Abstract
Objective
Adiponectin receptors 1 and 2 (AdipoR1/R2) mediate the effects of adiponectin on glucose and lipid metabolism in vivo. We examined whether AdipoR1 and/or AdipoR2 mRNA expression in human adipose tissue is fat-depot specific. We also studied whether their expression in visceral and subcutaneous fat depots is associated with metabolic parameters and whether their expression is regulated by intensive physical exercise.
Research design and methods
We determined metabolic parameters and assessed AdipoR1 and R2 mRNA expression using quantitative real-time PCR in adipose tissue in an observational study of 153 subjects, and an interventional study of 60 subjects (20 each with normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes) before and after intensive physical training for 4 weeks.
Results
AdipoR1 and R2 mRNA expression is not significantly different between omental and subcutaneous fat, but their expression is several fold lower in adipose tissue than in muscle. AdipoR2 mRNA expression in visceral fat is highly correlated with its expression in subcutaneous fat. AdipoR2 mRNA expression in both visceral and subcutaneous fat is positively associated with circulating adiponectin and HDL levels but negatively associated with obesity as well as parameters of insulin resistance, glycemia and other lipid levels before and after adjustment for fat mass. Physical training for 4 weeks resulted in increased AdipoR1 and AdipoR2 mRNA expression in subcutaneous fat.
Conclusions
AdipoR2 mRNA expression in fat is negatively associated with insulin resistance and metabolic parameters independently of obesity, and may mediate the improvement of insulin resistance in response to exercise.
Keywords: Adiponectin, AdipoR1, AdipoR2, adipose tissue, obesity, diabetes, exercise training
Introduction
Adiponectin is an adipose tissue-secreted cytokine which acts as a key modulator of insulin sensitivity(1,2), and glucose and lipid metabolism(3), and has pronounced anti-atherosclerotic effects(4,5). The beneficial effects of this highly abundant 244-amino acid protein hormone (circulating at ∼10μg/ml concentration in human serum and accounting for approximately 0.01% of total plasma protein) are predominantly mediated by two cell-membrane receptors, AdipoR1 and AdipoR2(6).
AdipoR1 is a high-affinity receptor for globular adiponectin and studies in mice have shown that it is ubiquitously expressed (6-10) but most abundantly in skeletal muscle. AdipoR2 is predominantly expressed in liver and has intermediate affinity for both full-length and globular adiponectin (6,8). Simultaneous disruption of both AdipoR1 and R2 abolished adiponectin binding and actions, resulting in increased tissue triglyceride content, inflammation and oxidative stress, leading to insulin resistance and glucose intolerance in mice (9). Elevated expression of AdipoR1 and R2 has been associated with decreased plasma insulin levels in mice in either physiological (i.e., fasting) or pathological conditions (11). We have previously reported that prolonged exposure to high fat feeding decreases adiponectin and up-regulates expression of adiponectin receptors in mice (12).
Both adiponectin receptors are expressed in human adipocytes (13-15) and muscle cells (16). Moreover, we have demonstrated that adiponectin receptor expression in skeletal muscle is increased in conditions of insulin resistance and type 2 diabetes, and an exercise intervention for 4 weeks, which improves metabolic parameters also increases circulating adiponectin levels and up-regulates expression of both adiponectin receptors in skeletal muscle(16). It was recently shown, that improvement of insulin sensitivity by thiazolidinediones is not related to AdipoR1/R2 expression changes (15).
Here, we first explored associations of AdipoR1 and/or R2 expression in visceral and subcutaneous fat with metabolic parameters and insulin sensitivity in paired samples of omental and subcutaneous adipose tissue from 153 subjects with a wide range of obesity, body fat distribution, insulin sensitivity, and glucose tolerance in the context of a cross-sectional study. We further tested the hypothesis that AdipoR1 and/or R2 mRNA expression in subcutaneous adipose tissue is up-regulated by the previously described intensive exercise regimen for 4 weeks in 60 subjects with varying degrees of insulin resistance (16).
Research Design and Methods
Cross-sectional study
Paired samples of visceral and subcutaneous adipose tissue were obtained from 153 consecutively enrolled Caucasian men (n=75) and women (n=78) who underwent open abdominal surgery for gastric banding, cholecystectomy, appendectomy, weight reduction surgery, abdominal injuries, or explorative laparotomy. Percentage body fat was measured by dual-energy X-ray absorptiometry (DEXA). In addition, abdominal visceral and subcutaneous fat area was calculated using computed tomography scans at the level of L4-L5 as described previously (17,18). Areas of subcutaneous and intraabdominal adipose tissue (attenuation range of −30 to −190 Hounsfield units) were counted using ImageAccess software (Image, Glattburg, Switzerland). In obese subjects only (BMI>30kg/m2), the ratio of intra-abdominal visceral divided by abdominal subcutaneous fat area was calculated as described previously (17-19).
Using oral glucose tolerance tests (OGTTs), we identified 67 individuals with either type 2 diabetes (n=36) or impaired glucose tolerance (IGT, n=31). All subjects had a stable weight with no fluctuations of >2% of the body weight for at least 3 months before surgery. Patients with malignant diseases or any acute or chronic inflammatory disease as determined by a leucocyte count >7.0 × 109 /l, C-reactive protein >5.0mg/dl or clinical signs of infection were excluded from the study. Samples of visceral and subcutaneous adipose tissue were immediately frozen in liquid nitrogen after explantation. The study was approved by the ethics committee of the University of Leipzig. All subjects gave written informed consent before taking part in the study. Insulin sensitivity was assessed with the euglycemic-hyperinsulinemic clamp method as previously described (16,20). Basal blood samples were taken after an overnight fast. Plasma insulin was measured with an enzyme immunometric assay for the IMMULITE automated analyzer (Diagnostic Products, Los Angeles, CA,). Plasma adiponectin levels were assessed by radioimmunoassay (Linco Research, St. Charles, MO).
Exercise interventional study
We studied 60 Caucasian men and women with no acute or chronic inflammatory disease, alcohol or drug abuse, or diabetic retinopathy or nephropathy. These subjects, which have not been included into the cross-sectional study, were categorized into groups of normal glucose tolerance (NGT) (n=20, 9 men, 11 women), impaired glucose tolerance (IGT) (n=20, 9 men, 11 women), and type 2 diabetes (T2D) (n=20, 11 men, 9 women). All subjects were enrolled in 60 minutes of supervised physical training sessions 3 days per week as previously described (16). At baseline and after 4 weeks of training (48 hours after the last training session), subcutaneous adipose tissue and blood samples were obtained in the fasting state, and dual-energy X-ray absorptiometry analyses and measurements of anthropometric parameters were performed. All baseline blood samples and adipose tissue samples were collected between 8 and 10am after an overnight fast. Subcutaneous adipose tissue samples were immediately frozen in liquid nitrogen after explantation. The study was approved by the ethics committee, and all subjects gave written informed consent.
Analysis of AdipoR1/R2 mRNA expression in adipose tissue
Human AdipoR1 and AdipoR2 gene expression was measured by quantitative real-time PCR in a fluorescent temperature cycler using the TaqMan assay, and fluorescence was detected on an ABI PRISM 7000 sequence detector (Applied Biosystems, Darmstadt, Germany) as described previously (16). The following primers were used: human AdipoR1: TTC TTC CTC ATG GCT GTG ATG T (sense) and AAG AAG CGC TCA GGA ATT CG (antisense); human AdipoR2: ATA GGG CAG ATA GGC TGG TTG A (sense) and GGA TCC GGG CAG CAT ACA (antisense); human 18s rRNA: TGC CAT GTC TAA GTA CGC ACG (sense); TTG ATA GGG CAG ACG TTC GA (antisense).
Statistical analyses
In both, the cross-sectional and interventional studies, comparisons of descriptive characteristics, expressed as mean ± SE or means with 95% CIs, were conducted using one-way ANOVA with Bonferroni corrected post hoc tests and were repeated using nonparametric Kruskal-Wallis tests. Nonparametric Spearman correlation coefficients were calculated to examine the cross-sectional associations of adiponectin and its receptors with anthropometric and insulin resistance-related parameters. Analyses were repeated adjusting for age, body fat and sex. For the interventional study, post hoc comparisons of baseline and after-training measures, expressed as mean ± SE, were conducted using paired t tests within groups of glucose tolerance (NGT, IGT and T2D). Differences in change between groups in measurements were compared by one-way ANOVA with Bonferroni corrected post hoc tests.
Results
We present descriptive characteristics of participants in the cross-sectional study (n=153) and exercise intervention study (n=60) in Table 1. In lean and healthy subjects, AdipoR2 gene expression in adipose tissue was higher than that of AdipoR1 (P<0.05), and expressions of both AdipoR1 and AdipoR2 was substantially lower in subcutaneous adipose tissue than in muscle (P<0.05 for both) (Figure 1A). In addition, visceral and subcutaneous AdipoR2 expression were highly interrelated (Table 2) and both were significantly higher in lean than in obese subjects and in subjects with NGT compared to subjects with IGT or type 2 diabetes (Figure 1B). Expression of AdipoR1 did not differ between lean, subcutaneous obese or visceral obese subjects in either depot (Figure 1A). Subcutaneous AdipoR1 mRNA expression was lower in subjects with NGT compared with IGT or type 2 diabetes (Figure 1B). Age was negatively correlated with circulating adiponectin levels (r = -0.18, P = 0.033) as well as AdipoR2 expression in both subcutaneous (r = -0.30, P < 0.001) and visceral adipose tissue (r =-0.32, P < 0.001). The associations of adiponectin receptor gene expression with insulin resistance and obesity were similar when subjects were stratified by age in two categories, i.e. younger or older than 60 years (data not shown).
Table 1.
Descriptive and metabolic characteristics, along with adiponectin and receptor gene expression, from a cross-sectional study of n=153 subjects categorized as Lean, Subcutaneous (SC) Obese or Visceral (visc) Obese and n=60 subjects categorized in groups of normal glucose tolerance (NGT), impaired glucose tolerance (IGT) and type 2 diabetes (T2D) from separate exercise intervention trial at baseline. Data are expressed as means ± SE and compared using ANOVA with Bonferroni corrections for post-hoc tests.
| Variable | Cross-sectional study | Exercise intervention study | ||||
|---|---|---|---|---|---|---|
| Lean (n=58) | SC obese (n=58) | Visc obese (n=37) | NGT (n=20) | IGT (n=20) | T2D (n=20) | |
| Male (n) / Female (n) | 28 / 30 | 28 / 30 | 19 / 18 | 9 / 11 | 9 / 11 | 11 / 9 |
| Age (y) | 50.2 ± 2.1 | 55.3 ± 1.7 | 64.4 ± 1.9***++ | 32.8 ± 2.5 | 56.0 ± 3.6*** | 53.1 ± 1.5*** |
| Anthropometric | ||||||
| BMI (kg/m2) | 23.9 ± 0.2 | 35.9 ± 0.9*** | 33.6 ± 1.0*** | 24.3 ± 0.3 | 29.8 ± 0.9*** | 31.4 ± 0.7*** |
| WHR | 0.85 ± 0.02 | 1.05 ± 0.02*** | 1.13 ± 0.02***++ | 0.84 ± 0.02 | 1.21 ± 0.04*** | 1.28 ± 0.03*** |
| Body Fat (%) | 21.6 ± 0.4 | 41.1 ± 1.2*** | 32.9 ± 1.1***+++ | 24.5 ± 0.7 | 34.9 ± 1.9*** | 38.2 ± 1.8*** |
| Visceral Fat Area (cm2) | 60.9 ± 2.5 | 146.6 ± 4.1*** | 272.7 ± 10.2***+++ | -- | -- | -- |
| SC Fat Area (cm2) | 77.1 ± 3.9 | 620.5 ± 39.7*** | 381.4 ± 28.7***+++ | -- | -- | -- |
| CT ratio | 0.83 ± 0.03 | 0.48 ± 0.04*** | 0.84 ± 0.03+++ | -- | -- | -- |
| Metabolic | ||||||
| Fasting plasma insulin (pmol/l) | 28.0 ± 1.5 | 170.4 ± 17.2*** | 198.0 ± 18.5*** | 66.0 ± 8.0 | 695.0 ± 110*** | 319.0 ± 48*++ |
| Fasting plasma glucose (mmol/l) | 5.4 ± 0.1 | 5.9 ± 0.19** | 5.8 ± 0.2 | 5.2 ± 0.1 | 5.7 ± 0.12* | 6.2 ± 0.1***++ |
| 2h OGTT glucose (mmol/l) | 5.9 ± 0.1 | 6.8 ± 0.30* | 7.4 ± 0.39** | 6.0 ± 0.2 | 9.4 ± 0.20*** | 13.1 ± 0.3***+++ |
| WBGU (μmol/kg/min) | 97.2 ± 1.0 | 57.4 ± 4.0*** | 38.6 ± 4.7***++ | 75.9 ± 3.8 | 18.7 ± 2.0*** | 21.5 ± 2.1*** |
| Lipids | ||||||
| Total cholesterol (mg/dl) | 201.0 ± 4.8 | 202.2 ± 3.9 | 224.2 ± 4.8**++ | 178.7 ± 4.3 | 206.5 ± 4.6** | 216.6 ± 6.2*** |
| HDL cholesterol (mg/dl) | 62.0 ± 2.5 | 48.6 ± 1.9*** | 41.6 ± 3.0*** | 46.4 ± 1.9 | 63.4 ± 2.7*** | 56.8 ± 2.7* |
| LDL cholesterol (mg/dl) | 107.1 ± 3.9 | 110.6 ± 3.7 | 136.5 ± 4.4***+++ | 90.5 ± 3.9 | 124.5 ± 4.6*** | 127.6 ± 7.3*** |
| FFA (mmol) | 0.30 ± 0.02 | 0.66 ± 0.05*** | 0.78 ± 0.05*** | 0.41 ± 0.04 | 0.54 ± 0.06 | 0.56 ± 0.06 |
P<0.05,
P<0.01,
P<0.001 versus Lean group (cross-sectional study) or NGT group (intervention study)
P<0.05,
P<0.01,
P<0.001 versus SC Obese group (cross-sectional study) or IGT group (intervention study)
Figure 1.
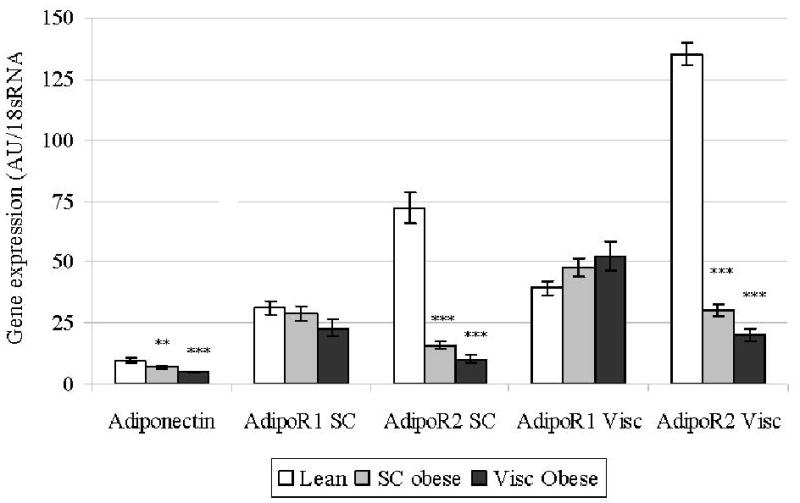
Figure 1A). Adiponectin and receptor gene expression in visceral and subcutaneous (SC) adipose tissues at baseline in subjects with lean, subcutaneous obese and visceral obese for cross-sectional study. * P<0.05, ** P<0.01, ** P<0.001 versus lean;
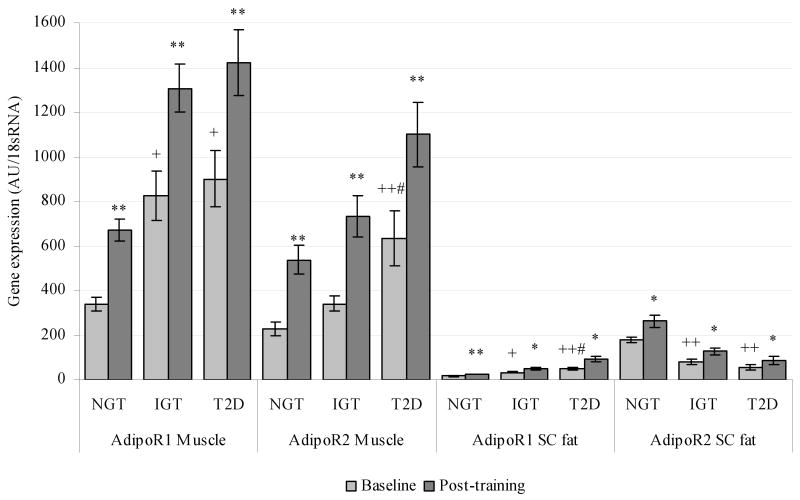
Figure1B). Adiponectin receptor gene expression in skeletal muscle and subcutaneous (SC) adipose tissue at baseline and after intensive physical training in subjects with normal glucose tolerance (NGT), impaired glucose tolerance (IGT) and type 2 diabetes (T2D) for interventional study. * P<0.01, ** P<0.001 versus baseline; + P<0.01, ++ P<0.001 versus NGT; # P<0.05 versus IGT.
Table 2.
Spearman correlation matrix of gene expression of AdipoR1 and AdipoR2 in visceral (Vis) and subcutaneous (SC) adipose tissue with study variables for cross-sectional study subjects (n=153). WBGU, whole body glucose uptake during the steady state of euglycemic-hyperinsulinemic clamp.
| Variable | Adiponectin | AdipoR1 SC | AdipoR2 SC | AdipoR1 Vis | AdipoR2 Vis | |||
|---|---|---|---|---|---|---|---|---|
| a. Age adjusted | ||||||||
| BMI | -0.34*** | -0.10 | -0.63*** | 0.12 | -0.68*** | |||
| WHR | -0.39*** | -0.23** | -0.49*** | 0.06 | -0.46*** | |||
| Body Fat % | -0.36*** | -0.16 | -0.50*** | 0.12 | -0.65*** | |||
| Fasting plasma glucose | -0.23** | -0.01 | -0.30*** | -0.06 | -0.32*** | |||
| Fasting plasma insulin | -0.36*** | -0.11 | -0.60*** | 0.11 | -0.67*** | |||
| 2hr OGTT glucose | -0.20* | -0.13 | -0.22* | -0.10 | -0.19* | |||
| WGBU | 0.42*** | 0.25** | 0.63*** | 0.03 | 0.69*** | |||
| Total cholesterol | -0.15 | -0.02 | -0.18* | 0.05 | -0.21** | |||
| HDL cholesterol | 0.29** | 0.15 | 0.32*** | -0.11 | 0.35*** | |||
| LDL cholesterol | -0.19* | -0.05 | -0.21** | 0.03 | -0.31*** | |||
| FFA | -0.33*** | -0.06 | -0.41*** | 0.28*** | -0.50*** | |||
| Adiponectin | 0.13 | 0.29** | -0.02 | 0.36*** | ||||
| AdipoR1 SC fat | 0.08 | 0.22** | 0.15 | |||||
| AdipoR2 SC fat | -0.04 | 0.80*** | ||||||
| AdipoR1 Visceral fat | -0.04 | |||||||
| b. Age, gender, and body fat adjusted | ||||||||
| BMI | -0.04 | 0.09 | -0.45*** | 0.01 | -0.33*** | |||
| WHR | -0.24** | -0.15 | -0.35*** | -0.08 | -0.27** | |||
| Fasting plasma glucose | -0.16 | 0.04 | -0.21* | -0.10 | -0.22** | |||
| Fasting plasma insulin | -0.19* | -0.01 | -0.42*** | 0.05 | -0.45*** | |||
| 2hr OGTT glucose | -0.14 | -0.11 | -0.15 | -0.15 | -0.11 | |||
| WGBU | 0.29** | 0.17 | 0.48*** | 0.08 | 0.49*** | |||
| Total cholesterol | -0.10 | 0.01 | -0.12 | 0.03 | -0.14 | |||
| HDL cholesterol | 0.18* | 0.11 | 0.20* | -0.07 | 0.22** | |||
| LDL cholesterol | -0.13 | -0.03 | -0.14 | 0.02 | -0.24** | |||
| FFA | -0.19* | 0.02 | -0.21* | 0.27** | -0.24** | |||
| Adiponectin | 0.06 | 0.14 | 0.03 | 0.19* | ||||
| AdipoR1 SC fat | 0.00 | 0.26** | 0.07 | |||||
| AdipoR2 SC fat | 0.03 | 0.72*** | ||||||
| AdipoR1 Visceral fat | 0.04 | |||||||
P<0.05,
P<0.01,
P<0.001
We then examined age-adjusted associations of adiponectin receptor expression in fat with anthropometric and metabolic variables among subjects enrolled in the cross-sectional study (Table 2). Serum adiponectin was significantly and negatively correlated to adiposity measures, IGT, and dyslipidemia. After adjustments for age, sex and percent body fat, adiponectin remained negatively associated with waist-to-hip ratio (WHR) and free fatty acids and positively associated with HDL cholesterol and insulin sensitivity (clamp). Expression of AdipoR1 in visceral fat was only correlated with subcutaneous AdipoR1 mRNA expression and free fatty acid serum concentrations beyond the effects of age, sex and percent body fat (Table 2). In contrast, AdipoR2 expression in both fat depots showed strong correlations with adiponectin, HDL cholesterol, and measures of adiposity, IGT, and dyslipidemia (Table 2). Among subjects in the interventional study, moderate correlations between AdipoR1 expression in subcutaneous fat and adiposity/IGT (r=0.5 for BMI, WHR, percent body fat, and 2hr OGTT glucose) disappeared after adjusting for sex and percent body fat. Associations of AdipoR2 expression in subcutaneous fat with the studied metabolic variables were very similar both in direction and magnitude with those reported for the cross-sectional study (data not shown).
Associations between AdipoR2 expression and metabolic variables also remained significant upon adjusting for age, sex and body fat. To eliminate a potential effect of type 2 diabetes (n=26 of 153, 17%), we performed additional analyses excluding type 2 diabetic subjects. Neither mean AdipoR2 mRNA expression differences, nor statistically significant associations were altered by excluding subjects with type 2 diabetes from the analyses (data not shown).
Four weeks of intensive physical training resulted in significant improvements of body weight, percent body fat, insulin sensitivity and circulating adiponectin, leading to increases in skeletal muscle AdipoR1/R2 mRNA expression (16). We report here that the same intervention led to an increased expression of AdipoR1/R2 in subcutaneous fat (Figure 1B). Subcutaneous AdipoR1 expression was elevated by 63% in subjects with NGT, by 48% in participants with IGT, and by 89% in type 2 diabetic patients. Similarly, AdipoR2 expression in subcutaneous fat was significantly higher, with increases of 48, 58, and 57% for the same subgroups, respectively. These changes in receptor expression were independent of decreases in body weight (data not shown, adjusted P<0.05 for both in all groups). Finally, the increase of AdipoR2 in subcutaneous fat is significantly and positively correlated to the increases of AdipoR2 in skeletal muscle (R2 =0.34, P<0.01).
Conclusions
Adiponectin has become widely accepted as a key regulator of insulin sensitivity and metabolism(1-5), but the physiological regulation and role of adiponectin receptors, AdipoR1 and AdipoR2, in mediating the beneficial effects of adiponectin in humans remain to be fully elucidated. We confirm age, gender and BMI-independent relationships between circulating adiponectin and measures of obesity and insulin sensitivity, supporting previous reports that adiponectin is related to central adiposity and improved insulin sensitivity beyond associations with total body fat (21). In this study, we confirm, using quantitative real-time PCR that both receptors are expressed in subcutaneous and visceral adipose tissue, but at levels significantly lower than in skeletal muscle. We also demonstrate that expression of AdipoR2 is several fold higher than that of AdipoR1 in fat. It correlates negatively with obesity, lipid level, glycemia and insulin resistance, and is significantly decreased in states of insulin resistance including obesity and type 2 diabetes.
It was recently shown that in elderly men, high adiponectin levels are associated with increased all-cause and cardiovascular disease mortality (22). However, subgroup analyses in individuals aged > 60 years revealed similar associations of adiponectin receptor gene expression with insulin resistance and obesity compared to younger subjects. In parallel to our previous analysis of skeletal muscle AdipoR1/R2 mRNA expression (16), 4-weeks of intensive exercise training significantly elevated gene expression of both AdipoR1/R2 in subcutaneous adipose tissue in subjects with NGT, IGT, and type 2 diabetes, with comparable relative increases across groups. However, because of the lack of association between baseline AdipoR1 expression and most parameters in the cross-sectional study, the potential mechanisms causing increased AdipoR1 expression in response to exercise remain elusive. Therefore, more sophisticated study designs are necessary to elucidate the causal factors for training-induced AdipoR1 expression changes.
Only a limited number of studies have examined adiponectin receptor expression in adipose tissue in a small number of humans and have yielded inconclusive results (13,15, 23). A recent cross-sectional study reported, similar to our study, decreased AdipoR2 expression, but no differences in AdipoR1 expression in intra-abdominal adipose tissue among obese subjects with and without diabetes compared to lean controls (23). Our study confirms these findings in visceral adipose samples from a larger study sample (n=153) and extends previous results by demonstrating similar associations in subcutaneous fat.
Similar to our study, a recent weight-loss intervention study of lean and obese women consistently reported reduced AdipoR2 gene expression in subcutaneous adipose tissue from obese versus lean women (13) and reported significant decreases in AdipoR1 with obesity. In contrast to our and other previous (23) findings, however, this study did not find decreased AdipoR2 mRNA expression in visceral fat. These differences may be due to the exclusion of males and a lower mean age of this study population (13). We confirm the data referring to AdipoR2 expression in subcutaneous adipose tissue, utilizing a longer study, and consisting with that previous study (13), we show that serum adiponectin and adiponectin receptor expressions increase with exercise in both lean and obese subjects independent of glycemic control. Moreover, in the exercise intervention study, we also show that AdipoR1 expression in subcutaneous adipose tissue is ∼20-fold lower and AdipoR2 ∼1.5-fold less than in muscle in humans.
Although AdipoR1/R2 gene expression increases both in skeletal muscle and subcutaneous fat with intensive exercise, mRNA expression of both receptors is higher and shows greater improvements with exercise training in skeletal muscle than in subcutaneous adipose tissue. It was recently shown, that insulin-sensitizing effects of thiazolidinediones are not linked to AdipoR1/R2 expression changes (15), suggesting that mechanisms other than improved insulin sensitivity causing increased AdipoR1/R2 mRNA levels in response to training. Additional paracrine or autocrine adiponectin effects in adipose tissue may explain the different aspects of AdipoR1/R2 mRNA expression regulation between fat and muscle. We further investigated whether adiponectin receptor mRNA expression varies in relation to differences in fat distribution and glycemic control. Based on computed tomography scanning measurements (L4-L5) of abdominal visceral and subcutaneous fat areas, obese subjects were further categorized as predominantly visceral or subcutaneous obese, with predominantly visceral obesity defined as a ratio of visceral-to-subcutaneous fat area > 0.5, as previously described (17,18). Independent of fat distribution, AdipoR2 mRNA was reduced in subcutaneous and visceral fat among subjects with obesity compared to lean individuals. No such differences were found for AdipoR1 mRNA expression among all groups. Similarly, AdipoR2 mRNA expression in subcutaneous fat was reduced in patients with IGT and type 2 diabetes, whereas AdipoR1 mRNA was higher in IGT and type 2 diabetic patients. It has been previously suggested that AdipoR2 may play a more substantial role in the pathogenesis of type 2 diabetes (23-25). This hypothesis is supported by the significant associations of AdipoR2 mRNA expression in adipose tissue with measures of obesity and diabetes in this study. Dysregulation of AdipoR2 expression (6,23) in adipose tissue may promote accumulation of lipids due to reduced adiponectin action. Future studies are needed to fully clarify the potentially distinct roles of AdipoR1 and AdipoR2 in regulating the action of adiponectin in various tissues and metabolic conditions.
In summary, we show that AdipoR2 mRNA expression in subcutaneous and visceral adipose tissue is reduced in states of obesity and type 2 diabetes. In contrast to AdipoR1 mRNA expression, AdipoR2 mRNA expression in fat correlates with circulating adiponectin levels, lipid levels, parameters of insulin sensitivity and glycemic control. An exercise intervention for 4 weeks resulted in increased expression of both adiponectin and adiponectin receptors which may thus mediate the beneficial effects of exercise on insulin resistance, glycemia and lipidemia.
Acknowledgments
This work was supported by grants from Deutsche Forschungsgemeinschaft (BL 580/3-1 to M.B.), the Clinical Research group “Atherobesity” KFO 152 (project BL 833/1-1 to M.B.) and the Interdisciplinary Center of Clinical Research Leipzig at the Faculty of Medicine of the University of Leipzig (project B24 to M.B.), by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant R01 58785 (to C.S.M.), and in part, by National Institutes of Health (NIDDK) Grant P30 DK 57521 (“The Metabolic Physiology Core”), the Humboldt Foundation, and by a discretionary grant from Beth Israel Deaconess Medical Center (to C.S.M.).
Reference List
- 1.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 2.Pajvani UB, Scherer PE. Adiponectin: systemic contributor to insulin sensitivity. Curr Diab Rep. 2003;3:207–213. doi: 10.1007/s11892-003-0065-2. [DOI] [PubMed] [Google Scholar]
- 3.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 4.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–635. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 5.Kobashi C, Urakaze M, Kishida M, Kibayashi E, Kobayashi H, Kihara S, Funahashi T, Takata M, Temaru R, Sato A, Yamazaki K, Nakamura N, Kobayashi M. Adiponectin inhibits endothelial synthesis of interleukin-8. Circ Res. 2005;97:1245–1252. doi: 10.1161/01.RES.0000194328.57164.36. [DOI] [PubMed] [Google Scholar]
- 6.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein BJ, Scalia R. Adiponectin: A novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. 2004;89:2563–2568. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- 9.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 10.Kharroubi I, Rasschaert J, Eizirik DL, Cnop M. Expression of adiponectin receptors in pancreatic beta cells. Biochem Biophys Res Commun. 2003;312:1118–1122. doi: 10.1016/j.bbrc.2003.11.042. [DOI] [PubMed] [Google Scholar]
- 11.Tsuchida A, Yamauchi T, Ito Y, Hada Y, Maki T, Takekawa S, Kamon J, Kobayashi M, Suzuki R, Hara K, Kubota N, Terauchi Y, Froguel P, Nakae J, Kasuga M, Accili D, Tobe K, Ueki K, Nagai R, Kadowaki T. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem. 2004;279:30817–30822. doi: 10.1074/jbc.M402367200. [DOI] [PubMed] [Google Scholar]
- 12.Bullen JW, Jr, Bluher S, Kelesidis T, Mantzoros CS. Regulation of adiponectin and its receptors in response to development of diet induced obesity in mice. Am J Physiol Endocrinol Metab. 2007;292:E1079–E1086. doi: 10.1152/ajpendo.00245.2006. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen MS, Lihn AS, Pedersen SB, Bruun JM, Rasmussen M, Richelsen B. Adiponectin receptors in human adipose tissue: effects of obesity, weight loss, and fat depots. Obesity (Silver Spring) 2006;14:28–35. doi: 10.1038/oby.2006.5. [DOI] [PubMed] [Google Scholar]
- 14.Fasshauer M, Klein J, Kralisch S, Klier M, Lossner U, Bluher M, Paschke R. Growth hormone is a positive regulator of adiponectin receptor 2 in 3T3-L1 adipocytes. FEBS Lett. 2004;558:27–32. doi: 10.1016/S0014-5793(03)01525-4. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Tonelli J, Kishore P, Owen R, Goodman E, Scherer PE, Hawkins M. Insulin-sensitizing effects of thiazolidinediones are not linked to adiponectin receptor expression in human fat or muscle. Am J Physiol. 2007;292:E1301–E1307. doi: 10.1152/ajpendo.00312.2006. [DOI] [PubMed] [Google Scholar]
- 16.Blüher M, Bullen JW, Jr, Lee JH, Kralisch S, Fasshauer M, Kloting N, Niebauer J, Schon MR, Williams CJ, Mantzoros CS. Circulating adiponectin and expression of adiponectin receptors in human skeletal muscle: associations with metabolic parameters and insulin resistance and regulation by physical training. J Clin Endocrinol Metab. 2006;91:2310–2316. doi: 10.1210/jc.2005-2556. [DOI] [PubMed] [Google Scholar]
- 17.Berndt J, Kloting N, Kralisch S, Kovacs P, Fasshauer M, Schon MR, Stumvoll M, Bluher M. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005;54:2911–2916. doi: 10.2337/diabetes.54.10.2911. [DOI] [PubMed] [Google Scholar]
- 18.Blüher M, Engeli S, Kloting N, Berndt J, Fasshauer M, Batkai S, Pacher P, Schon MR, Jordan J, Stumvoll M. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes. 2006;55:3053–3060. doi: 10.2337/db06-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujiioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism. 1987;36:54–59. doi: 10.1016/0026-0495(87)90063-1. [DOI] [PubMed] [Google Scholar]
- 20.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 21.Mantzoros CS, Li T, Manson JE, Meigs JB, Hu FB. Circulating adiponectin levels are associated with better glycemic control, more favorable lipid profile, and reduced inflammation in women with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:4542–4548. doi: 10.1210/jc.2005-0372. [DOI] [PubMed] [Google Scholar]
- 22.Wannamethee SG, Whincup PH, Lennon L, Sattar N. Circulating adiponectin levels and mortality in elderly men with and without cardiovascular disease and heart failure. Arch Intern Med. 2007;167:1510–1517. doi: 10.1001/archinte.167.14.1510. [DOI] [PubMed] [Google Scholar]
- 23.Morinigo R, Musri M, Vidal J, Casamitjana R, Delgado S, Lacy AM, Ayuso C, Gomis R, Corominola H. Intra-abdominal fat adiponectin receptors expression and cardiovascular metabolic risk factors in obesity and diabetes. Obes Surg. 2006;16:745–751. doi: 10.1381/096089206777346736. [DOI] [PubMed] [Google Scholar]
- 24.Bauche IB, it El MS, Rezsohazy R, Funahashi T, Maeda N, Miranda LM, Brichard SM. Adiponectin downregulates its own production and the expression of its AdipoR2 receptor in transgenic mice. Biochem Biophys Res Commun. 2006;345:1414–1424. doi: 10.1016/j.bbrc.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 25.Damcott CM, Ott SH, Pollin TI, Reinhart LJ, Wang J, O'connell JR, Mitchell BD, Shuldiner AR. Genetic variation in adiponectin receptor 1 and adiponectin receptor 2 is associated with type 2 diabetes in the Old Order Amish. Diabetes. 2005;54:2245–2250. doi: 10.2337/diabetes.54.7.2245. [DOI] [PubMed] [Google Scholar]