Abstract
The progressive loss of laminin 5 and the α6β4 integrin is a characteristic of the transition of prostatic intraepithelial neoplasia (PIN) to invasive human prostate cancer. Our objective was to determine if the loss of the interaction with laminin 5 would influence the ability of human epithelial cells to respond to DNA damage. Three cellular damage responses to ionizing radiation (IR) were analyzed including G2 progression, cdc2 phosphorylation, and cell survival. The adhesion of normal human prostate epithelial cells to laminin 5 amplified the G2 arrest induced by IR, and depends on a known cell binding domain of laminin 5. The alteration of G2 arrest was confirmed by an inhibition of phospho-cdc2 nuclear translocation. In contrast, a prostate epithelial cancer cell line blocked in G2 independent of adhesion to laminin 5. The survival of these cell lines in response to IR was unaffected by adhesion to laminin 5. These results suggest that cell adhesion to laminin 5 in normal cells will amplify the IR induced G2 cell cycle progression block without altering cell survival. The loss of laminin 5 and the α6β4 integrin in PIN lesions may contribute to the selection and progression of genetically unstable cell types via attenuation of a DNA damage induced G2 arrest.
Keywords: laminin 5, α6β4 integrin, cell cycle, ionizing radiation, phospho-cdc2
INTRODUCTION
Prostate cancer is the most commonly diagnosed cancer and second most prevalent cause of cancer death in men in the United States [1,2]. Limited understanding is available concerning the development and progression of prostate cancer despite its prevalence. Neoplastic changes are first apparent in prostatic intraepithelial neoplasia (PIN) and it is thought that PIN may be a precursor to prostate cancer [3–9]. PIN lesions are characterized by an attenuated basal lamina, enlarged nuclei, and an increased number of luminal type cells. The loss of basement membrane constituents and the basal cell phenotype are characteristics of prostate cancer. The alteration of the basement membrane in the gland and the basal cell phenotype may allow malignant cells to invade and migrate through the tissue [10–12].
Laminin 5 (α3β2γ2) is a member of a family of extracellular matrix (ECM) glycoproteins that are expressed in the basal lamina of several tissues including the prostate [13]. The adhesion of normal epithelial cells to the ECM, in particular laminin 5, through the formation of hemidesmosomal complexes with the α6β4 integrin, is known to be a critical component for continued cell growth, survival, and genome stability [14–20]. Laminin 5 is downregulated or lost in several squamous and epithelial carcinomas including the prostate [10,21–24]. In our previous studies, we have found that the loss of laminin 5 and the α6β4 integrin (laminin receptor) was a characteristic feature accompanying the transition of normal prostate epithelial glands to PIN lesions to invasive prostate cancer [4,6,7].
The loss of laminin 5, and subsequent loss of the hemidesmosome, may contribute to the instability of the epithelial cell functions within the PIN lesions leading to selection and progression to invasive carcinoma via loss of a coordinated cellular damage response.
Prostate cancer arises primarily in older men and evidence of oxidative DNA damage increases with age in prostate tissue [25,26]. Most cell division occurs in the basal cell layer [27] suggesting that basal cells that accumulate damage may give rise to altered cells. DNA damage response elements appear to be activated in early stages of tumorogenesis, indicating their importance in preventing cancer progression [28]. Recent reports suggest that malignant prostate cell lines have defective DNA repair mechanisms lending support to the idea that prostate cancer progression occurs due to aberrant DNA repair [29]. Loss of adhesion in prostate cancer cell lines has also been associated with impaired DNA damage repair [30]. Theα6β4 integrin is a potential candidate as a target for cancer prevention if loss of specific components contributes to progression of genetically unstable cells. The early events associated with loss of laminin 5 and the α6β4 integrin in prostate cancer progression may contribute to the progression of a genetically unstable cell phenotype.
Cell cycle progression is regulated through signaling pathways controlled by integrin-mediated adhesion to the ECM and receptor binding of growth factors [31–33]. G1 phase and S phase [34] are influenced by integrins and it has been recently shown that adhesion of cells to fibronectin through integrin receptors plays a role in regulating the G2 phase and entry into mitosis [35,36]. Signaling events through cell adhesion to laminin 5 through the α6β4 integrin may affect cell cycle regulation after DNA damage.
We hypothesized that the loss of adhesion to laminin 5 through the α6β4 integrin would decrease the cells ability to respond to DNA damage. Ionizing radiation (IR) was used to generate DNA damage in normal and cancer epithelial cell lines and its effects on G2 progression and cell survival were investigated under conditions of laminin 5 adhesion.
MATERIALS AND METHODS
Cell Culture
The normal prostate cell line, PrEC, and prostate cancer cell line, PC3, were purchased from Clonetics (San Diego, CA). PrEC cells are primary epithelial cells isolated from normal prostate tissue with limited proliferation and limited passages. PrEC cells were maintained in PrEGM medium (Clonetics, San Diego, CA) and PC3 cells were maintained in Dulbecco’s modified Eagle medium (DMEM; Invitrogen, Carlesbad, CA) supplemented with 10% fetal bovine serum. The normal breast epithelial cell line, MCF10A (American Type Culture Collection, Manassas, VA) was maintained in IMDM (Invitrogen, Carlesbad, CA) supplemented with 5% v/v horse serum, 1% v/v penicillin/streptomycin, 100 ng/ml cholera toxin, 20 ng/ml epidermal growth factor (EGF), and 500 ng/ml hydrocortisone. HaCat cells, a normal immortalized keratinocyte cell line, were maintained in DMEM supplemented with 10% fetal bovine serum. All cell lines were incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2. Cells were irradiated at 1, 2, 3, 5, or 7 Gy at room temperature using γ radiation from a 60Co source.
Cell Cycle Assays
Tissue culture plates were coated with laminin 5 from sterile filtered conditioned HaCat serum-free media for 2 hr and washed with PBS. PC3 or PrEC cells were placed into 35 mm laminin 5 coated dishes at 85,000 cells/well, and irradiated 72 hr later with 0, 0.25, 1, 2, 5, or 7 Gy in triplicates for each dose. Cells were harvested with trypsin 48 hr later and suspended in Krishan buffer (0.1% sodium citrate, 0.02 mg/ml RNAse, 0.3% NP-40 and 0.05% propidium iodide) for 30 min on ice. DNA content was assessed with flow cytometry using a FACs Star Plus flow cytometer (Becton-Dickenson).
For antibody blocking experiments, tissue culture plates were coated at a concentration of 10 μg/ml with BM165 (Dr. Robert Burgeson, Mass General Hospital, Harvard Medical School), or mouse IgG (MsIgG; Chemicon, Temecula, CA) in PBS for 2 hr at room temperature. Tissue culture plates were rinsed with PBS, blocked with 1% BSA for 1 hr, rinsed, and coated with laminin 5 for 2 hr, using conditioned HaCat serum-free media. MCF10A cells in serum media were seeded onto uncoated tissue culture dishes, or dishes coated with BM165 or MsIgG, 90 min before irradiation. Cells were irradiated at 0.5, 1, 1.5, or 2 Gy, and harvested at 2, 4, 6, 8, or 24 hr post irradiation. Cell cycle checkpoint analysis was performed by incorporating Bromo-deoxy-uridine (BrdU; Molecular Probes, Eugene, OR) into DNA during S phase of the cell cycle. Cells were incubated in 10 μM BrdU for 30 min prior to harvesting cells. Cells were harvested by trypsinization, centrifuged, and fixed in 60% ethanol in cold PBS. Cells were digested in 0.04% pepsin, in 0.1% HCl for 1 hr at 37°C, followed by incubation in 2N HCl diluted 1:10 for 30 min at 37 °C. NaBorate (0.1M) was added to the HCl, cells vortexed, centrifuged, and solution aspirated. FITC conjugated BrdU antibody (Molecular Probes, Eugene, OR) was added at 5 μl/100 μl in PBST (Dulbecco’s PBS, 0.5% Tween, 0.5% BSA) for 1 hr at room temperature. Cells were rinsed in PBST, and incubated in 10 mg/ml Ribonuclease A (Sigma-Aldrich, St. Louis, MO) and 1 μg/ml propidium iodide for 30 min at 37°C. Twenty thousand events were collected per sample using a FACs Star Plus flow cytometer (Becton-Dickenson) and the percentage of cells in G0/G1, S, and G2/M phases of the cell cycle was assessed with Cellquest software. Accumulation of cells in G2/M phase after ionizing radiation was interpreted as an increase in number of cells in G2 arrest [37,38].
Western Blotting
MCF10A cells were trypsinized and seeded on tissue culture plastic, or BM165, or MsIgG coated tissue culture plates. Cells were irradiated at 2 Gy after 1.5 hr and harvested 4 hr post irradiation. Cells were lysed in ice-cold RIPA buffer (50 mM Tris-HCL, 150 mM NaCl, 1% NP40, 0.5% sodium deoxycholate, and 0.1% SDS, pH 7.5) with 1 μg/ml each of leupeptin, aprotinin, sodium vanidate, and 10 μg/ml phenylmethyl sufonyl fluoride (PMSF). Western analysis was performed using anti-p53 antibody (clone DO-7; Dako, Carpinteria, CA) at 1:1,000 and anti-mouse HRP secondary antibody at 1:50,000. Signal was detected with Perkin-Elmer Western Lightening. For cdc2 and phospho-cdc2 detection, cytoplasm and nuclear extracts were isolated using NePer buffer kit (Pierce, Rockford, IL) instead of RIPA buffer. Protein (200 μg) was incubated with cdc2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and 35 μl of a 50% v/v protein G-Sepharose beads overnight at 4°C. Proteins were detected using phospho-cdc2 (Cell Signaling, Beverly, MA) and cdc2 antibodies.
Survival Assay
PC3, PrEC, or MCF10A cells were seeded onto 96-well plates at 50,000 or 80,000 cells/well. Cells were irradiated at 1, 3, 5, or 7 Gy and harvested 5 or 7 days after irradiation. Cell survival was estimated using the XTT assay (Roche Diagnostics, Germany) following the protocol provided by Roche Diagnostics. Briefly, XTT labeling reagent was combined with electron-coupling reagent. XTT solution (50 μl) was added per 100 μl media for each well. Cells were incubated for 6 hr in a humidified chamber. An orange formazan solution was formed by cleavage of the yellow tetrazolium salt XTT by metabolically active cells. Absorbance was measured at 490 nm using an ELX 800 microplate reader (Bio Tek Instruments, Winooski, VT).
RESULTS
Laminin 5 Amplifies the G2 Arrest in Normal Prostate Epithelial Cells
Normal prostate epithelial cells, PrEC, and prostate cancer cells, PC3, were tested for their ability to arrest in G1, S, or G2/M phase of the cell cycle in the presence or absence of laminin 5. PreC cells and PC3 cells were seeded onto tissue culture plastic or a laminin 5 coated surface, and irradiated at 1, 2, 3, 5, or 7 Gy. The number of cells in G1, S, or G2/M phase of the cell cycle was quantified with flow cytometry. A ratio of the number of treated cells to control cells was recorded for each dose. The number of cells in G1 phase was similar for tissue culture plastic and laminin 5 treatments and was not affected by irradiation (Fig. 1A). The number of cells in S phase did not change with increasing radiation dose for cells on tissue culture plastic, but the number of S phase cells on laminin 5 decreased with increasing radiation dose compared to cells on tissue culture plastic (Fig. 1B). Exposure to IR increased the number of cells accumulated in G2/M phase of the cell cycle for cells on tissue culture plastic and laminin 5 (Fig. 1C). However, an amplification of cells in G2/M phase was observed in cells on laminin 5 compared to cells on tissue culture plastic for all IR doses. Cells on laminin 5 had a higher ratio of cells in G2/M than cells on tissue culture plastic for each IR dose. At 2 Gy, the number of cells in G2/M was nearly double on laminin 5 compared to tissue culture plastic.
Fig. 1.
XX.
Adhesion to laminin 5 did not amplify the response to IR in PC3, prostate cancer cells. The number of cells in G1 phase for tissue culture plastic, and laminin 5 treatments decreased with increasing radiation dose (Fig. 2A). The number of cells in S phase was unaffected by increasing IR dose in cells on laminin 5 and tissue culture plastic (Fig. 2B). G2 arrest increased with increasing IR dose on laminin 5 and tissue culture plastic (Fig. 2C). A comparison of the G2/M response between PrEC and PC3 cells shows that PrEC cells responded to ionizing radiation with an amplification in the G2 block in cells on laminin 5 compared with cells on tissue culture plastic. PC3 cells did not show an amplification in the G2 block with laminin 5 (Fig. 1C, Fig. 2C).
Fig. 2.
XX.
In order to investigate the influence of ligand receptor binding through the α6β4 integrin with laminin 5, the normal breast epithelial cell line, MCF10A, was used for further experiments on adhesion-mediated DNA damage responses. Cell cycle distribution was analyzed using BrDU incorporation and propidium iodide to more accurately quantify DNA synthesis and cell cycle distribution for MCF10A cells. MCF10A cells, which produce laminin 5 and α6β4 integrin, were seeded onto a tissue culture plastic surface and cells were irradiated at 2 Gy. Cell cycle progression was determined by measuring BrdU incorporation in irradiated or unirradiated cells. Cells seeded on tissue culture plastic were irradiated at 2 Gy, and BrdU incorporated into DNA for 30 min before harvesting. MCF10A cells grown on tissue culture plastic were assessed for the ability to block in G2 at 8 hr post irradiation at 0.5, 1, 1.5, or 2 Gy. The percentage of cells in G2/M increased with increasing IR dose from 20% at 0 Gy to 45% at 2 Gy (Fig. 3).
Fig. 3.
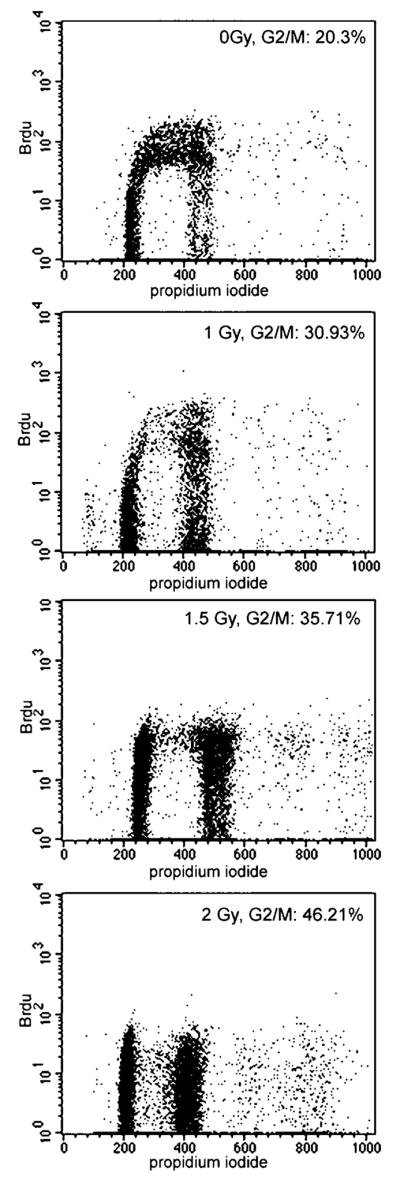
XX.
Blocking Laminin 5 Adhesion Abrogates the G2 Arrest in MCF10ACells
BM165 is a function-inhibitory antibody that binds to the G-domain of the α3 chain of laminin 5 [39]. Cells were seeded onto tissue culture plastic, MsIgG control antibody, or BM165 antibody surfaces and irradiated at 2 Gy, 90 min later. At the time of irradiation, cells were attached and spread on tissue culture plastic or MsIgG control antibody surfaces (Fig. 4A, B). The majority of cells seeded on the BM165 treated surface were not attached and spread (Fig. 4C). Cells were irradiated at 2 Gy and harvested 8 hr later. Cells in all treatments were adherent and spread at 8 hr. FACs analysis of BrdU incorporation in cells, seeded on tissue culture plastic or MsIgG, indicates a 5–15% increase in G2/M phase after ionizing radiation. Cells seeded on BM165 treated surfaces had a 5–10% higher initial percentage of cells in G2/M phase than unirradiated cells seeded on tissue culture plastic or MsIgG. However, after ionizing radiation cells on BM165 did not have an increased percentage of cells in G2/M phase over untreated cells (Fig. 4D).
Fig. 4.
XX.
P53 Upregulation Occursin IR Treated MCF10ACells Independent of Adhesion Conditions
P53 expression in MCF10A cells was observed to determine if the loss of an increased G 2 arrest in cells seeded on BM165 coated surfaces was p53-dependent. P53 expression in irradiated and unirradiated cells was determined in MCF10A cells seeded on tissue culture plastic, BM165, or MsIgG. Cells were irradiated at 2 Gy and harvested 4 or 6 hr post irradiation. P53 expression was not affected by adhesion to tissue culture plastic, MsIgG, or BM165 surfaces. P53 was expressed at very low levels in unirradiated cells and upregulated in irradiated cells on all three surfaces (Fig. 5A).
Fig. 5.
XX.
BM165 Uncouples Phospho-cdc2 (Tyr15) Regulation From the G2 Arrest Response
Activation of cdc2 through dephosphorylation of the Tyr15 and Thr14 sites is required for entry into mitosis [40]. Inactivation of cdc2 via phosphorylation of Tyr15 after IR was examined in MCF10A cells exposed to irradiation. MCF10A cells were seeded on tissue culture plastic or a B M165 antibody surface, irradiated at 2 Gy, and harvested 4 hr post irradiation. Phospho-cdc2 expression was low in unirradiated cells seeded on tissue culture plastic and expression increased in the nucleus and cytoplasm after IR (Fig. 5B). Phospho-cdc2 was expressed in the cytoplasm and nucleus of unirradiated cells seeded on B M165. In irradiated cells the level of phospho-cdc2 expression in cytoplasm extracts was similar to the level observed in unirradiated cells. Expression of phospho-cdc2 was observed in nuclear extracts of cells on a BM165 surface compared to unirradiated cells on BM165 and irradiated cells seeded on tissue culture plastic.
Cell Survival After IR Unaffected by Adhesion to Laminin 5
Cell survival after irradiation was assessed in PrEC, PC3, or MCF10A cells that were attached to tissue culture plastic or laminin 5. Cells were irradiated at 1, 3, 5, or 7 Gy and harvested 5 or 7 days post irradiation. Survival was measured using the XTT assay. Cell survival decreased in a dose-dependent manner for PrEC, PC3, and MCF10A cell lines. Attachment to laminin 5 did not increase the percentage of cell survival over cells attached to tissue culture plastic in any of the cell lines (Fig. 6).
Fig. 6.
XX
DISCUSSION
Laminin 5 amplifies the G2 arrest after IR in normal prostate cells (PrEC), but did not affect G2 arrest in the prostate cancer cell line, PC3. To further investigate the role of laminin 5 adhesion in cellular DNA damage response, the breast epithelial cell line, MCF10A, which produces laminin 5 and forms hemidesmosome like structures with α6β4, was chosen for further studies. Adhesion to laminin 5 enables the normal epithelial cell lines PrEC and MCF10A to block progression through the cell cycle after DNA damage from ionizing radiation. These results imply that loss of laminin 5 during prostate cancer may, in part, lead to the loss of the ability of cells to respond to DNA damage via an inability to arrest in G2 phase of the cell cycle.
We next investigated expression of cell cycle proteins p53 and cdc2. Cell division is a highly regulated process that is modulated in response to adverse conditions including ionizing radiation [40,41]. Signaling via adhesion of integrins to laminin 5 is known to be involved in progression through the G1 phase of the cell cycle [32,33,42] and other integrin/ligand interactions in the G2/M transition [35,43]. Our data support adhesion-mediated regulation of the G2 phase through the integrin ligand laminin 5.
P53, tumor suppressor protein, is a molecular switch between cell fates, and mediates cell cycle and apoptosis through cell signaling. P53-dependent and independent mechanisms are in place to enable cells to respond to DNA damage and block the cell division cycle [44–47]. Our results show that in MCF10A cells, which express p53, ionizing radiation influences the G2 arrest in a p53-independent manner. Cells seeded on tissue culture plastic or BM165-treated surfaces showed equal upregulation of the p53 protein following IR.
Cdc2 is an essential cell cycle regulator that associates with cyclinB, forming a complex essential for the transition from G2 phase to M phase [47]. The cyclinB/cdc2 complex is activated by dephosphorylation on Tyr15 and Thr14 and shuttles between the nucleus and cytoplasm during interphase and translocates to the nucleus at mitosis [41]. G2/M cell cycle arrest can occur independent of p53 expression [48,49]. Under normal conditions, MCF10A cells have the ability to arrest in G2 phase and show increased inactivated cdc2 complex located in the nucleus after IR. MCF10A cells prevented from binding to laminin 5 by the functional inhibitory antibody, BM165, did not exhibit an amplification in G2 arrest following IR and did not localize phospho-cdc2 to the nucleus. These results show that loss of adhesion to laminin 5 via the α6β4 integrin prevents an amplified G2 arrest response after DNA damage at least, in part, by altering the location of phospho-cdc2. Cell adhesion has been shown to influence the nuclear/cytoplasmic distribution of MAP kinases [50] and paxillin [51], and our results suggest the possibility of integrin-mediated influence over nuclear translocation of cell cycle proteins.
Interactions between the extracellular matrix and integrins are known to enhance cell survival [52–54]. However, it has been reported that in some cell lines, adhesion does not confer increased survival after DNA damage [43] and G2 arrest responses can occur without altering cell survival [55]. In our study, adhesion to laminin 5 did not influence cell survival in PrEC, PC3, and MCF10A cells exposed to ionizing radiation. The loss of laminin 5 adhesion decreased the G2 arrest response without altering cell survival. These results suggest the interesting possibility that the decreased ability to arrest in G2 (via loss of laminin 5 adhesion) without affecting survival could contribute to the emergence of a genetically unstable population. The amplification of the G2 arrest by laminin 5 suggests that adhesion is, in part, responsible for normal repair. It has been hypothesized that prostate cancer arises from a stem-cell-like cell population within the basal cell population with a mixed phenotype of basal and luminal cell characteristics [56,57]. Adhesion-mediated amplification of G2 arrest would explain the apparent multi-focal nature of the disease as an instability induced by the inability of the stem cells within the basal cell population to repair radical induced DNA damage. In support of this idea, it has been reported that age-related radical-induced DNA damage is linked to prostate cancer [26]. It is interesting to note that the basal cell population is highly resistant to apoptosis, since there is an accumulation of bcl2 [53]. Thus, the basal cells are uniquely positioned to harbor and accumulate mutations that are not lethal but would eventually lead to instability. The resulting instability of epithelial cells may be kept in check by a stable microenvironment [58], including the adhesion of basal cells to the basement membrane. The loss of α6β4 integrin and laminin 5 in basal cells may facilitate progression of an unstable cell phenotype. Stabilization of the α6β4 integrin/laminin 5 interaction may delay or prevent genomic instability in prostate tissue.
Acknowledgments
We thank Norma Seaver and Debbie Sakiestewa for their assistance with flow cytometry, and Ewa Sikorski for technical assistance.
Grant sponsor: NIH; Grant numbers: T32CA09213, CA75152, CA78447; Grant sponsor: NASA; Grant number: NAG5-4452; Grant sponsor: DOE; Grant number: ER63240.
References
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics. CA Cancer J Clin. 2004;54(1):8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Oakley-Girvan I, Kolonel LN, Gallagher RP, Wu AH, Felberg A, Whittemore AS. Stage at diagnosis and survival in a multiethnic cohort of prostate cancer patients. Am J Public Health. 2003;93(10):1753–1759. doi: 10.2105/ajph.93.10.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brawer MK. Prostatic intraepithelial neoplasia: A premalignant lesion. Hum Pathol. 1992;23(3):242–248. doi: 10.1016/0046-8177(92)90104-b. [DOI] [PubMed] [Google Scholar]
- 4.Nagle RB, Hao J, Knox JD, Dalkin BL, Clark V, Cress AE. Expression of hemidesmosomal and extracellular matrix proteins by normal and malignant human prostate tissue. Am J Pathol. 1995;146(6):1498–1507. [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs ME, Brawer MK, Rennels MA, Nagle RB. The relationship of basement membrane to histologic grade of human prostatic carcinoma. Mod Pathol. 1989;2(2):105–111. [PubMed] [Google Scholar]
- 6.Cress AE, Rabinovitz I, Zhu W, Nagle RB. The alpha 6 beta 1 and alpha 6 beta 4 integrins in human prostate cancer progression. Cancer Metastasis Rev. 1995;14(3):219–228. doi: 10.1007/BF00690293. [DOI] [PubMed] [Google Scholar]
- 7.Davis TL, Cress AE, Dalkin BL, Nagle RB. Unique expression pattern of the alpha6beta4 integrin and laminin-5 in human prostate carcinoma. Prostate. 2001;46(3):240–248. doi: 10.1002/1097-0045(20010215)46:3<240::aid-pros1029>3.0.co;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabinovitz I, Nagle RB, Cress AE. Integrin alpha 6 expression in human prostate carcinoma cells is associated with a migratory and invasive phenotype in vitro and in vivo. Clin Exp Metastasis. 1995;13(6):481–491. doi: 10.1007/BF00118187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witkowski CM, Rabinovitz I, Nagle RB, Affinito KS, Cress AE. Characterization of integrin subunits, cellular adhesion and tumorgenicity of four human prostate cell lines. J Cancer Res Clin Oncol. 1993;119(11):637–644. doi: 10.1007/BF01215981. [DOI] [PubMed] [Google Scholar]
- 10.Hao J, Yang Y, McDaniel KM, Dalkin BL, Cress AE, Nagle RB. Differential expression of laminin 5 (alpha 3 beta 3 gamma 2) by human malignant and normal prostate. Am J Pathol. 1996;149(4):1341–1349. [PMC free article] [PubMed] [Google Scholar]
- 11.Savoia P, Trusolino L, Pepino E, Cremona O, Marchisio PC. Expression and topography of integrins and basement membrane proteins in epidermal carcinomas: Basal but not squamous cell carcinomas display loss of alpha 6 beta 4 and BM-600/nicein. J Invest Dermatol. 1993;101(3):352–358. doi: 10.1111/1523-1747.ep12365531. [DOI] [PubMed] [Google Scholar]
- 12.Bonkhoff H, Stein U, Remberger K. Differential expression of alpha 6 and alpha 2 very late antigen integrins in the normal, hyperplastic, and neoplastic prostate: Simultaneous demonstration of cell surface receptors and their extracellular ligands. Hum Pathol. 1993;24(3):243–248. doi: 10.1016/0046-8177(93)90033-d. [DOI] [PubMed] [Google Scholar]
- 13.Burgeson RE, Chiquet M, Deutzmann R, Ekblom P, Engel J, Kleinman H, Martin GR, Meneguzzi G, Paulsson M, Sanes J, et al. A new nomenclature for the laminins. Matrix Biol. 1994;14(3):209–211. doi: 10.1016/0945-053x(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 14.Mercurio AM, Rabinovitz I, Shaw LM. The alpha 6 beta 4 integrin and epithelial cell migration. Curr Opin Cell Biol. 2001;13(5):541–545. doi: 10.1016/s0955-0674(00)00249-0. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen BP, Ren XD, Schwartz MA, Carter WG. Ligation of integrin alpha 3beta 1 by laminin 5 at the wound edge activates Rho-dependent adhesion of leading keratinocytes on collagen. J Biol Chem. 2001;276(47):43860–43870. doi: 10.1074/jbc.M103404200. [DOI] [PubMed] [Google Scholar]
- 16.Belkin AM, Stepp MA. Integrins as receptors for laminins. Microsc Res Tech. 2000;51(3):280–301. doi: 10.1002/1097-0029(20001101)51:3<280::AID-JEMT7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Okura M, Imamoto A. Focal adhesions require catalytic activity of Src family kinases to mediate integrin-matrix adhesion. Mol Cell Biol. 2002;22(4):1203–1217. doi: 10.1128/MCB.22.4.1203-1217.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mercurio AM, Rabinovitz I. Towards a mechanistic understanding of tumor invasion–lessons from the alpha6beta 4 integrin. Semin Cancer Biol. 2001;11(2):129–141. doi: 10.1006/scbi.2000.0364. [DOI] [PubMed] [Google Scholar]
- 19.Rabinovitz I, Mercurio AM. The integrin alpha 6 beta 4 and the biology of carcinoma. Biochem Cell Biol. 1996;74(6):811–821. doi: 10.1139/o96-087. [DOI] [PubMed] [Google Scholar]
- 20.Colognato H, Yurchenco PD. Form and function: The laminin family of heterotrimers. Dev Dyn. 2000;218(2):213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 21.Mizushima H, Miyagi Y, Kikkawa Y, Yamanaka N, Yasumitsu H, Misugi K, Miyazaki K. Differential expression of laminin-5/ladsin subunits in human tissues and cancer cell lines and their induction by tumor promoter and growth factors. J Biochem (Tokyo) 1996;120(6):1196–1202. doi: 10.1093/oxfordjournals.jbchem.a021541. [DOI] [PubMed] [Google Scholar]
- 22.Koshikawa N, Moriyama K, Takamura H, Mizushima H, Nagashima Y, Yanoma S, Miyazaki K. Overexpression of laminin gamma2 chain monomer in invading gastric carcinoma cells. Cancer Res. 1999;59(21):5596–5601. [PubMed] [Google Scholar]
- 23.Hao J, Jackson L, Calaluce R, McDaniel K, Dalkin BL, Nagle RB. Investigation into the mechanism of the loss of laminin 5 (alpha3beta3gamma2) expression in prostate cancer. Am J Pathol. 2001;158(3):1129–1135. doi: 10.1016/s0002-9440(10)64060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin KJ, Kwan CP, Nagasaki K, Zhang X, O’Hare MJ, Kaelin CM, Burgeson RE, Pardee AB, Sager R. Down-regulation of laminin-5 in breast carcinoma cells. Mol Med. 1998;4(9):602–613. [PMC free article] [PubMed] [Google Scholar]
- 25.Trzeciak AR, Nyaga SG, Jaruga P, Lohani A, Dizdaroglu M, Evans MK. Cellular repair of oxidatively induced DNA base lesions is defective in prostate cancer cell lines, PC-3 and DU-145. Carcinogenesis. 2004;25(8):1359–1370. doi: 10.1093/carcin/bgh144. [DOI] [PubMed] [Google Scholar]
- 26.Malins DC, Johnson PM, Wheeler TM, Barker EA, Polissar NL, Vinson MA. Age-related radical-induced DNA damage is linked to prostate cancer. Cancer Res. 2001;61(16):6025–6028. [PubMed] [Google Scholar]
- 27.Bonkhoff H, Stein U, Remberger K. The proliferative function of basal cells in the normal and hyperplastic human prostate. Prostate. 1994;24(3):114–118. doi: 10.1002/pros.2990240303. [DOI] [PubMed] [Google Scholar]
- 28.Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, Orntoft T, Lukas J, Bartek J. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434(7035):864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 29.Fan R, Kumaravel TS, Jalali F, Marrano P, Squire JA, Bristow RG. Defective DNA strand break repair after DNA damage in prostate cancer cells: Implications for genetic instability and prostate cancer progression. Cancer Res. 2004;64(23):8526–8533. doi: 10.1158/0008-5472.CAN-04-1601. [DOI] [PubMed] [Google Scholar]
- 30.Wang JY, Ho T, Trojanek J, Chintapalli J, Grabacka M, Stoklosa T, Garcia FU, Skorski T, Reiss K. Impaired homologous recombination DNA repair and enhanced sensitivity to DNA damage in prostate cancer cells exposed to anchorage-independence. Oncogene. 2005 doi: 10.1038/sj.onc.1208537. [DOI] [PubMed] [Google Scholar]
- 31.Assoian RK, Schwartz MA. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G(1) phase cell-cycle progression. Curr Opin Genet Dev. 2001;11(1):48–53. doi: 10.1016/s0959-437x(00)00155-6. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz MA, Assoian RK. Integrins and cell proliferation: Regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J Cell Sci. 2001;114(Pt 14):2553–2560. doi: 10.1242/jcs.114.14.2553. [DOI] [PubMed] [Google Scholar]
- 33.Welsh CF, Roovers K, Villanueva J, Liu YQ, Schwartz MA, Assoian RK. Timing of cyclin D1 expression within G1 phase is controlled by Rho. Nat Cell Biol. 2001;3(11):950–957. doi: 10.1038/ncb1101-950. [DOI] [PubMed] [Google Scholar]
- 34.Klekotka PA, Santoro SA, Wang H, Zutter MM. Specific residues within the alpha 2 integrin subunit cytoplasmic domain regulate migration and cell cycle progression via distinct MAPK pathways. J Biol Chem. 2001;276(34):32353–32361. doi: 10.1074/jbc.M101921200. [DOI] [PubMed] [Google Scholar]
- 35.Cordes N, van Beuningen D. Cell adhesion to the extracellular matrix protein fibronectin modulates radiation-dependent G2 phase arrest involving integrin-linked kinase (ILK) and glycogen synthase kinase-3beta (GSK-3beta) in vitro. Br J Cancer. 2003;88(9):1470–1479. doi: 10.1038/sj.bjc.6600912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cordes N, Beinke C. Fibronectin alters cell survival and intracellular signaling of confluent A549 cultures after irradiation. Cancer Biol Ther. 2004;3(1) [PubMed] [Google Scholar]
- 37.Maity A, McKenna WG, Muschel RJ. The molecular basis for cell cycle delays following ionizing radiation: A review. Radiother Oncol. 1994;31(1):1–13. doi: 10.1016/0167-8140(94)90408-1. [DOI] [PubMed] [Google Scholar]
- 38.Holgersson A, Heiden T, Castro J, Edgren MR, Lewensohn R, Meijer AE. Different G2/M accumulation in M059J and M059K cells after exposure to DNA double-strand break-inducing agents. Int J Radiat Oncol Biol Phys. 2005;61(3):915–921. doi: 10.1016/j.ijrobp.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 39.Rousselle P, Lunstrum GP, Keene DR, Burgeson RE. Kalinin—An epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991;114(3):567–576. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smits VA, Medema RH. Checking out the G(2)/M transition. Biochim Biophys Acta. 2001;1519(1–2):1–12. doi: 10.1016/s0167-4781(01)00204-4. [DOI] [PubMed] [Google Scholar]
- 41.Pines J. Four-dimensional control of the cell cycle. Nat Cell Biol. 1999;1(3):E73–79. doi: 10.1038/11041. [DOI] [PubMed] [Google Scholar]
- 42.Mettouchi A, Klein S, Guo W, Lopez-Lago M, Lemichez E, Westwick JK, Giancotti FG. Integrin-specific activation of Rac controls progression through the G(1) phase of the cell cycle. Mol Cell. 2001;8(1):115–127. doi: 10.1016/s1097-2765(01)00285-4. [DOI] [PubMed] [Google Scholar]
- 43.Cordes N, Hansmeier B, Beinke C, Meineke V, van Beuningen D. Irradiation differentially affects substratum-dependent survival, adhesion, and invasion of glioblastoma cell lines. Br J Cancer. 2003;89(11):2122–2132. doi: 10.1038/sj.bjc.6601429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Girinsky T, Koumenis C, Graeber TG, Peehl DM, Giaccia AJ. Attenuated response of P53 and P21 in primary cultures of human prostatic epithelial-cells exposed to DNA-damaging agents. Cancer Res. 1995;55(17):3726–3731. [PubMed] [Google Scholar]
- 45.O’Connell MJ, Walworth NC, Carr AM. The G2-phase DNA-damage checkpoint. Trends Cell Biol. 2000;10(7):296–303. doi: 10.1016/s0962-8924(00)01773-6. [DOI] [PubMed] [Google Scholar]
- 46.Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20(15):1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- 47.Azzam EI, de Toledo SM, Pykett MJ, Nagasawa H, Little JB. CDC2 is down-regulated by ionizing radiation in a p53-dependent manner. Cell Growth Differ. 1997;8(11):1161–1169. [PubMed] [Google Scholar]
- 48.Huynh H, Nguyen TH, Panasci L, Do P. 2-Chloroethyl-3-sarcosinamide-1-nitrosourea (SarCNU) inhibits prostate carcinoma cell growth via p53-dependent and p53-independent pathways. Cancer. 2004;101(12):2881–2891. doi: 10.1002/cncr.20670. [DOI] [PubMed] [Google Scholar]
- 49.Wang XW, Zhan Q, Coursen JD, Khan MA, Kontny HU, Yu L, Hollander MC, O’Connor PM, Fornace AJ, Jr, Harris CC. GADD45 induction of a G2/M cell cycle checkpoint. Proc Natl Acad Sci USA. 1999;96(7):3706–3711. doi: 10.1073/pnas.96.7.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aplin AE, Hogan BP, Tomeu J, Juliano RL. Cell adhesion differentially regulates the nucleocytoplasmic distribution of active MAP kinases. J Cell Sci. 2002;115(Pt 13):2781–2790. doi: 10.1242/jcs.115.13.2781. [DOI] [PubMed] [Google Scholar]
- 51.Ogawa M, Hiraoka Y, Aiso S. Nuclear translocation of Xenopus laevis paxillin. Biochem Biophys Res Commun. 2003;304(4):676–683. doi: 10.1016/s0006-291x(03)00640-5. [DOI] [PubMed] [Google Scholar]
- 52.Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): Role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93(5):1658–1667. [PMC free article] [PubMed] [Google Scholar]
- 53.Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9(5):701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 54.Fornaro M, Plescia J, Chheang S, Tallini G, Zhu YM, King M, Altieri DC, Languino LR. Fibronectin protects prostate cancer cells from tumor necrosis factor alpha-induced apoptosis via the AKT/Survivin pathway. J Biol Chem. 2003 doi: 10.1074/jbc.M307627200. [DOI] [PubMed] [Google Scholar]
- 55.Yan T, Schupp JE, Hwang HS, Wagner MW, Berry SE, Strickfaden S, Veigl ML, Sedwick WD, Boothman DA, Kinsella TJ. Loss of DNA mismatch repair imparts defective cdc2 signaling and G(2) arrest responses without altering survival after ionizing radiation. Cancer Res. 2001;61(22):8290–8297. [PubMed] [Google Scholar]
- 56.Hudson DL, Guy AT, Fry P, O’Hare MJ, Watt FM, Masters JRW. Epithelial cell differentiation pathways in the human prostate: Identification of intermediate phenotypes by keratin expression. J Histochem Cytochem. 2001;49(2):271–278. doi: 10.1177/002215540104900214. [DOI] [PubMed] [Google Scholar]
- 57.Bonkhoff H. Role of the basal cells in premalignant changes of the human prostate: A stem cell concept for the development of prostate cancer. Eur Urol. 1996;30(2):201–205. doi: 10.1159/000474170. [DOI] [PubMed] [Google Scholar]
- 58.Bissell MJ, Labarge MA. Context, tissue plasticity, and cancer: Are tumor stem cells also regulated by the microenvironment? Cancer Cell. 2005;7(1):17–23. doi: 10.1016/j.ccr.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]







