Abstract
Regulatory T cells (Treg) are critically involved in maintaining immunological tolerance, but this potent suppression must be quenched to allow the generation of adaptive immune responses. Here we report that type 1 sphingosine-1-phosphate (S1P) receptor (S1P1) delivers an intrinsic negative signal to restrain thymic generation, peripheral maintenance and suppressive activity of Treg cells. Combining loss- and gain-of-function genetic approaches, we found that S1P1 blocked the differentiation of thymic Treg precursors and function of mature Treg cells, and affected Treg-mediated immune tolerance. S1P1 induced the selective activation of the Akt-mTOR pathway to impede Treg development and function. Dynamic regulation of S1P1 contributed to lymphocyte priming and immune homeostasis. Thus, by antagonizing Treg-mediated immune suppression, the lipid-activated S1P1-Akt-mTOR pathway orchestrates adaptive immune responses.
Keywords: T cells, tolerance, signal transduction
INTRODUCTION
Regulatory T cells (Treg cells) play a central role in the maintenance of immune tolerance1-5. Treg cells are produced mainly in the thymus and require expression of the transcription factor Foxp3. Thymic development of Treg cells is dependent upon signals transduced by T cell receptor (TCR) engagement with self-peptide-MHC complexes. In addition, costimulatory factors (such as CD28) and cytokines including interleukin 2 (IL-2) and transforming growth factor-β (TGF-β) contribute to the induction of Foxp3 and thymic development of Treg cells6,7. Following their maturation and release into the periphery, Treg cells employ diverse mechanisms to mediate immune suppression. These mechanisms include production of inhibitory cytokines, modulation of dendritic cell maturation and function and killing or metabolic disruption of target cells8. While Treg activity is essential for immune tolerance and prevention of autoimmunity, the potent Treg-mediated suppression may abrogate adaptive immune responses and render the host susceptible to infection and cancer. How Treg development and activity are controlled to establish protective immunity without pathological anti-self reactivity is an open question. Stimulation of antigen-presenting cells through Toll-like receptors (TLRs) has been implicated in the reversal of Treg suppressive activity9,10. In addition, direct activation of TLR2 and TLR8 expressed by Treg cells can also down-modulate Treg activity11-13. Despite these studies, precisely how Treg cells are regulated to effect immune function and tolerance remains poorly understood.
Sphingosine 1-phosphate (S1P) is a natural lysophospholipid with micromolar concentration in the plasma14-16. S1P signals through five known G protein-coupled receptors (S1P1-S1P5). FTY720, a new class of immunosuppressants in clinical trials for transplantation tolerance and multiple sclerosis, sequestrates T cells in lymphoid organs by acting on four of the five S1P receptors17, 18. Genetic approaches to alter the function of S1P1 (also known as Edg1, http://www.signaling-gateway.org/molecule/query?afcsid=A000813) indicate that S1P1 is the main S1P receptor that regulates T cell trafficking. T cells from S1P1-deficient mice fail to egress from thymus19, 20, while S1P1-transgenic T cells preferentially distribute to the blood rather than lymphoid organs21, 22. Thus, S1P1 is critical for the egress of T cells from lymphoid organs. S1P1 primarily couples with pertussis toxin-sensitive Gi proteins. Major pathways downstream of S1P1 include activation of the kinase cascades involving Ras-Erk and phosphoinositide 3-kinase (PI(3)K)-Akt [http://www.signaling-gateway.org/molecule/query?afcsid=A000249; http://www.signaling-gateway.org/molecule/query?afcsid=A000250; http://www.signaling-gateway.org/molecule/query?afcsid=A000251], calcium mobilization, and actin cytoskeletal rearrangement23.
S1P1 is expressed on Treg cells but its functional significance has not been directly addressed24. Given the limitations of the pharmacological inhibitors, we chose to use two complementary genetic approaches, by eliminating and enhancing S1P1 function selectively in T cells. Our results showed that loss of S1P1 function resulted in enhanced thymic differentiation and suppressive activity of Treg cells. Conversely, increased S1P1 signaling led to reduced development and function of Treg cells in vitro and in vivo, and more importantly, development of spontaneous autoimmunity due to defects in Treg cells. Thus, S1P1 negatively regulates both thymic generation and suppressive activity of Treg cells. We further demonstrated that the function of S1P1 in Treg cells is mediated by the downstream Akt-mTOR pathway. Finally, S1P1 expression was differentially regulated in Treg cells as compared with conventional T cells (Tconv cells), suggesting that S1P1 coordinates the responses of Treg and Tconv cells to effect a productive and self-controlled immune response.
RESULTS
S1P1 signaling reduces the thymic Treg population
To investigate the intrinsic function of S1P1 in T cells, we crossed mice carrying a conditional S1P1 allele (S1pr1fl/fl)20 with CD4-Cre transgenic mice to delete the floxed S1pr1 allele specifically in T cells (S1P1-KO mice). Compared with wild-type controls, S1P1-KO mice showed accumulation of mature single-positive thymocytes and substantial reduction of T cells in the periphery (Supplementary Fig. 1a online). Real-time PCR analysis indicated efficient deletion of the S1pr1 gene in thymocytes (Supplementary Fig. 1b). These findings are consistent with a role for S1P1 in thymocyte egress19, 20. To assess the requirement of S1P1 in the development of naturally occurring Foxp3+ Treg cells, we examined the expression of Foxp3 in mature CD4 single-positive (CD4SP) thymocytes. As compared with wild-type mice, S1P1-KO mice contained elevated numbers of the thymic Treg population expressing Foxp3, CTLA4 and GITR (Fig. 1a and Supplementary Fig. 1c). Thus, S1P1 deficiency causes expansion of the thymic Treg cell population.
Figure 1. S1P1 negatively regulates thymic Foxp3+ Treg population.
(a,b) Flow cytometry of total and gated CD4SP thymocytes isolated from wild-type (WT) control, S1P1-KO (a) and S1P1-Tg mice (b). Panels on the right show the proportions and absolute numbers of Foxp3+ CD4SP Treg cells. Data are the mean (+s.d.) of 8-14 mice of each genotype from 7 experiments. (c) Foxp3 expression in bone marrow chimeras following retroviral transduction of S1P1. Bone marrow stem cells from WT mice were transduced with retrovirus expressing S1P1 (S1P1-GFP) or empty vector (GFP), and transferred into sublethally irradiated Rag1-/- mice. At 6-8 weeks after reconstitution, Foxp3 expression was analyzed in gated CD4SP thymocytes. Data are representative of 2 independent experiments. (d) Expression of Foxp3 in mixed bone marrow chimeras. Bone marrow stem cells from WT (CD45.1+) and S1P1-KO or S1P1-Tg mice (CD45.2+) were mixed at 1:1, and transferred into Rag1-/- mice to generate mixed bone marrow chimeras. At 6-8 weeks after reconstitution, Foxp3 expression was analyzed in CD4SP thymocytes, and cells from different donors were distinguished by their CD45.1 and CD45.2 expression. Data are representative of 3 independent experiments. *, P < 0.001 (Student’s t-test).
Is S1P1 sufficient to affect the thymic Treg population? We analyzed two independent lines of transgenic mice expressing the S1pr1 gene under the control of the human CD2 promoter-enhancer that results in increased expression and function of S1P1 in T cells (S1P1-Tg mice)21. Transgenic mice had a severe reduction of thymic Foxp3+ CD4SP cells as compared with wild-type controls (Fig. 1b). As a separate gain-of-function approach, we transduced bone marrow (BM) stem cells with a retrovirus expressing S1P1, and implanted them into alymphoid Rag1-/- mice. Following reconstitution, Foxp3+ thymocytes expressing S1P1 were one-third the number of those which expressed the empty vector (Fig. 1c). Therefore, increased S1P1 function reduces the thymic Treg population.
The defect in S1P1-expressing cells in the BM chimeras suggests a cell-autonomous effect of S1P1 on Treg cells, because the presence of non-transduced cells in the same host failed to rescue the defect by providing necessary trans-acting factors. We further tested this notion by constructing mixed BM chimeras derived from a 1:1 mixture of wild-type BM (CD45.1+) cells and S1P1-KO or S1P1-Tg cells (CD45.2+). Compared with the co-transferred CD45.1+ cells, S1P1-KO and S1P1-Tg BM developed into elevated and reduced thymic Foxp3+ Treg cells, respectively (Fig. 1d). We therefore conclude that S1P1 has a cell-autonomous effect in inhibiting the thymic Treg population.
S1P1 inhibits thymic Treg development
Altered thymic Treg cells in S1P1-KO and S1P1-Tg mice might be due to the effects of S1P1 on Treg egress, differentiation or both. To distinguish these possibilities, we first analyzed the trafficking of S1P1-KO Foxp3+ CD4SP thymocytes relative to Foxp3− Tconv cells. When adoptively transferred, wild-type Tconv cells distributed to different lymphoid organs including spleen, lymph nodes and blood, while S1P1-KO Tconv cells were able to enter lymphoid organs but unable to exit into blood, as reported19. S1P1-KO Treg cells exhibited a similar defect as S1P1-KO Tconv cells (Supplementary Fig. 2 online). Thus, S1P1 is required for egress of both Treg and Tconv cells, but this function is unlikely to account for the selective expansion of Treg cells in S1P1-KO thymus. Further supporting this, CD4+CD8+ double-positive thymocytes, which do not egress into the periphery, contained altered Foxp3+ populations in S1P1-KO and S1P1-Tg mice (Supplementary Fig. 1d).
We used two strategies to directly assess an intrinsic role of S1P1 in thymic Treg differentiation. First, we analyzed Foxp3+ thymocytes in mice 3-5 days after birth, a period critical for the initial generation of Treg cells in wild-type mice, before substantial numbers of Treg cells appear in the periphery25. Consequently, the effects of thymocyte emigration are not expected to affect thymic Treg population at this stage. We found the total CD4SP population was not significantly altered in the thymus from neonatal S1P1-KO or S1P1-Tg mice (Supplementary Fig. 3 online), unlike those in adult mice. However, in these neonatal animals, thymic Treg cells were still altered with an increase in S1P1-KO thymus and a reciprocal decrease in S1P1-Tg thymus (Supplementary Fig. 3).
Second, we used fetal thymus organ culture (FTOC), which allowed de novo differentiation of thymocytes including Treg cells, to obviate the effects of differential thymocyte egress or peripheral Treg cells homing to the thymus. We cultured thymus isolated from E16.5 embryos in vitro, and found no differences in thymocyte numbers or the distribution of CD4 and CD8 expression among different groups. However, the Foxp3+ CD4SP population was significantly increased in S1P1-KO cells and decreased in S1P1-Tg cells (Fig. 2a). These findings collectively demonstrate that S1P1 plays an intrinsic negative role in thymic Treg development.
Figure 2. S1P1 blocks thymic differentiation of Treg cells.
(a) Flow cytometry of total and gated CD4SP thymocytes isolated from WT control, S1P1-KO and S1P1-Tg FTOC. Panels on the right show the proportions and absolute numbers of Foxp3+ CD4SP Treg cells with the mean (+s.d.) calculated from ≥8 mice of each genotype. (b) Flow cytometry of gated CD4SP thymocytes from WT control, S1P1-KO and S1P1-Tg mice. Panels on the right show the proportions and absolute numbers of the CD4+CD25+Foxp3− precursor population, with the mean (+s.d.) calculated from ≥8 mice of each genotype. (c) Induction of Foxp3 expression in the CD4+CD25+Foxp3− population in vitro. CD4+CD25+Foxp3− cells were purified and stimulated with medium alone, IL-2 or IL-15 for 20 h, and induction of Foxp3 expression was measured by flow cytometry. The lower panel shows an IL-2 dependent dose response curve. Data are representative of 5 independent experiments. *, P < 0.05; **, P < 0.01 (Student’s t-test).
S1P1 blocks differentiation of CD4+CD25+ Foxp3− cells
We hypothesized that S1P1 affects thymic Treg development by acting on a precursor population(s). Previous studies suggest that Foxp3+ Treg cells develop from the putative precursors (CD4+CD25+Foxp3−) that are poised to express Foxp3 without TCR engagement, requiring only IL-2 or IL-15 stimulation26, 27. Remarkably, the CD4+CD25+Foxp3− population was greatly reduced in S1P1-KO thymus but significantly increased in S1P1-Tg thymus (Fig. 2b), suggesting that S1P1 may act on these cells to restrain their further differentiation into mature Foxp3-expressing Treg cells.
To directly test this, we crossed S1P1-KO and S1P1-Tg mice with Foxp3gfp knockin mice that express green fluorescent protein (GFP) regulated by the Foxp3 control elements28. We sorted thymic CD4+CD25+Foxp3− population and cultured them with IL-2 or IL-15. Under these conditions, no significant differences were observed in the apoptosis of these cells (data not shown). Foxp3 induction was substantially elevated in S1P1-KO cells, while a reciprocal change was observed in S1P1-Tg cells, irrespective of the stimuli and doses used (Fig. 2c). Therefore, S1P1 blocks the differentiation of CD4+CD25+Foxp3− cells into mature Treg cells.
Altered homeostasis and function of S1P1-KO Treg cells
Our results thus far have identified a negative role for S1P1 in thymic differentiation of Treg cells. We then examined whether S1P1 affects homeostasis and suppressive activity of Treg cells in the periphery. In S1P1-KO peripheral lymphoid organs, there was a selective increase of the Treg population marked by the expression of Foxp3 (Fig. 3a), CD25, GITR and CTLA4 (Fig. 3b), in line with the thymic alterations. Nonetheless, very few S1P1-KO peripheral cells could be isolated due to blocked thymocyte egress. For functional studies, we sorted Foxp3+ CD4SP thymocytes, which possess suppressive activity similar to peripheral Treg cells2. Although S1P1 deficiency resulted in increased numbers of Foxp3+ CD4SP cells, Foxp3 expression on a per cell basis was comparable between wild-type and S1P1-KO cells (Supplementary Fig. 4 online). In an in vitro T cell suppression assay, proliferation of the target Foxp3− Tconv cells from wild-type mice was tested in the presence of wild-type or S1P1-deficient Foxp3+ thymic Treg cells. S1P1-KO Treg cells showed a significantly increased capacity than wild-type Treg cells to suppress Tconv cell proliferation and IL-2 production (Fig. 3c). We examined the proliferation of Treg cells alone but no significant proliferation was observed in either wild-type or S1P1-KO Foxp3+ cells (data not shown), suggesting that the difference is not due to the differential proliferation of Treg cells.
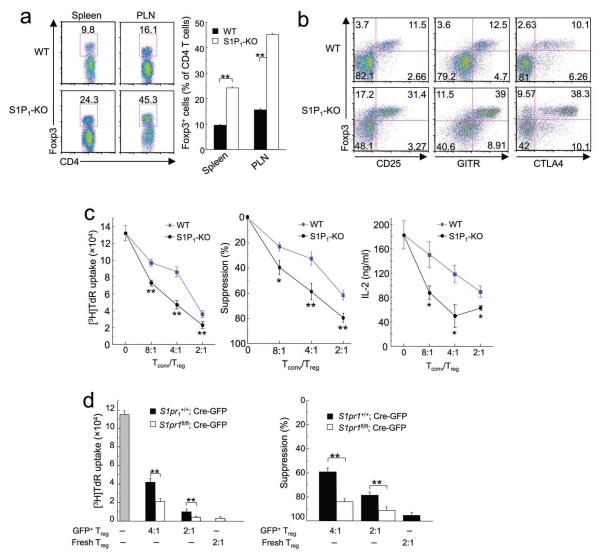
Figure 3. Enhanced peripheral population and suppressive activity of S1P1-KO Treg cells.
(a) Flow cytometry of gated CD4 T cells from the spleen and peripheral lymph nodes (PLN) of WT and S1P1-KO mice. The panel on the right shows the proportions of Foxp3+ Treg cells among total CD4+ T cell population, with the mean (+s.d.) calculated from 4 mice of each genotype. (b) Flow cytometry analysis of Treg markers (Foxp3, CD25, GITR and CTLA4) in PLN of WT and S1P1-KO mice. Data are representative of 2 independent experiments. Similar findings were observed in other peripheral lymphoid organs (not shown). (c) In vitro T-cell suppression assays using Foxp3+ CD4SP cells from WT and S1P1-KO mice. The left panel shows a representative proliferative assay of 4 independent experiments, the middle panel is the percentage of suppression with the mean (±s.d.) calculated from 4 experiments, and the right panel shows a representative of 2 independent experiments measuring IL-2 production. (d) In vitro T-cell suppression assays using S1P1-deleted peripheral Treg cells. Foxp3+ Treg cells from the periphery of S1pr1+/+ and S1pr1fl/fl mice were transduced with Cre-expressing retrovirus (Cre-GFP), and sorted GFP+ Treg cells were used in the T-cell suppression assays with different Tconv and Treg ratios; freshly isolated Treg cells were used as a comparison. The left panel is a representative of 3 independent experiments, and the right panel shows the percentage of suppression with the mean (+s.d.) calculated from 3 experiments. *, P < 0.01; **, P < 0.001 (Student’s t-test).
Given the altered differentiation of Foxp3+ cells in the S1P1-KO thymus, it remains possible that the increased suppressive activity of S1P1-KO Treg cells is secondary to defective thymic development. Hence, we transduced Treg cells from the periphery of S1pr1fl/fl mice with Cre-expressing retrovirus to acutely delete the S1pr1 gene in vitro. S1pr1-deleted cells showed a greater suppressive activity than Cre-expressing wild-type controls (Fig. 3d). In contrast, there was no difference in the suppressive activity between the non-transduced (GFP−) cells of both genotypes (data not shown). Therefore, S1P1 deficiency directly potentiates Treg suppressive activity.
Impaired suppressive activity of S1P1-Tg Treg cells
We next asked whether ectopic S1P1 expression is sufficient to alter Treg suppressive activity. In S1P1-Tg mice, peripheral Treg populations were largely normal (Supplementary Figs. 5a,b online), suggesting that compensatory mechanisms in the periphery might overcome defects in thymic development. However, in the chimeras generated by the mixture of wild-type and S1P1-Tg BM cells, Treg cells derived from S1P1-Tg BM were selectively reduced as compared with co-transferred wild-type counterparts (Supplementary Fig. 5c). Thus, S1P1-Tg Treg cells have a competitive disadvantage in their peripheral maintenance. In T cell suppression assays, S1P1-Tg Treg cells were much less efficient at suppressing the responses of Tconv cells. Specifically, proliferation and cell cycle progression of target cells were significantly higher in the presence of S1P1-Tg Treg cells as compared with wild-type Treg cells (Fig. 4a and Supplementary Fig. 6a online). Further, S1P1-Tg Treg cells had a greatly reduced capacity to inhibit the IL-2 production of target cells (Supplementary Fig. 6b). To demonstrate that such an altered Treg activity is a direct result of S1P1 function rather than secondary to defective thymic development, we transduced wild-type Treg cells with S1P1-expressing retrovirus. Expression of S1P1 substantially impaired Treg suppressive activity (Fig. 4b), highlighting an intrinsic inhibitory effect of S1P1 on Treg function.
Figure 4. Reduced suppressive activity of S1P1-Tg Treg cells in vitro and in vivo.
(a) In vitro T-cell suppression assays using Foxp3+ Treg cells from WT and S1P1-Tg mice. The left panel shows a representative proliferative assay of 8 independent experiments, and the right panel is the percentage of suppression with the mean (±s.d.) calculated from 8 experiments. (b) In vitro T-cell suppression assays using Foxp3+ Treg cells transduced with S1P1-expressing retrovirus. WT Treg cells were transduced with S1P1-expressing (S1P1-GFP) and empty vector (GFP) retroviruses, and sorted GFP+ Treg cells were used in the T-cell suppression assays with different Tconv and Treg ratios. The left panel shows a representative proliferative assay of 5 independent experiments, and the right panel is the percentage of suppression with the mean (+s.d.) calculated from 5 experiments. (c-e) Failure of S1P1-Tg Treg cells to control colitis in vivo. Tconv cells were transferred alone or in combination with WT or S1P1-Tg Treg cells into Rag1-/- mice. (c) Changes in body weight after transfer. (d) Histology scores of experimental mice. (e) Representative colon histology. Data are the mean (+s.d.) of 5 mice of each genotype and are representative of 2 independent experiments. *, P < 0.001 (Student’s t-test).
We next employed two approaches to investigate whether S1P1 controls Treg suppressive activity in vivo. First, we used a model of colitis induced by the transfer of Tconv cells into lymphopenic hosts; this disease can be prevented by the cotransfer of Treg cells (Supplementary Fig. 7a online)29. Transfer of wild-type Tconv cells resulted in severe weight loss (data not shown), and co-transfer of Treg cells from wild-type, but not S1P1-Tg mice, prevented bodyweight loss (Fig. 4c). Mice were euthanized 10 weeks after transfer and lesions in the colon and cecum were assessed. Recipients of Tconv and wild-type Treg co-transferred cells did not develop prominent colitis whereas recipients of Tconv alone or those of Tconv and S1P1-Tg Treg cell co-transferred cells developed severe colitis (Fig. 4d). The distribution of Treg cells in the colon and lymphoid organs was comparable between wild-type and S1P1-Tg Treg cell transfer groups (Supplementary Figs. 7b,c). Thus, S1P1-Tg Treg cells did not have obvious defects in the expansion and/or homeostasis in this model, suggesting that the inability of these cells to control colitis was most likely due to their impaired suppressive activity.
Second, we examined the importance of S1P1 in controlling systemic autoimmune diseases caused by Treg deficiency. We constructed mixed BM chimeras by transferring BM cells from Foxp3-deficient Scurfy (sf) mice and S1P1-Tg or wild-type mice into Rag1-/- recipients. In the resulting chimeras, only the S1P1-Tg or wild-type BM cells can give rise to Foxp3+ Treg cells (Supplementary Fig. 8a online). In the chimeras that received sf BM alone, T cells were overtly activated and there were prominent inflammation and lymphocytic infiltration in the liver, lung and colon. In contrast, chimeras that received sf and wild-type BM cells exhibited minimal T cell activation and tissue inflammation. Strikingly, chimeras that received sf and S1P1-Tg BM cells showed profound T cell activation and inflammatory diseases that were indistinguishable from sf alone chimeras (Supplementary Figs. 8b,c). Thus, S1P1-Tg Treg cells fail to mediate immune tolerance in vivo.
Defects in S1P1-Tg Treg cells cause autoimmunity
The impaired suppressive activity of Treg cells from S1P1-Tg mice prompted us to examine whether homeostasis of the immune system was altered in these mice. S1P1-Tg mice had increased numbers of activated CD62LloCD44hi effector or memory T cells, which became more prominent when the mice aged (Fig. 5a). Further, Tconv cells from S1P1-Tg mice, even at young age, were hyper-proliferative to TCR stimulation (Fig. 5b), suggesting a lower threshold for activation. To examine whether this results in altered self-tolerance, we measured amounts of autoantibodies. Increased titers of anti-nuclear and anti-dsDNA antibodies were detected in the sera of aged S1P1-Tg mice (Fig. 5c), indicating autoimmune reactions. Because a predominant TH1 cytokine response especially interferon-γ (IFN-γ) is associated with the pathogenesis of lupus, a prototypical systemic autoimmune disease30, we determined whether activated T cells in S1P1-Tg mice were differentiated into a TH1 phenotype. S1P1-Tg cells produced more IFN-γ but less IL-4 as compared to controls (Fig. 5d). Consistently, serum titers of TH1-dependent IgG2a antibodies, but not TH2-dependent IgG1 antibodies, were significantly higher in S1P1-Tg mice (Fig. 5e). Together, increased S1P1 signaling in T cells leads to their spontaneous activation and differentiation into a TH1 phenotype and breakdown of immune tolerance.
Figure 5. S1P1-Tg mice show disrupted immune homeostasis and develop age-related autoimmunity due to defects in the Treg compartment.
(a-e) Analysis of WT and S1P1-Tg mice. (a) Flow cytometry of T cell activation markers from peripheral lymphoid organs of aged mice (10 months). MLN, mesenteric lymph nodes. Data are representative of 6 independent experiments. (b) Proliferative response to TCR stimulation of Tconv cells from WT and S1P1-Tg mice (2 months). Data are representative of 6 independent experiments. (c) Titers of anti-nuclear antigen and anti-ds DNA antibodies of aged mice (10 months). Data are the mean (±s.d.) of >10 mice of each genotype and are representative of 4 independent experiments. (d) Effector cytokine production of activated T cells from WT and S1P1-Tg mice (5-6 months). Data are representative of 2 independent experiments. (e) Serum titers of IgG1 and IgG2a (5-6 months). Data are the mean of 5 mice of each genotype and are representative of 3 independent experiments. (f-h) Analysis of WT and S1P1-Tg T cells in the mixed BM chimeras (6-9 months after reconstitution), including expression of activation markers (f), proliferation (g), and effector cytokine production (h). Data are representative of 3 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student’s t-test).
We reasoned that autoimmunity in S1P1-Tg mice could be due to an intrinsic defect in Tconv cells, or the impaired suppressive activity of Treg cells. To distinguish these possibilities, we generated BM chimeras by transferring wild-type and S1P1-Tg BM cells alone or in combination into Rag1-/- mice. As expected, T cells from mice given S1P1-Tg BM alone were spontaneously activated and hyper-responsive to TCR stimulation (data not shown). In contrast, in the mixed chimeras containing both S1P1-Tg and wild-type cells, S1P1-Tg Tconv cells were not spontaneously activated or hyper-responsive to TCR stimulation, and produced normal amounts of IFN-γ and IL-4 (Figs. 5f-h). Therefore, activation of Tconv cells in S1P1-Tg mice was non-cell-autonomous and could be rescued by the presence of wild-type cells. Next we determined whether providing S1P1-Tg mice with wild-type Treg cells alone could prevent the T cell activation phenotypes. We injected 3-day old S1P1-Tg mice with wild-type Treg cells, a condition that results in the population of donor Treg cells31. Following neonatal transfer of Treg cells into S1P1-Tg mice, Tconv cells in the recipients showed normal homeostasis and proliferation (Supplementary Fig. 9 online). We conclude that the impaired Treg compartment in S1P1-Tg mice accounts for the disrupted immune homeostasis.
S1P1 signals through Akt-mTOR to affect Treg cells
To investigate mechanisms mediating S1P1 function, we stimulated thymic Treg precursors (CD4+CD25+Foxp3−) with IL-2, which induced their differentiation into Treg cells (Fig. 2c)26. Activation of IL-2 downstream pathways, including Akt, Erk and STAT5, was examined by flow cytometry for the phosphorylated individual proteins. As expected, IL-2 activated all of the three pathways in wild-type Treg precursors. In contrast, S1P1-Tg cells activated Erk and STAT5 pathways similarly as wild-type cells but showed substantially elevated activation of Akt and phosphorylation of S6 ribosomal protein, a well-established target of the Akt-mTOR pathway (Fig. 6a).
Figure 6. S1P1 induces activation of Akt-mTOR to inhibit Treg development and function.
(a) IL-2 activated signaling pathways in thymic Treg precursors from WT and S1P1-Tg mice. CD4+CD25+Foxp3− cells were purified and stimulated with medium alone or IL-2, and activation of Akt, STAT5, Erk and S6 ribosomal protein (S6) were examined by flow cytometry using phospho-specific antibodies. Data are representative of 4 independent experiments. (b) Effects of drug treatments on IL-2 induced Foxp3 expression in Treg precursors. CD4+CD25+Foxp3− cells were treated with U0126, LY294002 and Rapamycin for 30 minutes, followed by IL-2 stimulation. Data are representative of 3 independent experiments. (c) IL-2 activated signaling pathways in peripheral Treg cells from WT and S1P1-Tg mice. Treg cells were stimulated with medium alone or IL-2, and activation of Akt, STAT5, Erk and S6 ribosomal protein (S6) were examined by flow cytometry using phospho-specific antibodies. Data are representative of 5 independent experiments. (d) Suppressive activity of Treg cells transduced with dn-Akt retrovirus. WT and S1P1-Tg Treg cells were transduced with control (MiT) and dn-Akt expressing (dn-Akt) retroviruses (non-transduced cells are shown on the right as a comparison), and transduced cells were sorted and used in the T-cell suppression assays with different Tconv and Treg ratios. Data are representative of 3 independent experiments. *, P < 0.001 (Student’s t-test).
We noted that Akt has recently been shown to inhibit Foxp3 induction in vitro and upon thymic injection32, 33. Does increased Akt activity mediate S1P1 functions in Treg differentiation? To this end, we stimulated wild-type and S1P1-Tg thymic Treg precursors with IL-2 in the presence of LY294002 (an inhibitor of PI(3)K, upstream of Akt) or rapamycin (an inhibitor of mTOR, downstream of Akt). LY294002 and rapamycin potentiated Foxp3 induction of S1P1-Tg Treg precursors similar to wild-type cells (Fig. 6b). Treatment with additional PI(3)K or mTOR inhibitors including PI-103 and NVP-BEZ235 (ref.34) had similar effects as rapamycin in restoring the Treg differentiation of S1P1-Tg cells (Supplementary Fig. 10 online), indicating the specific involvement of the Akt-mTOR pathway in S1P1 signaling. In contrast, treatment with U0126 (an inhibitor of Erk) did not affect Treg differentiation (Fig. 6b). Therefore, the increased Akt activity in S1P1-Tg Treg precursors results in their defective differentiation.
Next, we determined the signaling pathways activated by increased S1P1 expression in peripheral Treg cells. Upon stimulation with IL-2, peripheral Treg cells from S1P1-Tg mice showed normal activation of Erk and STAT5 but increased phosphorylation of Akt and S6 ribosomal protein (Fig. 6c). A similar pattern was observed when S1P1-Tg Treg cells were stimulated with anti-CD3 plus anti-CD28 (Supplementary Fig. 11 online). We then treated peripheral S1P1-Tg Treg cells with pharmacological inhibitors and found that LY294002, rapamycin, PI-103 or NVP-BEZ235, but not U0126, enhanced the suppressive activity of these cells comparable to that of similarly treated wild-type Treg cells (Supplementary Fig. 12 online). Moreover, blocking Akt activation by expression of dominant-negative Akt (dn-Akt) in S1P1-Tg cells completely restored their suppressive activity (Fig. 6d). We conclude that increased S1P1 function leads to excessive Akt activity which accounts for impaired Treg differentiation and function.
S1P1 is necessary for Akt activation in Treg cells
Whereas the results above indicated that S1P1 is sufficient to activate the Akt pathway to antagonize Treg development and function, it remains unclear whether S1P1 is necessary for Akt activation in Treg cells. To address this issue, we stimulated Treg precursors from wild-type or S1P1-KO mice with IL-2. As compared with wild-type cells, S1P1-KO cells exhibited substantially reduced Akt phosphorylation, but no change on STAT5 or Erk, following IL-2 stimulation (Fig. 7a). Similarly, S1P1 deficiency decreased Akt activation in mature thymic Treg cells (Fig. 7b). Moreover, after acute deletion of S1P1 from peripheral Treg cells in vitro, less Akt activation resulted following IL-2 or anti-CD3 plus anti-CD28 stimulation (Supplementary Figs. 13a,b online). Therefore, S1P1 is required to mediate IL-2 and TCR-induced Akt activation in Treg cells.
Figure 7. S1P1 is necessary for Akt activation in Treg cells.
(a,b) IL-2 activated signaling pathways in thymic Treg precursors (a) and Foxp3+ reg Treg cells (b) from WT and S1P1-KO mice. Purified CD4+CD25+Foxp3− cells (a) or CD4+Foxp3+ cells (b) were stimulated with medium alone or IL-2, and activation of Akt, STAT5 and Erk were examined by flow cytometry using phospho-specific antibodies. Data are representative of 3 independent experiments. (c) Suppressive activity of Treg cells transduced with Cre-GFP retrovirus alone or in combination with constitutively active Akt. Foxp3+ Treg cells from the periphery of S1pr1+/+ and S1pr1fl/fl mice were transduced with Cre-expressing retrovirus (Cre-GFP) alone or in combination with empty control (MiT) or constitutively active Akt (ca-Akt) retroviruses, and transduced cells were sorted and used in the T-cell suppression assays with different Tconv and Treg ratios. Data are representative of 2 independent experiments. *, P < 0.001 (Student’s t-test).
In addition to S1P1, Treg cells also express all of the other four receptors for S1P24. Exposure of T cells to S1P results in the activation of Akt35, although whether this is mediated by S1P1 is not known. We treated wild-type and S1P1-deficient Treg cells with S1P and examined the downstream signaling pathways. In both thymic Foxp3+ CD4SP cells and peripheral Treg cells after acute deletion of S1P1, activation of Akt, but not Erk, was substantially reduced as compared with wild-type counterparts (Supplementary Fig. 13c). Thus, among the S1P receptors, S1P1 plays an important role in mediating S1P-induced activation of Akt in Treg cells.
We then determined whether the reduced Akt activity accounts for the enhanced suppressive activity of S1P1-KO Treg cells. We transduced Treg cells from S1pr1fl/fl mice with Cre-GFP retrovirus to delete S1pr1, together with retrovirus expressing constitutively active Akt (Thy1.1 marked). Constitutive activation of Akt modestly reduced the suppressive activity of wild-type Treg cells, but severely decreased that of S1P1-deficient Treg cells (Fig. 7c). Therefore, the reduced Akt activity is responsible for the enhanced suppressive activity of S1P1-KO Treg cells.
Function and signaling of S1P1 in Tconv cells
Our data above have delineated that S1P1, by activating Akt, negatively regulates development and function of Treg cells. What are the function and signaling mechanisms of S1P1 in Tconv cells? Using mixed BM chimeras and neonatal transfer of Treg cells (Figs. 5f-h and Supplementary Fig. 9), we did not observe an intrinsic defect of Tconv cells from S1P1-Tg mice. To further extend this observation, we used retroviral systems to ectopically express S1P1 or delete S1pr1 in Foxp3− Tconv cells, but did not observe altered proliferation of these cells (Supplementary Figs. 14a,b online). Moreover, anti-CD3 induced similar degrees of proliferation between Foxp3− CD4SP thymocytes from wild-type and S1P1-KO mice (Supplementary Fig. 14c). Thus, S1P1 does not affect the proliferative response of Tconv cells.
To address the signaling mechanisms of S1P1 in Tconv cells, we activated wild-type and S1P1-Tg Tconv cells with IL-2 or anti-CD3 plus anti-CD28. Activation of Akt, Erk and STAT5 were comparable between these cells (Supplementary Fig. 15 online). Similarly, in S1P1-deficient Tconv cells, IL-2 and TCR induced normal activation of Akt (Supplementary Figs. 16a,b online). Thus, unlike in Treg cells, S1P1 is dispensable for TCR or IL-2 induced Akt activation in Tconv cells. To ensure that Tconv cells have no general defects in S1P1 signaling, we stimulated S1P1-sufficient and deficient Tconv cells with S1P. S1P1 deficiency resulted in reduced S1P-induced Akt activation (Supplementary Fig. 16c). Therefore, whereas Tconv cells use S1P1 to mediate Akt activation in response to S1P, the activation of Akt in response to TCR or IL-2 occurs independently of S1P1 in these cells.
The differential function of S1P1 in Treg and Tconv cells prompted us to examine whether S1P1 expression differs between these T cell subsets. S1pr1 mRNA can be detected in various immune cells, including Treg and Tconv cells (Supplementary Fig. 17 online). Following stimulation with TCR and IL-2, S1pr1 mRNA was decreased abruptly in Tconv cells, as reported21, 22, whereas it was downregulated gradually in Treg cells (Fig. 8a). Expression of KLF2, a transcription factor essential for S1P1 expression in thymocytes36, exhibited a similar pattern as that of S1P1 (Supplementary Fig. 18 online), suggesting that KLF2 contributes to the differential regulation of S1P1 between peripheral Treg and Tconv cells. To examine whether this differential regulation of S1P1 occurs in vivo, ovalbumin-specific OT-II TCR-transgenic Treg and Tconv cells were transferred to wild-type hosts, followed by antigen immunization. Two days after in vivo activation, downregulation of S1pr1 in Tconv cells was much more pronounced than that in Treg cells (Fig. 8b). These findings indicate that S1P1 function and expression are differentially regulated between Treg and Tconv cells. We propose that dynamic regulation of S1P1 expression contributes to lymphocyte priming and the maintenance of immune homeostasis (Supplementary Fig. 19 online).
Figure 8. Differential regulation of S1P1 expression in Treg and Tconv cells.
(a) Treg and Tconv cells from WT mice were stimulated with anti-CD3, anti-CD28 and IL-2, and S1pr1 mRNA expression was analyzed by quantitative PCR. Data are representative of 3 independent experiments. (b) OT-II TCR-transgenic mice were crossed with Foxp3gfp knockin mice, and sorted Treg and Tconv cells from these mice were transferred into C57BL/6 mice, followed by s.c. immunization with ovalbumin emulsified in CFA. Two days after immunization, Treg and Tconv cells from the draining lymph nodes were purified based on GFP expression, and S1pr1 mRNA expression was analyzed in each T-cell subset. Data are representative of 2 independent experiments.
DISSCUSION
Recent work on Treg cell biology has mainly focused on mechanisms of Treg-mediated immune suppression4, 8. How the development and function of Treg cells are regulated remains poorly understood. Here we report that S1P1 is an intrinsic negative regulator of thymic differentiation, peripheral maintenance and suppressive activity of Treg cells, and such functions are mediated by the downstream Akt-mTOR pathway. To our knowledge, S1P1 is the first receptor that negatively regulates these diverse physiological processes of Treg cells. Moreover, among the regulatory mechanisms in Treg cells, the S1P1 pathway is unique in that it couples trafficking and intrinsic development and function of Treg cells, and coordinates the immune responses mediated by Treg and Tconv cells.
Using animal models with deficient and enhanced S1P1 functions, we identified an inhibitory role for S1P1 on the thymic Treg population. Although S1P1 facilitates thymic egress of Treg cells, an intrinsic function for S1P1 to block thymic Treg differentiation was revealed by our analyses of neonatal thymi, FTOC and more importantly, of the CD4+CD25+Foxp3− population. Such a population has been postulated to act as precursors for thymic Treg cells, although genetic evidence is lacking26. The reciprocity of the alterations in CD4+CD25+Foxp3− and CD4+CD25+Foxp3+ populations observed in both S1P1-KO and S1P1-Tg mice provides key genetic evidence supporting the definition of CD4+CD25+Foxp3− cells as bona fide Treg precursors 26. Collectively, these findings demonstrate that S1P1 delivers a crucial negative signal for thymic development of Treg cells. Previous studies have shown that S1P1 expression is upregulated during thymocyte maturation19. Such upregulation in Treg cells likely serves as a molecular “switch” to restrain Treg cell differentiation (thus maintaining a proper balance between the Treg and Tconv populations) and to facilitate their release to the periphery, thereby coordinating the development and egress of Treg cells in the thymus.
Once Treg cells are released from thymus into secondary lymphoid organs, they modulate immunity to both self and foreign antigens. However, excessive Treg-mediated suppression may render the host susceptible to infection and cancer. Signaling through TLRs expressed in Treg or dendritic cells has been implicated in the negative control of Treg suppressive activity9-12, although the mechanisms of action remain unclear. Using multiple in vitro and in vivo systems, we demonstrated that S1P1 is a critical negative regulator of Treg function. S1P1 is highly expressed in both naïve Treg and Tconv cells. At an early stage of immune activation, S1P1 expression is largely maintained in Treg cells, resulting in a low suppressive activity and high mobility of these cells. This serves to prevent premature Treg-mediated suppression in order for an immune response to initiate. In contrast, S1P1 is rapidly and profoundly downregulated in Tconv cells to mediate their sequestration in the draining lymph nodes to engage an efficient interaction with antigen-presenting cells19, 21. The overall function of S1P1 in Treg and Tconv cells at this stage is to promote a productive immune response. At a late stage of immune activation, S1P1 is downregulated in Treg cells to release Treg-mediated suppression, thereby preventing an exuberant immune response caused by Tconv cells. In contrast, S1P1 is slowly recovered in Tconv cells to allow their egress into peripheral tissues19, 38. It remains unclear how Treg-mediated suppression is attenuated during acute infection to establish protective immunity9, 11, 39, 40, and our findings suggest that coordination of Treg and Tconv responses by S1P1 is crucial for a productive and self-controlled immune response.
To identify the molecular mechanisms mediating S1P1 functions in Treg cells, we examined signaling pathways activated by S1P1. We found a specific role for S1P1 in the activation of Akt, but not Erk or STAT5, in thymic Treg precursors and mature Treg cells after TCR of IL-2 stimulation. Notably, the Akt-mTOR pathway have recently been implicated in blocking Treg differentiation32, 33, but the cellular factors responsible for activating Akt have not been established. Our studies identified that S1P1 is a key receptor that activates Akt-mTOR. Importantly, restoration of proper Akt-mTOR activity corrected the defects caused by S1P1 loss and gain of function. Therefore, activation of Akt-mTOR by S1P1 mediates the negative effects of S1P1 on Treg development and function.
We further identified a fundamental difference in S1P1 signaling between Treg and Tconv cells. S1P1 does not affect Tconv cell proliferative response, and is dispensable for IL-2 or TCR-induced Akt activation in Tconv cells, unlike Treg cells. In contrast, S1P1 is required for Akt activation in response to S1P stimulation in both Treg and Tconv cells. It appears that S1P1 is selectively coupled to TCR and cytokine receptors for the activation of Akt signaling in Treg but not Tconv cells. In support of this model, transactivation of S1P1 by growth factor receptors has been observed with distinct mechanisms in a cell context-specific manner41. Notably, Tconv and Treg cells have different ability to activate Akt in response to TCR and IL-2 stimulation42, 43, and our studies on S1P1 further highlight the distinct mechanisms in Akt signaling between these two T-cell subsets.
In addition to S1P1, Treg cells also express all of the other four receptors for S1P24, the expression of which was not substantially altered in S1P1-KO and S1P1-Tg Treg cells (data not shown). Whether and how S1P1 interacts with its natural ligand S1P in Treg cells will be an important area of investigation. FTY720, the new generation immunosuppressive drug that targets S1P1, has been shown to enhance Treg activity24, 44, 45. These effects caused by FTY720 appear to be similar as S1P1 deficiency, suggesting that FTY720 may act as an antagonist to inactivate S1P1 in Treg cells. However, mechanisms of action of FTY720 are complex because it can serve as an agonist for four of the five known S1P receptors17, 18. Moreover, FTY720 possesses immunomodulatory activities independent of S1P receptors46. Given the limitation of the pharmacological approaches, we used genetic systems to specifically target the S1P1 pathway in T cells, and unequivocally revealed an intrinsic negative role for S1P1 in Treg development and function.
In summary, S1P1 delivers a key negative signal for the development, maintenance and function of Treg cells. The function of S1P1 is primarily mediated by Akt-mTOR in Treg cells. Our studies highlight that Treg cells are regulated by more than surface expression of TCR and co-stimulatory molecules and limited production of cytokines. Rather, the development and function of these cells are further shaped by an abundant circulatory lipid. Interestingly, FTY720 and Rapamycin, two new promising immunosuppressants for transplantation and autoimmune disease, target S1P1 and Akt-mTOR, respectively. Our studies suggest that a shared mechanism may contribute to immunomodulatory functions of these drugs. The S1P1-Akt-mTOR pathway in Treg cells may be explored to develop novel therapeutics for autoimmunity, cancer and infection.
METHODS
Mice and bone marrow chimeras
Mice of S1pr1fl 20, CD4-Cre 47, S1P1-Tg 21 and Foxp3gfp knockin 28 have been described previously, and have been backcrossed to the C57BL/6 background extensively. WT controls for S1P1-KO (CD4-Cre; S1pr1fl/fl) included Cre+ mice (CD4-Cre; S1pr1+/+) to account for Cre effects; controls for S1P1-Tg mice were transgene-negative littermates. C57BL/6, CD45.1, Thy1.1, Rag1-/-, OT-II, and Scurfy mice (all on the C57BL/6 background) were purchased from the Jackson Laboratory. Mice at 6-10 weeks old were used unless otherwise noted. Bone marrow chimeras were generated by transferring 1-2 × 107 T cell-depleted bone marrow cells into sublethally irradiated (5 Gy) alymphoid Rag1-/- mice, as described previously47. All mice were kept in specific pathogen—free conditions in Animal Resource Center at St. Jude. Animal protocols were approved by Institutional Animal Care and Use Committee of St. Jude.
Cell purification and flow cytometry
Lymphocytes were isolated from the thymi, spleens and lymph nodes of mice and sorted on a MoFlow (Beckman-Coulter) or Reflection (i-Cyt). Treg and Tconv cells were sorted based on CD4+CD45RBloCD25+ and CD4+CD45RBhiCD25− expression, respectively; alternatively and whenever possible, mice crossed with Foxp3gfp knockin were used from which CD4+CD45RBloGFP+ and CD4+CD45RBhiGFP− populations were sorted for Treg and Tconv cells, respectively. For flow cytometry analysis of surface markers, cells were stained with antibodies (all from eBioscience) in PBS containing 2% (wt/vol) BSA. Flow cytometry analysis of intracellular Foxp3 (FJK-16s; eBioscience), CTLA-4 (UC10-4F10-11), IFN-γ (XMG1.2) and IL-4 (11B11; all three antibodies from BD Biosciences) were performed per manufactures’ instructions. For detection of phosphorylated signaling proteins, purified cells were activated with IL-2 or anti-CD3 (145-2C11; BD Biosciences) and anti-CD28 (37.51; BD Biosciences), immediately fixed with Phosflow perm buffer (BD Biosciences), permeabilized with Phosflow lyse/fix buffer (BD Biosciences), and stained with PE or APC directly conjugated antibodies for phospho-Akt (pSer 473) (D9E; Cell Signaling Technology), phospho-Erk (pThr202/pTyr204) (20A; BD Biosciences), phospho-STAT5 (pTyr694) (47; BD Biosciences) and phospho-S6 (pSer235/236) (D57.2. 2E; Cell Signaling Technology). Flow cytometry data were acquired on an upgraded 5-color FACScan (Becton Dickinson), and analyzed using FlowJo software (Treestar). Cell numbers of various populations were calculated by multiplying the total cell number with the percentages of each individual population from the same mouse, and then averaged.
T-cell culture, activation and Treg suppression assays
T cells were cultured in Bruff’s medium supplemented with 10% FBS and 1% penicillin-streptomycin as described previously47. For measurement of T cell activation, sorted Tconv cells (5×104) were cultured in 96-well flat-bottom plates, and stimulated with various doses of anti-CD3 (145-2C11) and/or anti-CD28 (37.51) in the presence of irradiated splenocytes as antigen-presenting cells for 72 h. For in vitro T cell suppression assay, sorted Tconv (5×104) and Treg cells (at different ratios with Tconv cells) were cultured in 96-well flat-bottom plates along with 2 μg/ml anti-CD3 (145-2C11) and irradiated splenocytes for 72 h. T cell proliferation was determined by pulsing with [3H]thymidine at 1 μCi per well for the last 12–16 h of culture, or by carboxyfluorescein diacetate succinimidyl diester (CFSE) labeling according to the manufacturer’s protocols (Invitrogen-Molecular Probes). IL-2 production was analyzed by bioplex assays (BioRad). For drug treatment, cells were pre-incubated with vehicle, 5 μM U0126, 10 μM Ly294002 or 100 nM rapamycin (all from Calbiochem) for 1 h before stimulation.
Retroviral transduction
S1pr1 and Cre cDNAs were cloned into the mouse stem cell virus retroviral vector (MSCV) upstream of an internal ribosome entry site (IRES)-EGFP expression cassette. Retroviral constructs expressing dn-Akt and ca-Akt with linked Thy1.1 marker (MSCV-IRES-Thy1.1, abbreviated as MiT)48 were kindly provided by David Hildeman (U. of Cincinnati). Phoenix-Eco packaging cells were transfected with Lipofectamine (Invitrogen), and recombinant retroviruses were collected 48 and 72 h after transfection. Treg cells were stimulated with 5 μg/ml of anti-CD3 (145-2C11), 5 μg/ml of anti-CD28 (37.51) and 100 U/ml of IL-2 for 48 h and then were transduced with retroviruses by ‘spin inoculation’ (650 g for 1 h), as described47. Cells were cultured for an additional 5 d before being sorted according to EGFP or Thy1.1 expression, and used for T-cell suppression assays. Deletion of the S1pr1 gene by Cre expression in S1pr1fl/fl cells was confirmed by quantitative PCR analysis (data not shown). In certain experiments, double transduction was used in which Treg cells were transduced with Cre-GFP retrovirus at 48 h, and then with empty MiT vector or MiT expressing ca-Akt retrovirus 6 h later. For transduction of bone marrow stem cells, mice were injected with 5-fluorouracil (0.15 mg/g) and euthanized 2 days later. Bone marrow cells were harvested and expanded with IL-6 (50 ng/ml), IL-3 (20 ng/ml) and SCF (50 ng/ml) (all from R&D Systems) for 2 days before they were transduced with retrovirus as above. At 24 h after transduction, the cells were harvested and injected into irradiated Rag1-/- mice.
Measurement of serum antibodies
The titers of autoantibodies and immunoglobulin subclasses were determined with kits from Alpha Diagnostic International and Southern Biotechnology Associates, respectively.
Fetal thymus organ culture (FTOC)
Fetal thymus lobes were dissected from E16.5 embryos, and cultured on sponge-supported filter membranes at an interphase between 5% CO2-humidified air and T cell culture medium for approximately 7 days to induce thymocyte differentiation. The cell culture medium contained undetectable numbers of thymocytes (data not shown).
In vivo migration assays
Purified CD4SP thymocytes from WT or S1P1-KO mice were labeled with CFSE, mixed with approximately same numbers of control WT cells (Thy1.1+), and transferred into C57BL/6 recipient mice (Thy1.2+). The co-transferred Thy1.1+ cells served as an internal control to normalize the transfer and detection efficiencies among different recipients. At 24 h, lymphocytes were prepared from blood, spleen, PLN and Peyer’s patches of recipient mice, and stained with Foxp3 (FJK-16s), Thy1.1 (HIS51) and CD4 (RM4-5) antibodies (all from eBioscience). Donor experimental cells, co-transferred internal control cells, and recipient CD4+ T cells were distinguished by CFSE+Thy1.2+, CFSE−Thy1.1+ and CFSE−Thy1.2+, respectively. Donor experimental cells were further divided into Foxp3− Tconv and Foxp3+ Treg cells. The results were expressed as a ratio between WT or S1P1-KO T cells and the cotransferred Thy1.1+ internal control cells, as previously described21.
Neonatal transfer of Treg cells
Treg cells (CD45.1+) were injected intraperitoneally into WT 3-day-old and S1P1-Tg neonatal mice (CD45.2+). At 8 weeks after transfer, the recipient T lymphocytes were analyzed by staining of the congenic markers CD45.1 and CD45.2.
Colitis model
Rag1-/- mice were injected intraperitoneally with 4×105 Tconv cells (CD45.1+) alone or in combination with 2×105 Treg cells (CD45.2+) cells. Mice were weighed and assessed for clinical signs of colitis weekly, and were euthanized 9-10 weeks after transfer. Colons were sectioned, fixed in 10% neutral buffered formalin and processed routinely, and 4-μm sections cut and stained with H&E or Alcian blue/Periodic acid Schiff (PAS). T cells were visualized using a goat anti-CD3 polyclonal antisera (Santa Cruz) and diaminobenzidine chromagen with haematoxylin as a counterstain. Treg cells were visualized with rat anti-Foxp3 clone FJK-16s antibody (eBioscience). Pathology of the colon was scored blindly by an experienced pathologist (K.B.) using a semi-quantitative scale of zero to five. In summary, grade 0 was assigned when no changes were observed; grade 1, minimal inflammatory infiltrates present in the lamina propria with or without mild mucosal hyperplasia; grade 2, mild inflammation in the lamina propria with occasional extension into the submucosa, focal erosions, minimal to mild mucosal hyperplasia and minimal to moderate mucin depletion; grade 3, mild to moderate inflammation in the lamina propria and submucosa occasionally transmural with ulceration and moderate mucosal hyperplasia and mucin depletion; grade 4, marked inflammatory infiltrates commonly transmural with ulceration, marked mucosal hyperplasia and mucin depletion, and multifocal crypt necrosis; grade 5, marked transmural inflammation with ulceration, widespread crypt necrosis and loss of intestinal glands. Blood, spleen, peripheral and mesenteric lymph nodes were also removed, and cells were counted and subsequently stained for CD4 and Foxp3 to determine Tconv and Treg numbers in these organs.
Quantitative RT-PCR
RNA was extracted with RNeasy kit (Qiagen), and cDNA was synthesized with Superscript III reverse transcriptase (Invitrogen). An ABI 7900 Real-time PCR system was used for quantitative PCR, with primer and probe sets from Applied Biosystems (S1pr1, Mm00514644_m1; Foxp3, Mm00475156_m1); results were analyzed with SDS 2.1 software. The cycling threshold value of the endogenous control gene (Hprt1) was subtracted from the cycling threshold value of each target gene to generate the change in cycling threshold (ΔCT). The relative expression of each target gene is expressed as the ‘fold change’ relative to that of wild-type unstimulated samples (2-ΔΔCT), as described49.
Statistical analysis
P values were calculated using Student’s t-test. P values of less than 0.05 were considered significant. All error bars in graphs represent s.d. calculated from at least 3 replicates.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge A. Rudensky (U. of Washington) for Foxp3gfp knockin mice, S. Shrestha for help with genotyping, M. McGargill for help with the FTOC procedure, R. Cross and G. Lennon for cell sorting, and D. Green and Dario Vignali for scientific discussions and sharing important reagents. This work was supported in part by National Institutes of Health, the Arthritis Foundation and Arthritis National Research Foundation, and ALSAC (H.C.), and the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R.L.P.).
REFERENCES
- 1.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 3.Campbell DJ, Ziegler SF. FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol. 2007;7:305–310. doi: 10.1038/nri2061. [DOI] [PubMed] [Google Scholar]
- 4.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shevach EM, et al. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 6.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, et al. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 8.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Huang CT, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5:508–515. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 11.Peng G, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 12.Sutmuller RP, et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116:485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:7048–7053. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 15.Rosen H, et al. Modulating tone: the overture of S1P receptor immunotherapeutics. Immunol Rev. 2008;223:221–235. doi: 10.1111/j.1600-065X.2008.00645.x. [DOI] [PubMed] [Google Scholar]
- 16.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandala S, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 18.Brinkmann V, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 19.Matloubian M, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 20.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J. Biol. Chem. 2004;279:15396–15401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 21.Chi H, Flavell RA. Cutting edge: regulation of T cell trafficking and primary immune responses by sphingosine 1-phosphate receptor 1. J Immunol. 2005;174:2485–2488. doi: 10.4049/jimmunol.174.5.2485. [DOI] [PubMed] [Google Scholar]
- 22.Graler MH, Huang MC, Watson S, Goetzl EJ. Immunological effects of transgenic constitutive expression of the type 1 sphingosine 1-phosphate receptor by mouse lymphocytes. J Immunol. 2005;174:1997–2003. doi: 10.4049/jimmunol.174.4.1997. [DOI] [PubMed] [Google Scholar]
- 23.Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- 24.Sawicka E, et al. The sphingosine 1-phosphate receptor agonist FTY720 differentially affects the sequestration of CD4+/CD25+ T-regulatory cells and enhances their functional activity. J Immunol. 2005;175:7973–7980. doi: 10.4049/jimmunol.175.12.7973. [DOI] [PubMed] [Google Scholar]
- 25.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202:901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burchill MA, et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28:112–121. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Powrie F, Correa-Oliveira R, Mauze S, Coffman RL. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J Exp Med. 1994;179:589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Theofilopoulos AN, Koundouris S, Kono DH, Lawson BR. The role of IFN-gamma in systemic lupus erythematosus: a challenge to the Th1/Th2 paradigm in autoimmunity. Arthritis Res. 2001;3:136–141. doi: 10.1186/ar290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 32.Sauer S, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knight ZA, et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125:733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nombela-Arrieta C, et al. A central role for DOCK2 during interstitial lymphocyte motility and sphingosine-1-phosphate-mediated egress. J Exp Med. 2007;204:497–510. doi: 10.1084/jem.20061780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlson CM, et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 37.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Ledgerwood LG, et al. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat Immunol. 2008;9:42–53. doi: 10.1038/ni1534. [DOI] [PubMed] [Google Scholar]
- 39.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 40.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bensinger SJ, et al. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5287–5296. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crellin NK, Garcia RV, Levings MK. Altered activation of AKT is required for the suppressive function of human CD4+CD25+ T regulatory cells. Blood. 2007;109:2014–2022. doi: 10.1182/blood-2006-07-035279. [DOI] [PubMed] [Google Scholar]
- 44.Sehrawat S, Rouse BT. Anti-Inflammatory Effects of FTY720 against Viral-Induced Immunopathology: Role of Drug-Induced Conversion of T Cells to Become Foxp3+ Regulators. J Immunol. 2008;180:7636–7647. doi: 10.4049/jimmunol.180.11.7636. [DOI] [PubMed] [Google Scholar]
- 45.Daniel C, et al. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J Immunol. 2007;178:2458–2468. doi: 10.4049/jimmunol.178.4.2458. [DOI] [PubMed] [Google Scholar]
- 46.Payne SG, et al. The immunosuppressant drug FTY720 inhibits cytosolic phospholipase A2 independently of sphingosine-1-phosphate receptors. Blood. 2007;109:1077–1085. doi: 10.1182/blood-2006-03-011437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wan YY, Chi H, Xie M, Schneider MD, Flavell RA. The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat Immunol. 2006;7:851–858. doi: 10.1038/ni1355. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell TC, et al. Immunological adjuvants promote activated T cell survival via induction of Bcl-3. Nat Immunol. 2001;2:397–402. doi: 10.1038/87692. [DOI] [PubMed] [Google Scholar]
- 49.Menon S, et al. COP9 signalosome subunit 8 is essential for peripheral T cell homeostasis and antigen receptor-induced entry into the cell cycle from quiescence. Nat Immunol. 2007;8:1236–1245. doi: 10.1038/ni1514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.