Melanocyte biology
Melanocytes are an intraepidermal population of dendritic cells responsible for the production of melanin, a pigment that varies from yellow to brown to black pigment that after transfer to neighboring keratinocytes acts both as an endogenous screen and a buffering system against harmful ultraviolet (UV) wavelengths in sunlight [1]. Skin pigmentation has both individual and societal implications. The cosmetic desire for increased pigmentation (tanning) has resulted in many deleterious alterations including hastened skin ageing with wrinkles and poikiloderma and an increase in lentigines, melanocytic nevi, and melanoma. Focal or widespread loss of normal pigmentation not only renders individuals extraordinarily vulnerable to the harmful effects of sunlight (eg, increased risk of skin cancer in albinism), but it can also result in severe emotional stress and, in some societies, ostracism and discrimination (eg, vitiligo).
Melanocytes are derived from the neural crest and are located along the basal layer of the epidermis and within the hair follicle, predominately the basal layer of the hair bulb matrix [1,2]. By the 50th day of intrauterine life, melanocytes can be detected in the epidermis; their migration to the epidermis and survival is dependent on receptor tyrosine kinase (RTK) c-Kit and its ligand stem cell factor (SCF) within the epidermis [3,4]. Mutations of the c-Kit gene lead to patches of hypopigmentation caused by lack of melanocyte migration, termed piebaldism [5]. Another important signaling molecule in melanocyte migration and development is Wnt5a, which signals via the Frizzled-5 receptor [6]. Overexpression of Wnt5a/Frizzled is found in melanomas and associated with increased cell motility and invasiveness [7,8].
Skin keratinocytes obtain melanin pigment from melanocytes, and keratinocytes provide the necessary microenvironment for melanocyte survival, proliferation, differentiation, and migration via production of ligands that interact with melanocyte receptors [1,9-11]. The epidermal melanin unit denotes the symbiotic relationship between one melanocyte transporting melanin via its dendritic processes to approximately 36 keratinocytes [10]. Melanocytes are located on the basement membrane among basal keratinocytes at ratio of 1 melanocyte per 5 basal keratinocytes in hematoxylin and eosin—stained histologic sections. This balance is maintained through regulated induction of melanocyte division. During childhood as the skin surface expands, throughout adulthood to maintain melanocyte numbers, and in response to exposure to sunlight or skin wounding, melanocytes are stimulated to proliferate at a low rate. Melanocyte proliferation entails uncoupling from keratinocytes, loss of their dendrites, cell division, migration along the basement membrane, then recoupling with keratinocytes to form the epidermal melanin unit. Keratinocytes regulate melanocyte growth and expression of melanocyte cell surface receptors via cell adhesion and growth factors, which include E-cadherin, P-cadherin, and desmoglein that are regulated through growth factors such as hepatocyte growth factor (HGF), platelet-derived growth factor (PDGF), and endothelin-1 (produced by fibroblasts or keratinocytes). Morphogens such as Notch receptors and their ligands also play a role in maintaining melanocyte function and morphology [12]. Loss of keratinocyte regulation characterizes the development of melanoma and is seen in the down-regulation of E-and P-cadherins, up-regulation of melanocyte-melanocyte and melanocyte cell-fibroblast adhesion molecules such as Mel-CAM and N-cadherin, expression of cell-matrix adhesion molecules such as αvβ3 integrins and increased elaboration of metallo-proteinases [10]. The importance in growth factor signaling in producing the malignant phenotype has been shown in experimental models where increased expression of basic fibroblastic growth factor (bFGF), HGF, SCF, and endothelin-3 coupled with UV radiation produced invasive and in situ—like tumors [13,14].
Melanins are polymorphous and multifunctional biopolymers, whose biosynthesis involves a metabolic pathway beginning with the oxidation of tyrosine to L-DOPA, followed by a series of divergent steps that give rise to a brown-black pigment (eumelanin) composed predominantly of indolic units and a yellow to reddish-brown pigment (pheomelanin) having a backbone of benzothiazine units [1,2]. Most of human skin and hair pigmentation involves a combination of these pathways giving rise to mixtures of varying composition [1,2]. The phenotypic expression of this is generally classified according to the clinical Fitzpatrick skin types 1 through 6 and emphasizes the inverse relationship between the degree of pigmentation and solar damage to the skin, including photocarcinogenesis. The functions of melanin pigments include protection from UV light, control of vitamin D3 synthesis, and local thermoregulation [1,15,16]. Melanogenesis is under complex regulatory control by multiple agents interacting via pathways activated by receptor-dependent and -independent mechanisms, in hormonal, autocrine, paracrine, or intracrine fashion [1]. Because of the multidirectional nature and heterogeneous character of the melanogenesis-modifying agents, its controlling factors are not organized into simple linear sequences, but they interact instead in a multidimensional network, with extensive functional overlapping with connections arranged both in series and in parallel [1,2]. The most important positive regulator of melanogenesis is the MC1 receptor with its ligands melanocortins and ACTH, whereas among the negative regulators, agouti protein stands out, determining intensity of melanogenesis and also the type of melanin synthesized [1,17]. Solar UV light is one of the main culprits in the etiology of skin cancers, and skin pigmentation and melanin content are principal determinants of the susceptibility to melanoma and other sun-induced skin cancers [1, 18-22]. In general, individuals with fair skin who burn rather than tan when exposed to sun are at high risk for melanoma [19,21]. Mutations and polymorphisms of melanocortin-1 receptor type 1 gene (MCR1) play a role in skin cancer [23-25]. Other important risk factors for skin cancer include DNA repair capacity, because melanoma patients have a lower DNA repair capacity than the general population, which may be in part related to loss-of-function mutations in the MCR1[26,27]. Indeed, there exists a strong association between BRAF mutations and germline defects in melanocortin receptor type 1 (MC1R) and incidence of melanoma with no evidence of chronic sun damage [27].
Melanoma, like other cancers, arises because of accumulation of mutations in genes crucial for cell proliferation, cell differentiation, and cell death [28-30]. The factors influencing development and progression of melanoma include tumor initiation (mutations, loss of heterozygosity, gene amplification, gain and loss of chromosomes, gene silencing by methylation), growth (loss of cell cycle control, growth factors, neovascularization), resistance to apoptosis (inactivation of cell death pathways, gain of anti-apoptotic and survival factors), invasion and metastasis (cell motility, cell adhesion, proteolytic enzymes), and escape from immune surveillance (loss or gain of immune regulators) [29]. Melanocyte receptors that engage with tumor suppressor, oncogene, cell adhesion and cell-motility, and apoptotic pathways are all potential targets for oncogenic events or therapeutic agents.
Tumor suppressor pathways most commonly inactivated in melanoma are the p16INK4/cyclin-dependent kinases 4 and 6/retinoblastoma protein and p14ARF/human double minute/p53 pathways, which control the G1 stage of the cell cycle [31,32]. Ras and its effector pathways Raf-MAPK kinase-ERK and PI3K-Akt are most commonly activated [33,34]. Melanoma also shares many characteristics in common with developmental precursors, stem cells, or melanoblasts, to melanocytes that include activation of developmental signaling pathways [12,35,36]. Specifically, signaling by receptor tyrosine kinases (RTK) (eg, c-Kit), the Wnt signaling pathway, melanocortin signaling pathway (α-MSH/MC1-R/cAMP), as well as loss of the p16INK4a cyclin-dependent kinase inhibitor are pathways that impact on the expression or function of Mitf, which plays an essential role in melanocyte development and survival [1,37,38]. Lastly, down-regulation of death receptors such as Fas also play a role in melanoma progression[39,40].
Cell surface melanocyte receptors
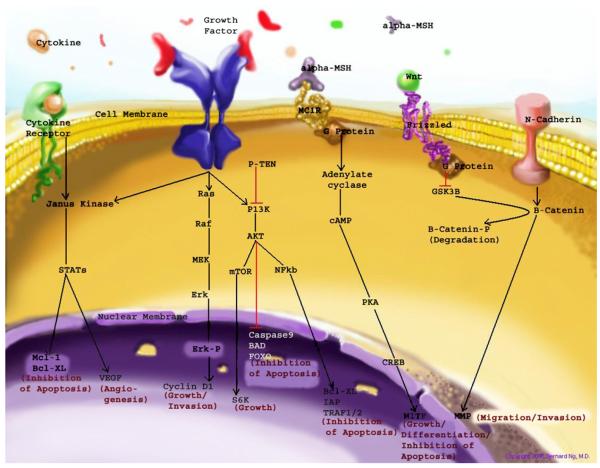
Fig. 1 outlines key membrane bound melanocyte receptors and cell cycle pathways involved in melanocyte physiology and/or are activated or disrupted in melanoma. Table 1 lists melanocyte receptors that have both clinical relevance and potential as therapeutic targets to augment normal melanocyte function or manage melanocytic pathology. Among those are receptors coupled to G-proteins (G-protein coupled receptors [GPCR]) or having tyrosine kinases activities (receptor tyrosine kinases [RTK]). Other receptors of importance are death receptors, nuclear receptors such as the vitamin D receptor, and cell adhesion molecules, which can activate critical cell cycle, survival, and migration pathways.
Fig. 1.
Outline of the major cell surface receptor-mediated signaling pathways important in the differentiation, survival, and function of melanocytes. In melanoma, regulatory disruption via activating mutations or gene amplifications of oncogenes or deletion, epigenetic silencing, or inactivating mutations of tumor suppressor genes of either upstream or down stream constituents of these pathways can be commonly found. Mitogen-activated protein kinase (MAPK) pathway and phosphatidylinositol 3 kinase (PI3K)-Akt pathway are 2 crucial pathways activated by numerous growth factors and cell surface receptors. Binding of growth factors to their respective receptors leads to, via adapter proteins (not shown), activation of RAS proteins, which phosphorylate the mitogen-activated protein kinase (MEK) kinases, which then act on extracellular-related kinase (ERK) kinases. ERK kinases also interact with the PI3K-AKT pathway. Phosporylated ERK kinases (ERK-P) translocate to the nucleus and activate transcription factors, which promote cell cycle progression and proliferation. The PI3K-AKT pathway mediates cell survival signaling via growth factors such as PDGF, NGF, and IGF-1. Phosphatase and tensin homolog (PTEN) inhibits growth factor signaling by inactivating phosphatidylinositol triphosphate (PIP3) generated by PI3K. Activated PI3K converts the plasma membrane lipid phosphatidylinositol 4,5-bisphosphonate to PIP3, which acts as a second messenger leading to the phosphorylation AKT and subsequent up-regulation of cell cycle, growth, and survival proteins. AKT can also up-regulate mTOR (mammalian target of rapamycin), S6K, and NFκβ leading to cell growth and inhibition of apoptosis. Wnt-Frizzled-β-catenin pathway. In the Wnt-Frizzled-β-catenin pathway, β-catenin plays a central role in cell adhesion and cell signaling. Wnt ligands activate the G-protein—coupled receptor, Frizzled, which blocks the breakdown of β-catenin by inactivation of the kinase GSK3. β-catenin accumulates in the cytoplasm and translocates to the nucleus, where it binds to LEF—TCF transcription factors and increases the expression of several genes, including microphthalmia transcription factor (MITF) important in melanocyte survival and matrix metalloproteinases (MMP) crucial for cell invasion. JAK/Stat pathway can be activated by cytokines as well as growth factors such as EGF. JAK-STAT pathway is mediated by Janus kinase (JAK), a tyrosine kinase that phosphorylates STAT proteins localized to the plasma membrane. Phosphorylated STAT proteins are translocated to the nucleus where they activate gene transcription leading to inhibition of apoptosis and angiogenesis. α-MSH-MC1R or Microphthalmia-associated transcription factor (MITF) pathway. MITF is regulated at both the transcriptional level via activation of melanocortin receptor 1 (MCR1) pathway and at the posttranslation level via ERK phosphorylation (not shown). MC1R activates cyclic AMP (cAMP), which activates the camp response-element binding protein (CREB) leading to increased expression of MITF. MITF stimulates melanin production and cell cycle arrest in normal melanocytes, and in melanoma cells, it protects against apoptosis and adds chemotherapy resistance. (Courtesy of B. NG, MD, Albany, NY; Copyright © 2006).
Table 1.
Melanocyte receptors: clinical relevance and potential therapeutic strategies
| Receptors | Function | Pathologic mechanism | Antagonist | Agonist | Clinical target |
|---|---|---|---|---|---|
| αvβ3 integrin | Dimer that forms cell-matrix adhesion molecule. |
Increased expression in melanoma, facilitating invasion and angiogenesis |
Radiolabeled conjugates, Cilengitide (EMD 121,974) |
Metastatic melanoma | |
| c-Kit | MPAK and AKT activation; regulates cell motility, survival, and differentiation. |
Activating mutation in minority of melanomas, mostly mucosal or lentigo maligna. |
Imatinib, gefinitib, erlotinib |
SCF | Metastatic melanoma |
| CRH-R1 | Regulates proliferation, differentiation, and immune functions. |
Not described | Antalarmin, a-helical CRH |
CRH, urocortin and modified CRH and urocortin peptides |
Metastatic melanoma |
| Death receptors, DR3, DR4, Fas |
Death receptors induce apoptosis. |
Loss of expression or mutation contributes to immune evasion. |
Fas-Ligand, TRAIL; HGS-ETR1, -2, -2J PRO1762, CD95-Fc, TRA-8 |
Metastatic melanoma | |
| Endothelin receptor | GPCR induces melanocyte proliferation and melanin synthesis. |
Down-regulated in melanoma. |
Bosentan | EDN-1, EDN-2, EDN-3 | Metastatic melanoma |
| Frizzled | G protein coupled receptor activates AKT pathway, regulates motility. |
Activated in melanoma because of increased expression of Wnt5a leads to melanoma invasiveness and motility. |
Wnt5a | Metastatic melanoma | |
| Melanocortin receptor (MC1R) |
Pigmentation, anti-inflammatory, anti-pyretic. |
Gene polymorphisms affect hair and skin color and response to UVR. |
Agouti protein | α-MSH = ACTH > β-MSH>> γ-MSH, radiolabeled α-MSH conjugate |
Tanning response, melanoma |
| Melatonin receptors | GPCR and nuclear receptors aid in homeostasis and protect against radiation damage. |
Loss of melatonin or its receptors may facilitate melanoma development and progression. |
Melatonin | Melanoma | |
| MelCam | Cell-cell adhesion molecule. | Up-regulated in melanoma, aids in metastatic spread and growth at distant sites. |
Blocking antibodies | Metastatic melanoma | |
| Metabotropic glutamate receptor (Grm1) |
GPCR, which activates MPAK and AKT pathways. |
Possibly up-regulated in melanoma. |
Bay 36-7620 | Glutamate | Metastatic melanoma |
| N-cadherin | Cell-cell adhesion- melancoyte-fibroblast adhesion. |
Increased expression in melanoma. |
Blocking antibodies | Metastatic melanoma | |
| Notch receptors | MPAK and AKT activation regulates cell growth, survival, and differentiation. |
Up-regulates melanoma cell adhesion via N-cadherin. |
Jagged 1-2, Delta1-3 | Metastatic melanoma | |
| Receptor protein kinases | MPAK and AKT activation regulates cell growth, survival and differentiation. |
Activated or mutated in some melanomas by paracrine and autocrine mechanisms. |
Imatinib, gefinitib, erlotinib | bFGF, HGF, IGF-1, VEGF |
Metastatic melanoma |
| VEGF receptor | RTK, activates MPAK pathway and leads to angio-/lymphogenesis |
Increase expression in melanoma along with VEGF |
BAY 43-9006 (sorafenib) | VEGF-1, -2,-3 | Metastatic melanoma |
| Vitamin D receptor | Regulates cell growth, survival. |
Polymorphosisms related to risk for melanoma and melanoma survival. |
Vitamin D3 | Melanoma |
Novel pharmacologic agent in development or clinical trials indicated in bold italics.
Abbreviations: SCF, stem cell factor; UVR, ultraviolet radiation; VEGFR, vascular endothelial growth factor receptor.
G-protein coupled receptors
G-protein-coupled receptors, which initiate cellular signaling, constitute the largest class of cell-surface localized receptors whose genes account for about 5% of the human genome [41]. The ligands of GPCRs are chemically diverse and include neurotransmitters, hormones, phospholipids, odorants, and, in phototransduction, photons. Receptor agonists, in some cases, can activate multiple members of GPCR families, such as the neurotransmitter catecholamines that can activate nine distinct members of the adrenergic family of GPCRs. Cytoplasmic domains of activated GPCRs transduce signals to activate guanine nucleotide exchange of one or more heterotrimeric G proteins found on the inner leaflet of the cell membrane. Activation stimulates the exchange of bound GDP for GTP by the G-protein α (Gα) subunits and the functional dissociation of the Gβγ complex. Gα and the Gβγ complexes then act to regulate the activity of members of the larger family of G-protein effector molecules, including adenylyl cyclase, phospholipase Cb, cyclic nucleotide phosphodiesterases, and various ion channels. Among the numerous factors regulating melanocyte/melanoma behavior, the proopiomelanocortin (POMC)-derived peptides MSH, ACTH, and β-endorphin appear to be the most important [1,2,11,17]. In this context, and of particular interest, is the demonstration of MC1R activity up-regulation by UV light, indicating a novel site for physicochemical interactions with the environment [1,22]. Other important regulators of normal and malignant melanocyte activities include endothelines, histamine, eocosanoids, catecholamines, corticotrophin-releasing hormone (CRH), serotonin, and melatonin acting through corresponding GPCRs [1,11,17,42,43]. There is also substantial overlapping in the regulation of melanocyte behavior by the above factors [1].
GPCRs are considered among the most desirable targets for drug development, and many GPCRs, such as endothelin receptors, chemokine receptors, metabotropic glutamate receptors, and protease-activated receptors have been regulated in human cancers [44]. Using microarray data, some GPCRs, such as endothelin receptor A (ENRA), may be involved in early tumor progression, and others, such as CXCR4, may play a critical role in tumor invasion and metastasis [44].
Receptors for proopiomelanocortin-derived peptides
The precursor protein POMC produces many biologically active peptides via a series of enzymatic steps in a tissue-specific manner, yielding α, β, γ-MSH, ACTH) and β-endorphin [17]. The α, β, γ-MSH and ACTH peptides bind with different affinities to the extracellular G-protein—coupled MCR1-5 [1,17,45]. Of those, the most important role in the regulation of the melanocyte behavior is played by MCR1. Although there are reports showing MCR2 expression on melanocytes [46,47], its role in normal and malignant melanocytes is unclear. Concerning β-endorphin, recent reports have shown that the cutaneous β-endorphin/μ-opiate receptor system is functionally active via its ability to up-regulate melanocyte dendricity, proliferation, and pigmentation [48].
Melanocortin receptor type
The MC1R (activated with similar efficiency by α-MSH and ACTH) shows widespread cutaneous expression and influence behavior of both melanocytes and keratinocytes as well as the skin immune system [1,17,26,45,49]. Mutations in the MC1R gene lead to fair skin and red hair in humans, which is also seen with inactivating human POMC gene mutations. MC1R mutant receptor expression changing the receptor activity is also listed as one of etiologic factors responsible for an increased incidence of the melanoma and nonmelanoma skin cancers [23,24].
The MC1R has pleiotropic effects including the modulation of a wide range of immune actions such as anti-inflammatory actions, expression of adhesion molecules, and expression inflammatory transcription factors (down-regulation of NFκB)[1,50]. It has been proposed that these actions would be consistent with a cytoprotective role for this hormone in protecting skin cells from exogenous stress, such as ultraviolet radiation (UVR), exposure to biological agents, or oxidative stress. In addition to actions on normal skin cells, MC1R ligands also modulate both cutaneous and uveal melanoma cell behavior. With respect to melanoma, it is intriguing to see reports showing that while α-MSH has the potential to retard metastatic spread it can also reduce the immune response against melanoma (for example through down-regulation of adhesion molecules) [45]. Thus, role of the MC1R extends far beyond cutaneous and hair pigmentation and includes modulation of immune system, cell viability, cell differentiation, and ligand-induced activation of detoxification system in melanocytes [1,26,45]. However, progression of melanoma may owe at least some of its success to the “protective” role of α-MSH [45].
Cutaneous pigmentation is determined by the amounts of eumelanin and pheomelanin synthesized by epidermal melanocytes, which is also regulated by MC1R [1]. The human MC1R gene is highly polymorphic, and certain allelic variants of the gene are associated with red hair phenotype, melanoma, and nonmelanoma skin cancer [24,51]. The impact of specific polymorphisms in the MC1R on the responses of melanocytes to melanotropins and UV radiation have been investigated [26,52]. Human melanocytes homozygous for Arg160Trp, heterozygous for Arg160Trp and Asp294His or for Arg151Cys and Asp294His substitutions, but not melanocytes homozygous for Val92Met substitution, in the MC1R, showed a significantly reduced response to its specific ligands [53,54]. Presence of a non-functional MC1R increased in their sensitivity to the cytotoxic effect of UV radiation, suggesting that loss-of-function mutations in the MC1R gene sensitize melanocytes to the DNA damaging effects of UV radiation, thereby increasing risk of melanoma development [53].
Targeting melanocortin receptors
Both natural-occurring and synthetic peptides have been used as inhibitors or agonists to identify the pathophysiologic role of MCR in different tissues [55]. For systemic processes, MCR inhibitors, mainly via prevention of NFkB activation, have been proposed as modulators of the immune system to dampen damage from inflammation, ischemia and infection, or as suppressors of appetite to prevent obesity. Radioconjugates of a cyclic peptide analog of α-MSH (eg, coupled with 111In-DOTA) are being studied as selective agents for early detection of melanoma using positron-emission tomography imaging and as potential therapeutic agents [56,57]. The Y86 conjugate appears promising based on high selective uptake in B16 melanoma with rapid clearance from normal tissues [57].
Corticotrophin-releasing hormone receptor
Melanocytes and melanoma cells express G protein—coupled CRH-R1 responding to CRH and urocortin peptides (exogenous or produced locally) through activation of cAMP, IP3, and Camediated pathways to modify the melanocyte phenotype [30,58,59]. In both normal and immortalized melanocytes, CRH inhibited cell proliferation in serum-containing medium, while inhibiting early and late apoptosis in serum free media [58]. Concerning melanoma cells, the effect was heterogenous depending on cell line [58,60]. The variability in CHR action on melanoma cells could be explained by co-expression of alternatively spliced CRH-R1 isoforms on the same cells that would modify the action of the CRH-R1α isoform [30,59]. Of significance, an antimelanoma effect for selective CRH-R1 agonists has already been observed in experimental models of melanoma in vivo [60]. Accordingly, selective targeting of CRH-R1 has been proposed for the treatment of malignant tumors that include melanoma (patent WO0153777).
Endothelin receptors
Endothelins (EDN-1, EDN-2, EDN-3) are paracrine signal peptides that bind to at least two subtypes of GPCR (EDNRA and EDNRB) [36,61,62]. Endothelins induce normal human melanocyte proliferate, produce melanin, and affect chemokinesis, chemotaxis, and dendricity. Keratinocytes produce EDN-1 after exposure to UVB. In melanoma cell lines, EDN induces down-regulation of E-cadherin expression and up-regulates N-cadherin, increases αvβ3 integrin expression and tumor proteolytic activity; these events enhance melanoma cell adhesion, migration, and invasiveness. The selective blockade of EDNRB results in inhibition of focal adhesion kinase (FAK) and mitogen-activated protein kinase (MAPK) phosphorylation and cell proliferation induced by EDN [62]. EDN-1 also acts as a autocrine/paracrine growth factor or an antiapoptotic factor in human cancers, and blockade of ET-1 receptors can sensitize human tumor cells to apoptosis [26,63]. In an experimental study, bosentan, a dual EDNR(A/B)-receptor antagonist, decreased melanoma cell viability and DNA synthesis and induced melanoma cell apoptosis in defined human melanoma cells [63]. In addition, the effects of bosentan and alkylating agents were additive in melanoma cells [63].
Melatonin receptors
Melatonin is an indole with pleiotropic bio-activities, which are mediated through interactions with high-affinity membrane bound or nuclear receptors or through nonreceptor actions. Because melatonin receptors are expressed in melanocytes and melanoma cells, these have the potential to mediate phenotypic actions on cellular proliferation and differentiation [16,42,43,64]. In addition, its receptor-independent activity suggests that melatonin could also have a protective role against UV-induced pathology [42,43,65]. Both biosynthetic and biodegradative pathways for melatonin have been initially characterized in whole human skin and in melanoma cells [42,43,66,67].
Melatonin has also been reported to exhibit tumorostatic properties in different tumor models that include melanomas [42,43]. In addition, several clinical studies have reported positive results with melatonin in patients with metastatic malignant melanoma. Most recently, melatonin has been shown to have tumorostatic effect in human melanoma cell lines of different behavior [65]. The intensity of the oncostatic response to melatonin was related to the cell-line—specific pattern of melatonin cell surface and nuclear receptor expressions [65]. Thus, targeting melatonin receptors may represent a realistic approach in melanoma therapy [42].
Frizzled-5 receptor
Frizzled receptors are GPCR that transduce signals upon Wnt binding leading to stabilization of β-catenin. In the absence of Wnt signaling, cytoplasmic β-catenin is degraded via an ubiquitin-mediated pathway, after phosphorylation by glycogen synthase kinase-β (GSK3β) [68]. Upon stabilization, β-catenin accumulates in the cytoplasm, some of which translocates into the nucleus where it participates in transcription via ternary complex factors (TCFs), lymphoid-specific DNA-binding proteins, regulating genes involved in development and tumorigenesis such as c-Myc, matrix metalloproteinase, and cyclin D1 [68]. In melanoma, gene expression profiling has shown increased expression of Wnt5a/Frizzled in subsets of tumors [69]; this increased expression of Wnt and its receptor Frizzled correlates with histologic features of invasiveness [7,70].
Metabotropic glutamate receptor 1
Metabotropic glutamate receptor 1 (GRM1) is GPCR, the ligand of which is glutamate that was identified as aberrantly expressed in transgenic mouse model of spontaneous melanoma [71]. Its activity, whether agonist or constitutively induced, can be blocked by the inhibitor BAY 36-7620; thus, this receptor could be a target for therapy if it plays a role in human melanomas.
Chemokine receptors
Chemokines belong to a superfamily of small, cytokine-like molecules characterized by four conserved cysteine residues and by their capacity for binding to particular GPCR [72]. Chemokines regulate the directional migration of leukocytes; thus, they play an important role in infection and inflammation. These molecules also play a broader role in the biological process as exemplified by the CXC chemokine receptor CXCR4 and its ligand CXCL12 (also known as stromal-derived factor-1): CXCR4 is linked to human immunodeficiency virus-1 (HIV-1) infection (serving as a coreceptor required for entry of HIV-1 into T cells), hematopoietic stem cell mobilization [73], and developmental processes such as embryogenesis, organogenesis, and angiogenesis. In addition, CXCR4 has been implicated in tumor progression where it is highly expressed in breast cancer cells. In vivo neutralization of CXCR4 by monoclonal antibodies (mAbs) significantly impaired metastasis of breast cancers to regional lymph nodes and lung, indicating the involvement of CXCR4 in selective metastases of breast cancer to certain organs [74]. In melanoma [75], expression of CXCR4 in vivo selectively enhances the metastatic potential of melanoma cells to lung, but not to other organs. For primary cutaneous melanoma, CXCR4 is a prognostic marker indicative of a high risk of relapse; therefore, patients expressing this receptor warrant close follow-up [76]. Based on the above, chemokine receptors would be an attractive target to regulate the migration of tumor cells in vivo. Small molecular inhibitors of CXCR4 such as AMD3100 (byciclam) that are available orally, have been developed and may be of value in the treatment of metastatic melanoma [77].
Receptor tyrosine kinases
Protein receptor kinases, which catalyze the phosphorylation of specific tyrosine residues on their substrate proteins is another major cell signaling paradigm. Protein receptor kinases are composed of both receptor tyrosine kinases (RTKs) and nonreceptor tyrosine kinases (NRTKs). These enzymes are involved in cellular signaling pathways that regulate key cell functions such as cellular proliferation, differentiation, and apoptosis [41]. Examples of RTKs include the cell surface receptors for insulin (IR), insulinlike growth factor-I (IGF-IR), and epidermal growth factor (EGFR). NRTKs are made up of nine distinct families: Src, Jak, Abl, Fak, Fps, Csk, Syk, Pyk2, and Btk. These families share protein—protein interaction domains (eg, SH2 [Src homology 2] and SH3 domains) that mediate many of their actions. Protein tyrosine receptor kinases have an extracellular ligand binding domain, a transmembrane domain, and an intracellular catalytic domain. Activation of the receptor is achieved by ligand binding to the extracellular domain, which induces dimerization of the receptors. Receptors then are able to autophosphorylate tyrosine residues outside the catalytic domain, which form SH2 or phosphotyrosine binding (PTB) sites. The SH2 and PTB domains serve as docking sites for the recognition and recruitment of SH2-domain—containing proteins (eg, NRTK Src and Ras).
c-Kit (CD117)
C-Kit is an RTK for SCF, which is a growth factor for melanocytes that affects melanogenesis, proliferation, migration, and survival. Activation of c-Kit significantly promotes migration of the melanocytes both in vitro and in vivo, suggesting that, in mammalian melanocytes, activation of the cKit is primarily responsible for transmission of promigration signals, which may antagonize proliferation and melanogenesis [78]. In melanoma, the unregulated activity of c-Kit may be caused by overexpression, autocrine loops, or mutational activation; thus, targeting c-Kit with antagonists such as imatinib (Gleevec) is a potentially exploitable target in metastatic melanoma. Nevertheless, a recent clinical trial report concluded that imatinib as a single agent had no activity in patients with relapsed, refractory metastatic melanoma [79]. It appears that c-Kit was expressed at low levels on only a small minority of metastatic melanomas accounting for, in part, the lack of imatinib activity.
Vascular endothelial growth factor, hepatocyte growth factor, insulinlike growth factor-1, basic fibroblastic growth factor, and eye-derived growth factor receptors
All of these growth factor receptors have a tyrosine kinase activity and activate signaling pathways that alter gene expression patterns and induce proliferation. In many cancers, both the overexpression of the growth factor and the receptor, besides mutations at the cytoplasmic tyrosine kinase domain, contribute to constitutive signaling; thus, these receptors make attractive targets for targeted therapies [80]. For example, in the transition from radial to vertical growth phase, melanoma as well as angiogenesis is heralded by both the expression and release of vascular endothelial growth factor (VEGF), which facilitates both growth of new blood and the tumor [29,81,82]; inhibition of VEGF receptor (VEGFR) by sorafenib (formally known as BAY 43-9006 [83,84] in combination with antibodies that block VEGF such as bevacizumab might represent important combination therapy in metastatic melanoma patients. Early clinical development of sorafenib given with carboplatin and paclitaxel to patients with relapsed, refractory metastatic melanoma shows striking activity with prolonged progression-free survival (PFS; median PFS, 10 months) [85,86]. Randomized phase III clinical trials are underway in North America, Europe, and Australia evaluating this combination in metastatic melanoma.
Death receptors
Death receptors are transmembrane proteins that belong to the tumor necrosis factor (TNF) family of receptors [87]. They are activated by specific extracellular or type II transmembrane family member ligands, that rapidly trigger cellular death via an intracellular proteolytic cascade, which leads to irreversible cleavage of proteins involved maintenance of vital cell functions [39,87]. In melanocytes, 3 death receptor family members appear to be prominent: Fas (CD95) and TNF-related apoptosis-inducing ligand cell receptors 1 and 2 (TRAIL-R1 [DR-4] and TRAIL-R2 [DR-5], also known as DR4/TNFRSF10A and DR5/TNFRSF10B, respectively) [40,88,89]. Agents that can trigger Fas or TRAIL-mediated cell death may prove effective in the treatment of metastatic melanoma [89,90]. Blockade of expression of death receptor ligands, such as PD-L1 (programmed death-ligand #1, member of the B7 family of costimulatory molecules), which appear to inhibit human tumor-specific T cell responses, is also another target for melanoma therapy [91].
Hersey and associates have characterized death receptor expression by human melanoma in detail and most melanoma lines examined express DR4 and DR5 [92,93]. However, many melanoma lines that express DR4/DR5 are not susceptible to TRAIL-mediated apoptosis suggesting additional determinants that down-regulate sensitivity such as protein kinase C (PKC) activation [92]. Recent data confirm DR5 expression at relatively high levels in approximately 75% of primary cutaneous melanoma; interestingly, DR4 expression was significantly lower in both primary lesions and metastatic melanoma [88]. Novel therapeutics (recombinant ligands and monoclonal antibodies) specific for DR4/DR5 are currently in early-phase clinical trials. Fas (also known as APO-1 or CD95) is a type II membrane receptor of the TNF family and is known to be the receptor for Fas ligand (FasL). FasL engagement (or monoclonal antibody crosslinking) of cell surface Fas triggers tumor cell death by apoptosis in a variety of experimental systems. Expression of Fas by human cutaneous melanomas has been documented in approximately 60% of cases [94]; only a small minority (3 of 44 primary cutaneous melanomas) had detectable mutations in Fas [95]. A survey of 13 established human melanoma lines showed low-level Fas expression in 5 of 13 lines [40]; however, this has been challenged because another group has reported high cell surface Fas expression on 15 of 17 melanoma lines examined [96]. The significance of these findings remains unclear.
Cell adhesion receptors
Melanocyte homeostasis is also governed by intercellular communication via cell-cell adhesions and cell-extracellular matrix (ECM) adhesion [10,97]. In this manner, transmembrane cell adhesion receptors influence whether a cell remains quiescent or proliferates, differentiates, or undergoes apoptosis; thus, the nature of cell-cell and cell-ECM interactions are integral in the regulation of homeostasis and tissue phenotype [98]. Alterations in the expression and/or adhesive interactions of cell adhesion receptors can trigger unchecked proliferation and altered invasive properties of melanocytes. In the development of melanoma, accumulating evidence has shown the importance of the tumor microenvironment on the behavior of malignant cells, where interactions between melanoma cells and stromal cells create a context that promotes the transitions from normal to benign to in situ to locally invasive to metastatic lesions [10,29,99]. Alterations in cell adhesion receptors are important in the processes underlying these transitions. Specifically, melanoma progression is associated with the loss of E-cadherin function and with up-regulation or induction of N-cadherin, MelCAM, and integrin αVβ3.
Cadherins
These calcium-dependent transmembrane proteins modulate cell-cell adhesion [100]. Alterations in the form and expression of cadherins appear critical in melanoma progression. Frequently, a switch from E- to N-cadherin expression occurs during the transition from radial to vertical growth phase. Loss of E-cadherin expression appears to be an important way that melanocytic cells detach from basal keratinocytes. Re-expression of E-cadherin in melanoma cells reduces their tumorigenicity in vivo [101]. N-cadherin expression is frequently up-regulated in melanoma cells and allows for interactions with fibroblasts and endothelial cells in the dermis [102]. In this manner, enhanced N-cadherin expression promotes melanoma cell migration and survival.
αvβ3-integrin
Integrins are heterodimeric transmembrane glycoproteins that participate in cell adhesion, migration, and proliferation. In particular the vitronectin/fibronectin receptor αvβ3 is up-regulated in melanoma progression [103]. Expression of the β3 subunit is controlled by the RAF-MEK-ERK1/2 pathway that is highly active in melanoma cells because of either mutational activation of BRAF or RAS [104]. Antagonists to αvβ3 integrin can block tumor-associated angiogeneisis and cause tumor regression [105]. This integrin has an Arg-Gly-Asp (RGD) binding site that can be targeted by radiolabeled peptides [56,105] or inhibited by the cyclic peptide cilengitide (EMD 121,974) [106]. Expression of αVβ3 integrin allows melanoma cells to bind to and localize matrix metalloproteinase (MMP)-2 (aka, type 4 collagenase/gelatinase A), which is suspected to augment the metastatic capability of melanoma cells by degrading the basement membrane zone and surrounding tissues. The development of agents that can block these interactions, such as antibodies, could stop metastatic spread of melanoma [107]. Recent studies have generated αvβ3-targeting nanoparticle to deliver a kinase-defective form of C-RAF to angiogenic blood vessels in mice [108]. It would be of considerable interest if a similar nanoparticle could be generated to block B-RAF signaling in αvβ3-expressing melanoma cells.
MelCAM
MelCAM, a member of the IgG superfamily of cell adhesion molecules [100], is another good marker of tumor progression in human melanoma [109-111]. It has been primarily linked to a role in invasion and metastasis. MelCAM expression in nontumorigenic melanoma cells mediates invasion of melanoma cells in a human skin reconstruct system [112] and tumorigenicity in nude mice [113]. Additionally, reduced MelCAM expression or antibody-mediated function blocking of Mel-CAM inhibits melanoma cell tumorigenicity and metastasis [112,114]. MelCAM participates in both homotypic and heterophilic interactions, although in the latter case the nature of the ligand remains to be defined [107,115,116]. MelCAM has a reciprocal regulation loop with the serine/threonine kinase, AKT. Inhibition of elevated AKT activity in human melanoma cell lines substantially reduced the expression of MelCAM; conversely, overexpression of constitutively active AKT up-regulated the levels of MelCAM [117]. In addition, overexpression of MelCAM in melanoma cells activated endogenous AKT and inhibited the proapoptotic protein BAD, leading to increased survival under stress conditions. Studies on melanoma cells implanted in nude mice have shown blocking antibodies to MelCAM inhibit cell growth and metastasis [114]. Therefore, the MelCAM-AKT signaling axis in melanoma is a potential target for therapy.
Notch receptors
In humans, the Notch family consists of 4 transmembrane receptors (Notch1-4) and 5 ligands (Jagged 1-2, Delta1-3). Notch receptors 1 and 4 may be up-regulated in melanoma [118,119]. Ligand binding leads to metalloproteinase- and γ-secretase—mediated proteolysis and cleavage of Notch1 intracellular from the plasma membrane [120,121]. Notch1 is translocated to the nucleus, where it associates with transcription factors RBP-Jk/CSL and mastermind-like (MAML) to form a heteromeric complex that mediates the transcription of genes in the hairy enhancer of split (HES). Notch receptors and their ligands regulate cell fate specification, differentiation, proliferation, and survival during cell-cell contact. In melanoma, Notch1 signaling drives the vertical growth phase of melanoma toward more aggressive phenotypes via activation of MAPK and PI3K-Akt pathways, increased tumor cell adhesion, and expression of N-cadherin [121]. Blocking of Notch signaling involved in melanoma progression may be a potential target of therapy in metastatic melanoma. Initial studies addressing this issue have shown that a γ-secretase inhibitor compound induced apoptosis in melanoma cell lines [122].
Nuclear receptors
Estrogen androgen receptors
Normal melanocytes and melanoma cells have been shown to express functional receptors for steroidal sex hormones (reviewed in [1]). Androgen action appears to stimulate melanin pigmentation; however, cell culture studies showed conflicting results. Nevertheless, melanocytes from genital skin do express androgen receptors and can transform testosterone to dihydrotestosterone (DHT). Similarly, genital melanocytes do express functional estrogen receptors; however, estrogen has shown inconsistent effects on proliferation and tyrosinase activity of cultured human foreskin melanocyte [1]. On the other hand, patients with elevated serum estrogen concentrations tend to have increased skin pigmentation, suggesting that estradiol may be involved in the pathogenesis of melasma (chloasma). These diverse reports suggest that the effects of androgens and estrogens on melanocyte activity may be influenced by multiple factors, such as culture conditions, sex, age, and anatomic localization [1]. Concerning melanoma, it is know that its incidence rates rise in women until about age 50, and recent studies have found a correlation between estrogen receptor α (ERα) and melanoma progression [123]. Additional experimental studies are required to determine whether estrogen and androgen receptors could serve as a target for melanoma therapy.
Glucocorticoids receptors
Both inhibition and stimulation of melanin synthesis have been reported after glucocorticoid treatment, and this class of nuclear receptors may also be involved in melanocyte development (reviewed in [1]). The glucocorticoid receptors (GR) are expressed in the majority of human melanomas, with higher expression levels in metastatic sites, and, notably, glucocorticoids can inhibit growth of experimental melanomas in mice and GR-expressing human melanoma cells in culture [1]. However, potent immunosuppressive properties of glucorticoids could serve as a limiting factor in such a therapeutic approach, but one study found that glucocorticoids did not affect adoptive cell transfer (ACT)-based immunotherapy regimen in transplanted B16 melanoma [124]. Finally, epidemiologic studies showed that glucocorticoid-based therapy appeared to be protective against melanoma incidence in a Mediterranean population [125]. Thus, glucocorticoids and their receptors could be of help in melanoma therapy; however, further studies are needed to better define this issue.
Vitamin D3 receptors
Vitamin D3 is formed in the skin by UVB-mediated photolysis of 7-dehydrocholesterol with following thermal isomerization [126]. To exert its bioregulatory action, vitamin D3 must be converted to its active form 1,25-dihydoxycholecalciferol locally (skin) or at the systemic level (liver and kidney) [126]. 1,25-dihydroxyvitamin D3 [1,25(OH)2D3; calcitriol] is inactivated through hydroxylation at position 24 [126]. 1,25(OH)2D3 has pleiotropic activities that, in addition to regulation of calcium metabolism, also acts as a potent regulator of cell growth, differentiation, apoptosis, and immune and endocrine functions [126-129]. The signaling pathway of 1,25(OH)2D3 uses the vitamin D nuclear receptor (VDR), which is a transcription factor for 1,25(OH)2D3 target genes that have a major inhibitory effect on the G1/S checkpoint of the cell cycle by up-regulating the cyclin-dependent kinase inhibitors p27 and p21, and by inhibiting cyclin D1. Indirect mechanisms include up-regulation of transforming growth factor-beta and down-regulation of the epidermal growth factor receptor. 1,25(OH)2D3 may induce apoptosis either indirectly through effects on the IGFR and TNF-α or more directly via the Bcl-2 family system, the ceramide pathway, the death receptors (eg, Fas), and the stress-activated protein kinase pathways (Jun N terminal kinase and p38). Taking into consideration well-documented antitumorigenic properties of vitamin D3, it is becoming evident that targeting VDR may serve as a potential adjuvant therapy of melanoma [130]. Specifically, melanocytes and melanoma cells do express functionally active VDR, which apparently mediate vitamin D3 antimelanoma effects (reviewed in [1,16]). Thus, the antiproliferative and prodifferentiation effects of 1,25(OH)2D3 have been shown in cultured melanocytes, MM cells, and MM xenografts. Furthermore, melanoma patients may present with lower levels of 1,25(OH)2D3 in their sera [131], and polymorphisms at the VDR has been suggest to influence susceptibility to malignant melanoma [132,133]. Finally, epidemiologic studies by some investigators found that melanoma patients do better if exposed to solar radiation after removal of the tumor, an indirect indication of a beneficial role for vitamin D metabolites during disease progression [134]. However, other investigators have questioned this correlation [135]. Nevertheless, there is sufficient experimental evidence for the use of vitamin D3 or its analogs in melanoma therapy. Unfortunately, potential use of vitamin D in therapy of melanoma may be limited, because of well-documented toxicity (hypercalcemia) of vitamin D3 derivatives when used at pharmacologic concentrations. Nevertheless, this cytoxicity (hypercalcemic effect) can be decreased or eliminated by cleavage or modification of the side chain [136]. Most recently, it has been uncovered that the classical enzyme of steroidogenesis cytochrome P450scc can also cleave the side chain of 7-DHC to produce 7-dehydropregnenolone (7-DHP) identifying a novel metabolic pathway producing 5,7 diene hydroxysteroids that after UVB exposure could generate secosteroidal vitamin D—like (VitDL) products [137,138]. Moreover, vitamin D3, ergosterol (provitamin D2) and vitamin D2 are additionally metabolized by the same P450scc producing hydroxyderivatives [42,139,140], some of which induce differentiation and inhibit proliferation of skin cells [139,141]. Thus, new family of vitamin D derivatives was identified that could be tested for its potential antimelanoma activity.
Summary
Cell surface and nuclear receptors and adhesion molecules are important regulators of critical pathways in normal and pathologic melanocytes. Antagonists and agonists of these proteins offer a novel, disease-specific means of targeted therapy. Much of the current development of anticancer therapies tries to target causative proteins in a specific manner to minimize side effects, some melanocyte receptors discussed in this review may become the targets for therapy of metastatic melanoma in the near future.
Acknowledgments
Reviewed work was supported in part by National Institutes of Health (NIH) grants AR047079 and AR052190 from National Institute of Arthritis and Muskuloskeletal and Skin Disease and University of Tennessee Cancer pilot grant to Andrzej Slominski, and NIH grant R01-GM067893 to Andrew Aplin.
References
- [1].Slominski A, Tobin DJ, Shibahara S, et al. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84(4):1155–228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- [2].Slominski A, Wortsman J, Plonka PM, et al. Hair follicle pigmentation. J Invest Dermatol. 2005;124(1):13–21. doi: 10.1111/j.0022-202X.2004.23528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Holbrook KA. Melanocytes in human embryonic skin and fetal skin: review and new findings. Pigment Cell Res. 1998;1(Suppl):6–17. [Google Scholar]
- [4].Yoshida H, Kunisada T, Grimm T, et al. Review: melanocyte migration and survival controlled by SCF/c-kit expression. J Invest Dermatol Symp Proc. 2001;6(1):1–5. doi: 10.1046/j.0022-202x.2001.00006.x. [DOI] [PubMed] [Google Scholar]
- [5].Fleischman RA, Saltman DL, Stastny V, et al. Deletion of the c-kit protooncogene in the human developmental defect piebald trait. Proc Natl Acad Sci U S A. 1991;88(23):10885–9. doi: 10.1073/pnas.88.23.10885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Christiansen JH, Coles EG, Wilkinson DG. Molecular control of neural crest formation, migration and differentiation. Curr Opin Cell Biol. 2000;12(6):719–24. doi: 10.1016/s0955-0674(00)00158-7. [DOI] [PubMed] [Google Scholar]
- [7].Weeraratna AT, Jiang Y, Hostetter G, et al. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1(3):279–88. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- [8].Bachmann IM, Straume O, Puntervoll HE, et al. Importance of P-cadherin, beta-catenin, and Wnt5a/frizzled for progression of melanocytic tumors and prognosis in cutaneous melanoma. Clin Cancer Res. 2005;11(24 Pt 1):8606–14. doi: 10.1158/1078-0432.CCR-05-0011. [DOI] [PubMed] [Google Scholar]
- [9].Kunisada T, Yamazaki H, Hayashi SI. Review: ligands for receptor tyrosine kinases expressed in the skin as environmental factors for melanocyte development. J Investig Dermatol Symp Proc. 2001;6(1):6–9. doi: 10.1046/j.0022-202x.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- [10].Haass NK, Herlyn M. Normal human melanocyte homeostasis as a paradigm for understanding melanoma. J Investig Dermatol Symp Proc. 2005;10(2):153–63. doi: 10.1111/j.1087-0024.2005.200407.x. [DOI] [PubMed] [Google Scholar]
- [11].Slominski A, Paus R, Schadendorf D. Melanocytes as “sensory” and regulatory cells in the epidermis. J Theor Biol. 1993;164(1):103–20. doi: 10.1006/jtbi.1993.1142. [DOI] [PubMed] [Google Scholar]
- [12].Moriyama M, Osawa M, Mak SS, et al. Notch signaling via Hes1 transcription factor maintains survival of melanoblasts and melanocyte stem cells. J Cell Biol. 2006;173(3):333–9. doi: 10.1083/jcb.200509084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Berking C, Takemoto R, Satyamoorthy K, et al. Induction of melanoma phenotypes in human skin by growth factors and ultraviolet B. Cancer Res. 2004;64(3):807–11. doi: 10.1158/0008-5472.can-03-3438. [DOI] [PubMed] [Google Scholar]
- [14].Noonan FP, Dudek J, Merlino G, et al. Animal models of melanoma: an HGF/SF transgenic mouse model may facilitate experimental access to UV initiating events. Pigment Cell Res. 2003;16(1):16–25. doi: 10.1034/j.1600-0749.2003.00014.x. [DOI] [PubMed] [Google Scholar]
- [15].Wood JM, Jimbow K, Boissy RE, et al. What’s the use of generating melanin? Exp Dermatol. 1999;8(2):153–64. doi: 10.1111/j.1600-0625.1999.tb00365.x. [DOI] [PubMed] [Google Scholar]
- [16].Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21(5):457–87. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- [17].Slominski A, Wortsman J, Luger T, et al. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80(3):979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- [18].Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(Suppl 61):1–6. doi: 10.1046/j.1365-2133.146.s61.2.x. [DOI] [PubMed] [Google Scholar]
- [19].Gilchrest BA, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340(17):1341–8. doi: 10.1056/NEJM199904293401707. [DOI] [PubMed] [Google Scholar]
- [20].Kennedy C, ter Huurne J, Berkhout M, et al. Melanocortin 1 receptor (MC1R) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair color. J Invest Dermatol. 2001;117(2):294–300. doi: 10.1046/j.0022-202x.2001.01421.x. [DOI] [PubMed] [Google Scholar]
- [21].Carlson JA, Slominski A, Linette GP, et al. Biomarkers in melanoma: predisposition, screening and diagnosis. Expert Rev Mol Diagn. 2003;3(2):163–84. doi: 10.1586/14737159.3.2.163. [DOI] [PubMed] [Google Scholar]
- [22].Slominski A, Pawelek J. Animals under the sun: effects of ultraviolet radiation on mammalian skin. Clin Dermatol. 1998;16(4):503–15. doi: 10.1016/s0738-081x(98)00023-6. [DOI] [PubMed] [Google Scholar]
- [23].Han J, Kraft P, Colditz GA, et al. Melanocortin 1 receptor variants and skin cancer risk. Int J Cancer. 2006;119(8):1976–84. doi: 10.1002/ijc.22074. [DOI] [PubMed] [Google Scholar]
- [24].Rees JL. The genetics of sun sensitivity in humans. Am J Hum Genet. 2004;75(5):739–51. doi: 10.1086/425285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hauser JE, Kadekaro AL, Kavanagh RJ, et al. Melanin content and MC1R function independently affect UVR-induced DNA damage in cultured human melanocytes. Pigment Cell Res. 2006;19(4):303–14. doi: 10.1111/j.1600-0749.2006.00315.x. [DOI] [PubMed] [Google Scholar]
- [26].Kadekaro AL, Wakamatsu K, Ito S, et al. Cutaneous photoprotection and melanoma susceptibility: reaching beyond melanin content to the frontiers of DNA repair. Front Biosci. 2006;11:2157–73. doi: 10.2741/1958. [DOI] [PubMed] [Google Scholar]
- [27].Landi MT, Bauer J, Pfeiffer RM, et al. MC1R germline variants confer risk for BRAF-mutant melanoma. Science. 2006;313(5786):521–2. doi: 10.1126/science.1127515. [DOI] [PubMed] [Google Scholar]
- [28].Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- [29].Carlson JA, Ross JS, Slominski A, et al. Molecular diagnostics in melanoma. J Am Acad Dermatol. 2005;52(5):743–75. doi: 10.1016/j.jaad.2004.08.034. [quiz: 775–8] [DOI] [PubMed] [Google Scholar]
- [30].Slominski A, Wortsman J, Pisarchik A, et al. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15(10):1678–93. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- [31].Chin L. The genetics of malignant melanoma: lessons from mouse and man. Nat Rev Cancer. 2003;3(8):559–70. doi: 10.1038/nrc1145. [DOI] [PubMed] [Google Scholar]
- [32].Sharpless E, Chin L. The INK4a/ARF locus and melanoma. Oncogene. 2003;22(20):3092–8. doi: 10.1038/sj.onc.1206461. [DOI] [PubMed] [Google Scholar]
- [33].Daniotti M, Oggionni M, Ranzani T, et al. BRAF alterations are associated with complex mutational profiles in malignant melanoma. Oncogene. 2004;23(35):5968–77. doi: 10.1038/sj.onc.1207780. [DOI] [PubMed] [Google Scholar]
- [34].Gorden A, Osman I, Gai W, et al. Analysis of BRAF and N-RAS mutations in metastatic melanoma tissues. Cancer Res. 2003;63(14):3955–7. [PubMed] [Google Scholar]
- [35].Grichnik JM, Burch JA, Schulteis RD, et al. Melanoma, a tumor based on a mutant stem cell? J Invest Dermatol. 2006;126(1):142–53. doi: 10.1038/sj.jid.5700017. [DOI] [PubMed] [Google Scholar]
- [36].Lahav R. Endothelin receptor B is required for the expansion of melanocyte precursors and malignant melanoma. Int J Dev Biol. 2005;49(2–3):173–80. doi: 10.1387/ijdb.041951rl. [DOI] [PubMed] [Google Scholar]
- [37].Goding CR. Melanocyte development and malignant melanoma. Forum (Genova) 2000;10(3):176–87. [PubMed] [Google Scholar]
- [38].Widlund HR, Fisher DE. Microphthalamia-associated transcription factor: a critical regulator of pigment cell development and survival. Oncogene. 2003;22(20):3035–41. doi: 10.1038/sj.onc.1206443. [DOI] [PubMed] [Google Scholar]
- [39].Wehrli P, Viard I, Bullani R, et al. Death receptors in cutaneous biology and disease. J Invest Dermatol. 2000;115(2):141–8. doi: 10.1046/j.1523-1747.2000.00037.x. [DOI] [PubMed] [Google Scholar]
- [40].Bullani RR, Wehrli P, Viard-Leveugle I, et al. Frequent downregulation of Fas (CD95) expression and function in melanoma. Melanoma Res. 2002;12(3):263–70. doi: 10.1097/00008390-200206000-00010. [DOI] [PubMed] [Google Scholar]
- [41].Gavi S, Shumay E, Wang HY, et al. G-protein-coupled receptors and tyrosine kinases: crossroads in cell signaling and regulation. Trends Endocrinol Metab. 2006;17(2):48–54. doi: 10.1016/j.tem.2006.01.006. [DOI] [PubMed] [Google Scholar]
- [42].Slominski A, Fischer TW, Zmijewski MA, et al. On the role of melatonin in skin physiology and pathology. Endocrine. 2005;27(2):137–48. doi: 10.1385/ENDO:27:2:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005;19(2):176–94. doi: 10.1096/fj.04-2079rev. [DOI] [PubMed] [Google Scholar]
- [44].Li S, Huang S, Peng SB. Overexpression of G protein-coupled receptors in cancer cells: involvement in tumor progression. Int J Oncol. 2005;27(5):1329–39. [PubMed] [Google Scholar]
- [45].Eves PC, MacNeil S, Haycock JW. alpha-Melanocyte stimulating hormone, inflammation and human melanoma. Peptides. 2006;27(2):444–52. doi: 10.1016/j.peptides.2005.01.027. [DOI] [PubMed] [Google Scholar]
- [46].Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J Clin Endocrinol Metab. 1996;81(7):2746–9. doi: 10.1210/jcem.81.7.8675607. [DOI] [PubMed] [Google Scholar]
- [47].Hunt G, Donatien PD, Lunec J, et al. Cultured human melanocytes respond to MSH peptides and ACTH. Pigment Cell Res. 1994;7(4):217–21. doi: 10.1111/j.1600-0749.1994.tb00052.x. [DOI] [PubMed] [Google Scholar]
- [48].Kauser S, Schallreuter KU, Thody AJ, et al. Regulation of human epidermal melanocyte biology by beta-endorphin. J Invest Dermatol. 2003;120(6):1073–80. doi: 10.1046/j.1523-1747.2003.12242.x. [DOI] [PubMed] [Google Scholar]
- [49].Manna SK, Sarkar A, Sreenivasan Y. Alpha-melanocyte-stimulating hormone down-regulates CXC receptors through activation of neutrophil elastase. Eur J Immunol. 2006;36(3):754–69. doi: 10.1002/eji.200535209. [DOI] [PubMed] [Google Scholar]
- [50].Moustafa M, Szabo M, Ghanem GE, et al. Inhibition of tumor necrosis factor-alpha stimulated NFkappaB/p65 in human keratinocytes by alpha-melanocyte stimulating hormone and adrenocorticotropic hormone peptides. J Invest Dermatol. 2002;119(6):1244–53. doi: 10.1046/j.1523-1747.2002.19602.x. [DOI] [PubMed] [Google Scholar]
- [51].Healy E. Melanocortin 1 receptor variants, pigmentation, and skin cancer susceptibility. Photodermatol Photoimmunol Photomed. 2004;20(6):283–8. doi: 10.1111/j.1600-0781.2004.00132.x. [DOI] [PubMed] [Google Scholar]
- [52].Abdel-Malek ZA, Kadekaro AL, Kavanagh RJ, et al. Melanoma prevention strategy based on using tetrapeptide {alpha}-MSH analogs that protect human melanocytes from UV-induced DNA damage and cytotoxicity. FASEB J. 2006;20(9):1561–3. doi: 10.1096/fj.05-5655fje. [DOI] [PubMed] [Google Scholar]
- [53].Scott MC, Wakamatsu K, Ito S, et al. Human melanocortin 1 receptor variants, receptor function and melanocyte response to UV radiation. J Cell Sci. 2002;115(Pt 11):2349–55. doi: 10.1242/jcs.115.11.2349. [DOI] [PubMed] [Google Scholar]
- [54].Abdel-Malek Z, Scott MC, Suzuki I, et al. The melanocortin-1 receptor is a key regulator of human cutaneous pigmentation. Pigment Cell Res. 2000;13(Suppl 8):156–62. doi: 10.1034/j.1600-0749.13.s8.28.x. [DOI] [PubMed] [Google Scholar]
- [55].Getting SJ. Targeting melanocortin receptors as potential novel therapeutics. Pharmacol Ther. 2006;111(1):1–15. doi: 10.1016/j.pharmthera.2005.06.022. [DOI] [PubMed] [Google Scholar]
- [56].Weiner RE, Thakur ML. Radiolabeled peptides in oncology: role in diagnosis and treatment. BioDrugs. 2005;19(3):145–63. doi: 10.2165/00063030-200519030-00002. [DOI] [PubMed] [Google Scholar]
- [57].McQuade P, Miao Y, Yoo J, et al. Imaging of melanoma using 64Cu- and 86Y-DOTA-ReCCMSH(Arg11), a cyclized peptide analogue of alpha-MSH. J Med Chem. 2005;48(8):2985–92. doi: 10.1021/jm0490282. [DOI] [PubMed] [Google Scholar]
- [58].Slominski A, Zbytek B, Pisarchik A, et al. CRH functions as a growth factor/cytokine in the skin. J Cell Physiol. 2006;206(3):780–91. doi: 10.1002/jcp.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Slominski A, Zbytek B, Zmijewski M, et al. Corticotropin releasing hormone and the skin. Front Biosci. 2006;11:2230–48. doi: 10.2741/1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Carlson KW, Nawy SS, Wei ET, et al. Inhibition of mouse melanoma cell proliferation by corticotropin-releasing hormone and its analogs. Anticancer Res. 2001;21(2A):1173–9. [PubMed] [Google Scholar]
- [61].Eberle J, Weitmann S, Thieck O, et al. Downregulation of endothelin B receptor in human melanoma cell lines parallel to differentiation genes. J Invest Dermatol. 1999;112(6):925–32. doi: 10.1046/j.1523-1747.1999.00598.x. [DOI] [PubMed] [Google Scholar]
- [62].Rosano L, Spinella F, Genovesi G, et al. Endothelin-B receptor blockade inhibits molecular effectors of melanoma cell progression. J Cardiovasc Pharmacol. 2004;44:S136–9. doi: 10.1097/01.fjc.0000166247.35992.dd. [DOI] [PubMed] [Google Scholar]
- [63].Berger Y, Bernasconi CC, Juillerat-Jeanneret L. Targeting the endothelin axis in human melanoma: combination of endothelin receptor antagonism and alkylating agents. Exp Biol Med (Maywood) 2006;231(6):1111–9. [PubMed] [Google Scholar]
- [64].Slominski A, Pisarchik A, Zbytek B, et al. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J Cell Physiol. 2003;196(1):144–53. doi: 10.1002/jcp.10287. [DOI] [PubMed] [Google Scholar]
- [65].Fisher T, Zmijewski M, Zbytek B, et al. Oncostatic effects of indole melatonin and expression of its cytosolic and nuclear receptor in cultured human melanoma cell lines. Int J Oncol. 2006;29:665–72. doi: 10.3892/ijo.29.3.665. [DOI] [PubMed] [Google Scholar]
- [66].Slominski A, Pisarchik A, Semak I, et al. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 2002;16(8):896–8. doi: 10.1096/fj.01-0952fje. [DOI] [PubMed] [Google Scholar]
- [67].Slominski A, Semak I, Pisarchik A, et al. Conversion of L-tryptophan to serotonin and melatonin in melanoma cells. FEBS Lett. 2002;511:102–6. doi: 10.1016/s0014-5793(01)03319-1. [DOI] [PubMed] [Google Scholar]
- [68].Weeraratna AT. A Wnt-er wonderland—the complexity of Wnt signaling in melanoma. Cancer Metastasis Rev. 2005;24(2):237–50. doi: 10.1007/s10555-005-1574-z. [DOI] [PubMed] [Google Scholar]
- [69].Bittner M, Meltzer P, Chen Y, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406(6795):536–40. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- [70].Pham K, Milovanovic T, Barr RJ, et al. Wnt ligand expression in malignant melanoma: pilot study indicating correlation with histopathological features. Mol Pathol. 2003;56(5):280–5. doi: 10.1136/mp.56.5.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Namkoong J, Martino JJ, Chen S. From existing therapies to novel targets: a current view on melanoma. Front Biosci. 2006;11:2081–92. doi: 10.2741/1951. [DOI] [PubMed] [Google Scholar]
- [72].Baggiolini M. Chemokines in pathology and medicine. J Intern Med. 2001;250(2):91–104. doi: 10.1046/j.1365-2796.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- [73].Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201(8):1307–18. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- [75].Murakami T, Maki W, Cardones AR, et al. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res. 2002;62(24):7328–34. [PubMed] [Google Scholar]
- [76].Scala S, Ottaiano A, Ascierto PA, et al. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res. 2005;11(5):1835–41. doi: 10.1158/1078-0432.CCR-04-1887. [DOI] [PubMed] [Google Scholar]
- [77].Scala S, Giuliano P, Ascierto PA, et al. Human melanoma metastases express functional CXCR4. Clin Cancer Res. 2006;12(8):2427–33. doi: 10.1158/1078-0432.CCR-05-1940. [DOI] [PubMed] [Google Scholar]
- [78].Alexeev V, Yoon K. Distinctive role of the cKit receptor tyrosine kinase signaling in mammalian melanocytes. J Invest Dermatol. 2006;126(5):1102–10. doi: 10.1038/sj.jid.5700125. [DOI] [PubMed] [Google Scholar]
- [79].Wyman K, Atkins MB, Prieto V, et al. Multicenter Phase II trial of high-dose imatinib mesylate in metastatic melanoma: significant toxicity with no clinical efficacy. Cancer. 2006;106(9):2005–11. doi: 10.1002/cncr.21834. [DOI] [PubMed] [Google Scholar]
- [80].Perona R. Cell signalling: growth factors and tyrosine kinase receptors. Clin Transl Oncol. 2006;8(2):77–82. doi: 10.1007/s12094-006-0162-1. [DOI] [PubMed] [Google Scholar]
- [81].Simonetti O, Lucarini G, Brancorsini D, et al. Immunohistochemical expression of vascular endothelial growth factor, matrix metalloproteinase 2, and matrix metalloproteinase 9 in cutaneous melanocytic lesions. Cancer. 2002;95(9):1963–70. doi: 10.1002/cncr.10888. [DOI] [PubMed] [Google Scholar]
- [82].Graells J, Vinyals A, Figueras A, et al. Overproduction of VEGF concomitantly expressed with its receptors promotes growth and survival of melanoma cells through MAPK and PI3K signaling. J Invest Dermatol. 2004;123(6):1151–61. doi: 10.1111/j.0022-202X.2004.23460.x. [DOI] [PubMed] [Google Scholar]
- [83].Adnane L, Trail PA, Taylor I, et al. Sorafenib (BAY 43-9006, Nexavar((R))), a Dual-Action Inhibitor That Targets RAF/MEK/ERK Pathway in Tumor Cells and Tyrosine Kinases VEGFR/PDGFR in Tumor Vasculature. Methods Enzymol. 2005;407:597–612. doi: 10.1016/S0076-6879(05)07047-3. [DOI] [PubMed] [Google Scholar]
- [84].Sharma V, Kaur I, Kumar B. Calcipotriol versus coal tar: a prospective randomized study in stable plaque psoriasis. Int J Dermatol. 2003;42(10):834–8. doi: 10.1046/j.1365-4362.2003.01974.x. [DOI] [PubMed] [Google Scholar]
- [85].Flaherty KT, Brose M, Schuchter L, et al. Phase I/II trial of BAY 43-9006, carboplatin (C) and paclitaxel (P) demonstrates preliminary antitumor activity in the expansion cohort of patients with metastatic melanoma. J Clin Oncol. 2004;22(14S):7507. [Google Scholar]
- [86].Flaherty KT. Chemotherapy and targeted therapy combinations in advanced melanoma. Clin Cancer Res. 2006;12(7 Pt 2):2366s–70s. doi: 10.1158/1078-0432.CCR-05-2505. [DOI] [PubMed] [Google Scholar]
- [87].Bouralexis S, Findlay DM, Evdokiou A. Death to the bad guys: targeting cancer via Apo2L/TRAIL. Apoptosis. 2005;10(1):35–51. doi: 10.1007/s10495-005-6060-0. [DOI] [PubMed] [Google Scholar]
- [88].McCarthy MM, Divito KA, Sznol M, et al. Expression of tumor necrosis factor-related apoptosis-inducing ligand receptors 1 and 2 in melanoma. Clin Cancer Res. 2006;12(12):3856–63. doi: 10.1158/1078-0432.CCR-06-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kurbanov BM, Geilen CC, Fecker LF, et al. Efficient TRAIL-R1/DR4-mediated apoptosis in melanoma cells by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) J Invest Dermatol. 2005;125(5):1010–9. doi: 10.1111/j.0022-202X.2005.23900.x. [DOI] [PubMed] [Google Scholar]
- [90].Fischer U, Schulze-Osthoff K. Apoptosis-based therapies and drug targets. Cell Death Differ. 2005;12(Suppl 1):942–61. doi: 10.1038/sj.cdd.4401556. [DOI] [PubMed] [Google Scholar]
- [91].Blank C, Kuball J, Voelkl S, et al. Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro. Int J Cancer. 2006;119(2):317–27. doi: 10.1002/ijc.21775. [DOI] [PubMed] [Google Scholar]
- [92].Wu JJ, Zhang XD, Gillespie S, et al. Selection for TRAIL resistance results in melanoma cells with high proliferative potential. FEBS Lett. 2005;579(9):1940–4. doi: 10.1016/j.febslet.2005.02.041. [DOI] [PubMed] [Google Scholar]
- [93].Zhang XD, Franco A, Myers K, et al. Relation of TNF-related apoptosis-inducing ligand (TRAIL) receptor and FLICE-inhibitory protein expression to TRAIL-induced apoptosis of melanoma. Cancer Res. 1999;59(11):2747–53. [PubMed] [Google Scholar]
- [94].Redondo P, Solano T, Vazquez B, et al. Fas and Fas ligand: expression and soluble circulating levels in cutaneous malignant melanoma. Br J Dermatol. 2002;147(1):80–6. doi: 10.1046/j.1365-2133.2002.04745.x. [DOI] [PubMed] [Google Scholar]
- [95].Shin MS, Park WS, Kim SY, et al. Alterations of Fas (Apo-1/CD95) gene in cutaneous malignant melanoma. Am J Pathol. 1999;154(6):1785–91. doi: 10.1016/S0002-9440(10)65434-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Eberle AN, Froidevaux S. Radiolabeled alpha-melanocyte-stimulating hormone analogs for receptor-mediated targeting of melanoma: from tritium to indium. J Mol Recognit. 2003;16(5):248–54. doi: 10.1002/jmr.633. [DOI] [PubMed] [Google Scholar]
- [97].Haass NK, Smalley KS, Li L, et al. Adhesion, migration and communication in melanocytes and melanoma. Pigment Cell Res. 2005;18(3):150–9. doi: 10.1111/j.1600-0749.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- [98].Bissell MJ, Radisky DC, Rizki A, et al. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70(9–10):537–46. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Ruiter D, Bogenrieder T, Elder D, et al. Melanoma-stroma interactions: structural and functional aspects. Lancet Oncol. 2002;3(1):35–43. doi: 10.1016/s1470-2045(01)00620-9. [DOI] [PubMed] [Google Scholar]
- [100].Aplin AE, Howe A, Alahari SK, et al. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev. 1998;50(2):197–263. [PubMed] [Google Scholar]
- [101].Hsu M, Andl T, Li G, et al. Cadherin repertoire determines partner-specific gap junctional communication during melanoma progression. J Cell Sci. 2000;113(Pt 9):1535–42. doi: 10.1242/jcs.113.9.1535. [DOI] [PubMed] [Google Scholar]
- [102].Hsu MY, Wheelock MJ, Johnson KR, et al. Shifts in cadherin profiles between human normal melanocytes and melanomas. J Investig Dermatol Symp Proc. 1996;1(2):188–94. [PubMed] [Google Scholar]
- [103].Albelda SM, Mette SA, Elder DE, et al. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 1990;50(20):6757–64. [PubMed] [Google Scholar]
- [104].Woods D, Cherwinski H, Venetsanakos E, et al. Induction of beta3-integrin gene expression by sustained activation of the Ras-regulated Raf-MEK-extracellular signal-regulated kinase signaling pathway. Mol Cell Biol. 2001;21(9):3192–205. doi: 10.1128/MCB.21.9.3192-3205.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Haubner R, Wester HJ, Burkhart F, et al. Glycosylated RGD-containing peptides: tracer for tumor targeting and angiogenesis imaging with improved biokinetics. J Nucl Med. 2001;42(2):326–36. [PubMed] [Google Scholar]
- [106].Eskens FA, Dumez H, Hoekstra R, et al. Phase I and pharmacokinetic study of continuous twice weekly intravenous administration of Cilengitide (EMD 121974), a novel inhibitor of the integrins alphavbeta3 and alphavbeta5 in patients with advanced solid tumours. Eur J Cancer. 2003;39(7):917–26. doi: 10.1016/s0959-8049(03)00057-1. [DOI] [PubMed] [Google Scholar]
- [107].McGary EC, Lev DC, Bar-Eli M. Cellular adhesion pathways and metastatic potential of human melanoma. Cancer Biol Ther. 2002;1(5):459–65. doi: 10.4161/cbt.1.5.158. [DOI] [PubMed] [Google Scholar]
- [108].Hood JD, Bednarski M, Frausto R, et al. Tumor regression by targeted gene delivery to the neovasculature. Science. 2002;296(5577):2404–7. doi: 10.1126/science.1070200. [DOI] [PubMed] [Google Scholar]
- [109].Lehmann JM, Riethmuller G, Johnson JP. MUC18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamily. Proc Natl Acad Sci U S A. 1989;86(24):9891–5. doi: 10.1073/pnas.86.24.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Shih IM, Elder DE, Hsu MY, et al. Regulation of Mel-CAM/MUC18 expression on melanocytes of different stages of tumor progression by normal keratinocytes. Am J Pathol. 1994;145(4):837–45. [PMC free article] [PubMed] [Google Scholar]
- [111].Shih IM, Elder DE, Speicher D, et al. Isolation and functional characterization of the A32 melanoma-associated antigen. Cancer Res. 1994;54(9):2514–20. [PubMed] [Google Scholar]
- [112].Satyamoorthy K, Muyrers J, Meier F, et al. Mel-CAM-specific genetic suppressor elements inhibit melanoma growth and invasion through loss of gap junctional communication. Oncogene. 2001;20(34):4676–84. doi: 10.1038/sj.onc.1204616. [DOI] [PubMed] [Google Scholar]
- [113].Xie S, Luca M, Huang S, et al. Expression of MCAM/MUC18 by human melanoma cells leads to increased tumor growth and metastasis. Cancer Res. 1997;57(11):2295–303. [PubMed] [Google Scholar]
- [114].Mills L, Tellez C, Huang S, et al. Fully human antibodies to MCAM/MUC18 inhibit tumor growth and metastasis of human melanoma. Cancer Res. 2002;62(17):5106–14. [PubMed] [Google Scholar]
- [115].Shih IM, Speicher D, Hsu MY, et al. Melanoma cell-cell interactions are mediated through heterophilic Mel-CAM/ligand adhesion. Cancer Res. 1997;57(17):3835–40. [PubMed] [Google Scholar]
- [116].Johnson JP, Bar-Eli M, Jansen B, et al. Melanoma progression-associated glycoprotein MUC18/MCAM mediates homotypic cell adhesion through interaction with a heterophilic ligand. Int J Cancer. 1997;73(5):769–74. doi: 10.1002/(sici)1097-0215(19971127)73:5<769::aid-ijc26>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- [117].Li G, Kalabis J, Xu X, et al. Reciprocal regulation of MelCAM and AKT in human melanoma. Oncogene. 2003;22(44):6891–9. doi: 10.1038/sj.onc.1206819. [DOI] [PubMed] [Google Scholar]
- [118].Nickoloff BJ, Hendrix MJ, Pollock PM, et al. Notch and NOXA-related pathways in melanoma cells. J Investig Dermatol Symp Proc. 2005;10(2):95–104. doi: 10.1111/j.1087-0024.2005.200404.x. [DOI] [PubMed] [Google Scholar]
- [119].Nickoloff BJ, Osborne BA, Miele L. Notch signaling as a therapeutic target in cancer: a new approach to the development of cell fate modifying agents. Oncogene. 2003;22(42):6598–608. doi: 10.1038/sj.onc.1206758. [DOI] [PubMed] [Google Scholar]
- [120].Miele L. Notch signaling. Clin Cancer Res. 2006;12(4):1074–9. doi: 10.1158/1078-0432.CCR-05-2570. [DOI] [PubMed] [Google Scholar]
- [121].Liu ZJ, Xiao M, Balint K, et al. Notch1 signaling promotes primary melanoma progression by activating mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt pathways and up-regulating N-cadherin expression. Cancer Res. 2006;66(8):4182–90. doi: 10.1158/0008-5472.CAN-05-3589. [DOI] [PubMed] [Google Scholar]
- [122].Qin JZ, Stennett L, Bacon P, et al. p53-independent NOXA induction overcomes apoptotic resistance of malignant melanomas. Mol Cancer Ther. 2004;3(8):895–902. [PubMed] [Google Scholar]
- [123].Mori T, Martinez SR, O’Day SJ, et al. Estrogen receptor-alpha methylation predicts melanoma progression. Cancer Res. 2006;66(13):6692–8. doi: 10.1158/0008-5472.CAN-06-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Hinrichs CS, Palmer DC, Rosenberg SA, et al. Glucocorticoids do not inhibit antitumor activity of activated CD8+ T cells. J Immunother. 2005;28(6):517–24. doi: 10.1097/01.cji.0000177999.95831.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Landi MT, Baccarelli A, Calista D, et al. Glucocorticoid use and melanoma risk. Int J Cancer. 2001;94(2):302–3. doi: 10.1002/ijc.1468. [DOI] [PubMed] [Google Scholar]
- [126].Holick MF. Vitamin D: a millenium perspective. J Cell Biochem. 2003;88(2):296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- [127].Holick MF. Evolution and function of vitamin D. Recent Results Cancer Res. 2003;164:3–28. doi: 10.1007/978-3-642-55580-0_1. [DOI] [PubMed] [Google Scholar]
- [128].Bikle DD. Vitamin D and skin cancer. J Nutr. 2004;134(Suppl 12):3472S–8S. doi: 10.1093/jn/134.12.3472S. [DOI] [PubMed] [Google Scholar]
- [129].Bikle DD, Oda Y, Xie Z. Vitamin D and skin cancer: a problem in gene regulation. J Steroid Biochem Mol Biol. 2005 doi: 10.1016/j.jsbmb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- [130].Osborne JE, Hutchinson PE. Vitamin D and systemic cancer: is this relevant to malignant melanoma? Br J Dermatol. 2002;147(2):197–213. doi: 10.1046/j.1365-2133.2002.04960.x. [DOI] [PubMed] [Google Scholar]
- [131].Cornwell ML, Comstock GW, Holick MF, et al. Prediagnostic serum levels of 1,25-dihydroxyvitamin D and malignant melanoma. Photodermatol Photoimmunol Photomed. 1992;9(3):109–12. [PubMed] [Google Scholar]
- [132].Hutchinson PE, Osborne JE, Lear JT, et al. Vitamin D receptor polymorphisms are associated with altered prognosis in patients with malignant melanoma. Clin Cancer Res. 2000;6(2):498–504. [PubMed] [Google Scholar]
- [133].Halsall JA, Osborne JE, Potter L, et al. A novel polymorphism in the 1A promoter region of the vitamin D receptor is associated with altered susceptibility and prognosis in malignant melanoma. Br J Cancer. 2004;91(4):765–70. doi: 10.1038/sj.bjc.6602006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Berwick M, Armstrong BK, Ben-Porat L, et al. Sun exposure and mortality from melanoma. J Natl Cancer Inst. 2005;97(3):195–9. doi: 10.1093/jnci/dji019. [DOI] [PubMed] [Google Scholar]
- [135].Lim HW, Gilchrest BA, Cooper KD, et al. Sunlight, tanning booths, and vitamin D. J Am Acad Dermatol. 2005;52(5):868–76. doi: 10.1016/j.jaad.2005.03.015. [DOI] [PubMed] [Google Scholar]
- [136].Holick MF, Garabedian M, Schnoes HK, et al. Relationship of 25-hydroxyvitamin D3 side chain structure to biological activity. J Biol Chem. 1975;250(1):226–30. [PubMed] [Google Scholar]
- [137].Slominski A, Zjawiony J, Wortsman J, et al. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271(21):4178–88. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Slominski A. Neuroendocrine system of the skin. Dermatology. 2005;211(3):199–208. doi: 10.1159/000087012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Slominski A, Semak I, Zjawiony J, et al. Enzymatic metabolism of ergosterol by cytochrome p450scc to biologically active 17alpha, 24-dihydroxyergosterol. Chem Biol. 2005;12(8):931–9. doi: 10.1016/j.chembiol.2005.06.010. [DOI] [PubMed] [Google Scholar]
- [140].Slominski A, Semak I, Zjawiony J, et al. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005;272(16):4080–90. doi: 10.1111/j.1742-4658.2005.04819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Slominski A, Semak I, Wortsman J, et al. An alternative pathway of vitamin D metabolism. FEBS J. 2006;273(13):2891–901. doi: 10.1111/j.1742-4658.2006.05302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]