Abstract
Autism is a neurodevelopmental disorder that is often comorbid with seizures. Gamma-aminobutyric acid (GABA) is the main inhibitory neurotransmitter in brain. GABAB receptors play an important role in maintaining excitatory/inhibitory balance in brain and alterations may lead to seizures. We compared levels of GABAB receptor subunits GABBR1 and GABBR2 in cerebellum, BA9 and BA40 of subjects with autism and matched controls. Levels of GABBR1 were significantly decreased in BA9, BA40, and cerebellum, while GABBR2 was significantly reduced in the cerebellum. The presence of seizure disorder did not have a significant impact on the observed reductions in GABAB receptor subunit expression. Decreases in GABAB receptor subunits may help explain the presence of seizures that are often comorbid with autism, as well as cognitive difficulties prevalent in autism.
Keywords: GABBR1, GABBR2, autism, cerebellum, BA9, BA40
Introduction
Autism is a neurodevelopmental disorder characterized by impairments in social functioning, stereotypica, including mental retardation [2], and seizure disorders, including epilepsy [3], are often comorbid with autism.
Gamma-aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the brain. There are three classes of GABA receptors: GABAA, GABAB, and GABAC. GABAB receptors are present in the thalamus, cerebellum, hippocampus, cerebral cortex, and interpenduncular nucleus and are coupled via G proteins to membrane K+ and Ca++ channels and to adenylate cyclase in humans [4]. GABAB receptors are heterodimeric, formed from two subunits: GABAB receptor 1 (GABBR1) and GABAB receptor 2 (GABBR2) [5]. GABAB receptors contribute to synaptic events in the mammalian brain presynaptically by facilitating the release of neurtransmitters, including glutamate and GABA, and postsynaptically by generating inhibitory potentials [4, 6]. As a result, GABAB receptors play an important role in maintaining an excitatory/inhibitory balance in the brain, and alterations in GABAB expression may lead to the development of seizures [7, 8].
There is preliminary evidence that polymorphisms of GABBR1 are associated with schizophrenia [9] and obsessive compulsive disorder [10], while the association of GABBR1 polymorphisms with temporal lobe epilepsy is inconclusive [11, 12]. Similarly, GABBR2 has also been associated with temporal lobe epilepsy [11], and multiple laboratories have demonstrated altered expression of GABBR1 and GABBR2 in animal models for seizure disorders [13-15].
We investigated whether GABAB subunits are altered in brains of subjects with autism by measuring expression of GABBR1 and GABBR2 in a well-characterized group of age, sex, and postmortem-interval (PMI) matched brain samples from subjects with autism and matched controls in cerebellum, superior frontal (Brodmann's Area 9 (BA9)) and parietal (BA40) cortices, areas that are involved in the pathology of autism.
Materials and Methods
Tissue preparation
All experimental procedures were approved by the Institutional Review Board of the University of Minnesota School of Medicine. Postmortem blocks of parietal cortex (BA40), superior frontal cortex (BA9), and cerebellum (lobar origin unknown) were obtained from the Autism Research Foundation and several brain banks (the NICHD Brain and Tissue Bank for Developmental Disorders; TARF; the Harvard Brain Tissue Resource Center, which is supported in part by PHS grant number R24 MH068855; the Brain Endowment Bank, which is funded in part by the National Parkinson Foundation, Inc., Miami, Florida; and the Autism Tissue Program). These samples, which have been used by our laboratory previously, are some of the most well-characterized and most-studied brain collections used by multiple groups (for review, [16]). All samples were stored at -80°C until use. Samples were derived from three groups of subjects (cerebellum: N=4-6 from subjects with autism, N=8 from control subjects; BA9: N=4-5 from subjects with autism, N=3 from control subjects; BA40: N=5-8 from subjects with autism, N=6 from control subjects). Consent from next of kin was given to the respective institutions. DSM-IV diagnoses were established prior to death by neurologists and psychiatrists using information from all available medical records and from family interviews. Details regarding the subject selection, diagnostic process, and tissue processing were collected by the Autism Research Foundation. Samples were matched for age, gender, and PMI. All demographic information is listed in Table 1. Seven out of nine subjects with autism had seizure disorder, and all subjects with autism displayed varying degrees of mental retardation (personal communication from Dr. Margaret Bauman). None of the controls had any known history of neuropsychiatric disorders, seizure disorder, or mental retardation.
Table 1.
Demographic Data for Subjects with Autism and Controls
| Case | Dx | Sex | Age | PMI (Hrs.) | Ethnicity | Medication History | Cause of Death | MR* | Seizure* | Brain Areas |
|---|---|---|---|---|---|---|---|---|---|---|
| B1078 | Autistic | M | 22 | 14.3 | Caucasian | Dilantin, Tegretol, Phenobarbital, Theodure Cefobid, | Asphyxia | Yes | Yes | A40 |
| B1045 | Autistic | M | 28 | 16.3 | Caucasian | Urecholine, Duracef | Cardiac arrest | Yes | Yes | Cer, A40 |
| B5000 | Autistic | M | 27 | 8.3 | Caucasian | Synthroid | Drowning | Yes | No | Cer |
| B1401 | Autistic | F | 21 | 20.6 | Caucasian | Tetracycline | Pneumonia, sepsis | Yes | Yes | Cer, A9, A40 |
| B1664 | Autistic | M | 20 | 15 | Caucasian | Vitamins B, C | Perforation of ulcer; asphyxia | Yes | Yes | Cer, A9, A40 |
| B2825 | Autistic | M | 19 | 9.5 | Caucasian | None | Seizure | Yes | Yes | Cer, A9, A40 |
| B3511 | Autistic | M | 29 | 15 | Caucasian | None | Hit by train | Yes | Yes | Cer, A9, A40 |
| B3845 | Autistic | M | 30 | 28.4 | Caucasian | Mellaril, Phenobarbital, Dilantin | Shock; acute pancreatitis | Yes | Yes | A9, A40 |
| B1484 | Autistic | M | 19 | 15 | Caucasian | None | Burns | Yes | No | A9, A40 |
| B3829 | Control | M | 22 | 24.3 | Caucasian | None | MVA | No | No | Cer |
| B4267 | Control | M | 26 | 20 | African-American | None | MVA | No | No | Cer |
| B4268 | Control | M | 30 | 22 | African-American | None | Cardio-myopathy | No | No | Cer, A40 |
| B4269 | Control | M | 28 | 24 | Caucasian | Lidocaine 12.0 mg/L found in blood | Areterio-sclerotic cardiovascular disease | No | No | Cer, A9, A40 |
| B4272 | Control | M | 19 | 17 | Caucasian | None | Accident; chest injuries | No | No | Cer |
| B4275 | Control | M | 20 | 16 | Caucasian | None | Accident | No | No | Cer, A9, A40 |
| B4279 | Control | F | 20 | 21 | Caucasian | None | MVA | No | No | Cer |
| B4362 | Control | M | 30 | 20 | African-American | None | MVA | No | No | Cer, A9 |
| B4101 | Control | M | 24 | 5 | Unknown | None | Gun shot wound | No | No | Cer, A40 |
| B4271 | Control | M | 19 | 21 | African-American | EtOH, Advil, Amoxapine | Epiglottitis | No | No | A40 |
| B4363 | Control | M | 21 | 9 | Caucasian | None | MVA | No | No | Cer, A40 |
Dx, diagnosis; Hrs, hours; PMI, postmortem interval; M, male; F, female; EtOH, alcohol; MVA, motor vehicle accident; MR, Mental retardation
Communication from Dr. M. Bauman.
SDS-PAGE and Western Blotting
Brain tissue (~40 mg per subject) was cut and placed on ice in lysis buffer (3 μl/mg of tissue) [20mM Tris pH 8.0, 0.2 mM EDTA, 150 mM NaCl, 3% Igepal.NP40 (v/v), 1% sodium deoxycholate (w/v), 0.1% SDS (w/v), 50 μl/ml leupeptin, 0.2 mM PMSF, 1 mM sodium orthovanadate, and aprotinin (Sigma, St. Louis, MO; A6279, 30μl/ml buffer)]. Tissue samples were homogenized using a Kontes hand pestle (Kimble-Kontes, Vineland, NJ, USA) while the temperature was maintained at 4°C. Following homogenization, an additional 1 μl of PMSF (0.2 mM) was added to each sample, and the samples were incubated on ice for 30 min. The homogenates were centrifuged for 20 min at 10,000 X g at 4°C. Supernatants were collected and assayed for total protein using the Bradford method (BioRad, Richmond, CA). Samples were stored at -86°C until used. Samples were mixed with denaturing SDS sample buffer (20% glycerol, 100 mM Tris pH 6.8, 0.05% w/v Bromophenol blue, 2.5% SDS (w/v), 5% β-mercaptoethanol) and denatured by heating at 100°C for 5 minutes. SDS polyacrylamide gels were prepared with standard Laemmli solutions (BioRad) (resolving: 6%, stacking: 5%). Sixty μg of protein per lane was loaded onto the gel and electrophoresed for 15 min at 75V followed by 55 min at 150V at room temperature (RT). The proteins were electrotransferred onto nitrocellulose membranes for 2 hr at 300mAmp at 4°C. Protein blots were blocked with 0.2% I-Block (Tropix, Bedford, MA, USA) in PBS with 0.3% Tween 20 for 1hr at RT. The blots were then incubated with anti-GABBR1 (NB300-160, Novus Biologicals (Littleton, CO) 1:1,000), or anti-GABBR2 (56311, QED Bioscience Inc. (San Diego, CA) 1:1,000) for 20 hr at 4°C. Blots were subsequently washed with 0.3% Tween-PBS for 30 minutes, then incubated in secondary antibody for 1 hr at RT (A-9169, Sigma, goat anti-rabbit IgG 1:80,000). Blots were washed twice for 15 minutes with 0.3% Tween-PBS. The immune complexes were visualized using the ECL Plus detection system (Amersham Pharmacia Biotech, Arlington Hts., IL) and exposed to Hyperfilm ECL (Amersham Pharmacia Biotech). Sample densities were analyzed blind to diagnostic nature of the tissue using a BioRad densitometer and the BioRad Multi Analyst software. The molecular weights of approximately 108 kDa (GABBR1) and 105 kDa (GABBR2) immunoreactive bands were quantified with background subtraction. Results obtained are based on at least two independent experiments.
Statistical Analysis
All protein measurements for subjects with autism and control subjects were normalized against β-actin (Table 2). Possible confounding variables were compared between controls and autistics. Neither age nor PMI were found to be statistically different between groups (t(40)=0.94, p=0.35, t(40)=0.38, p=0.71, respectively) with effect sizes for the differences of 0.33 or smaller. We also examined gender and found no significant difference between the two groups (chi-square=0.91, p=0.34). Given this overall lack of difference on potential confounds, we chose to conduct group comparisons (independent group t-tests) without covariates. Significance criteria was set at p<0.05 and all tests were two-tailed. There was a significant difference on seizure status between groups. None of the controls had seizures, whereas 75% of the subjects with autism did (chi-square=28.6, p<0.001). Therefore, we conducted a second analysis examining subjects with autism comorbid with seizure disorder vs. controls using independent group t-tests. Significance criteria was again set at p<0.05 and all tests were two-tailed.
Table 2.
Expression of GABBR1 and GABBR2 in Cerebellum, BA40, and BA9 in Subjects with Autism vs. Controls
| Cerebellum | Control | Autistic | Change | P* |
|---|---|---|---|---|
| GABBR1 / β-Actin | 0.051±0.018 | 0.017±0.006 | ↓ 67% | 0.0049 |
| GABBR2 / β-Actin | 0.068±0.028 | 0.037±0.012 | ↓ 46% | 0.026 |
| BA40 | Control | Autistic | Change | P |
|---|---|---|---|---|
| GABBR1 / β-Actin | 0.079±0.049 | 0.023±0.026 | ↓ 71% | 0.019 |
| GABBR2 / β-Actin | 0.29±0.27 | 0.061±0.038 | ↓ 79% | 0.104 |
| BA9 | Control | Autistic | Change | P |
|---|---|---|---|---|
| GABBR1 / β-Actin | 0.076±0.023 | 0.023±0.024 | ↓ 70% | 0.021 |
| GABBR2 / β-Actin | 0.115±0.016 | 0.053±0.042 | ↓ 54% | 0.064 |
two-tailed independent group t-test
Results
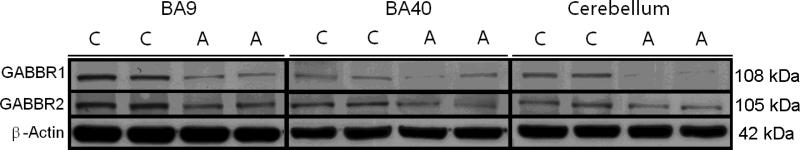
All GABAB western-blotting experiments were normalized against β-actin and are shown as ratios of the various GABAB subunits to β-actin. In cerebellum, GABBR1 (108 kDa) was significantly reduced by 67% (p<0.0049) in subjects with autism. GABBR2 (105 kDa) was also significantly reduced in subjects with autism (46%, p<0.026) when compared with controls (Figure 1, Table 2). GABBR1 was significantly reduced in both BA40 (71%, p<0.019) and in BA9 (70%, p<0.021) in subjects with autism. In contrast, GABBR2 was not significantly altered in either area (Figure 1, Table 2), despite nonsignificant trends for reduction in both brain areas.
Figure 1.
Representative samples of GABBR1 (108 kDa), GABBR2 (105 kDa), and β-Actin (42 kDa) in BA, BA40, and cerebellum of subjects with autism (A) and matched controls (C).
There was a significant difference on seizure status between subjects with autism and controls (chi-square=28.6, p<0.001). However, presence of seizure disorder in subjects with autism did not have an impact on the observed significant reductions of GABBR1 and GABBR2 (Table 3).
Table 3.
Impact of Seizure on Expression of GABBR1 and GABBR2 in Cerebellum, BA40, and BA9 in Subjects with Autism vs. Control
| Cerebellum | Control | Autistic | Change | P* |
|---|---|---|---|---|
| GABBR1 / β-Actin | 0.051±0.018 | 0.015±0.005 | ↓ 71% | 0.013 |
| GABBR2 / β-Actin | 0.068±0.028 | 0.037±0.013 | ↓ 46% | 0.050 |
| BA40 | Control | Autistic | Change | P |
|---|---|---|---|---|
| GABBR1 / β-Actin | 0.079±0.049 | 0.026±0.027 | ↓ 67% | 0.034 |
| GABBR2 / β-Actin | 0.29±0.27 | 0.061±0.038 | ↓ 79% | 0.104 |
| BA9 | Control | Autistic | Change | P |
|---|---|---|---|---|
| GABBR1 / β-Actin | 0.076±0.023 | 0.028±0.024 | ↓ 63% | 0.044 |
| GABBR2 / β-Actin | 0.115±0.016 | 0.067±0.038 | ↓ 42% | 0.115 |
two-tailed independent group t-test
Discussion
Our results are the first to demonstrate changes in GABAB subunits in subjects with autism. We found that GABBR1 was significantly decreased in cerebellum, BA9, and BA40, while GABBR2 was significantly altered in cerebellum only. When comparing subjects with autism comorbid with seizure disorder vs. controls, there was no loss of significance, indicating that seizure disorder did not have a significant effect on the observed results.
Multiple laboratories have demonstrated altered expression of GABBR1 and GABBR2 in animal models for seizure disorders [13-15]. Han et al. [15] found that as a result of multiple seizures, there was a long-term decrease in GABBR1 (15 days) and GABBR2 (30 days) expression in rat hippocampus [15]. Following an injection of kainic acid into the dorsal hippocampus in mouse model of temporal lobe epilepsy, Straessle et al. [13] observed a rapid decline in GABBR1 and GABBR2 in CA1, CA3c, and hilus [13]. A study by Princivalle et al. [14] of the corticothalamic circuit, using Genetic Absence Epilepsy Rats from Strasbourg (GARES), found increased GABBR1 and GABBR2 protein expression in somatosensory cortex, ventrobasal, and reticular thalamic nuclei [14]. Moreover, knockout mice lacking GABBR1 exhibit epilepsy, enhanced prepulse inhibition, impaired memory, and die prematurely largely as a result of development of generalized seizures [17,18]. Taken together, these animal studies suggest that the changes in the number GABAB receptors may lead to epilepsy, due to changes in transmitter release (presynaptic) and inhibition (postsynaptic). There are limited studies of the expression of GABAB receptors in humans. Princivalle et al. [11] demonstrated altered expression for GABBR1A, GABBR1B, and GABBR2 in the hippocampus of subjects with temporal lobe epilepsy [11]. While we did not include hippocampus, making comparisons to the animal models difficult, we did demonstrate pervasive GABAB receptor downregulation, which may have a profound affect on the excitatory/inhibitory balance in the brains of subjects with autism and thus, involvement in the development of seizures.
The occurrence of seizure disorders with autism has been estimated anywhere from 4% to 44% [3]. This wide range is thought to be due to the heterogeneity of clinical populations [19]. For example, seizures are more frequent in individuals with autism that display mental retardation, and the type of language disorder is also relevant to the association of autism and seizure disorders [19]. Eplieptiform activity interferes with cognition by causing disturbances of vigilance, shifting attention, and sudden language difficulties [20], and these phenomena may also occur in children with autism and epilepsy. It may be that the regression in language and behavior frequently observed between ages two and three in children with autism may be due to epileptiform activity [19]. Alterations in GABAB receptors may partially explain the seizure disorders associated with autism. One of our subjects with autism died from seizures (Table 2), and, in total, seven of our subjects with autism were comorbid with seizure disorders. Levels of GABBR1 and GABBR2 proteins that were previously significantly different remained so using this analysis, indicating that presence of seizures did not account for reductions in levels of GABAB receptors. Future studies should include additional areas including the hippocampus and thalamus, and include data from children with autism with and without seizure disorder. Finally, decreases in levels of glutamic acid decarboxylase 65/67 kDa proteins in subjects with autism may be part of the GABAergic deficits seen in autism [21, 22] and be connected mechanistically to the repression in GABAB receptors observed here.
Conclusion
Our laboratory is the first to show significant decreases in GABBR1 and GABBR2 subunits in subjects with autism when compared to normal controls. These changes may help explain the presence of seizure disorders and cognitive abnormalities in subjects with autism.
Acknowledgements
Human tissue was obtained from the NICHD Brain and Tissue Bank for Developmental Disorders; TARF; the Harvard Brain Tissue Resource Center, which is supported in part by PHS grant number R24 MH068855; the Brain Endowment Bank, which is funded in part by the National Parkinson Foundation, Inc., Miami, Florida; and the Autism Tissue Program and is gratefully acknowledged. Grant support by National Institute of Child Health and Human Development (#5R01HD052074-01A2) to SHF is gratefully acknowledged.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. APA Press; Washington, DC: 1994. [Google Scholar]
- 2.Fombonne E. Past and future perspectives on autism epidemiology. In: Moldin SO, Rubenstein JLR, editors. Understanding autism from basic neuroscience to treatment. CRC/Taylor and Francis; Boca Raton, FL: 2006. pp. 25–48. [Google Scholar]
- 3.Tuchman R, Rapin I. Epilepsy in autism. Lancet Neurol. 2002;1:352–358. doi: 10.1016/s1474-4422(02)00160-6. [DOI] [PubMed] [Google Scholar]
- 4.Bowery NG. GABAB receptors structure and function. In: Martin DL, Olsen RW, editors. GABA in the nervous system: the view at fifty years. Lippincott, Williams and Wilkins; Philadelphia, PA: 2000. pp. 233–244. [Google Scholar]
- 5.Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, et al. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 6.Kuriyama K, Hirouchi M, Kimura H. Neurochemical and molecular pharmacological aspects of the GABA(B) receptor. Neurochem Res. 2000;25:1233–1239. doi: 10.1023/a:1007640027977. [DOI] [PubMed] [Google Scholar]
- 7.Leung LS, Canning KJ, Shen B. Hippocampal afterdischarges after GABA(B)-receptor blockade in the freely moving rat. Epilepsia. 2005;46:203–216. doi: 10.1111/j.0013-9580.2005.35804.x. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Olinger AB, Dassow MS, Abel MS. Up-regulation of GABA(B) receptor mRNA and protein in the hippocampus of cocaine-and lidocaine-kindled rats. Neuroscience. 2003;118:451–462. doi: 10.1016/s0306-4522(02)00995-8. [DOI] [PubMed] [Google Scholar]
- 9.Zai G, King N, Wong GW, Barr CL, Kennedy JL. Possible association between the gamma-aminobutyric acid type B receptor 1 (GABBR1) gene and schizophrenia. Eur Neuropsychopharmacol. 2005;15:347–352. doi: 10.1016/j.euroneuro.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Zai G, Arnold P, Burroughs E, Barr CL, Richter MA, Kennedy JL. Evidence for the gamma-amino-butyric acid type B receptor 1 (GABBR1) gene as a susceptibility factor in obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2005;134:25–29. doi: 10.1002/ajmg.b.30152. [DOI] [PubMed] [Google Scholar]
- 11.Princivalle AP, Duncan JS, Thom M, Bowery NG. GABA(B1a), GABA(B1b) AND GABA(B2) mRNA variants expression in hippocampus resected from patients with temporal lobe epilepsy. Neuroscience. 2003;122:975–984. doi: 10.1016/j.neuroscience.2003.08.044. [DOI] [PubMed] [Google Scholar]
- 12.Tan NC, Heron SE, Scheffer IE, Berkovic SF, Mulley JC. Is variation in the GABA(B) receptor 1 gene associated with temporal lobe epilepsy? Epilepsia. 2005;46:778–780. doi: 10.1111/j.1528-1167.2005.49004.x. [DOI] [PubMed] [Google Scholar]
- 13.Straessle A, Loup F, Arabadzisz D, Ohning GV, Fritschy JM. Rapid and long-term alterations of hippocampal GABAB receptors in a mouse model of temporal lobe epilepsy. Eur J Neurosci. 2003;18:2213–2226. doi: 10.1046/j.1460-9568.2003.02964.x. [DOI] [PubMed] [Google Scholar]
- 14.Princivalle AP, Richards DA, Duncan JS, Spreafico R, Bowery NG. Modification of GABA(B1) and GABA(B2) receptor subunits in the somatosensory cerebral cortex and thalamus of rats with absence seizures (GAERS) Epilepsy Res. 2003;55:39–51. doi: 10.1016/s0920-1211(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 15.Han Y, Qin J, Bu DF, Chang XZ, Yang ZX. Successive alterations of hippocampal gamma-aminobutyric acid B receptor subunits in a rat model of febrile seizure. Life Sci. 2006;78:2944–2952. doi: 10.1016/j.lfs.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 16.Palmen SJ, van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. 2004;127:2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- 17.Prosser HM, Gill CH, Hirst WD, Grau E, Robbins M, Calver A, et al. Epileptogenesis and enhanced prepulse inhibition in GABA(B1)-deficient mice. Mol Cell Neurosci. 2001;17:1059–1070. doi: 10.1006/mcne.2001.0995. [DOI] [PubMed] [Google Scholar]
- 18.Schuler V, Lüscher C, Blanchet C, Klix N, Sansig G, Klebs K, et al. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)) Neuron. 2001;31:47–58. doi: 10.1016/s0896-6273(01)00345-2. [DOI] [PubMed] [Google Scholar]
- 19.Canitano R. Epilepsy in autism spectrum disorders. Eur Child Adolesc Psychiatry. 2007;16:61–66. doi: 10.1007/s00787-006-0563-2. [DOI] [PubMed] [Google Scholar]
- 20.Binnie CD. Significance and management of transitory cognitive impairment due to subclinical EEG discharges in children. Brain Dev. 1993;15:23–30. doi: 10.1016/0387-7604(93)90003-q. [DOI] [PubMed] [Google Scholar]
- 21.Fatemi SH, Halt A, Stary J, Kanodia R, Schulz SC, Realmuto G. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in parietal and cerebellar cortices of autistic subjects. Biol Psychiatry. 2002;52:805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- 22.Yip J, Soghomonian JJ, Blatt GJ. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathol. 2007;113:559–568. doi: 10.1007/s00401-006-0176-3. [DOI] [PubMed] [Google Scholar]