dose).
Table 2.
Number of sessions and session blocks for each condition for each participant.
| Partic. | Schedule | Number of sessions | ||||
| V | 0.6 | 1.2 | 1.8 | Total | ||
| 02 | Conc VI 12-s VI 60-s | 5 | 4 | 5 | 6 | 20 |
| Conc VI 20-s VI 20-s | 4 | 5 | 3 | 6 | 18 | |
| Conc VI 60-s VI 12-s | 5 | 3 | 4 | 3 | 15 | |
| Conc VI 12-s (PUN) VI 60-s | 3 | 4 | 5 | 5 | 17 | |
| Conc VI 20-s (PUN) VI 20-s | 4 | 3 | 5 | 4 | 16 | |
| Conc VI 60-s (PUN) VI 12-s | 3 | 4 | 3 | 5 | 15 | |
| Total sessions within dose | 24 | 23 | 25 | 29 | 101 | |
| Session blocks | 4 | 4 | 5 | 5 | 18 | |
| 04 | Conc VI 12-s VI 60-s | 5 | 6 | 4 | 3 | 18 |
| Conc VI 20-s VI 20-s | 8 | 3 | 4 | 4 | 19 | |
| Conc VI 60-s VI 12-s | 5 | 7 | 8 | 5 | 25 | |
| Conc VI 12-s (PUN) VI 60-s | 4 | 4 | 3 | 3 | 14 | |
| Conc VI 20-s (PUN) VI 20-s | 6 | 3 | 3 | 4 | 16 | |
| Conc VI 60-s (PUN) VI 12-s | 9 | 8 | 4 | 5 | 26 | |
| Total sessions within dose | 37 | 31 | 26 | 24 | 118 | |
| Session blocks | 8 | 5 | 4 | 4 | 21 | |
| 05 | Conc VI 12-s VI 60-s | 7 | 12 | 9 | 3 | 31 |
| Conc VI 20-s VI 20-s | 4 | 3 | 4 | 3 | 14 | |
| Conc VI 60-s VI 12-s | 5 | 5 | 3 | 6 | 19 | |
| Conc VI 12-s (PUN) VI 60-s | 3 | 5 | 7 | 8 | 23 | |
| Conc VI 20-s (PUN) VI 20-s | 3 | 7 | 3 | 7 | 20 | |
| Conc VI 60-s (PUN) VI 12-s | 6 | 11 | 7 | 8 | 32 | |
| Total sessions within dose | 28 | 43 | 33 | 35 | 139 | |
| Session blocks | 5 | 8 | 6 | 7 | 26 | |
| 06 | Conc VI 12-s VI 60-s | 8 | 9 | 10 | 16 | 43 |
| Conc VI 20-s VI 20-s | 21 | 8 | 12 | 7 | 48 | |
| Conc VI 60-s VI 12-s | 8 | 12 | 24 | 11 | 55 | |
| Conc VI 12-s (PUN) VI 60-s | 7 | 7 | 9 | 5 | 28 | |
| Conc VI 20-s (PUN) VI 20-s | 10 | 5 | 6 | 4 | 25 | |
| Conc VI 60-s (PUN) VI 12-s | 7 | 10 | 5 | 7 | 29 | |
| Total sessions within dose | 61 | 51 | 66 | 50 | 228 | |
| Session blocks | 11 | 9 | 12 | 8 | 40 | |
| 08 | Conc VI 12-s VI 60-s | 3 | 4 | 5 | 3 | 15 |
| Conc VI 20-s VI 20-s | 3 | 3 | 3 | 3 | 12 | |
| Conc VI 60-s VI 12-s | 3 | 3 | 3 | 3 | 12 | |
| Conc VI 12-s (PUN) VI 60-s | 3 | 4 | 3 | 5 | 15 | |
| Conc VI 20-s (PUN) VI 20-s | 3 | 3 | 3 | 3 | 12 | |
| Conc VI 60-s (PUN) VI 12-s | 4 | 3 | 3 | 3 | 13 | |
| Total sessions within dose | 19 | 20 | 20 | 20 | 79 | |
| Session blocks | 3 | 4 | 4 | 4 | 15 | |
After a session block was completed, participants were required to stay in the laboratory until BACs were 0.02 or below. They were placed in the “recovery room” in which they studied, slept, played games on the computer, or conversed with others.
Analysis
Dependent variables included the number of responses, reinforcers, and, for the punished alternative, punishers delivered on alternative A or B. Overall response rates on each alternative (responses per min) were determined by dividing the number of responses on each alternative by 8 (since session duration was 8 min long). Local response rates also were determined by dividing the number of responses on each alternative by the time spent on the respective alternative (see Appendix for these time intervals), but no strong effects of ethanol or punishment were found on local rates; therefore, overall response rates will be reported here. Obtained reinforcers (total delivered), as opposed to net reinforcers (points delivered minus points lost through punishment) were used.
Reinforcer and response ratios were constructed by dividing the number of responses, or reinforcers, on alternative A by that for alternative B. The log of the response ratio was then expressed as a linear function of the log reinforcer ratio and this log-transformed version was applied to Equation 1 using linear regression. Response and reinforcer ratios from the last three consecutive stable sessions of each concurrent schedule were used for the analyses. The free parameters c (sensitivity) and log k (bias) were the dependent variables of interest. Though the punishment schedule was superimposed on alternative A for 3 participants and on B for 2 participants (see Table 1), all data will be reported as though the punisher appeared on alternative A for clarity of presentation.
Bias values for punishment under baseline conditions were compared using both obtained and net reinforcers in Rasmussen and Newland (2008). The former yielded a range of bias values that were slightly, though not significantly, lower than the net values. We used obtained reinforcers in the present study because it slightly enhanced the range of bias values, thereby limiting potential floor or restriction-of-range effects.
RESULTS
The appendix contains the mean number of responses per session on alternatives A and B, mean response rate on alternative A (responses/8), and mean obtained reinforcers earned on each alternative for each participant under each drug dose and concurrent schedule. Means for each represent the data from the last three stable sessions for each condition. As the appendix shows, under the conc VI 12-s VI 60-s schedule (no-punishment), response rate on alternative A during placebo sessions varied between 32 and 97 responses per min, and decreased to 2 to 14 under punishment; under conc VI 20-s VI 20-s schedule (no punishment), rate ranged between 16 and 40 responses per min, and decreased to 1 to 22 under punishment; under the conc VI 60-s VI 12-s schedule (no punishment), rates varied between 3 and 32 in the no-punishment condition, and were suppressed to 1 to 7 responses per minute in the punishment condition. In all placebo conditions, then, response rates in the punishment conditions were lower than in the no-punishment condition.
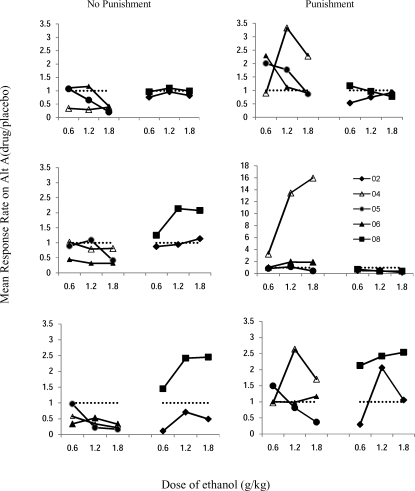
Figure 1 shows mean response rate on alternative A for each dose as a proportion of placebo (drug/vehicle) for each subject across all concurrent schedules. Ethanol reduced response rates in the no-punishment condition in conc VI 12-s VI 60-s (top), conc VI 60-s VI 12-s (bottom), and, to some extent, conc VI 20-s VI 20-s (middle) for Participants 04, 05, and 06. For Participant 02, response rate changed unsystematically, and for Participant 08, rate increases were observed in conc VI 20-s VI 20-s and conc VI 60-s 12-s. In the punishment condition, ethanol-related response rate increases were observed for Participants 04, 05, and 06. Participant 02 showed a dose-dependent increase under the conc VI 60-s VI 12-s schedule. (Note the scaling for the conc VI 20-s VI 20-s punishment condition, middle right panel. Because Participant 04 showed a 16-fold increase in rate, it reduces the ability to see the rate increase for Participant 05.) For Participant 08 there was a rate increase under the VI 60-s VI 12-s schedule (bottom right) equivalent to that seen in the nonpunishment component. Otherwise, there were few rate increases noted for Participants 02 and 08.
Fig 1.
Response rate (drug/vehicle) as a function of dose of ethanol for each schedule. The left column represents the no-punishment condition; the right column, the punishment condition. Top panels: conc VI 12-s VI 60-s; middle panels: conc VI 20-s VI 20-s, bottom panels: conc VI 60-s VI 12-s. Each participant is represented by a different symbol. The dotted horizontal line shows no change from placebo.
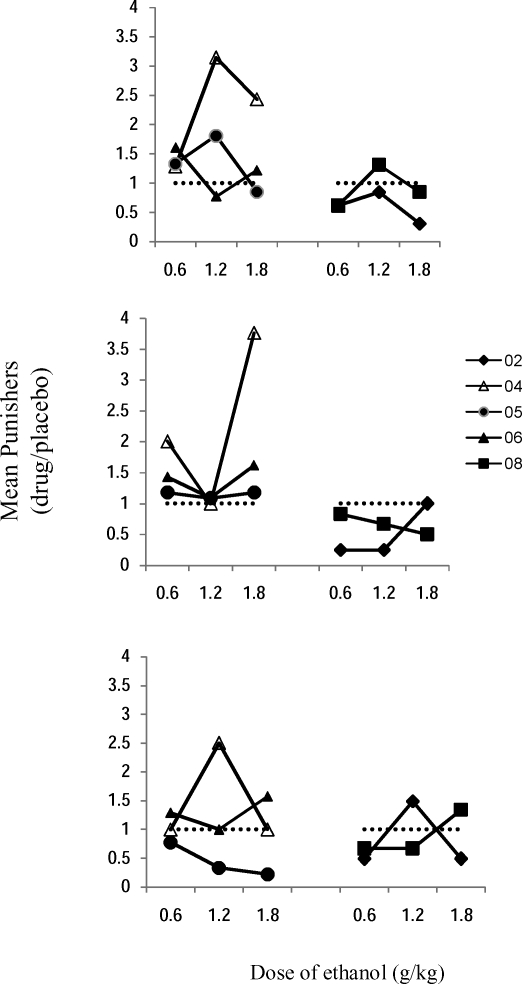
Figure 2 shows mean punishers as a function of ethanol dose for each participant across the three concurrent schedules as a proportion of baseline, i.e, the mean number of punishers earned under a particular drug dose divided by those earned under the placebo. For Participants 04, 05, and 06, ethanol-related increases were observed in the number of punishers across the three concurrent schedules, except for Participant 05, who showed a dose-dependent decrease under the conc VI 60-s VI 12-s schedule. The number of punishers earned was not greatly different, but usually smaller than placebo conditions, for Participants 02 and 08.
Fig 2.
Mean punishers as a function of dose of ethanol. Each participant is represented by a different symbol. Each datum is the mean number of punishers under a drug dose divided by those under the placebo. The dotted horizontal line represents no change from placebo. Top panel: conc VI 12-s VI 60-s; middle panel: conc VI 20-s VI 20-s, bottom panel: conc VI 60-s VI 12-s.
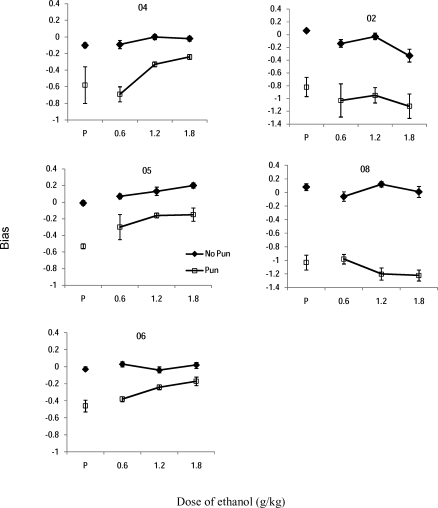
Figure 3 shows the value of log k, the bias parameter under placebo (P) conditions. Error bars represent the standard error of the estimate. Each panel represents a participant. The mean bias value in the no-punishment placebo condition (closed diamonds) was −0.03 (SEM = 0.03) for the group (see Table 3 for individual data), suggesting a very small amount of bias toward alternative B. Negative values of bias, ranging from −0.46 to −1.03 (M = −0.68, SEM = 0.11) were obtained under the punishment conditions (open diamonds) under placebo, suggesting a strong bias toward the unpunished alternative. A paired samples t-test confirmed a significant difference between no-punishment and punishment conditions for log k under placebo conditions, t(4) = −5.58, p < 0.01.
Fig 3.
Bias values for each participant as a function of dose of ethanol for no-punishment (closed diamonds) and punishment (open squares) conditions. Error bars represent one standard error of the estimate. In some instances, the error is so small that the data point covers it.
Table 3.
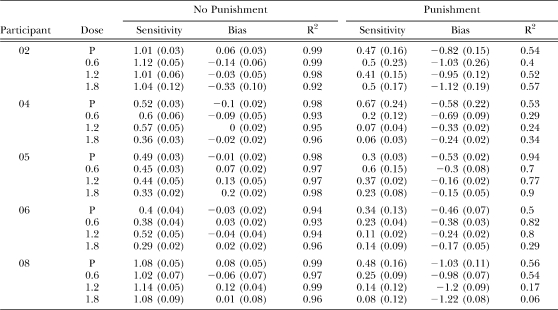
Parameters from the generalized matching equation for each participant. Standard errors of the estimate are given in parentheses.
| Participant | Dose | No Punishment | Punishment | ||||
| Sensitivity | Bias | R2 | Sensitivity | Bias | R2 | ||
| 02 | P | 1.01 (0.03) | 0.06 (0.03) | 0.99 | 0.47 (0.16) | −0.82 (0.15) | 0.54 |
| 0.6 | 1.12 (0.05) | −0.14 (0.06) | 0.99 | 0.5 (0.23) | −1.03 (0.26) | 0.4 | |
| 1.2 | 1.01 (0.06) | −0.03 (0.05) | 0.98 | 0.41 (0.15) | −0.95 (0.12) | 0.52 | |
| 1.8 | 1.04 (0.12) | −0.33 (0.10) | 0.92 | 0.5 (0.17) | −1.12 (0.19) | 0.57 | |
| 04 | P | 0.52 (0.03) | −0.1 (0.02) | 0.98 | 0.67 (0.24) | −0.58 (0.22) | 0.53 |
| 0.6 | 0.6 (0.06) | −0.09 (0.05) | 0.93 | 0.2 (0.12) | −0.69 (0.09) | 0.29 | |
| 1.2 | 0.57 (0.05) | 0 (0.02) | 0.95 | 0.07 (0.04) | −0.33 (0.02) | 0.24 | |
| 1.8 | 0.36 (0.03) | −0.02 (0.02) | 0.96 | 0.06 (0.03) | −0.24 (0.02) | 0.34 | |
| 05 | P | 0.49 (0.03) | −0.01 (0.02) | 0.98 | 0.3 (0.03) | −0.53 (0.02) | 0.94 |
| 0.6 | 0.45 (0.03) | 0.07 (0.02) | 0.97 | 0.6 (0.15) | −0.3 (0.08) | 0.7 | |
| 1.2 | 0.44 (0.05) | 0.13 (0.05) | 0.97 | 0.37 (0.02) | −0.16 (0.02) | 0.77 | |
| 1.8 | 0.33 (0.02) | 0.2 (0.02) | 0.98 | 0.23 (0.08) | −0.15 (0.05) | 0.9 | |
| 06 | P | 0.4 (0.04) | −0.03 (0.02) | 0.94 | 0.34 (0.13) | −0.46 (0.07) | 0.5 |
| 0.6 | 0.38 (0.04) | 0.03 (0.02) | 0.93 | 0.23 (0.04) | −0.38 (0.03) | 0.82 | |
| 1.2 | 0.52 (0.05) | −0.04 (0.04) | 0.94 | 0.11 (0.02) | −0.24 (0.02) | 0.8 | |
| 1.8 | 0.29 (0.02) | 0.02 (0.02) | 0.96 | 0.14 (0.09) | −0.17 (0.05) | 0.29 | |
| 08 | P | 1.08 (0.05) | 0.08 (0.05) | 0.99 | 0.48 (0.16) | −1.03 (0.11) | 0.56 |
| 0.6 | 1.02 (0.07) | −0.06 (0.07) | 0.97 | 0.25 (0.09) | −0.98 (0.07) | 0.54 | |
| 1.2 | 1.14 (0.05) | 0.12 (0.04) | 0.99 | 0.14 (0.12) | −1.2 (0.09) | 0.17 | |
| 1.8 | 1.08 (0.09) | 0.01 (0.08) | 0.96 | 0.08 (0.12) | −1.22 (0.08) | 0.06 | |
Figure 3 also shows the bias parameter under each dose of ethanol under no-punishment and punishment conditions. Table 3 shows corresponding parameter values for each individual. With ethanol, bias under the schedule with punishment diminished by at least half (a less negative value was obtained) as a function of ethanol dose for Participants 04, 05, and 06. Bias was unchanged for Participant 02 and became slightly more extreme for Participant 08. Bias under the schedule without punishment (closed diamonds) changed little with ethanol for all participants, though Participant 02 showed a slight bias toward alternative B at the highest dose.
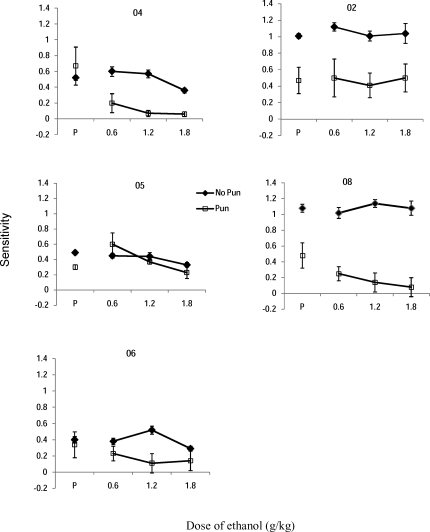
Figure 4 shows the sensitivity parameter under placebo (P) and each dose of ethanol (g/kg) under no-punishment (closed diamonds) and punishment conditions (open squares). Table 3 shows corresponding parameter values for each individual. Sensitivity values in the no-punishment placebo conditions were less than 1 (undermatching) for 3 participants (04, 05, 06) and approximated 1 (matching) for 2 participants (02 and 08). Imposing the punishment contingency decreased sensitivity for Participants 02, 05 and 08, but did not change sensitivity for the other 2 participants.
Fig 4.
Sensitivity values for each participant as a function of dose of ethanol for no-punishment (closed diamonds) and punishment (open squares) conditions. Error bars represent one standard error of the estimate. In some instances, the error is so small, the data point covers it.
Figure 4 also shows little to no ethanol-related change in sensitivity under the no-punishment condition; see Table 3 for individual values. An ethanol-related decrease in the sensitivity parameter was more pronounced under punishment conditions for Participants 04, 06, and 08. Ethanol appeared to have no effect on sensitivity to reinforcement for Participant 02 and a slight increase at one dose for Participant 05.
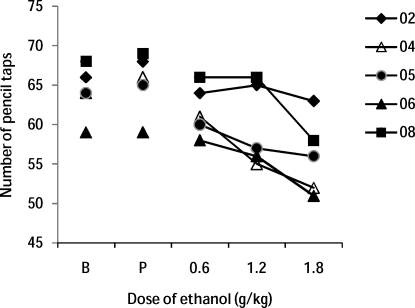
Figure 5 shows the number of pencil taps in a 60-s period as a function of dose of ethanol for each participant. Overall, ethanol decreased pencil tapping in a dose-dependent fashion, F(4, 24) = 3.71, p = 0.02, but individual differences were noted. Participants 04, 05, and 06 showed dose-related declines, while Participant 08 showed a decline only at the highest dose, and Participant 02 appeared insensitive to the doses of ethanol used.
Fig 5.
The number of pencil taps in 60 s for each participant as a function of dose of ethanol.
DISCUSSION
Stimuli signaling monetary gain functioned as a reinforcer and stimuli signaling monetary loss functioned as a punisher for all participants under concurrent schedule arrangements in the placebo condition. The generalized matching equation described the data well under placebo no-punishment conditions, with R2 values ranging between 0.94 and 0.99. When one alternative was punished, the R2 values under placebo conditions were lower, between 0.50 and 0.94. At first glance, it may seem that lower R2 values mean that the matching equation did not describe the data well in the punishment condition, but R2 is sensitive not just to the degree of scatter but also to the slope of the relationship. A shallow slope, for example, will not account for a large proportion of the variance, even if the error in the estimate is small. Because of the importance of the parameter estimates, we provided the standard error of the estimate (SEE) for the bias and sensitivity parameters (Heiman, 1992). The SEE for bias and sensitivity parameters was very small in the no-punishment condition (range 0.02–0.10), and in the punishment conditions they ranged from 0.02 to −0.24. In some instances, then, the error was greater for the punishment condition compared to the no-punishment condition, but overall, it was still quite low. The lower R2 values, then, can be attributed mostly to punishment flattening the slopes of the regression line (i.e., reducing sensitivity to reinforcement).
The parameter values from the generalized matching equation under placebo conditions in the current study were similar to those reported on nonplacebo baseline data of these same participants as described in an earlier report (Rasmussen & Newland, 2008). In the no-punishment placebo condition, bias values were near 0. The imposition of a punishment contingency did three things. First, it reduced response rate on the alternative associated with punishment and shifted behavior to the unpunished alternative. Second, it degraded reinforcement efficacy of the response alternative associated with punishment. This was evident in a sizeable shift in bias toward the unpunished alternative, even when point gain in the unpunished alternative was the same as point gain in the punished alternative (described further in Rasmussen & Newland, 2008). Third, it diminished the sensitivity of behavior to differences in reinforcement rates between the two conditions, as expressed in the reinforcer ratios. This is supported by the observation that 4 of the 5 participants exhibited lower sensitivity to the difference in reinforcement ratios under punishment compared to no-punishment conditions.
The data show two sets of effects after ethanol administration, one contributed by Participants 04, 05, and 06, and the other by Participants 02 and 08. These two patterns of data were observed in punished behavior under the concurrent schedules, in overall response rates, and in the pencil tapping test, and suggest individual differences in ethanol sensitivity. Data from Participants 04, 05 and 06 will be considered first.
Ethanol's effects were consistently expressed in the analysis of bias in these 3 participants. Under the no-punishment condition, bias was virtually unaffected by ethanol but under the punishment condition it was diminished in a consistent and dose-related fashion. This shift in responses reflected a greater allocation of behavior to the punished alternative after a behaviorally active dose was consumed by these participants. The change was substantial. For example, a change in bias from −0.46 to −0.17, as seen for Participant 6, means that under placebo conditions, the ratio of punished to unpunished responding was 1∶2.9 (the antilog of −0.46); while under ethanol it was 1∶1.4. This represents a nearly 2-fold shift in behavior toward the punished alternative under ethanol.
Ethanol produced a modest reduction in sensitivity to reinforcer ratios, as measured by the slope of the matching functions, for Participants 04, 05, and 06 in the no-punishment condition. A similar effect was also reported by Glautier, Bankart, Rigney, and Willner (1998), who found small changes in reinforcer sensitivity under 0.6 g/kg of ethanol in humans earning money under a single schedule of reinforcement. In contrast, ethanol produced large decreases in this sensitivity in the punishment condition for these same participants. These data suggest that ethanol's effects on sensitivity to reinforcer ratios are specific to punished behavior or are amplified by the presence of a punisher.
Ethanol's effects on bias were reflected in other measures, but there was intersubject variability in which measures were most affected. For example, ethanol frequently, but not always, increased response rates on the punished alternative, as well as the number of punishers, relative to placebo for Participants 04, 05 and 06. Moreover, ethanol generally, though not in every instance, reduced response rate under the no-punishment conditions for these same participants.
In most studies of antipunishment effects of ethanol, or other drugs, rate changes in punished responding are examined with single schedules of reinforcement or multiple schedules, in which each schedule component is presented in isolation (e.g., Koob et al., 1986; Liljequist & Engel, 1984; Vogel, 1980). The question of why ethanol's effects were reflected less consistently by specific rate measures here than in other studies of anxiolytic effects on punished responding remains open, but we do offer two observations. The first is the difference between human and nonhuman species in language ability, behavioral complexity, and control over reinforcement history, any of which could be important.
The second observation is that in most studies of nonhuman species, the choice presented is between lever-pressing that is supported by an effective reinforcer and the few response alternatives that are available in barren operant chambers. In such studies, the only behavioral option to lever-pressing is a poorly-defined set of response alternatives such as exploring or sitting, i.e., doing “something else.” The present study employed a concurrent arrangement, which presented two alternatives whose values, as determined by what reinforcer was used and the rate at which it was presented, could be manipulated and quantified explicitly. In addition, the values of the reinforcers and punishers were measured in the same units (cents lost or gained per min) and therefore directly comparable to each other (Rasmussen & Newland, 2008). The benefit of using concurrent schedules can be seen by comparing the consistent effects on bias with the effects on measures of response rates or of punishers delivered for the participants. For example, Participant 6 was clearly sensitive to ethanol, as noted in response rates and in pencil tapping, and ethanol produced a clear shift toward the punished alternative as noted above. This effect, however, was less consistently expressed in the rate on the punished alternative, but more on the overall allocation of behavior. Inspection of data in the appendix shows that for this subject ethanol also decreased rate on the unpunished alternative, which would be reflected in bias.
Bias captures a different and more global aspect of behavior than the other variables do since it is a measure of relative preference or overall allocation of behavior toward, in this case, the punished alternative. This measure is indifferent to whether that preference is expressed specifically in punishers delivered, overall rate on the punished alternative, or overall rate on the unpunished alternative. Bias may function here similarly to the way that responding in the initial link does in a concurrent chain. There, a choice is presented between two outcomes, and the outcomes can entail a different and even more complex reinforcement contingency. Preference for an outcome can at times be independent of response rate, a marker of response strength, that occurs in the terminal link after the outcome has been presented and that may be driven by more local contingencies (reviewed in Fantino & Romanowich, 2007; see also Fantino, 1965). The consistent ethanol-induced shift in bias by these participants reveals a stronger likelihood of selecting a punished outcome compared to vehicle sessions, even with inconsistencies in the particulars of whether this was related to the number of punishers delivered or response rates in the punished or unpunished alternatives.
The other 2 participants (02 and 08) showed some similarities to one another in behavior. First, they came under control of new contingencies faster (i.e., they experienced fewer session blocks) than the other 3 participants (see Table 2). Second, in the no-punishment condition, the bias and sensitivity values under the vehicle conditions appeared greater for these 2 participants than for the other 3 participants. For example, under placebo conditions, their slopes were slightly greater than one under no-punishment conditions. It is important to note, however, that in the placebo punishment conditions, their bias and sensitivity values were within the range of the other 3 participants. The absence of antipunishment effects for these 2 participants could be related to their behavior patterns during placebo conditions, but that explanation is not fully convincing as differences in matching were observed in the no-punishment condition.
Ethanol had little to no effect on bias or punished responding for Participants 02 and 08, but for these participants its effect on overall rate measures was also different. Total responding (the sum of all responses) was minimally affected by alcohol or produced only occasional rate increases (Participant 08). Pencil tapping was assessed separately from the concurrent schedule procedure, using a different apparatus (pencil and paper) and a separate, rate-based reinforcement contingency. Rapid, repetitive tapping is a simple psychomotor task that is sensitive to motor impairment associated with acute ethanol (Lindenschmidt et al., 1983) and diazepam administration (Palva, 1985) as well as neurotoxicant exposure, aging, or neurological impairment (Cousins et al., 1998; Mitchell et al., 2008; Nagasaki et al., 1988; Parks et al., 2003; Winneke et al., 1983; Ziv et al., 1999). The doses of ethanol used did not affect pencil tapping for Participant 02, and only the highest dose affected it for Participant 08. Thus these participants were less sensitive than the others to a variety of effects of ethanol.
The present study's results extend to humans the antipunishment effects of an anxiolytic drug that has previously been reported with laboratory animals. Ethanol shifted responses toward the punished alternative when given in a dose that was behaviorally active according to rates of overall responding or on a simple pencil-tapping task. Carlton et al. (1981), who also used a concurrent schedule arrangement, showed that 10 mg/kg of diazepam dose-dependently increased punished behavior in human participants in a concurrent schedule with conjoint schedule of punishment. While the data suggest that antipunishment effects are related to a general sensitivity to the doses of ethanol used, behavioral differences in baseline rates may also be important.
Ethanol has long been noted to have “disinhibitory” effects that may lead to socially inappropriate behavior in humans (see Julien, 2001). This effect has been captured and studied in some detail in laboratory settings designed to study inappropriate behavior, such as aggression (e.g., Cherek et al., 1985; Cherek et al., 1984; Dougherty et al., 1996). The results of those studies are interpretable by viewing inappropriate behavior as reflecting an increase in behavior suppressed by social punishers. Such an interpretation has been limited by the paucity of data showing that ethanol actually has antipunishment effects in humans. The present data give some credence to such interpretations by demonstrating the plausibility of antipunishment effects.
In summary, the generalized matching equation was able to quantify ethanol's antipunishment effects in humans. The antipunishment effects observed in the present study also extend the extant ethanol-related animal literature (e.g., Barrett et al., 1985; Glowa & Barrett, 1976; Koob et al., 1986; Vogel, 1980) to humans. This study also represents a first attempt to explicitly show that antipunishment may be a behavioral mechanism for alcohol-related behavioral disinhibition in humans.
Acknowledgments
Funding for these studies was provided by the Graduate School and Department of Psychology at Auburn University and Sigma Xi. Portions of this manuscript were presented at the Association for Behavior Analysis and the Southeastern Association for Behavior Analysis.
APPENDIX
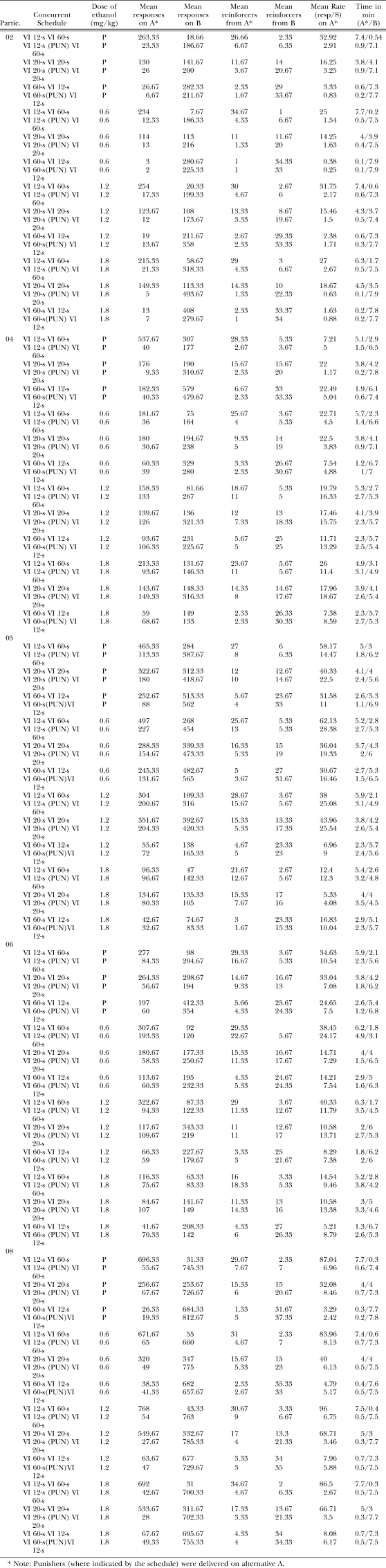
| Partic. | Concurrent Schedule | Dose of ethanol (mg/kg) | Mean responses on A* | Mean responses on B | Mean reinforcers from A* | Mean reinforcers from B | Mean Rate (resp/8) on A* | Time in min (A*/B) |
| 02 | VI 12-s VI 60-s | P | 263.33 | 18.66 | 26.66 | 2.33 | 32.92 | 7.4/0.54 |
| VI 12-s (PUN) VI 60-s | P | 23.33 | 186.67 | 6.67 | 6.33 | 2.91 | 0.9/7.1 | |
| VI 20-s VI 20-s | P | 130 | 141.67 | 11.67 | 14 | 16.25 | 3.8/4.1 | |
| VI 20-s (PUN) VI 20-s | P | 26 | 200 | 3.67 | 20.67 | 3.25 | 0.9/7.1 | |
| VI 60-s VI 12-s | P | 26.67 | 282.33 | 2.33 | 29 | 3.33 | 0.6/7.3 | |
| VI 60-s(PUN) VI 12-s | P | 6.67 | 211.67 | 1.67 | 33.67 | 0.83 | 0.2/7.7 | |
| VI 12-s VI 60-s | 0.6 | 234 | 7.67 | 34.67 | 1 | 25 | 7.7/0.2 | |
| VI 12-s (PUN) VI 60-s | 0.6 | 12.33 | 186.33 | 4.33 | 6.67 | 1.54 | 0.5/7.5 | |
| VI 20-s VI 20-s | 0.6 | 114 | 113 | 11 | 11.67 | 14.25 | 4/3.9 | |
| VI 20-s (PUN) VI 20-s | 0.6 | 13 | 216 | 1.33 | 20 | 1.63 | 0.4/7.5 | |
| VI 60-s VI 12-s | 0.6 | 3 | 280.67 | 1 | 34.33 | 0.38 | 0.1/7.9 | |
| VI 60-s(PUN) VI 12-s | 0.6 | 2 | 225.33 | 1 | 33 | 0.25 | 0.1/7.9 | |
| VI 12-s VI 60-s | 1.2 | 254 | 20.33 | 30 | 2.67 | 31.75 | 7.4/0.6 | |
| VI 12-s (PUN) VI 60-s | 1.2 | 17.33 | 199.33 | 4.67 | 6 | 2.17 | 0.6/7.3 | |
| VI 20-s VI 20-s | 1.2 | 123.67 | 108 | 13.33 | 8.67 | 15.46 | 4.3/3.7 | |
| VI 20-s (PUN) VI 20-s | 1.2 | 12 | 173.67 | 3.33 | 19.67 | 1.5 | 0.5/7.4 | |
| VI 60-s VI 12-s | 1.2 | 19 | 211.67 | 2.67 | 29.33 | 2.38 | 0.6/7.3 | |
| VI 60-s(PUN) VI 12-s | 1.2 | 13.67 | 358 | 2.33 | 33.33 | 1.71 | 0.3/7.7 | |
| VI 12-s VI 60-s | 1.8 | 215.33 | 58.67 | 29 | 3 | 27 | 6.3/1.7 | |
| VI 12-s (PUN) VI 60-s | 1.8 | 21.33 | 318.33 | 4.33 | 6.67 | 2.67 | 0.5/7.5 | |
| VI 20-s VI 20-s | 1.8 | 149.33 | 113.33 | 14.33 | 10 | 18.67 | 4.5/3.5 | |
| VI 20-s (PUN) VI 20-s | 1.8 | 5 | 493.67 | 1.33 | 22.33 | 0.63 | 0.1/7.9 | |
| VI 60-s VI 12-s | 1.8 | 13 | 408 | 2.33 | 33.37 | 1.63 | 0.2/7.8 | |
| VI 60-s(PUN) VI 12-s | 1.8 | 7 | 279.67 | 1 | 34 | 0.88 | 0.2/7.7 | |
| 04 | VI 12-s VI 60-s | P | 537.67 | 307 | 28.33 | 5.33 | 7.21 | 5.1/2.9 |
| VI 12-s (PUN) VI 60-s | P | 40 | 177 | 2.67 | 3.67 | 5 | 1.5/6.5 | |
| VI 20-s VI 20-s | P | 176 | 190 | 15.67 | 15.67 | 22 | 3.8/4.2 | |
| VI 20-s (PUN) VI 20-s | P | 9.33 | 310.67 | 2.33 | 20 | 1.17 | 0.2/7.8 | |
| VI 60-s VI 12-s | P | 182.33 | 579 | 6.67 | 33 | 22.49 | 1.9/6.1 | |
| VI 60-s(PUN) VI 12-s | P | 40.33 | 479.67 | 2.33 | 33.33 | 5.04 | 0.6/7.4 | |
| VI 12-s VI 60-s | 0.6 | 181.67 | 75 | 25.67 | 3.67 | 22.71 | 5.7/2.3 | |
| VI 12-s (PUN) VI 60-s | 0.6 | 36 | 164 | 4 | 5.33 | 4.5 | 1.4/6.6 | |
| VI 20-s VI 20-s | 0.6 | 180 | 194.67 | 9.33 | 14 | 22.5 | 3.8/4.1 | |
| VI 20-s (PUN) VI 20-s | 0.6 | 30.67 | 238 | 5 | 19 | 3.83 | 0.9/7.1 | |
| VI 60-s VI 12-s | 0.6 | 60.33 | 329 | 3.33 | 26.67 | 7.54 | 1.2/6.7 | |
| VI 60-s(PUN) VI 12-s | 0.6 | 39 | 280 | 2.33 | 30.67 | 4.88 | 1/7 | |
| VI 12-s VI 60-s | 1.2 | 158.33 | 81.66 | 18.67 | 5.33 | 19.79 | 5.3/2.7 | |
| VI 12-s (PUN) VI 60-s | 1.2 | 133 | 267 | 11 | 5 | 16.33 | 2.7/5.3 | |
| VI 20-s VI 20-s | 1.2 | 139.67 | 136 | 12 | 13 | 17.46 | 4.1/3.9 | |
| VI 20-s (PUN) VI 20-s | 1.2 | 126 | 321.33 | 7.33 | 18.33 | 15.75 | 2.3/5.7 | |
| VI 60-s VI 12-s | 1.2 | 93.67 | 231 | 5.67 | 25 | 11.71 | 2.3/5.7 | |
| VI 60-s(PUN) VI 12-s | 1.2 | 106.33 | 225.67 | 5 | 25 | 13.29 | 2.5/5.4 | |
| VI 12-s VI 60-s | 1.8 | 213.33 | 131.67 | 23.67 | 5.67 | 26 | 4.9/3.1 | |
| VI 12-s (PUN) VI 60-s | 1.8 | 93.67 | 146.33 | 11 | 5.67 | 11.4 | 3.1/4.9 | |
| VI 20-s VI 20-s | 1.8 | 143.67 | 148.33 | 14.33 | 14.67 | 17.96 | 3.9/4.1 | |
| VI 20-s (PUN) VI 20-s | 1.8 | 149.33 | 316.33 | 8 | 17.67 | 18.67 | 2.6/5.4 | |
| VI 60-s VI 12-s | 1.8 | 59 | 149 | 2.33 | 26.33 | 7.38 | 2.3/5.7 | |
| VI 60-s(PUN) VI 12-s | 1.8 | 68.67 | 133 | 2.33 | 30.33 | 8.59 | 2.7/5.3 | |
| 05 | ||||||||
| VI 12-s VI 60-s | P | 465.33 | 284 | 27 | 6 | 58.17 | 5/3 | |
| VI 12-s (PUN) VI 60-s | P | 113.33 | 387.67 | 8 | 6.33 | 14.47 | 1.8/6.2 | |
| VI 20-s VI 20-s | P | 322.67 | 312.33 | 12 | 12.67 | 40.33 | 4.1/4 | |
| VI 20-s (PUN) VI 20-s | P | 180 | 418.67 | 10 | 14.67 | 22.5 | 2.4/5.6 | |
| VI 60-s VI 12-s | P | 252.67 | 513.33 | 5.67 | 23.67 | 31.58 | 2.6/5.3 | |
| VI 60-s(PUN)VI 12-s | P | 88 | 562 | 4 | 33 | 11 | 1.1/6.9 | |
| VI 12-s VI 60-s | 0.6 | 497 | 268 | 25.67 | 5.33 | 62.13 | 5.2/2.8 | |
| VI 12-s (PUN) VI 60-s | 0.6 | 227 | 454 | 13 | 5.33 | 28.38 | 2.7/5.3 | |
| VI 20-s VI 20-s | 0.6 | 288.33 | 339.33 | 16.33 | 15 | 36.04 | 3.7/4.3 | |
| VI 20-s (PUN) VI 20-s | 0.6 | 154.67 | 473.33 | 5.33 | 19 | 19.33 | 2/6 | |
| VI 60-s VI 12-s | 0.6 | 245.33 | 482.67 | 5 | 27 | 30.67 | 2.7/5.3 | |
| VI 60-s(PUN)VI 12-s | 0.6 | 131.67 | 565 | 3.67 | 31.67 | 16.46 | 1.5/6.5 | |
| VI 12-s VI 60-s | 1.2 | 304 | 109.33 | 28.67 | 3.67 | 38 | 5.9/2.1 | |
| VI 12-s (PUN) VI 60-s | 1.2 | 200.67 | 316 | 15.67 | 5.67 | 25.08 | 3.1/4.9 | |
| VI 20-s VI 20-s | 1.2 | 351.67 | 392.67 | 15.33 | 13.33 | 43.96 | 3.8/4.2 | |
| VI 20-s (PUN) VI 20-s | 1.2 | 204.33 | 420.33 | 5.33 | 17.33 | 25.54 | 2.6/5.4 | |
| VI 60-s VI 12-s | 1.2 | 55.67 | 138 | 4.67 | 23.33 | 6.96 | 2.3/5.7 | |
| VI 60-s(PUN)VI 12-s | 1.2 | 72 | 165.33 | 5 | 23 | 9 | 2.4/5.6 | |
| VI 12-s VI 60-s | 1.8 | 96.33 | 47 | 21.67 | 2.67 | 12.4 | 5.4/2.6 | |
| VI 12-s (PUN) VI 60-s | 1.8 | 96.67 | 142.33 | 12.67 | 5.67 | 12.3 | 3.2/4.8 | |
| VI 20-s VI 20-s | 1.8 | 134.67 | 135.33 | 15.33 | 17 | 5.33 | 4/4 | |
| VI 20-s (PUN) VI 20-s | 1.8 | 80.33 | 105 | 7.67 | 16 | 4.08 | 3.5/4.5 | |
| VI 60-s VI 12-s | 1.8 | 42.67 | 74.67 | 3 | 23.33 | 16.83 | 2.9/5.1 | |
| VI 60-s(PUN)VI 12-s | 1.8 | 32.67 | 83.33 | 1.67 | 15.33 | 10.04 | 2.3/5.7 | |
| 06 | ||||||||
| VI 12-s VI 60-s | P | 277 | 98 | 29.33 | 3.67 | 34.63 | 5.9/2.1 | |
| VI 12-s (PUN) VI 60-s | P | 84.33 | 204.67 | 16.67 | 5.33 | 10.54 | 2.3/5.6 | |
| VI 20-s VI 20-s | P | 264.33 | 298.67 | 14.67 | 16.67 | 33.04 | 3.8/4.2 | |
| VI 20-s (PUN) VI 20-s | P | 56.67 | 194 | 9.33 | 13 | 7.08 | 1.8/6.2 | |
| VI 60-s VI 12-s | P | 197 | 412.33 | 5.66 | 25.67 | 24.65 | 2.6/5.4 | |
| VI 60-s (PUN) VI 12-s | P | 60 | 354 | 4.33 | 24.33 | 7.5 | 1.2/6.8 | |
| VI 12-s VI 60-s | 0.6 | 307.67 | 92 | 29.33 | 38.45 | 6.2/1.8 | ||
| VI 12-s (PUN) VI 60-s | 0.6 | 193.33 | 120 | 22.67 | 5.67 | 24.17 | 4.9/3.1 | |
| VI 20-s VI 20-s | 0.6 | 180.67 | 177.33 | 15.33 | 16.67 | 14.71 | 4/4 | |
| VI 20-s (PUN) VI 20-s | 0.6 | 58.33 | 250.67 | 11.33 | 17.67 | 7.29 | 1.5/6.5 | |
| VI 60-s VI 12-s | 0.6 | 113.67 | 195 | 4.33 | 24.67 | 14.21 | 2.9/5 | |
| VI 60-s (PUN) VI 12-s | 0.6 | 60.33 | 232.33 | 5.33 | 24.33 | 7.54 | 1.6/6.3 | |
| VI 12-s VI 60-s | 1.2 | 322.67 | 87.33 | 29 | 3.67 | 40.33 | 6.3/1.7 | |
| VI 12-s (PUN) VI 60-s | 1.2 | 94.33 | 122.33 | 11.33 | 12.67 | 11.79 | 3.5/4.5 | |
| VI 20-s VI 20-s | 1.2 | 117.67 | 343.33 | 11 | 12.67 | 10.58 | 2/6 | |
| VI 20-s (PUN) VI 20-s | 1.2 | 109.67 | 219 | 11 | 17 | 13.71 | 2.7/5.3 | |
| VI 60-s VI 12-s | 1.2 | 66.33 | 227.67 | 3.33 | 25 | 8.29 | 1.8/6.2 | |
| VI 60-s (PUN) VI 12-s | 1.2 | 59 | 179.67 | 3 | 21.67 | 7.38 | 2/6 | |
| VI 12-s VI 60-s | 1.8 | 116.33 | 63.33 | 16 | 3.33 | 14.54 | 5.2/2.8 | |
| VI 12-s (PUN) VI 60-s | 1.8 | 75.67 | 83.33 | 18.33 | 5.33 | 9.46 | 3.8/4.2 | |
| VI 20-s VI 20-s | 1.8 | 84.67 | 141.67 | 11.33 | 13 | 10.58 | 3/5 | |
| VI 20-s (PUN) VI 20-s | 1.8 | 107 | 149 | 14.33 | 16 | 13.38 | 3.3/4.6 | |
| VI 60-s VI 12-s | 1.8 | 41.67 | 208.33 | 4.33 | 27 | 5.21 | 1.3/6.7 | |
| VI 60-s (PUN) VI 12-s | 1.8 | 70.33 | 142 | 6 | 26.33 | 8.79 | 2.6/5.3 | |
| 08 | ||||||||
| VI 12-s VI 60-s | P | 696.33 | 31.33 | 29.67 | 2.33 | 87.04 | 7.7/0.3 | |
| VI 12-s (PUN) VI 60-s | P | 55.67 | 745.33 | 7.67 | 7 | 6.96 | 0.6/7.4 | |
| VI 20-s VI 20-s | P | 256.67 | 253.67 | 15.33 | 15 | 32.08 | 4/4 | |
| VI 20-s (PUN) VI 20-s | P | 67.67 | 726.67 | 6 | 20.67 | 8.46 | 0.7/7.3 | |
| VI 60-s VI 12-s | P | 26.33 | 684.33 | 1.33 | 31.67 | 3.29 | 0.3/7.7 | |
| VI 60-s(PUN)VI 12-s | P | 19.33 | 812.67 | 3 | 37.33 | 2.42 | 0.2/7.8 | |
| VI 12-s VI 60-s | 0.6 | 671.67 | 55 | 31 | 2.33 | 83.96 | 7.4/0.6 | |
| VI 12-s (PUN) VI 60-s | 0.6 | 65 | 660 | 4.67 | 7 | 8.13 | 0.7/7.3 | |
| VI 20-s VI 20-s | 0.6 | 320 | 347 | 15.67 | 15 | 40 | 4/4 | |
| VI 20-s (PUN) VI 20-s | 0.6 | 49 | 775 | 5.33 | 23 | 6.13 | 0.5/7.5 | |
| VI 60-s VI 12-s | 0.6 | 38.33 | 682 | 2.33 | 35.33 | 4.79 | 0.4/7.6 | |
| VI 60-s(PUN)VI 12-s | 0.6 | 41.33 | 657.67 | 2.67 | 33 | 5.17 | 0.5/7.5 | |
| VI 12-s VI 60-s | 1.2 | 768 | 43.33 | 30.67 | 3.33 | 96 | 7.5/0.4 | |
| VI 12-s (PUN) VI 60-s | 1.2 | 54 | 763 | 9 | 6.67 | 6.75 | 0.5/7.5 | |
| VI 20-s VI 20-s | 1.2 | 549.67 | 332.67 | 17 | 13.3 | 68.71 | 5/3 | |
| VI 20-s (PUN) VI 20-s | 1.2 | 27.67 | 785.33 | 4 | 21.33 | 3.46 | 0.3/7.7 | |
| VI 60-s VI 12-s | 1.2 | 63.67 | 677 | 3.33 | 34 | 7.96 | 0.7/7.3 | |
| VI 60-s(PUN)VI 12-s | 1.2 | 47 | 729.67 | 3 | 35 | 5.88 | 0.5/7.5 | |
| VI 12-s VI 60-s | 1.8 | 692 | 31 | 34.67 | 2 | 86.5 | 7.7/0.3 | |
| VI 12-s (PUN) VI 60-s | 1.8 | 42.67 | 700.33 | 4.67 | 6.33 | 2.67 | 0.5/7.5 | |
| VI 20-s VI 20-s | 1.8 | 533.67 | 311.67 | 17.33 | 13.67 | 66.71 | 5/3 | |
| VI 20-s (PUN) VI 20-s | 1.8 | 28 | 702.33 | 3.33 | 21.33 | 3.5 | 0.3/7.7 | |
| VI 60-s VI 12-s | 1.8 | 67.67 | 695.67 | 4.33 | 34 | 8.08 | 0.7/7.3 | |
| VI 60-s(PUN)VI 12-s | 1.8 | 49.33 | 755.33 | 4 | 34.33 | 6.17 | 0.5/7.5 | |
Note: Punishers (where indicated by the schedule) were delivered on alternative A.
REFERENCES
- Baum W. On two types of deviation from the matching law: bias and undermatching. Journal of the Experimental Analysis of Behavior. 1974;21:231–242. doi: 10.1901/jeab.1974.22-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J, Brady L, Witkin J. Behavioral studies with anxiolytic drugs. I. Interactions of the benzodiazepine antagonist Ro 15-1788 with chlordiazepoxide, pentobarbital and ethanol. Journal of Pharmacology & Experimental Therapeutics. 1985;233:554–559. [PubMed] [Google Scholar]
- Bradshaw C, Szabadi E, Bevan P. The effect of punishment on free-operant choice behavior in humans. Journal of the Experimental Analysis of Behavior. 1979;31:71–81. doi: 10.1901/jeab.1979.31-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton P, Siegel J, Murphee H, Cook L. Effects of diazepam on operant behavior in man. Psychopharmacology. 1981;73:314–317. doi: 10.1007/BF00426457. [DOI] [PubMed] [Google Scholar]
- Cherek D, Steinberg J, Manno B. Effects of ethanol on human aggressive behavior. Journal of Studies on Ethanol. 1985;46:321–327. doi: 10.15288/jsa.1985.46.321. [DOI] [PubMed] [Google Scholar]
- Cherek D, Steinberg J, Vines R. Low doses of ethanol affect human aggressive responding. Biological Psychiatry. 1984;19:263–267. [PubMed] [Google Scholar]
- Collins R.L, Parks G.A, Marlatt G.A. Social determinants of alcohol consumption: The effects of social interaction and model status on the self-administration of alcohol. Journal of Consulting and Clinical Psychology. 1985;53:189–200. doi: 10.1037//0022-006x.53.2.189. [DOI] [PubMed] [Google Scholar]
- Commissaris R. Conflict behaviors as animal models for the study of anxiety. In: van Haaren F, editor. Research methods in behavioral pharmacology. Amsterdam: Elsevier Science Publishers; 1993. pp. 443–466. In. [Google Scholar]
- Cook L, Davidson A.B. Effects of behaviorally active drugs in a conflict-punishment procedure in rats. In: Garattini S, Mussini E, Randall L.O, editors. The benzodiazepines. Raven Press; New York: 1973. pp. 327–345. [Google Scholar]
- Cousins M.S, Corrow C, Finn M, Salamone J.D. Temporal measures of human finger tapping: effects of age. Pharmacology, Biochemistry, and Behavior. 1998;59:445–449. doi: 10.1016/s0091-3057(97)00443-7. [DOI] [PubMed] [Google Scholar]
- Critchfield T.S, Paletz E.M, MacAleese K.R. Punishment in human choice: Direct or competitive suppression. Journal of the Experimental Analysis of Behavior. 2003;80:1–27. doi: 10.1901/jeab.2003.80-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison M, McCarthy D. The matching law: A research review. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Deluty M.Z, Church R.M. Time allocation matching between punishing situations. Journal of the Experimental Analysis of Behavior. 1978;29:191–198. doi: 10.1901/jeab.1978.29-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deVilliers P. Toward a quantitative theory of punishment. Journal of the Experimental Analysis of Behavior. 1980;33:15–25. doi: 10.1901/jeab.1980.33-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty D, Cherek D, Bennett R. The effects of ethanol on the aggressive responding of women. Journal of Studies on Ethanol. 1996;23:178–186. doi: 10.15288/jsa.1996.57.178. [DOI] [PubMed] [Google Scholar]
- Fantino E. Some data on the discriminative stimulus hypothesis of conditioned reinforcement. Psychological Record. 1965;15:409–415. [Google Scholar]
- Fantino E, Romanowich P. The effect of conditioned reinforcement rate on choice: A review. Journal of the Experimental Analysis of Behavior. 2007;87:409–421. doi: 10.1901/jeab.2007.44-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley J, Fantino E. The symmetrical law of effect and the matching relation in choice behavior. Journal of the Experimental Analysis of Behavior. 1978;29:37–60. doi: 10.1901/jeab.1978.29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshler M, Hoffman H.S. A progression for generating variable interval schedules. Journal of the Experimental Analysis of Behavior. 1962;5:529–530. doi: 10.1901/jeab.1962.5-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glautier S, Bankart J, Rigney U, Willner P. Multiple variable interval schedule behaviour in humans: Effects of ethanol, mood, and reinforcer size on responding maintained by monetary reinforcement. Behavioural Pharmacology. 1998;9:619–630. doi: 10.1097/00008877-199811000-00017. [DOI] [PubMed] [Google Scholar]
- Glowa J.R, Barrett J.E. Effects of ethanol on punished and unpunished responding of squirrel monkeys. Pharmacology, Biochemistry, and Behavior. 1976;4:169–173. doi: 10.1016/0091-3057(76)90010-1. [DOI] [PubMed] [Google Scholar]
- Heiman G.W. Basic statistics for the behavioral sciences. Boston: Houghton Mifflin Co; 1992. [Google Scholar]
- Herrnstein R. Relative and absolute strength of response as a function of reinforcement. Journal of the Experimental Analysis of Behavior. 1961;4:267–272. doi: 10.1901/jeab.1961.4-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, Andersson L. Variability of the blood/breath ethanol ratio in drinking drivers. Journal of Forensic Sciences. 1996;41:916–921. [PubMed] [Google Scholar]
- Julien R. A primer of drug action. Holt; New York: 2001. [Google Scholar]
- Kallmen H, Gustafson R. Ethanol and disinhibition. European Addiction Research. 1998;4:150–162. doi: 10.1159/000018948. [DOI] [PubMed] [Google Scholar]
- Katz R. Effects of punishment in an alternative response context as a function of relative reinforcement rate. Psychological Record. 1973;23:65–74. [Google Scholar]
- Klevin M.S, Koek W. Effects of benzodiazipine agonists on punished responding in pigeons and their relationship with clinical doses in humans. Psychopharmacology. 1999;141:206–212. doi: 10.1007/s002130050826. [DOI] [PubMed] [Google Scholar]
- Kollins S, Newland M, Critchfield T. Human sensitivity to reinforcement in operant choice: How much do consequences matter. Psychonomic Bulletin and Review. 1997;4:208–220. doi: 10.3758/BF03209395. [DOI] [PubMed] [Google Scholar]
- Koob G, Braestrup C, Britton K. The effects of FG 7142 and Ro 15-1788 on the release of punished responding produced by chlordiazepoxide and ethanol in the rat. Psychopharmacologia. 1986;90:173–178. doi: 10.1007/BF00181236. [DOI] [PubMed] [Google Scholar]
- Liljequist S, Engel J. The effects of GABA and benzodiazepine receptor antagonists on the anti-conflict actions of diazepam or ethanol. Pharmacology, Biochemistry, and Behavior. 1984;21:521–5. doi: 10.1016/s0091-3057(84)80033-7. [DOI] [PubMed] [Google Scholar]
- Lindenschmidt R, Brown D, Cerimele B, Walle T, Forney R.B. Combined effects of propranolol and ethanol on human psychomotor performance. Toxicology and Applied Pharmacology. 1983;67:117–21. doi: 10.1016/0041-008x(83)90250-8. [DOI] [PubMed] [Google Scholar]
- McCloskey T.C, Paul B.K, Commissaris R.L. Buspirone effects in an animal conflict procedure: Comparison with diazepam and phenobarbital. Pharmacology, Biochemistry & Behavior. 1987;27:171–5. doi: 10.1016/0091-3057(87)90492-8. [DOI] [PubMed] [Google Scholar]
- McMillan D.E, Leander J.D. Drugs and punished responding. V. Effects of drugs on responding suppressed by response-dependent and response-independent electric shock. Archives Internationales de Pharmacodynamie et de Therapie. 1975;213:22–7. [PubMed] [Google Scholar]
- Mitchell A.W, Goodman A.O, Silva A.H, Lazic S.E, Morton A.J, Barker R.A. Hand tappng: A simple, reproducible, and objective marker of motor dysfunction in Huntington's disease. Journal of Neurology. 2008;255:1145–1152. doi: 10.1007/s00415-008-0859-x. [DOI] [PubMed] [Google Scholar]
- Nagasaki H, Itoh H, Maruyama H, Hashizume K. Characteristic difficulty in rhythmic movement with aging and its relation to Parkinson's disease. Experimental Aging Research. 1988;14:171–176. doi: 10.1080/03610738808259744. [DOI] [PubMed] [Google Scholar]
- Palva E.S. Gender-related differences in diazepam effects on performance. Medical Biology. 1985;63:92–5. [PubMed] [Google Scholar]
- Parks M.H, Morgan V.L, Pickens D.R, Price R.R, Dietrich M.S, Nickel M.K, et al. Brain fMRI activation associated with self-paced finger tapping in chronic alcohol-dependent patients. Alcoholism: Clinical and Experimental Research. 2003;27:704–711. doi: 10.1097/01.ALC.0000062759.14944.CF. [DOI] [PubMed] [Google Scholar]
- Pierce W, Epling W. Choice, matching and human behavior: A review of the literature. The Behavior Analyst. 1983;6:57–76. doi: 10.1007/BF03391874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard G.T, Howard J.L. Effects of drugs on punished behavior. Preclinical test for anxiolytics. Pharmacology and Therapeutics. 1990;45:403–424. doi: 10.1016/0163-7258(90)90075-d. [DOI] [PubMed] [Google Scholar]
- Rasmussen E.B. Behavior-releasing effects of drugs: Antipunishment and anti-conflict procedures. Mexican Journal of Behavior Analysis. 2006;32:73–91. [Google Scholar]
- Rasmussen E.B, Newland M.C. Asymmetry of reinforcement and punishment in human choice. Journal of the Experimental Analysis of Behavior. 2008;89:157–168. doi: 10.1901/jeab.2008.89-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger D.J. Effects of buspirone and related compounds on suppressed operant responding in rats. Journal of Pharmacology & Experimental Therapeutics. 1990;254:420–426. [PubMed] [Google Scholar]
- Spealman R. Comparison of drug effects on responding punished by pressurized air or electric shock delivery in squirrel monkeys: pentobarbital, chlordiazepoxide, d-amphetamine and cocaine. Journal of Pharmacology & Experimental Therapeutics. 1979;209:309–315. [PubMed] [Google Scholar]
- Todorov J.C. Concurrent performance: Effect of punishment contingent on the switching response. Journal of the Experimental Analysis of Behavior. 1971;16:51–62. doi: 10.1901/jeab.1971.16-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haaren F, Anderson K. Effects of chlordiazepoxide, buspirone and cocaine on behavior suppressed by timeout. Behavioural Pharmacology. 1997;8:174–182. [PubMed] [Google Scholar]
- Vogel R. Attenuation of the effects of punishment by ethanol: Comparisons with chlordiazepoxide. Psychopharmacology. 1980;71:123–129. doi: 10.1007/BF00434399. [DOI] [PubMed] [Google Scholar]
- Winneke G, Dramer U, Brockhaus A, Ewers U, Kujanek G, Lechner H, et al. Neuropsychological studies in children with elevated tooth-lead concentrations. II. Extended study. Internal Archives of Occupational and Environmental Health. 1983;51:231–252. doi: 10.1007/BF00377755. [DOI] [PubMed] [Google Scholar]
- Wojnicki F, Barrett J. Anticonflict effects of buspirone and chlordiazepoxide in pigeons under a concurrent schedule of punishment and a changeover response. Psychopharmacology. 1993;112:26–33. doi: 10.1007/BF02247360. [DOI] [PubMed] [Google Scholar]
- Ziv I, Avraham M, Dabby R, Zoldan J, Djaldetti R, Melamed E. Early-occurrence of manual motor blocks in Parkinson's disease: A quantitative assessment. Acta Neurologica Scandinavica. 1999;99:106–111. doi: 10.1111/j.1600-0404.1999.tb00666.x. [DOI] [PubMed] [Google Scholar]