Abstract
Adrenarche is thought to be experienced only by humans and some Old World primates despite observed regression of an adrenal fetal zone and establishment of a functional zona reticularis (ZR) in other species like rhesus macaques. Adrenal differentiation remains poorly defined biochemically in nonhuman primates. The present studies defined ZR development in the neonatal rhesus by examining androgen synthetic capacity and factors affecting it in rhesus and marmoset adrenals. Western immunoblots examined expression of 17α-hydroxylase/17,20-lyase cytochrome P450 (P450c17), cytochrome b5 (b5), and 3β-hydroxysteroid dehydrogenase (3βHSD), among other key enzymes. 17,20-lyase activity was quantified in adrenal microsomes, as was the contribution of b5 to 17,20-lyase activity in microsomes and cell transfection experiments with rhesus and marmoset P450c17. Expression of b5 increased from birth to 3 months, and was positively correlated with age and 17,20-lyase activity in the rhesus. Recombinant b5 addition stimulated 17,20-lyase activity to an extent inversely proportional to endogenous levels in adrenal microsomes. Although 3βHSD expression also increased with age, P450c17, 21-hydroxylase cytochrome P450, and the redox partner, reduced nicotinamide adenine dinucleotide phosphate-cytochrome P450 oxidoreductase, did not; nor did recombinant cytochrome P450 oxidoreductase augment 17,20-lyase activity. Cotransfection with b5 induced a dose-dependent increase in dehydroepiandrosterone synthesis by both nonhuman primate P450c17 enzymes. We conclude that the increase in 17,20-lyase activity characteristic of an adrenarche in rhesus macaques is driven primarily by increased b5 expression, without the need for a decrease in 3βHSD, as suggested from human studies. The rhesus macaque is a relevant and accessible model for human ZR development and adrenal function.
Neonatal rhesus adrenal development includes a “biochemical adrenarche” marked by an increase in androgen synthetic capacity driven by increased cytochrome b5 in the zona reticularis.
The prepubertal increase in secretion of C-19 steroids [the so-called adrenal androgens dehydroepiandrosterone (DHEA) and its sulfate (DS)] from the adrenal cortex marks adrenarche. This landmark event is associated with the differentiation of the zona reticularis (ZR) of the adrenal cortex, and is generally considered to be experienced only by humans and some great apes (1,2). Although Old World primates like rhesus macaques and baboons develop a functional ZR after birth (3), they appear to lack a distinct prepubertal increase in adrenal androgen output (4,5,6). Some New World primates appear not to develop a ZR at all, and circulating levels of adrenal androgens remain very low after birth (7). Understanding the basis of the differences in adrenal maturation and adrenal androgen synthesis among primates is likely to provide significant insight into adrenarche in humans.
Androgen and cortisol synthesis in the primate adrenal cortex share a requirement for expression of several steroidogenic enzymes, one of the most important being 17α-hydroxylase/17,20-lyase cytochrome P450 (P450c17). In primates, P450c17 catalyzes 17α-hydroxylation of pregnenolone and progesterone, and 17–20 cleavage (17,20-lyase activity) of 17OH-pregnenolone but not 17OH-progesterone (8,9,10,11). Both activities are required for androgen (DHEA) synthesis, whereas 17α-hydroxylase only is required for cortisol production, which is promoted by the inefficiency of 17–20 cleavage of 17OH-progesterone (12). Because the increase in circulating adrenal androgens associated with adrenarche is not accompanied by increased cortisol secretion, it is probably not driven by increased P450c17 expression. Rather, it is believed that there is an increase in adrenal 17,20-lyase activity (13,14), independent of changes in levels of the P450c17 or the 17α-hydroxylase activity needed for cortisol (as well as androgen) production. The mechanisms involved in balancing 17α-hydroxylase and 17,20-lyase activities are complex but include changes in the levels of three proteins relative to P450c17 itself, namely reduced nicotinamide adenine dinucleotide phosphate-cytochrome P450 oxidoreductase (CPR), cytochrome b5 (b5), and 3β-hydroxysteroid dehydrogenase (3βHSD). The roles of CPR and b5 are well supported by experimental studies reconstituting enzyme activity using purified or recombinant proteins (15,16,17,18,19,20). The importance of 3βHSD is suggested by the observed decline in its expression in the developing human ZR (21) more than it is experimental results (22). However, few studies have been conducted into enzyme activities using human or nonhuman primate adrenal tissues during the period of ZR development (13,22); none focuses on the differential regulation of 17,20-lyase activity.
Studies in our laboratory have shown that the rhesus ZR is morphologically and functionally similar to that of the human adrenal, being high in b5 expression and lacking 3βHSD (23). More recently, we demonstrated that differentiation of the ZR in the rhesus macaque begins late in gestation (24), and is complete by 3 months of age (25). Therefore, the goal of the present studies was to further define ZR development in the rhesus by assessing expression of P450c17, CPR, b5, 3βHSD, and 21-hydroxylase cytochrome P450 (P450c21). 17,20-lyase activities of early neonatal rhesus and adult marmoset adrenal microsomal protein were also evaluated, supplemented or not with purified recombinant b5. Finally, DHEA synthesis was examined in intact cells expressing recombinant rhesus and marmoset P450c17 enzymes along with b5, to examine further its likely contribution to developmental changes in 17,20-lyase activity, and any fundamental difference in this regard between Old and New World primate species.
Materials and Methods
Materials
Unless otherwise stated, chemical reagents were obtained from Fisher Scientific (Pittsburgh, PA) and Sigma-Aldrich Corp. (St. Louis, MO).
Animals
Rhesus macaque adrenals were obtained opportunistically from approved projects at the California National Primate Research Center. Animals (n = 21; Table 1) ranged in age from 1 d old to 14 months old. Additional adrenals from adult male marmosets (n = 3) were obtained from the National Primate Research Center at the University of Wisconsin, Madison. Harvested adrenals were frozen and stored at −80 C until use.
Table 1.
Ages and numbers of animals from which adrenal glands were harvested
| Group | Ages | No. |
|---|---|---|
| Perinatal rhesus | 1–14 d | 5 |
| Neonatal rhesus | 1–3 months | 10 |
| Juvenile rhesus | 4–14 months | 6 |
| Adult male marmoset | 5–13 yr | 3 |
Microsome preparation
Tissue from each individual adrenal was homogenized on ice in KPO4 buffer [(pH 7.4), 0.1 m KPO4, 20% glycerol, 5 mm β-mercaptoethanol, 0.5 mm phenylmethylsulfonylfluoride, and 0.1 μg/ml aprotinin]. Premicrosomal pellets (15,000 × g, 10 min, 4 C) were discarded, and supernatants were centrifuged (100,000 × g, 1 h, 4 C). Microsomal pellets were resuspended in KPO4 buffer, and protein concentration was determined by the BCA Protein Assay Kit (Pierce, Rockford, IL). Microsomal preparations were stored at −80 C.
Western immunoblotting and analysis
Microsomal proteins (20 μg) were separated by electrophoresis on an 8% (CPR, P450c17, and 3βHSD) or 16% (P450c21 and b5) polyacrylamide gel (Bio-Rad Laboratories, Inc., Hercules, CA), and separated proteins were then transferred onto polyvinylidene difluoride membranes (Bio-Rad Laboratories). The standard curve used to determine microsomal b5 concentration consisted of recombinant rat b5 protein (0.01–1 pmol; generated in our own laboratory). Recombinant human P450c17 (1 pmol; our own laboratory), recombinant rat CPR (1 pmol; generously provided by Dr. Ron Estabrook, Department of Biochemistry, University of Texas Southwestern Medical Center, Dallas, TX), and a pool of male marmoset adrenal microsomes (3βHSD, P450c21) were used as standard for studies examining relative expression levels of the respective steroidogenic enzyme proteins. The appropriate standards were included in each gel probed. Membranes were incubated for 1 h at room temperature with the following primary antisera: b5 (1:5,000; polyclonal rabbit antihuman, raised in our laboratory against purified recombinant protein provided by Drs. Ron Estabrook and Manju Shet); P450c17 (1:2,000; polyclonal rabbit antihuman raised in our laboratory against purified recombinant protein); P450c21 (1:5,000; polyclonal rabbit antihuman, generously provided by Dr. Walter Miller, Department of Pediatrics, University of California San Francisco, San Francisco, CA); 3βHSD (1:5,000; polyclonal rabbit antihuman, generously provided by Dr. J. Ian Mason, Centre for Reproductive Biology, University of Edinburgh Medical School, Edinburgh, UK); and CPR (1:10,000; polyclonal rabbit antirat raised in our laboratory against purified recombinant protein provided by Dr. Ron Estabrook). Donkey antirabbit horseradish peroxidase-linked secondary antibody [b5, P450c21, 3βHSD, and CPR (1:10,000); and P450c17 (1:5,000)] and the ECL Western Analysis Kit (Amersham Biosciences Inc., Piscataway, NJ) were used for protein detection. Protein expression was quantified by densitometry after autoradiographic films were scanned.
17,20-Lyase activity
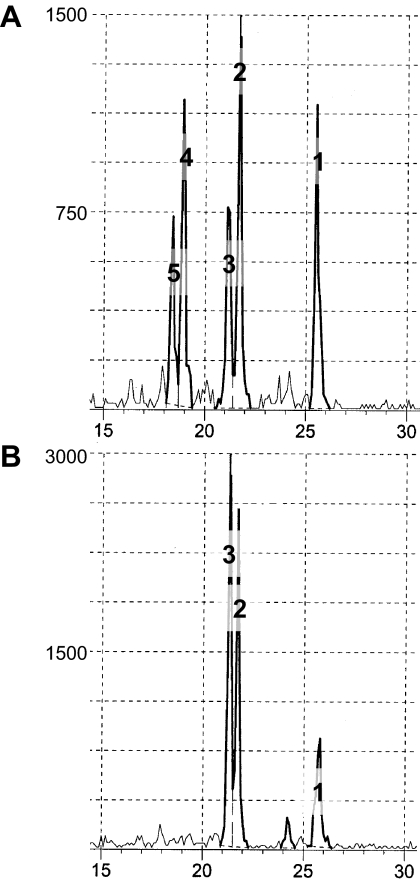
The 17,20-lyase activity of the microsomal fractions was measured radiometrically (26), as described by our laboratory (27), from the release of radiolabeled-acetate upon metabolism of [21-3H]17α-OH-pregnenolone (13.6 μCI/μmol, a generous gift from Drs. Vincent Njar and Angela Brodie, University of Maryland, Baltimore, MD). The reaction was linear over 60 min with microsomal protein concentrations between 10 and 60 μg. All subsequent studies used 20 μg microsomal protein and an incubation time of 60 min. Microsomal protein was incubated with assay buffer: 50 mm KPO4, 1 mm EDTA, 1 mm 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate], and 11.3 μm 21-3H-17α-OH-pregnenolone. To maintain a constant supply of reducing equivalents, a generating system consisting of 17 mm glucose-6-phosphate, 2 mm β-nicotinamide adenine dinucleotide phosphate, 1 mm dihydronicotinamide adenine dinucleotide phosphate, and 1 U glucose-6-phosphate dehydrogenase was also added. For studies examining the effect of recombinant protein addition, buffer, microsomal protein, and recombinant protein (10 pmole purified rat b5 or rat CPR) were mixed and incubated (60 min); after incubation, substrate and generating system were added. The final assay volume was incubated at 37 C (60 min), and the reaction was stopped with 30% trichloroacetic acid. Extraction of the organic phase was conducted with chloroform. Aqueous phase was mixed with 8.5% charcoal 0.85% dextran suspension, centrifuged for 30 min at 2000 × g, and quantified by liquid scintillation counting. The assay conditions for P450c17 and 3βHSD activities were assessed by the analysis of products of pregnenolone metabolism using HPLC. Synthesis of DHEA from pregnenolone [10 μm (Steraloids, Newport, RI); [7-3H]pregnenolone (PerkinElmer, Boston, MA] was confirmed in adult adrenal microsomes (100 μg, 2 h incubation) without (Fig. 1A) and in the presence of the 3βHSD inhibitor, trilostane (50 μm; Fig. 1B), as described previously (28). Trilostane inhibited synthesis of 11-deoxycortisol (Fig. 1B) and increased the accumulation of 17OH-pregnenolone by almost 20% but did not affect production of DHEA (P > 0.5; data not shown). Therefore, the determination of 17,20-lyase activity using [21-3H]17α-OH-pregnenolone was conducted without trilostane addition.
Figure 1.
HPLC elution profile (min, x-axis) of products (counts per minute, y-axis) from metabolism (2 h) of pregnenolone (10 μm) by adrenal microsomes (100 μg) from rhesus macaques, without (A) and with trilostane (50 μm) (B). A, Control incubation showing peaks of pregnenolone (1), DHEA (2), 17OH-pregnenolone (3), 11-deoxycortisol (4), and an unidentified steroid (5). B, Incubation with trilostane, showing pregnenolone (1) and accumulation of DHEA (2) and 17OH-pregnenolone (3), but no 11-deoxycortisol (4) or the unidentified peak (5). Note, similar amounts of pregnenolone were metabolized in A and B, but the scales differ.
P450c17 mammalian expression vectors
Marmoset and rhesus cDNA sequences encoding P450c17 (GenBank accession nos. AY746982 and AY746983, respectively), and a rat b5 cDNA (generously provided by Dr. Ron Estabrook, Department of Biochemistry, University of Texas Southwestern Medical Center, Dallas, TX), were cloned into the pBK-cytomegalovirus (CMV) mammalian expression vector (Stratagene, La Jolla, CA) after removal of the β-gal promoter. Rat b5 was inserted into pCMV-βgal by restriction digest. One clone containing the full-length P450c17 or b5 protein coding sequence in the correct orientation was grown and purified for transformation into mammalian cells using the QIAGEN EndoFree Plasmid Maxi Kit (QIAGEN, Inc., Valencia, CA). All mammalian expression vectors purified for transformation were verified for correct sequence using the BigDye reaction system (Applied Biosystems, Foster City, CA).
Transfecting human embryonic kidney (HEK)-293 cells
HEK-293 cells (American Type Culture Collection, Manassas, VA) were grown in MEMα (Invitrogen Corp., Carlsbad, CA) with penicillin/streptomycin (Invitrogen) and gentamycin with 10% fetal bovine serum. Cells were seeded into six- or 12-well plates for transfection using GeneJammer transfection reagent (Stratagene) essentially as described by the manufacturer. Cells were transfected with 1 μg constructs (wild-type rhesus or marmoset P450c17) or empty vector (containing the CMV promoter, but no insert), ± 0.1, 0.5, and 1 μg the rat b5 construct. P450c17 concentration was verified by Western immunoblot for protein correction (data not shown).
Steroid metabolism by transfected HEK-293 cells
Cells transfected with P450c17 ± b5 constructs were rinsed in 1 volume MEMα/penicillin/streptomycin/gentamycin. Cells were treated with 100,000 dpm [3H]17OH-pregnenolone (American Radiolabeled Chemicals, St. Louis, MO) in 20 μm 17OH-pregnenolone (Steraloids) in 1 volume MEMα with antibiotics. After 24 h, media were collected in 2 ml Cryovials and traced with 5 μm cold steroids (pregnenolone, 17OH-pregnenolone, DHEA; Steraloids) before being frozen, and each well was rinsed with 1 volume media before the plates were frozen. Media were stored at −70 C until assayed.
Detection of steroid products by thin-layer chromatography
Media from transfected cells were extracted twice with dichloromethane. The combined extracts were dried down at 37 C under air and reconstituted in 50 μl dichloromethane. The 50 μl sample was spotted to SilicaGel TLC plates (Merck & Co., Inc., Whitehouse Station, NJ). The plates were developed in 90:1 chloroform-ethyl acetate for 60 min, dried for 5 min, developed for an additional hour, and then dried for 5 min. Dry plates were sprayed with 18% sulfuric acid, baked for 5 min at 100 C; spots were isolated in approximately 1- × 1-cm squares. Squares were placed in empty borosilicate glass vials, 5 ml Insta-Gel Plus scintillation fluid (PerkinElmer) was added to each vial, and the vials were subsequently counted.
Statistics
Regression analysis was used to determine correlation coefficients among 17,20-lyase activity, stimulation of 17,20-lyase activity, protein levels, and age. Steroid levels were compared by one-way ANOVA followed by post hoc univariate F tests. All analyses were conducted using SAS 9.1 (SAS Institute Inc., Cary, NC).
Results
Changes in 17,20-lyase activity and P450c17 expression in neonatal rhesus adrenals
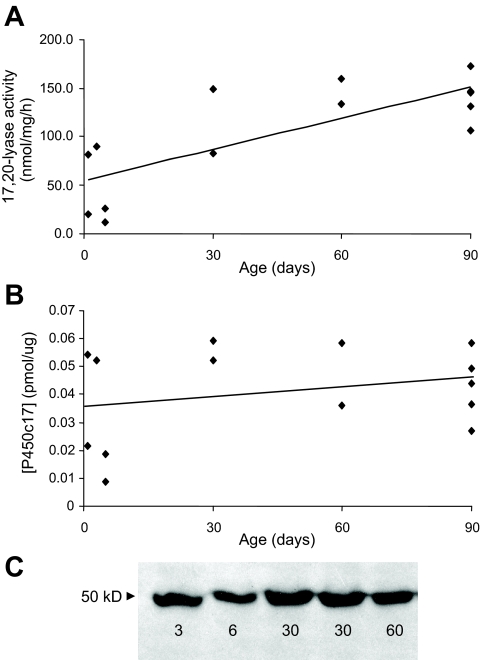
The 17,20-lyase activity of rhesus adrenal microsomes ranged from less than 20 to over 150 nmol/mg · h (observed in some samples from 2- and 3- month-old animals). Adrenal 17,20-lyase activity was positively correlated (R2 = 0.60; P < 0.01) with age during the first 3 months of life (Fig. 2A), and thereafter no significant relationships between 17,20-lyase activity and age were detected (data not shown). There was no corresponding change of P450c17 expression across the ages tested based on densitometric analysis (Fig 2B; P > 0.05) of Western immunoblots (Fig. 2C), suggesting that the early change in 17,20-lyase activity was not regulated at that level.
Figure 2.
Activity and expression of 17α-hydroxylase/17,20-lyase (P450c17) of rhesus adrenal microsomes from birth to 3 months of age. A, Adrenal microsomal (20 μg) 17,20-lyase activity (nmol/mg · h) measured by release of 3H-acetate from [21-3H]17α-OH-pregnenolone as described in Materials and Methods. Rhesus adrenal 17,20-lyase activity increased linearly during the first 3 months of life (R2 = 0.60; P < 0.01). B, Densitometric analysis of Western immunoblots for P450c17 (pmol/μg) expression in adrenal microsomes from perinatal and neonatal rhesus macaques. Concentrations were based on comparison with a recombinant human P450c17 standard (1 pmol included in each gel). Levels of P450c17 did not change with age (P > 0.05). C, Representative Western immunoblot of P450c17 expression in perinatal and neonatal rhesus adrenal microsomes (20 μg/lane) used for densitometry to detect age-related changes.
Changes in neonatal adrenal expression of b5 and other steroidogenic enzymes and accessory proteins
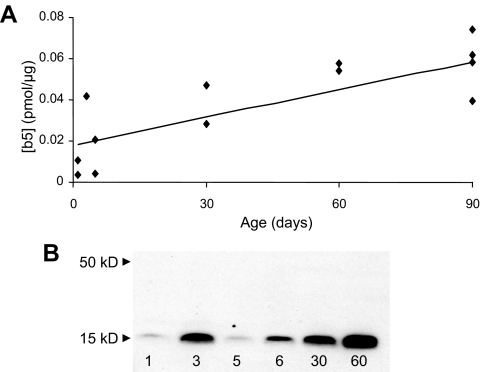
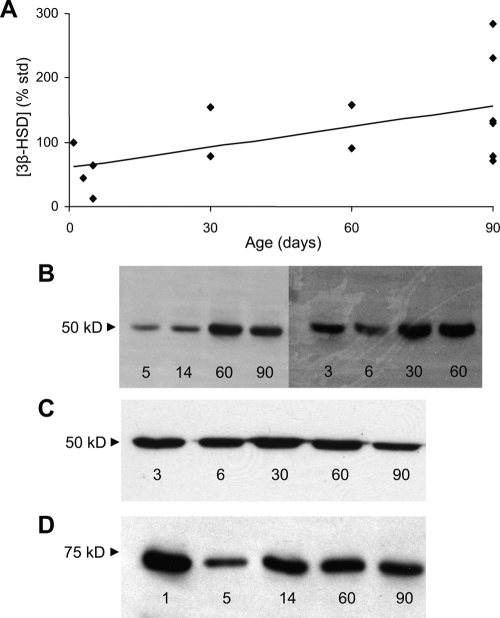
In contrast to P450c17, levels of b5 increased with age (R2 = 0.63; P < 0.01) during the first 3 months of life by more than 3- fold, based on densitometry estimates (Fig. 3A) of immunodetectable bands (Fig. 3B). 3βHSD levels also increased with age (Fig. 4A; R2 = 0.32; P < 0.05) during this developmental period by immunoblot analyses (Fig. 4B). However, there was no significant change in expression of P450c21 (Fig. 4C) or CPR (Fig. 4D).
Figure 3.
b5 expression in adrenal microsomes of neonatal rhesus macaques from birth to 3 months of age. A, Densitometric analysis of Western immunoblots of b5 (pmol/μg) expression in perinatal and neonatal rhesus adrenal microsomes. Band intensities were estimated by densitometry, and concentrations were based on recombinant rat b5 standard curves (0.01–1 pmol in each gel). Levels of b5 increased with age (R2 = 0.63; P < 0.01). B, Representative Western immunoblot of b5 expression in rhesus neonatal adrenal microsomes (20 μg/lane) used for densitometry to detect age-related changes.
Figure 4.
Expression of 3βHSD, P450c21, and reduced nicotinamide adenine dinucleotide phosphate-CPR in rhesus adrenal microsomes of neonatal rhesus macaques from birth to 3 months of age. A, Densitometric analysis of Western immunoblots of 3βHSD expression in perinatal and neonatal rhesus adrenal microsomes. Band intensities were expressed a percentage of a standard (20 μg from a pool of marmoset adrenal microsomes) included in each gel. Levels of 3βHSD increased with age (R2 = 0.32; P < 0.05). B, Representative Western immunoblot of 3βHSD used for densitometry to detect age-related changes in rhesus neonatal adrenal microsomes (20 μg/lane). C, Representative Western immunoblot of P450c21 used for densitometry. No age-related changes in P450c21 expression were found in rhesus neonatal adrenal microsomes (20 μg/lane). D, Representative Western immunoblot of CPR used for densitometry. No age-related changes in CPR expression were found in rhesus neonatal adrenal microsomes (20 μg/lane).
Investigation of the influence of steroidogenic enzyme and accessory protein levels on 17,20-lyase activity in primate adrenal microsomes: correlative analyses
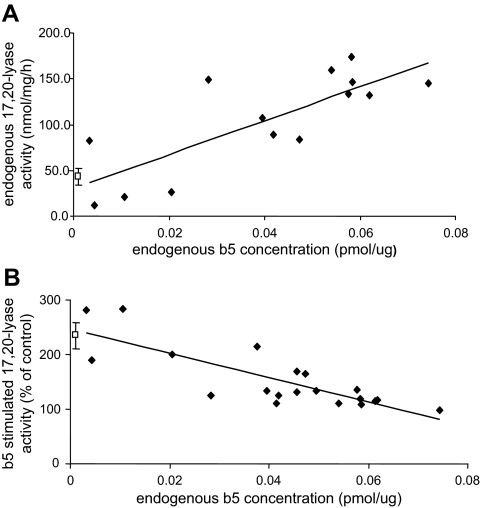
To further examine factors that influence 17,20-lyase activity during early life, expression of steroidogenic enzymes and accessory proteins were compared with 17,20-lyase activities for each sample. Previously determined levels of P450c17, P450c21, CPR, 3βHSD, and b5, based on densitometry along with respective standards, were compared with corresponding 17,20-lyase activities from rhesus adrenal microsomes (ages 1 d to 3 months). No significant relationships were found between 17,20-lyase activity and P450c17, P450c21, CPR, or 3βHSD levels (P > 0.05; data not shown). In contrast, 17,20-lyase activity was positively correlated with b5 concentration (Fig. 5A; R2 = 0.62; P < 0.01) in adrenal microsomes of animals from birth to 3 months old. Adult male marmoset adrenal samples were also used as negative controls because they do not develop a morphologically definable ZR, even in adulthood (28). As expected, b5 levels were not detectable by Western immunoblot (data not shown), and the 17,20-lyase activities of the male marmoset adrenal microsomes (42.61 ± 9.1 nmol/mg · h) were among the lowest detected in these studies (Fig. 5A).
Figure 5.
b5 levels and 17,20-lyase activity in neonatal rhesus adrenal microsomes. A, Correlation between rhesus adrenal microsomal 17,20-lyase activity (nmol/mg · h) and endogenous b5 concentration (pmol/μg). Samples represent adrenals from rhesus neonates 1 d old to 3 months of age. 17,20-lyase activity was positively correlated (R2 = 0.62; P < 0.01) with endogenous levels of b5. Minimal activity was observed in adult male marmoset adrenal microsomes (open square, n = 3, 20 μg/reaction), which are known not to express detectable levels of b5, and b5 expression was, therefore, assigned a nominal value (0.001 pmol/μg). B, Effects of addition of purified recombinant b5 (10 pmol) on 17,20-lyase activity (nmol/mg · h) in neonatal rhesus adrenal microsomes (20 μg/reaction). Stimulation of 17,20-lyase activity is expressed as a percentage of activity without added b5 (% control). The experiment included adult male marmoset adrenal microsomes (open square, n = 3, 20 μg/reaction), which are known not to express detectable levels of b5, and b5 expression was, therefore, assigned a nominal value (0.001 pmol/μg) for the purpose of calculation. The percent increase in 17,20-lyase activity upon addition of b5 was negatively correlated with endogenous concentration of b5 (R2 = 0.65; P < 0.01) with rhesus adrenal microsomes. Stimulation of 17,20-lyase activity was also observed with male marmoset microsomes (234.8 ± 24.5%).
Investigation of the influence of accessory protein concentration on 17,20-lyase activity in primate adrenal microsomes: effect of recombinant b5 and CPR
Studies were conducted to investigate the relationship between endogenous levels of accessory proteins and the capacity for stimulation of 17,20-lyase activity in rhesus adrenal microsomal protein. These experiments examined 17,20-lyase activity of rhesus adrenal microsomes (ages 1 d to 3 months old) and the effects of recombinant rat b5 (10 pmol) or rat CPR (10 pmol) on 17,20-lyase activity (control) calculated as a percentage of activity seen without additional redox partner support. Supplementation with b5 stimulated the 17,20-lyase activity of rhesus adrenal microsomes (Fig. 5B), but adding recombinant rat CPR had no effect (data not shown). In addition, endogenous b5 concentrations were negatively correlated (Fig. 5B; R2 = 0.65; P < 0.01) with the degree to which 17,20-lyase activity was stimulated by recombinant b5; the lower the endogenous level of b5, the greater17,20-lyase activity was stimulated by addition of recombinant b5 protein to a similar maximal level. Studies were also conducted with adult male marmoset adrenal microsomes in which endogenous 17,20-lyase activity was very low, and b5 levels were undetectable by immunoblot (nominally given 0.001 pmol/μg for purposes of analysis). Not only did recombinant b5 stimulate marmoset adrenal 17,20-lyase activity (Fig. 5B; 234.8 ± 24.5%), it stimulated to levels comparable to those seen in rhesus adrenal microsomes.
17,20-Lyase activity in cotransfected HEK-293 cells
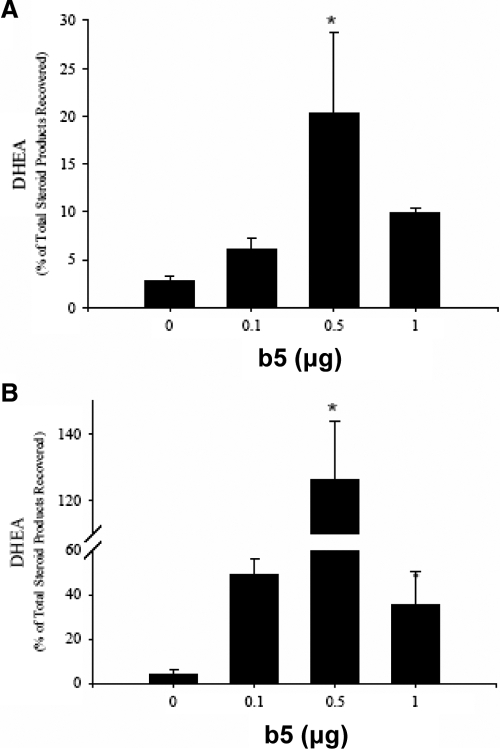
As an independent demonstration of the relative capacity of b5 to selectively increase lyase activity, increasing amounts of b5 expression plasmid were cotransfected with marmoset or rhesus P450c17 plasmids. b5 dose-dependently enhanced 17,20-lyase activity in both rhesus and marmoset wild-type constructs expressed in HEK293 cells (Fig. 6). Peak stimulation of DHEA synthesis was seen at the concentration of 0.5 μg b5 cotransfected with 1 μg primate P450c17, and DHEA production by cotransfected cells was significantly increased over cells transfected with P450c17 alone (P < 0.05). Cotransfection of b5 with even higher amounts of the b5 expression plasmid (1 μg b5 with 1 μg P450c17) resulted in submaximal lyase activity (Fig 6).
Figure 6.
Effect of coexpression of b5 on synthesis of DHEA by HEK293 cell transfected with expression plasmids encoding rhesus and marmoset P450c17. Increasing amounts of cotransfected b5 expression plasmid enhanced 17,20-lyase activity of marmoset (A) and rhesus (B) wild-type P450c17 transfected into HEK293 cells. Maximal DHEA synthesis was observed at 0.5 μg cotransfected b5 (*,P < 0.05). Further addition of b5, up to 1 μg, attenuated 17,20-lyase activity. Values were corrected for protein expression.
Discussion
Adrenarche in humans is marked by growth of axillary and pubic hair (14) and increased adrenal androgen concentrations, neither of which is readily apparent in rhesus macaques. However, adrenarche can be defined in ways more reflective of the underlying biology of the event that we believe is shared between human and nonhuman primates. For instance, adrenarche can be defined as the morphological differentiation of an inner ZR capable of androgen secretion. The ZR is prominent in Old World primates (3), and is the site of androgen synthesis in human (21,29,30) and rhesus adrenal glands (23). The rhesus adrenal also experiences a morphological transition similar to that associated with human adrenarche, but one that begins in utero (24), is concurrent with regression of the fetal zone (FZ), and is completed by 3 months of age (25). We, as others (5), have suggested that the concurrence of FZ regression with ZR differentiation, together with changes in DHEAS clearance postnatally (31), confounds the detection of an increase in adrenal androgen secretion that might otherwise accompany ZR maturation (25). However, adrenarche may also be defined biochemically, as an increase in adrenal 17,20-lyase activity (22) that is requisite for DHEA production. The present studies are the first to demonstrate that neonatal rhesus adrenal 17,20-lyase activity increases with differentiation of the ZR. More importantly, our data indicate that the increase in 17,20-lyase activity is positively correlated with b5 expression, as shown previously in marmoset adrenal tissues (32), and that adding recombinant b5 increases 17,20-lyase activity, especially in adrenals with low endogenous levels in both species. As in humans, steroidogenic and accessory protein expression is very zonal in the rhesus adrenal cortex (3,33). The ZR is the only adrenal zone that expresses b5, and the only zone lacking 3βHSD. Therefore, the microsomes as studied here have relatively less b5 and more 3βHSD than the ZR would itself by dilution from other cortical zones. This is likely to have minimized correlations among b5, P450c17, and 17,20-lyase, though these were still significant. The increase in expression of 3βHSD with age seen here must reflect changes in the ZG and ZF expression, not in the ZR based on our parallel immunohistochemical analyses (25). Although a consequence of microsome preparation and not strictly reflective of the ZR, the increase in 3βHSD nonetheless allowed some insight into its influence (or lack thereof) on 17,20-lyase activity. Despite these limitations, the data establish a clearer picture of the functional differentiation of androgen synthesizing capacity in the rhesus adrenal cortex between birth and 3 months of age and the central role of b5 in this transformation. Just as the same structural and functional changes are associated with adrenarche in humans, we believe a comparable process occurs in the rhesus (if earlier), though it has not previously been acknowledged to occur in this species (6).
Several molecular mechanisms are proposed to explain the differential regulation of 17,20-lyase activity. Increasing the ratio of CPR to P450c17 preferentially increased 17,20-lyase over 17α-hydroxylase activities of porcine P450c17 (34) but had no effect on rhesus adrenal microsomal 17,20-lyase activity in the current studies. A second mechanism relates to the accessory enzyme b5, which also selectively promotes 17,20-lyase activity (15,16,17), even if b5 itself is not redox active (20). The stimulatory effect of b5 on 17,20-lyase activity has been shown previously in numerous studies reconstituting activities with purified or overexpressed P450c17 proteins from various species (15,18,19,20,35,36,37) as well as in guinea pig adrenal microsomes (38). Our data also provide considerable support for the hypothesis that ZR maturation involves an increase in 17,20-lyase activity attributable primarily to increased expression of b5. We observed a significant correlation between b5 concentrations and 17,20-lyase activity, consistent with findings in androgen-secreting human adrenal adenomas (39,40). Furthermore, our data demonstrate that addition of b5, but not CPR, stimulated rhesus adrenal microsomal 17,20-lyase activity, and, importantly, the degree of stimulation was inversely proportional to endogenous levels of b5. The capacity of b5 to stimulate P450c17 appears to be well conserved, and the protein conserved enough to cross species. The 17,20-lyase activity of recombinant human P450c17 was stimulated by rat (19) as well as porcine (18) b5, and rat b5 can similarly support the bovine, porcine, and guinea pig P450c17 enzymes (37). The age-related decline in circulating adrenal androgen concentrations (41) is accompanied by a decrease in adrenal b5 expression (42). Male marmoset adrenal microsomes, lacking any detectable b5 expression (28), also exhibited a marked increase of 17,20-lyase activity with b5 addition, consistent with this conclusion. This further suggests that very low endogenous 17,20-lyase activity in New World primates may be due to the lack of endogenous b5 rather than by inherent differences in the activity of P450c17. Finally, nonsteroidogenic cells cotransfected with constructs encoding b5 and either rhesus or marmoset P450c17 secreted DHEA, and production was dependent on the level of cotransfected b5, consistent with similar studies using constructs to express recombinant human enzymes (43). These observations establish b5 expression as a fundamental element in the maturation of the ZR (23) as well as adrenal androgen synthetic potential of gonadal tissues (30), and that this same basic process underpins events in nonhuman primates as in humans.
A decline in 3βHSD expression within the developing ZR of the maturating adrenal cortex is also thought to be necessary for increasing adrenal androgen output (29,44). The data presented here argue that this is an association more than a cause, showing that 17,20-lyase in rhesus adrenal microsomes activity increased despite a concomitant increase in expression of 3βHSD. Furthermore, DHEA synthesis by rhesus adrenal microsomes was not increased in the presence of trilostane, a drug that effectively inhibited 3βHSD activity, increasing 17OH-pregnenolone accumulation and blocking synthesis of detectable 11-deoxycortisol. Endoh et al. (29) measured DHEA and cortisol production by cultured human fasciculata cells, with and without trilostane, and reported that trilostane markedly increased the ratio of DHEA to cortisol produced. However, no absolute concentrations of DHEA were reported, and our data show unequivocally that trilostane blocks virtually all 11-deoxycortisol and, thus, cortisol production in vitro. Therefore, it is not clear whether or not the altered DHEA to cortisol ratio reported by Endoh et al. (29) resulted from an increase in DHEA, or markedly decreased production of cortisol. The notion that 3βHSD activity can shunt steroid production away from androgens (45) is based on the greatly reduced capacity of human and nonhuman primate 17,20-lyase activity to cleave Δ4 substrates (3,11,28) but does not account for the generally low rates of 17,20-lyase compared with 17α-hydroxylase activity (13,22). This is not to deny that reduced expression of 3βHSD may contribute to increased androgen synthesis by adrenal tissues in some cases (46) when all else may be equal. Nonetheless, our data do suggest that when increased b5 expression occurs within the ZR, it is sufficient in itself to stimulate 17,20-lyase activity and may even be the most potent stimulus to consequent adrenal androgen production.
Despite morphological and biochemical evidence to the contrary, adrenarche has not been recognized in the rhesus because of a lack of data demonstrating a prepubertal increase in circulating concentrations of adrenal androgens. Longitudinal serum samples taken from two rhesus neonates were shown to exhibit an increase in circulating DHEAS from less than 100 μg/dl at 1 d old to a peak of more than 500 μg/dl at 2 months of age (3), and are consistent with the increase in 17,20-lyase reported here. Numerous studies have shown that circulating levels of DS in the rhesus macaque are at their highest levels immediately postpartum during the first few weeks of life (47,48), perhaps facilitated by a marked decrease of metabolic clearance (31). Old World primates such as the rhesus and baboon have much lower levels of DS than do humans, likely due to the 10-fold higher rates of glucuronidation in macaques (49) and 15-fold higher plasma clearance in baboons (50) compared with humans. As high as concentrations are in humans, it has been argued, nonetheless, that metabolism by alternative routes precludes earlier identification of increased androgen output in early childhood (51). The postpartum/neonatal decline in DS production in humans is attributable clearly to neonatal regression of the FZ (52,53,54) and accompanying decreased adrenal 17,20-lyase activity (22). The increased adrenal androgen levels often associated with adrenarche around 8–9 yr of age (55), or even earlier in some studies (51,56,57), are coincident with induction of ZR differentiation, which is delayed after FZ regression by an interval of years in humans (54,58,59). Our studies show that the events that contribute to the increases in adrenal androgen output associated with adrenarche in humans are mirrored in the neonatal rhesus. However, regression of the FZ and ZR differentiation are concurrent in the rhesus and occur over a much shorter period of 2–3 months. It is noteworthy that the condensed period of ZR development in the rhesus compared with humans also accords with their comparatively early onset of puberty, around 3 yr of age in this species (60). Therefore, in both humans and the rhesus, puberty is still experienced soon after adrenarche (as defined here), even though the events are apparently independent phenomena (61).
In summary, the present data emphasize the role of b5 as a crucial element in the regulation of adrenal androgen production. Unfortunately, very little is known or understood about how b5 expression is itself regulated, other than it is clearly developmental and tissue specific, and involves transcription factors that similarly enhance P450c17 expression (62). Although the interval is much faster in the rhesus than in humans, the increase in b5 accompanying ZR maturation is a still relatively slow event, which suggests slow increases in either trophic factors or the receptors responsive to them. The neonatal rhesus macaque and marmoset may provide valid, accessible animal models for the study of b5 expression, adrenal remodeling, and corresponding biochemical events that define adrenarche and androgen secretion in general (7,63,64,65). Such a model would prove valuable in investigation of the causes, which are largely unknown, and the long-lasting consequences of premature adrenarche, possibly polycystic ovarian syndrome (14) and hyperinsulinemia (55). Detailed studies of the hormonal events that occur during this dynamic period, and the molecular events that mediate their effects, are necessary for a more complete understanding of the adrenarche experienced by the rhesus. This would help to establish the rhesus, and other nonhuman primates like the marmoset, as appropriate and complimentary models for studies of adrenal development and pathophysiology.
Acknowledgments
The authors dedicate this paper to the memory of their co-author, collaborator, student, and friend, Dr. J. Christina Pattison, who passed away, tragically and unexpectedly, February 2, 2008. We thank Sona Santos, Dr. Alice Tarantal, and the staff of the California National Primate Research Center for collection of rhesus adrenal samples, Dr. Amy Usborne of the National Primate Research Center at the University of Wisconsin-Madison for providing marmoset adrenal tissue, Drs. Gary Henderson and Hongwu Jing for HPLC analysis, and Samantha Mapes for training in assay procedures.
Footnotes
J.C.P. was funded in part by National Institutes of Health Grant T32 HD 41921.
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 26, 2008
Abbreviations: b5, Cytochrome b5; CMV, cytomegalovirus; CPR, cytochrome P450 oxidoreductase; DHEA, dehydroepiandrosterone; DS, dehydroepiandrosterone sulfate; FZ, fetal zone; HEK, human embryonic kidney; 3βHSD, 3β-hydroxysteroid dehydrogenase; P450c21, 21-hydroxylase cytochrome P450; P450c17, 17α-hydroxylase/17,20-lyase cytochrome P450; ZR, zona reticularis.
References
- Cutler GJ, Glenn M, Bush M, Hodgen GD, Graham CE, Loriaux DL 1978 Adrenarche: a survey of rodents, domestic animals, and primates. Endocrinology 103:2112–2118 [DOI] [PubMed] [Google Scholar]
- Collins DC, Nadler RD, Preedy JRK 1981 Adrenarche in the great apes. Am J Primatol 1:344 (Abstract) [Google Scholar]
- Conley AJ, Pattison JC, Bird IM 2004 Variations in adrenal androgen production among (nonhuman) primates. Semin Reprod Med 22:311–326 [DOI] [PubMed] [Google Scholar]
- Smail PJ, Faiman C, Hobson WC, Fuller GB, Winter JS 1982 Further studies on adrenarche in nonhuman primates. Endocrinology 111:844–848 [DOI] [PubMed] [Google Scholar]
- Castracane VD, Cutler Jr GB, Loriaux DL 1981 Pubertal endocrinology of the baboon: adrenarche. Am J Physiol 241:E305–E309 [DOI] [PubMed] [Google Scholar]
- Arlt W, Martens JW, Song M, Wang JT, Auchus RJ, Miller WL 2002 Molecular evolution of adrenarche: structural and functional analysis of p450c17 from four primate species. Endocrinology 143:4665–4672 [DOI] [PubMed] [Google Scholar]
- Abbott DH, Bird IM 6 August 2008 Nonhuman primates as models for human adrenal androgen production: function and dysfunction. Rev Endocr Metab Disord 10.1007/5 11154-008-9099-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevold HR, Lorence MC, McCarthy JL, Trant JM, Kagimoto M, Waterman MR, Mason JI 1989 Rat P450(17 α) from testis: characterization of a full-length cDNA encoding a unique steroid hydroxylase capable of catalyzing both δ 4- and δ 5-steroid-17,20-lyase reactions. Mol Endocrinol 3:968–975 [DOI] [PubMed] [Google Scholar]
- Lin D, Black SM, Nagahama Y, Miller WL 1993 Steroid 17 α-hydroxylase and 17,20-lyase activities of P450c17: contributions of serine106 and P450 reductase. Endocrinology 132:2498–2506 [DOI] [PubMed] [Google Scholar]
- Brock BJ, Waterman MR 1999 Biochemical differences between rat and human cytochrome P450c17 support the different steroidogenic needs of these two species. Biochemistry 38:1598–1606 [DOI] [PubMed] [Google Scholar]
- Fluck CE, Miller WL, Auchus RJ 2003 The 17, 20-lyase activity of cytochrome p450c17 from human fetal testis favors the δ5 steroidogenic pathway. J Clin Endocrinol Metab 88:3762–3766 [DOI] [PubMed] [Google Scholar]
- Conley AJ, Bird IM 1997 The role of cytochrome P450 17 α-hydroxylase and 3 β-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the δ 5 and δ 4 pathways of steroidogenesis in mammals. Biol Reprod 56:789–799 [DOI] [PubMed] [Google Scholar]
- Couch RM, Muller J, Winter JS 1986 Regulation of the activities of 17-hydroxylase and 17,20-desmolase in the human adrenal cortex: kinetic analysis and inhibition by endogenous steroids. J Clin Endocrinol Metab 63:613–618 [DOI] [PubMed] [Google Scholar]
- Auchus RJ, Rainey WE 2004 Adrenarche—physiology, biochemistry and human disease. Clin Endocrinol (Oxf) 60:288–296 [DOI] [PubMed] [Google Scholar]
- Onoda M, Hall PF 1982 Cytochrome b5 stimulates purified testicular microsomal cytochrome P-450 (C21 side-chain cleavage). Biochem Biophys Res Commun 108:454–460 [DOI] [PubMed] [Google Scholar]
- Katagiri M, Suhara K, Shiroo M, Fujimura Y 1982 Role of cytochrome b5 in the cytochrome P-450-mediated C21-steroid 17,20-lyase reaction. Biochem Biophys Res Commun 108:379–384 [DOI] [PubMed] [Google Scholar]
- Shinzawa K, Kominami S, Takemori S 1985 Studies on cytochrome P-450 (P-450 17α,lyase) from guinea pig adrenal microsomes. Dual function of a single enzyme and effect of cytochrome b5. Biochim Biophys Acta 833:151–160 [PubMed] [Google Scholar]
- Lee-Robichaud P, Wright JN, Akhtar ME, Akhtar M 1995 Modulation of the activity of human 17 α-hydroxylase-17,20-lyase CYP17 by cytochrome b5: endocrinological and mechanistic implications. Biochem J 308(Pt 3):901–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri M, Kagawa N, Waterman MR 1995 The role of cytochrome b5 in the biosynthesis of androgens by human P450c17. Arch Biochem Biophys 317:343–347 [DOI] [PubMed] [Google Scholar]
- Auchus RJ, Lee TC, Miller WL 1998 Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem 273:3158–3165 [DOI] [PubMed] [Google Scholar]
- Narasaka T, Suzuki T, Moriya T, Sasano H 2001 Temporal and spatial distribution of corticosteroidogenic enzymes immunoreactivity in developing human adrenal. Mol Cell Endocrinol 174:111–120 [DOI] [PubMed] [Google Scholar]
- Schiebinger RJ, Albertson BD, Cassorla FG, Bowyer DW, Geelhoed GW, Cutler Jr GB, Loriaux DL 1981 The developmental changes in plasma adrenal androgens during infancy and adrenarche are associated with changing activities of adrenal microsomal 17-hydroxylase and 17,20-desmolase. J Clin Invest 67:1177–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapes S, Corbin CJ, Tarantal A, Conley A 1999 The primate adrenal zona reticularis is defined by expression of cytochrome b5, 17α-hydroxylase/17,20-lyase cytochrome P450 (P450c17) and NADPH-cytochrome P450 reductase (reductase) but not 3β-hydroxysteroid dehydrogenase/δ5-4 isomerase (3β-HSD). J Clin Endocrinol Metab 84:3382–3385 [DOI] [PubMed] [Google Scholar]
- Mapes S, Tarantal AF, Parker CR, Moran FM, Bahr JM, Pyter L, Conley AJ 2002 Adrenocortical cytochrome b5 expression during fetal development of the rhesus macaque. Endocrinology 143:1451–1458 [DOI] [PubMed] [Google Scholar]
- Nguyen A, Mapes S, Corbin C, Conley AJ 2008 Morphological adrenarche in rhesus macaques: development of the zona reticularis is concurrent with fetal zone regression in the early neonatal period. J Endocrinol 199:367–378 [DOI] [PubMed] [Google Scholar]
- Grigoryev DN, Kato K, Njar VC, Long BJ, Ling YZ, Wang X, Mohler J, Brodie AM 1999 Cytochrome P450c17-expressing Escherichia coli as a first-step screening system for 17α-hydroxylase-C17,20-lyase inhibitors. Anal Biochem 267:319–330 [DOI] [PubMed] [Google Scholar]
- Moran FM, Ford JJ, Corbin CJ, Mapes S, Njar VC, Brodie AM, Conley AJ 2002 Regulation of microsomal P450, redox partner proteins and steroidogenesis in the developing testes of the neonatal pig. Endocrinology 143:3361–3369 [DOI] [PubMed] [Google Scholar]
- Pattison JC, Abbott DH, Saltzman W, Nguyen AD, Henderson G, Jing H, Pryce CR, Allen AJ, Conley AJ, Bird IM 2005 Male marmoset monkeys express an adrenal fetal zone at birth, but not a zona reticularis in adulthood. Endocrinology 146:365–374 [DOI] [PubMed] [Google Scholar]
- Endoh A, Kristiansen SB, Casson PR, Buster JE, Hornsby PJ 1996 The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3β-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab 81:3558–3565 [DOI] [PubMed] [Google Scholar]
- Dharia S, Slane A, Jian M, Conner M, Conley AJ, Parker Jr CR 2004 Colocalization of P450c17 and cytochrome b5 in androgen-synthesizing tissues of the human. Biol Reprod 71:83–88 [DOI] [PubMed] [Google Scholar]
- Seron-Ferre M, Hess DL, Lindholm U, Jaffe RB 1986 Persistence of fetal zone function in the infant rhesus monkey adrenal gland. J Clin Endocrinol Metab 62:460–465 [DOI] [PubMed] [Google Scholar]
- Pattison JC, Saltzman W, Abbott DH, Hogan BK, Nguyen AD, Husen B, Einspanier A, Conley AJ, Bird IM 2007 Gender and gonadal status differences in zona reticularis expression in marmoset monkey adrenals: cytochrome b5 localization with respect to cytochrome P450 17,20-lyase activity. Mol Cell Endocrinol 265–266:93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AD, Conley AJ 2008 Adrenal androgens in humans and nonhuman primates: production, zonation and regulation. Endocr Dev 13:33–54 [DOI] [PubMed] [Google Scholar]
- Yanagibashi K, Hall PF 1986 Role of electron transport in the regulation of the lyase activity of C21 side-chain cleavage P-450 from porcine adrenal and testicular microsomes. J Biol Chem 261:8429–8433 [PubMed] [Google Scholar]
- Pandey AV, Miller WL 2005 Regulation of 17,20 lyase activity by cytochrome b5 and by serine phosphorylation of P450c17. J Biol Chem 280:13265–13271 [DOI] [PubMed] [Google Scholar]
- Naffin-Olivos JL, Auchus RJ 2006 Human cytochrome b5 requires residues E48 and E49 to stimulate the 17,20-lyase activity of cytochrome P450c17. Biochemistry 45:755–762 [DOI] [PubMed] [Google Scholar]
- Shet MS, Fisher CW, Tremblay Y, Belanger A, Conley AJ, Mason JI, Estabrook RW 2007 Comparison of the 17 α-hydroxylase/C17,20-lyase activities of porcine, guinea pig and bovine P450c17 using purified recombinant fusion proteins containing P450c17 linked to NADPH-P450 reductase. Drug Metab Rev 39:289–307 [DOI] [PubMed] [Google Scholar]
- Kominami S, Ogawa N, Morimune R, De-Ying H, Takemori S 1992 The role of cytochrome b5 in adrenal microsomal steroidogenesis. J Steroid Biochem Mol Biol 42:57–64 [DOI] [PubMed] [Google Scholar]
- Sakai Y, Yanase T, Hara T, Takayanagi R, Haji M, Nawata H 1994 In-vitro evidence for the regulation of 17,20-lyase activity by cytochrome b5 in adrenocortical adenomas from patients with Cushing's syndrome. Clin Endocrinol (Oxf) 40:205–209 [DOI] [PubMed] [Google Scholar]
- Yanase T, Sasano H, Yubisui T, Sakai Y, Takayanagi R, Nawata H 1998 Immunohistochemical study of cytochrome b5 in human adrenal gland and in adrenocortical adenomas from patients with Cushing's syndrome. Endocr J 45:89–95 [DOI] [PubMed] [Google Scholar]
- Dharia S, Parker Jr CR 2004 Adrenal androgens and aging. Semin Reprod Med 22:361–368 [DOI] [PubMed] [Google Scholar]
- Dharia S, Slane A, Jian M, Conner M, Conley AJ, Brissie RM, Parker Jr CR 2005 Effects of aging on cytochrome b5 expression in the human adrenal gland. J Clin Endocrinol Metab 90:4357–4361 [DOI] [PubMed] [Google Scholar]
- Soucy P, Luu-The V 2000 Conversion of pregnenolone to DHEA by human 17α-hydroxylase/17, 20-lyase (P450c17). Evidence that DHEA is produced from the released intermediate, 17α-hydroxypregnenolone. Eur J Biochem 267:3243–3247 [DOI] [PubMed] [Google Scholar]
- Gell JS, Carr BR, Sasano H, Atkins B, Margraf L, Mason JI, Rainey WE 1998 Adrenarche results from development of a 3β-hydroxysteroid dehydrogenase-deficient adrenal reticularis. J Clin Endocrinol Metab 83:3695–3701 [DOI] [PubMed] [Google Scholar]
- Sholl SA 1981 17-Hydroxyprogesterone metabolism in the monkey fetal adrenal: C17–20lyase and 21-hydroxylase activities. Steroids 38:221–228 [DOI] [PubMed] [Google Scholar]
- Sakai Y, Yanase T, Hara T, Takayanagi R, Haji M, Nawata H 1994 Mechanism of abnormal production of adrenal androgens in patients with adrenocortical adenomas and carcinomas. J Clin Endocrinol Metab 78:36–40 [DOI] [PubMed] [Google Scholar]
- Koritnik DR, Laherty RF, Rotten D, Jaffe RB 1983 A radioimmunoassay for dehydroepiandrosterone sulfate in the circulation of rhesus monkeys. Steroids 42:653–667 [DOI] [PubMed] [Google Scholar]
- Seron-Ferre M, Taylor NF, Rotten D, Koritnik DR, Jaffe RB 1983 Changes in fetal rhesus monkey plasma dehydroepiandrosterone sulfate: relationship to gestational age, adrenal weight and preterm delivery. J Clin Endocrinol Metab 57:1173–1178 [DOI] [PubMed] [Google Scholar]
- Guillemette C, Hum DW, Belanger A 1996 Levels of plasma C19 steroids and 5 α-reduced C19 steroid glucuronides in primates, rodents, and domestic animals. Am J Physiol 271(2 Pt 1):E348–E353 [DOI] [PubMed] [Google Scholar]
- Schut HA, Pepe GJ, Townsley JD 1978 Clearance and production of dehydroepiandrosterone and its sulfate in female baboons. Am J Physiol 235:E74–E77 [DOI] [PubMed] [Google Scholar]
- Remer T, Boye KR, Hartmann MF, Wudy SA 2005 Urinary markers of adrenarche: reference values in healthy subjects, aged 3–18 years. J Clin Endocrinol Metab 90:2015–2021 [DOI] [PubMed] [Google Scholar]
- Benner MC 1940 Studies on the involution of the fetal cortex of the adrenal glands. Am J Pathol 16:787–798 [PMC free article] [PubMed] [Google Scholar]
- Parker Jr CR 1999 Dehydroepiandrosterone and dehydroepiandrosterone sulfate production in the human adrenal during development and aging. Steroids 64:640–647 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, Carr BR, Rainey WE 2000 Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf) 53:739–747 [DOI] [PubMed] [Google Scholar]
- Ibanez L, DiMartino-Nardi J, Potau N, Saenger P 2000 Premature adrenarche—normal variant or forerunner of adult disease? Endocr Rev 21:671–696 [DOI] [PubMed] [Google Scholar]
- Palmert MR, Hayden DL, Mansfield MJ, Crigler Jr JF, Crowley Jr WF, Chandler DW, Boepple PA 2001 The longitudinal study of adrenal maturation during gonadal suppression: evidence that adrenarche is a gradual process. J Clin Endocrinol Metab 86:4536–4542 [DOI] [PubMed] [Google Scholar]
- Martin DD, Schweizer R, Schwarze CP, Elmlinger MW, Ranke MB, Binder G 2004 The early dehydroepiandrosterone sulfate rise of adrenarche and the delay of pubarche indicate primary ovarian failure in Turner syndrome. J Clin Endocrinol Metab 89:1164–1168 [DOI] [PubMed] [Google Scholar]
- de Peretti E, Forest MG 1976 Unconjugated dehydroepiandrosterone plasma levels in normal subjects from birth to adolescence in human: the use of a sensitive radioimmunoassay. J Clin Endocrinol Metab 43:982–991 [DOI] [PubMed] [Google Scholar]
- de Peretti E, Forest MG 1978 Pattern of plasma dehydroepiandrosterone sulfate levels in humans from birth to adulthood: evidence for testicular production. J Clin Endocrinol Metab 47:572–577 [DOI] [PubMed] [Google Scholar]
- Plant TM, Zorub DS 1982 The role of nongonadal restraint of gonadotropin secretion in the delay of the onset of puberty in the rhesus monkey (Macaca mulatta). J Anim Sci 55(Suppl 2):43–55 [PubMed] [Google Scholar]
- Sklar CA, Kaplan SL, Grumbach MM 1980 Evidence for dissociation between adrenarche and gonadarche: studies in patients with idiopathic precocious puberty, gonadal dysgenesis, isolated gonadotropin deficiency, and constitutionally delayed growth and adolescence. J Clin Endocrinol Metab 51:548–556 [DOI] [PubMed] [Google Scholar]
- Huang N, Dardis A, Miller WL 2005 Regulation of cytochrome b5 gene transcription by Sp3, GATA-6, and steroidogenic factor 1 in human adrenal NCI-H295A cells. Mol Endocrinol 19:2020–2034 [DOI] [PubMed] [Google Scholar]
- Abbott DH, Dumesic DA, Franks S 2002 Developmental origin of polycystic ovary syndrome—a hypothesis. J Endocrinol 174:1–5 [DOI] [PubMed] [Google Scholar]
- Abbott DH, Padmanabhan V, Dumesic DA 2006 Contributions of androgen and estrogen to fetal programming of ovarian dysfunction. Reprod Biol Endocrinol 4:17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott DH, Zhou R, Bird IM, Dumesic DA, Conley AJ 2008 Fetal programming of adrenal androgen excess: lessons from a nonhuman primate model of polycystic ovary syndrome. Endocr Dev 13:145–158 [DOI] [PMC free article] [PubMed] [Google Scholar]