Abstract
Objectives
Our objective was to evaluate the pharmacokinetics of nelfinavir (NFV) (625 mg tablets) 1250 mg twice daily during pregnancy and postpartum.
Methods
The participants were HIV-1-infected pregnant women enrolled in P1026s and receiving NFV (625 mg tablets) 1250 mg twice daily as part of routine clinical care. Intensive steady-state 12-h NFV pharmacokinetic profiles were performed during pregnancy and postpartum. The target NFV area under the plasma concentration–time curve (AUC0–12) was ≥ 10th percentile NFV AUC0–12 in non-pregnant historical controls (18.5 μg h/mL).
Results
Of 27 patients receiving NFV, pharmacokinetic data were available for four (second trimester), 27 (third trimester) and 22 (postpartum) patients. The NFV maximum concentration (Cmax), 12-h post-dose concentration (C12) and AUC0–12 were significantly lower during the third trimester compared to postpartum (P ≤ 0.03). The metabolite hydroxyl-tert-butylamide (M8) AUC0–12 and the M8/NFV AUC ratio were lower during the third trimester compared to postpartum (P<0.01). The NFV AUC0–12 exceeded the AUC0–12 target for 15/27 (56%) and 21/22 (95%) of third trimester and postpartum patients, respectively. The minimum concentration (Cmin) was above the suggested minimum trough concentration (0.8 μg/mL) in 15% (third trimester) and 18% (postpartum). The plasma viral load was <400 HIV-1 RNA copies/mL in 81% of patients at delivery.
Conclusions
These results suggest that higher doses of NFV should be considered during pregnancy.
Keywords: HIV, nelfinavir, pharmacokinetics, pregnancy
Introduction
Many HIV-infected pregnant women receive nelfinavir (NFV) as part of their antiretroviral (ARV) regimens, whether for treatment or as a component of combination ARV regimens for the prevention of mother-to-child transmission of HIV. During pregnancy, physiological changes can result in changes in the absorption, distribution, metabolism and elimination of drugs. Previous data indicated that NFV exposure was inadequate in most pregnant women receiving NFV as 250 mg tablets at a dose of 750 mg orally three times daily [1]. Subsequent studies suggested that NFV exposure remained inadequate with 1250 mg taken orally twice daily [2–4].
More recently, a new formulation of NFV as 625 mg tablets has been approved for use. In non-pregnant adults, the bioavailability of NFV is increased following administration of two 625 mg tablets compared to five 250 mg tablets [5]. However, the new formulation was designed for non-pregnant adults, and pharmacokinetic results in this population cannot necessarily be extrapolated to pregnant women. Therefore, as part of the Pediatric AIDS Clinical Trials Group (PACTG) Protocol 1026s (P1026s), NFV pharmacokinetic parameters during pregnancy were determined following administration of the newer 625 mg tablet formulation.
Patients and methods
P1026s is an ongoing, multi-centre, prospective study of ARV pharmacokinetics among HIV-infected pregnant women receiving ARVs for routine clinical care. The current analyses address only those women using NFV. P1026s is a sub-study of P1025, a prospective cohort study of HIV-infected pregnant women receiving care at PACTG sites. Institutional review boards approved both P1025 and P1026s at all participating sites. All participants provided written informed consent prior to participation in these studies.
Participants were eligible for inclusion in the NFV arm of P1026s if they met the following criteria: they were HIV-infected pregnant women ≥ 20 weeks’ gestation who were enrolled in P1025; they initiated NFV (625 mg tablets) at a dose of 1250 mg orally twice daily by 34 6/7 weeks of gestation; they were receiving this dose of NFV for at least 2 weeks prior to pharmacokinetic sampling; and they were planning to continue NFV until at least 6 weeks postpartum. Exclusion criteria were multiple-gestation pregnancies and clinical or laboratory toxicity that, in the opinion of the site investigator, would probably require a change in the ARV regimen during the study. The participant’s clinician determined the choice of ARVs, prescribed the drugs and remained responsible for clinical management throughout the study. Participants remained on study until the completion of postpartum pharmacokinetic sampling.
As part of P1025 patients were interviewed, physical examinations were performed and laboratory studies were conducted during pregnancy and postpartum. Data from P1025 accessed for this analysis include: demographic data (maternal age, ethnicity); concomitant ARVs; laboratory results [plasma HIV-1 RNA concentrations (viral loads) and CD4 T-cell counts]; infant birth data (gestational age, weight and length); and infant HIV infection status. Information collected as part of P1026s includes the patient’s height and weight on days of sampling, and adverse events experienced by patients.
On each sampling day, a medical history was obtained from the patient (including the time of the last two doses of NFV), a physical examination was performed and laboratory studies were obtained (including measurements of alanine aminotransferase, aspartate aminotransferase, bilirubin, creatinine, blood urea nitrogen, albumin and haemoglobin). Adverse events were reported according to the Division of AIDS (DAIDS)/National Institute of Allergy and Infectious Diseases (NIAID) Toxicity Table for Grading Severity of Adult Adverse Experiences (August 1992) (http://rcc/tech-res-intl.com). All adverse events were followed until resolution. The study team reviewed adverse event reports on monthly conference calls. The patient’s clinician was responsible for toxicity management.
Venipuncture for plasma samples for pharmacokinetic evaluation was performed at the following timepoints: second trimester (between 20 and 26 weeks of gestation), third trimester (between 30 and 36 weeks of gestation), at delivery and postpartum (between 6 and 12 weeks after delivery). Women who did not complete an antepartum evaluation were replaced in the study. Patients were receiving a stable ARV regimen for at least 2 weeks prior to pharmacokinetic sampling. Participants were instructed to take their NFV at the same times each day for the 3 days prior to and on the day of the antepartum and postpartum pharmacokinetic evaluations. Seven plasma samples were drawn at antepartum and postpartum pharmacokinetic evaluation visits, starting immediately before the morning NFV dose and at 1, 2, 4, 6, 8 and 12 h after the witnessed dose. The morning NFV dose was ingested immediately after a standardized meal of 1000 kcal with 50% fat provided at the site. To assess transplacental passage, NFV and metabolite concentrations were measured in maternal plasma and umbilical cord samples obtained at delivery.
Concentrations of NFV and its main active metabolite [hydroxyl-tert-butylamide (M8)] were determined simultaneously by high-performance liquid chromatography (HPLC) in the Pediatric Clinical Pharmacology Laboratory at the University of California, San Diego. Samples were stored at −20°C, and were assayed within 7–10 days of receipt. Briefly, plasma proteins were precipitated using acetonitrile (ACN) and supernatant injected directly onto a LUNA C-18 reversed phase HPLC column (Phenomenex Inc., Torrance, CA, USA). Drugs were separated isocratically using a mobile phase consisting of 10 mM potassium phosphate buffer pH 4.2: ACN (62:38 v/v). The flow rate was 1.2 mL/min and ultraviolet (UV) detection was at 206 nm. The detection limit for both NFV and M8 was 0.039 mg/mL. The mean inter- and intra-assay coefficients of variation based on validation data (quality control samples were run at multiple different concentrations over the control range of 0.039–8.5 μg/mL) revealed the lowest and highest values as follows: 5.2 ± 2.3% and 3.1 ± 2.1% for NFV, and 4.3 ± 1.8% and 3.3 ± 2.8% for M8 [5]. The laboratory is licensed by the State of California and is Clinical Laboratory Improvement Amendments (CLIA)-compliant, participating in the ACTG proficiency testing programme twice a year [6].
The concentration data were analysed by direct inspection to determine pre-dose concentration (Cpre-dose), maximum plasma concentration (Cmax), the corresponding time (Tmax), the minimum plasma concentration (Cmin), the corresponding time (Tmin) and the 12-h post-dose concentration (C12) for both NFV and M8. For concentrations below the assay limit of detection, a value of one half of the detection limit (0.0195 μg/mL for NFV and M8) was used in calculations of various ratios. The area under the plasma concentration–time curve during the dose interval [from time 0 to 12 h post-dose (AUC0–12)] for NFV and M8 were estimated using the trapezoidal rule. Apparent clearance (CL/F) from plasma for NFV was calculated as dose divided by AUC0–12. Apparent volume of distribution (Vd/F) was estimated by CL/F divided by the terminal slope of the curve (λz), and half-life (t1/2) was calculated as 0.693 divided by λz. The target NFV AUC0–12 was ≥ 10th percentile NFV AUC0–12 in non-pregnant historical controls (18.5 μg h/mL) [5]. The target Cmin for NFV was 0.8 μg/mL [7].
Both Vd/F and CL/F also were estimated using a one-compartment model in the software program WinNonlin® (version 5.0.1; Pharsight Corporation, Mountain View, CA, USA). Pharmacokinetic parameters derived from each approach were compared to assess the potential limitations of each methodology.
The difference in any pharmacokinetic parameter (third trimester vs. postpartum) was assessed using the Wilcoxon signed-rank test. For the comparison of third trimester vs. postpartum NFV and M8 pharmacokinetic parameters, we made all comparisons at the within-patient level, using 90% confidence intervals (CIs) for the geometric mean ratio of the pharmacokinetic exposure parameters during the third trimester of pregnancy vs. postpartum.
A true geometric mean ratio of 1 indicates equal geometric mean pharmacokinetic parameters for the two timepoints being compared. If the 90% CI is entirely outside the limits of (0.8, 1.25), the pharmacokinetic parameter is deemed different for the two timepoints. If, on the other hand, the 90% CI is entirely within the limits (0.8, 1.25), the parameter is not different between the two timepoints. If the 90% CI overlaps with (0.8, 1.25), these data alone do not support any conclusions regarding the pharmacokinetic parameter.
Results
P1026s enrolled 27 patients in the NFV arm between June 2005 and July 2006. Selected characteristics of study participants are shown in Table 1. All 27 patients received zidovudine (ZDV) and lamivudine (3TC) in addition to NFV.
Table 1.
Characteristics of the study population (n = 27 patients)
| Characteristic | n | n (%) | Median (range) |
|---|---|---|---|
| Maternal | |||
| Demographic characteristics (n = 27) | |||
| Age (years) at the time of the third trimester pharmacokinetic evaluation | 27 | 28.8 (15.2–39.1) | |
| Race/ethnicity | 27 | ||
| White, non-Hispanic | 2 (7.4) | ||
| Black, non-Hispanic | 14 (51.9) | ||
| Hispanic | 11 (40.7) | ||
| Second Trimester (n = 4) | |||
| Weight (kg) | 4 | 71.9 (61.4–86.5) | |
| Gestational age (weeks) | 4 | 23.9 (23.3–26.7) | |
| Duration (weeks) of receipt of nelfinavir before pharmacokinetic evaluation | 4 | 6.4 (2.3–130.4) | |
| CD4 lymphocyte count (cells/mL) | 3 | 565 (411–1045) | |
| Plasma HIV-1 RNA concentration (copies/mL) | 3 | 4370 (400–55 859) | |
| Plasma HIV-1 RNA concentration o400 copies/mL | 3 | ||
| Yes | 0 (0) | ||
| No | 3 (100) | ||
| Third trimester (n = 27) | |||
| Weight (kg) | 27 | 77.5 (56.0–176.1) | |
| Gestational age (weeks) | 27 | 33.9 (30.9–38.3) | |
| Duration (weeks) of receipt of nelfinavir before pharmacokinetic evaluation | 27 | 16.6 (2.9–138.4) | |
| CD4 lymphocyte count (cells/mL) | 26 | 509 (194–1208) | |
| Plasma HIV-1 RNA concentration (copies/mL) | 26 | 75 (10–787) | |
| Plasma HIV-1 RNA concentration o400 copies/mL | 26 | ||
| Yes | 23 (88.5) | ||
| No | 3 (11.5) | ||
| Delivery | |||
| Weight (kg) | 22 | 84.1 (57.6–176.0) | |
| CD4 lymphocyte count (cells/mL) | 26 | 497 (172–1530) | |
| Plasma HIV-1 RNA concentration (copies/mL) | 27 | 75 (10–9953) | |
| Plasma HIV-1 RNA concentration o400 copies/mL | 27 | ||
| Yes | 22 (81.5) | ||
| No | 5 (18.5) | ||
| Postpartum (n = 22) | |||
| Weight (kg) | 21 | 73.2 (46.0–174.0) | |
| Weeks after delivery | 22 | 8.2 (6.1–12.4) | |
| CD4 lymphocyte count (cells/mL) | 16 | 555 (172–1530) | |
| Plasma HIV-1 RNA concentration (copies/mL) | 15 | 75 (25–1650) | |
| Plasma HIV-1 RNA concentration o400 copies/mL | 15 | ||
| Yes | 12 (80.0) | ||
| No | 3 (20.0) | ||
| Infant (n = 27) | |||
| Birth weight (g) | 27 | 2920 (2145–3630) | |
| Length at birth (cm) | 27 | 49.5 (43.5–53.3) | |
| Gestational age (weeks) | 27 | 38 (36–40) | |
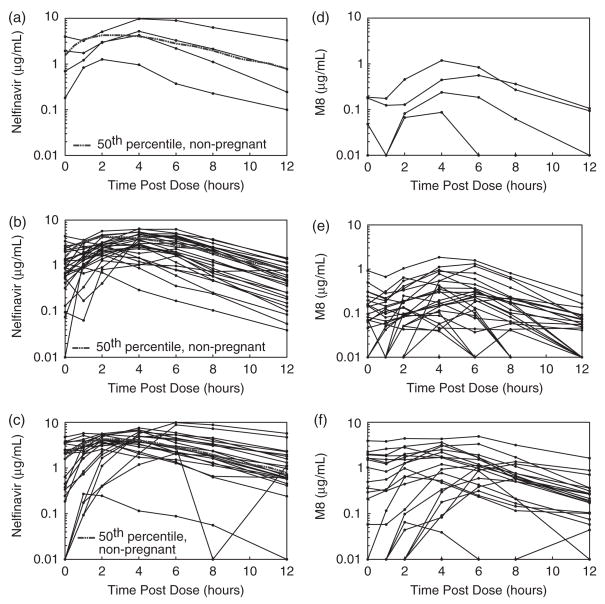
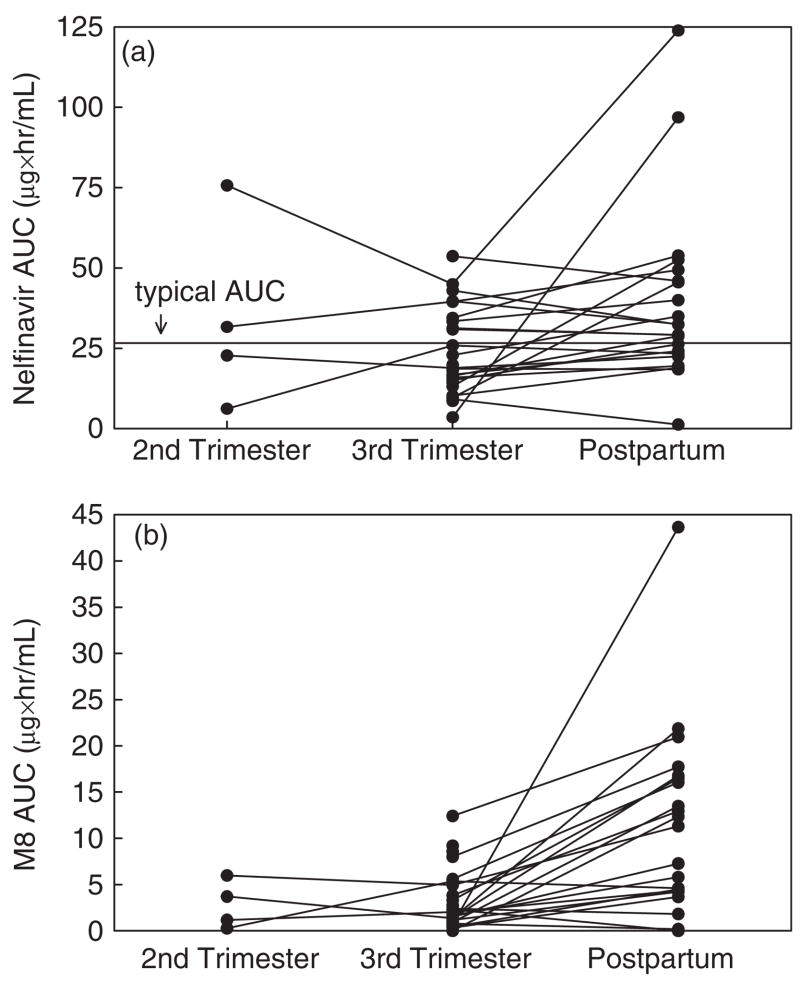
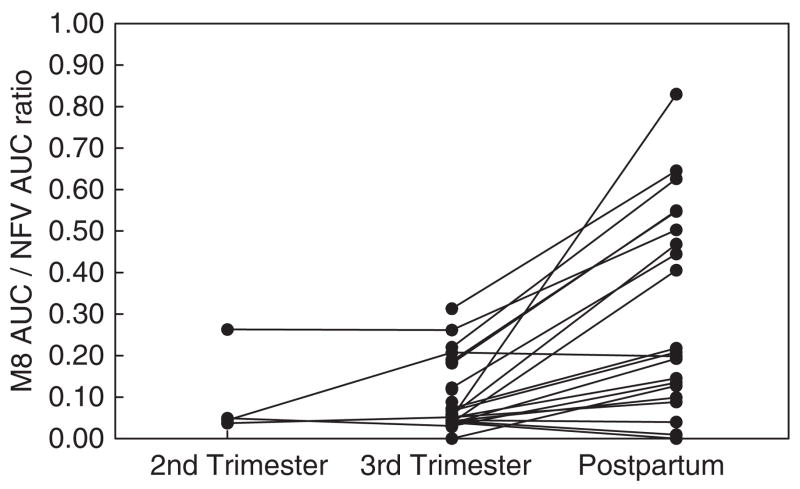
NFV and M8 pharmacokinetic data are shown in Table 2. The NFV Cmax, C12, AUC0–12 and t1/2 were significantly lower during the third trimester vs. postpartum (P ≤ 0.03). The M8 Cpre-dose, Cmax, C12 and Cmin were significantly lower during the third trimester vs. postpartum (P ≤ 0.01). Individual concentration–time curves of NFV and M8 plasma concentrations during pregnancy (second and third trimesters) and postpartum are shown in Fig. 1. Median NFV AUC0–12 was approximately 40% lower in the third trimester compared to postpartum (Fig. 2a). The M8 AUC0–12 and the M8/NFV AUC ratio were lower during the third trimester vs. postpartum (P<0.01) (Figs 2b and 3).
Table 2.
Pharmacokinetic parameters: nelfinavir and hydroxyl-tert-butylamide (M8)
| Second trimester: median (range) | Third trimester: median (range) | Postpartum: median (range) | |||
|---|---|---|---|---|---|
| n =4 | n =27 | n =22 | Ratio, third trimester/postpartum (90% CI) (n) | P-value | |
| Nelfinavir | |||||
| AUC0–12 (mg h/mL) | 27.3 (6.2–75.7) | 18.9 (3.6–53.7) | 30.8 (1.3–123.9) | 0.69 (0.49–0.98) (22) | 0.02 |
| Proportion with AUC0–12 | 3/4 (75%) | 15/27 (56%) | 21/22 (95%) | – | – |
| above 18.5 mg h/mL | |||||
| Cpre-dose (mg/mL) | 1.3 (0.2–4.0) | 0.9 (<0.039–4.4) | 1.1 (<0.039–4.8) | 1.64 (0.83–3.25) (22) | 0.71 |
| Cmax (mg/mL) | 4.7 (1.3–9.8) | 3.2 (0.9–6.5) | 4.6 (0.3–9.9) | 0.77 (0.58–1.03) (22) | 0.03 |
| Tmax (h) | 4 (2–4) | 4 (1–6) | 2 (0–6) | – | – |
| C12 (mg/mL) | 0.5 (<0.039–3.3) | 0.5 (0.04–1.5) | 0.8 (<0.039–5.7) | 0.48 (0.27–0.85) (21) | 0.01 |
| Cmin (mg/mL) | 0.5 (<0.039–3.2) | 0.3 (<0.039–1.5) | 0.4 (<0.039–2.3) | 1.11 (0.63–1.96) (22) | 0.48 |
| Proportion with Cmin | 1/4 (25%) | 4/27 (15%) | 4/22 (18%) | – | – |
| above 0.8 mg/mL | |||||
| Tmin (h) | 12 (1–12) | 12 (0–12) | 12 (0–12) | – | – |
| CL/F (L/h) | 47 (17–202) | 66 (23–347) | 41 (10–962) | 1.45 (1.02–2.05) (22) | 0.06 |
| Vd/F (L) | 150 (102–821) | 216 (92–1389) | 175 (94–4255) | 0.88 (0.60–1.30) (22) | 0.52 |
| t1/2 (h) | 2.8 (1.8–4.3) | 2.5 (1.6–4.9) | 3.5 (2.2–4.0) | 0.61 (0.47–0.79) (22) | 0.0002 |
| M8 | |||||
| AUC0–12 (mg h/mL) | 2.4 (0.3–6.0) | 1.5 (<0.234–12.4) | 11.3 (<0.234–43.7) | 0.25 (0.14–0.43) (21) | <0.0001 |
| Cpre-dose (mg/mL) | 0.1 (<0.039–0.2) | 0.1 (<0.039–0.9) | 0.3 (<0.039–3.9) | 0.42 (0.26–0.68) (21) | 0.01 |
| Cmax (mg/mL) | 0.4 (0.1–1.2) | 0.3 (0.1–1.9) | 1.2 (<0.039–4.9) | 0.33 (0.21–0.54) (20) | 0.0001 |
| Tmax (h) | 4 (4–6) | 4 (0–8) | 4 (0–8) | – | – |
| C12 (mg/mL) | 0.06 (<0.039–0.1) | 0.05 (<0.039–0.3) | 0.2 (<0.039–1.6) | 0.26 (0.16–0.44) (22) | 0.0002 |
| Cmin (mg/mL) | 0.06 (<0.039–0.1) | <0.039 (<0.039–0.3) | 0.09 (<0.039–1.6) | 0.42 (0.23–0.77) (22) | 0.002 |
| Tmin (h) | 12 (12–12) | 12 (0–12) | 12 (0–12) | – | – |
| M8/nelfinavir AUC ratio | 0.047 (0.037–0.26) | 0.06 (0.029–0.31) | 0.21 (0.01–0.83) | 0.36 (0.26–0.50) (21) | <0.0001 |
AUC0–12, area under the plasma concentration–time curve; C12, 12 h post-dose concentration; Cmax, maximum concentration; Cmin, minimum concentration; Cpre-dose, pre-dose concentration; CI, confidence interval; CL/F, oral clearance; t1/2, half-life; Tmax, time post-dose of maximum concentration; Tmin, time post-dose of minimum concentration; Vd/F, apparent volume of distribution.
Fig. 1.
Nelfinavir and hydroxyl-tert-butylamide (M8) plasma concentrations during pregnancy: (a) second trimester (n = 4), (b) third trimester (n = 27) and (c) postpartum (n = 22) (nelfinavir 1250 mg twice daily). Solid lines in (a), (b) and (c) represent individual nelfinavir profiles; the dashed line represents the typical (50th percentile) concentrations in non-pregnant historical controls. Solid lines in (d), (e) and (f) represent individual M8 profiles (d) during the second trimester (n = 4), (e) during the third trimester (n = 27) (f) and postpartum (n = 22).
Fig. 2.
Nelfinavir and hydroxyl-tert-butylamide (M8) area under the plasma concentration–time curves (AUCs). (a) Antepartum and postpartum nelfinavir AUCs, second trimester (n = 4), to third trimester (n = 27) to postpartum (n = 22) in the same patients. The horizontal line indicates the 50th percentile AUC in non-pregnant adults (26 μg h/mL). (b) Antepartum and postpartum M8 AUCs, second trimester (n = 4), to third trimester (n = 27) to postpartum (n = 22) in the same patients.
Fig. 3.
Hydroxyl-tert-butylamide (M8) area under the plasma concentration–time curve (AUC)/nelfinavir AUC ratio, antepartum and postpartum.
The NFV AUC0–12 exceeded the AUC0–12 target for only 15/27 (56%) of third trimester participants, in contrast to 21/22 (95%) of postpartum individuals. In the third trimester, 14/27 (52%) had Cpre-dose concentrations above the suggested minimum trough concentration of 0.8 μg/mL, but only 7/26 (27%) C12 were above this threshold. Postpartum, 11/22 (50%) of both the Cpre-dose and C12 were >0.8 μg/mL. Absorption lags, defined as 1 h post-dose concentrations lower than the pre-dose concentration and subsequent samples with increased concentrations, were observed in 2/4 (50%), 7/27 (26%) and 2/22 (9%) of individuals in the second trimester, third trimester and postpartum, respectively. In these patients, minimum concentrations occurred at some time between the pre-dose and the 12 h post-dose concentrations. Actual Cmin values were above the minimum trough target concentration of 0.8 μg/mL for only 4/27 (15%) of third trimester and 4/22 (18%) of postpartum patients.
The one-compartment analysis yielded similar NFV exposure parameters to the non-compartmental analysis. The one-compartment median (range) second trimester, third trimester and postpartum CL/F values were 48 L/h (12–202 L/h), 63 L/h (21–150 L/h) and 35 L/h (15–916 L/h), respectively. The corresponding one-compartment Vd/F estimated values were 146 L (59–389 L), 193 L (73–1141 L) and 160 L (71–3644 L).
NFV concentrations were measured in 21 patients at delivery. The NFV concentration at delivery exceeded the quantitation limit of the assay in 14 individuals, and the median maternal plasma NFV concentration was 0.26 μg/mL (range 0.067–1.62 μg/mL) at a median 7.8 h post-dose. The undetectable NFV concentrations were drawn at a median of 16 h post-dose. Cord blood NFV concentrations were measured in 24 patients. The NFV cord blood concentration exceeded the quantitation limit of the assay in 12 individuals, and the median cord blood NFV concentration was 0.11 μg/mL (range 0.04–1.23 μg/mL) at 15 h after the maternal dose. The undetectable cord samples were drawn a median of 8.5 h post-dose. For the 14 mothers with measurable NFV at the time of delivery and an adequate cord blood sample, NFV was above the limit of quantitation in only six of the cord blood samples. The median ratio of the concentration of NFV in cord blood to maternal plasma was 0.393 (range <0.039–1.83). M8 concentrations were measurable in seven maternal and seven cord blood samples, with median concentrations of 0.056 μg/mL (range 0.042–0.253 μg/mL) and 0.382 μg/mL (range 0.054–0.0685 μg/mL), respectively.
Of the 27 patients enrolled in the NFV arm of P1026s, two (7.4%) experienced one or more adverse events. One patient was treated with intravenous, and subsequently oral, antibiotic therapy for multiple skin abscesses (grade 4) because of methicillin-resistant Staphylococcus aureus on her thigh. Subsequently, she was diagnosed with grade 3 diarrhoea because of Clostridium difficile, for which she was treated with oral vancomycin. These adverse events were judged unrelated to NFV, and the NFV dosage was not modified. The other patient had grade 2 oligohydramnios at 38 weeks of gestation, classified as possibly related to her ARVs (ZDV, 3TC and NFV).
Plasma viral loads were below 400 HIV-1 RNA copies/mL for 23/26 (88%) patients during the third trimester, 22/27 (81%) patients at delivery and 12/15 (80%) patients postpartum. All 27 infants are uninfected.
Discussion
This is the first study of the pharmacokinetics of NFV during pregnancy with the new formulation of NFV (625 mg tablets) at a dosage of 1250 mg twice daily. NFV exposure during the third trimester of pregnancy was lower than postpartum, as evidenced by lower NFV Cmax, C12 and AUC0–12 and lower M8 AUC0–12 and M8/NFV AUC ratios. Also, the NFV AUC0–12 was below the target in 44% of participants during the third trimester, but in only 5% of patients postpartum. Finally, few (15% of third trimester and 18% of postpartum) participants had Cmin values above the suggested minimum trough concentration of 0.8 μg/mL. NFV was well tolerated, but only 81% of women had undetectable plasma viral loads at delivery.
Several studies of NFV pharmacokinetics during pregnancy have evaluated 250 mg tablets [1–4]. NFV exposure was inadequate in most pregnant women receiving NFV 750 mg three times daily [1], and thus a higher dose (1250 mg twice daily) was evaluated [1–4]. With NFV 1250 mg twice daily, NFV exposure was improved [1] but remained inadequate [2–4]. However, with 250 mg tablets, utilizing this dose required taking five tablets twice daily. Subsequently, the current formulation (625 mg tablets) was made available.
The lower NFV exposure during pregnancy vs. post-partum observed in our study is consistent with findings from other NFV pharmacokinetic studies using the previous formulation [1–4]. Reduced NFV exposure can be attributed to decreased bioavailability or to increased elimination and/or volume of distribution. NFV is metabolized by both CYP 2C19 and CYP 3A4. NFV is metabolized by CYP 2C19 into the active metabolite M8. Both NFV and M8 are metabolized by CYP 3A4 to inactive compounds that are excreted primarily in faeces. We observed a reduction in the M8/NFV ratio during pregnancy, which confirms the results of an earlier study and is consistent with a decrease during pregnancy in CYP 2C19 activity relative to CYP 3A4. Hirt et al. [8] suggested no change in metabolism of NFV to M8 by CYP 2C19, but induction of CYP 3A4 during pregnancy (resulting in increases in NFV and M8 elimination). Because of the possibility of sub-therapeutic concentrations of NFV, therapeutic drug monitoring has been proposed [3]. Based on the results of our study, the NFV dose should be increased during late pregnancy and, if not, then therapeutic drug monitoring should be utilized to guide dosing. The lower NFV exposure during the third trimester of pregnancy described in our study is consistent with the results of previous studies of other protease inhibitors. Specifically, saquinavir [9] and lopinavir [10] exposures were lower during late pregnancy compared to postpartum.
NFV was well tolerated by participants in this study; this finding is consistent with previous preclinical [11] and clinical [12] data. In 2007, the presence of ethylmethane-sulfonate (EMS), a process-related impurity formed during manufacture, was detected in NFV marketed in Europe. EMS is a potential human carcinogen, and is teratogenic, mutagenic and carcinogenic in animals. However, analyses of NFV marketed by Pfizer in the USA as Viracept (NFV mesylate) revealed substantially lower concentrations of EMS [13], and Viracept is still being used in the USA. Placental transfer of NFV appeared poor in our study, confirming results from earlier studies using the previous formulation of NFV [14,15]. However, although we did not observe any cases of HIV infection among the small number of infants in this study, only 81% of third trimester patients had undetectable viral loads. Recent studies have confirmed the relative inferiority of protease inhibitor-based (and, more specifically, NFV-based) regimens in terms of the proportion of women achieving undetectable viral loads and time to achieving viral suppression [16–18]. A relatively limited virological effect, combined with low NFV concentrations in the foetus, could result in lower effectiveness in preventing mother-to-child transmission of HIV in larger studies.
In summary, our data indicate that NFV exposure during pregnancy was lower than postpartum and that a large proportion of women failed to meet pharmacokinetic parameter targets. Given that NFV is highly protein-bound, future studies should determine the extent of any alternations in protein binding during pregnancy, because this information could affect interpretation of pharmacokinetic results. A higher dose of NFV will be evaluated among pregnant women in the next version of the P1026s protocol.
Acknowledgments
The authors thank the participants from the clinical centres. We appreciate the vital contributions of James Connor, Francesca Aweeka, Bradley W. Kosel, Kathleen Medvik, Maureen Shannon, Carol Elgie and Joanne Schiffhauer to this study. We thank the following site staff for their contributions: Arlene Bardeguez, Charmane Calipap-Ber-nardo, Linda Bettica (University of Medicine and Dentistry of New Jersey/University Hospital, Newark, NJ, USA); Laureen Kay, Anne Marie Regan (Boston Medical Center, MA, USA); Margaret A. Keller, Marie Beall, Spring Wettgen, Nicole Falgout (Harbor-UCLA Medical Center, Torrance, CA, USA); Shelly Buschur, Hunter Hammill, Chivon Jackson, Mary E. Paul (Texas Children’s Hospital, Houston, TX, USA); Julie Schmidt, Helen Cejton, James B. McAuley, Maureen Haak (Cook County Hospital, Chicago, IL, USA); Isaac Delke, Mobeen Rathore, Ana Alvarez, Ayesha Mirza (University of Florida Health Science Center, Jacksonville, FL, USA); Edwin Thorpe Jr, Nina K. Sublette, Katherine M. Knapp, Jill Utech (St Jude Regional Medical Center, Memphis, TN, USA); Katherine Luzuriaga, Sharon Cormier (University of Massachusetts Medical School, Worcester, MA, USA); Heather Charlton, Newana Beatty, Marilyn Crain, Robert Pass (University of Alabama at Birmingham, AL, USA); Ana Melendrez, Françoise Kramer, LaShonda Spencer, Yvonne Rodriguez (Los Angeles County and University of Southern California, Los Angeles, CA, USA).
Funding: This study was supported in part by the PACTG of the NIAID (grants U01 AI04189, U01 AI41089, UO1 AI27560-18, U01 AI32907), the General Clinical Research Center Units funded by the National Center for Research Resources (grants M01 RR00533, 5 M01 RR01271), the Pediatric/Perinatal HIV Clinical Trials Network of the National Institute of Child Health and Human Development (contract N01-HD-3-3365) and the Pediatric Pharmacology Research Unit Network of the National Institute for Child Health and Human Development (grant U01-HD-031318-11).
Footnotes
Presented in part at the 14th Conference on Retroviruses and Opportunistic Infections, Los Angeles, CA, February 2007 [Abstract 740].
References
- 1.Bryson YJ, Mirochnick M, Stek A, et al. Pharmacokinetics and safety of nelfinavir when used in combination with zidovudine and lamivudine in HIV-infected pregnant women: Pediatric AIDS Clinical Trials Group (PACTG) Protocol 353. HIV Clin Trials. 2008;9:115–125. doi: 10.1310/hct0902-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nellen JF, Schillevoort I, Wit FW, et al. Nelfinavir plasma concentrations are low during pregnancy. Clin Infect Dis. 2004;39:736–740. doi: 10.1086/422719. [DOI] [PubMed] [Google Scholar]
- 3.Van Heeswijk RPG, Khaliq Y, Gallicano KD, et al. The pharmacokinetics of nelfinavir and M8 during pregnancy and postpartum. Clin Pharmacol Ther. 2004;76:588–597. doi: 10.1016/j.clpt.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Villani P, Floridia M, Pirillo MF, et al. Pharmacokinetics of nelfinavir in HIV-1-infected pregnant and non-pregnant women. Br J Clin Pharmacol. 2006;62:309–315. doi: 10.1111/j.1365-2125.2006.02669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Food and Drug Administration. Nelfinavir Package Insert. La Jolla, CA: Agouron Pharmaceuticals Inc; 2007. [accessed 23 July 2008]. Available at: www.fda.gov/cder/foi/label/2007/020778s027,020779s048,021503s0091b1.pdf. [Google Scholar]
- 6.Holland DT, DiFrancesco R, Connor JD, Morse GD. Quality assurance program for pharmacokinetic assay of antiretrovirals: ACTG proficiency testing for pediatric and adult pharmacology support laboratories, 2003 to 2004, a requirement for therapeutic drug monitoring. Ther Drug Monit. 2006;28:367–374. doi: 10.1097/01.ftd.0000211817.58052.b8. [DOI] [PubMed] [Google Scholar]
- 7.DHHS panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 8.Hirt D, Treluyer J-M, Jullien V, et al. Pregnancy-related effects on nelfinavir – M8 pharmacokinetics: a population study with 133 women. Antimicrob Agents Chemother. 2006;50:2079–2086. doi: 10.1128/AAC.01596-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acosta EP, Zorrilla C, Van Dyke R, et al. Pharmacokinetics of saquinavir-SGC in HIV-infected pregnant women. HIV Clin Trials. 2001;2:460–465. doi: 10.1310/PUY3-5JWL-FX2B-98VU. [DOI] [PubMed] [Google Scholar]
- 10.Stek AM, Mirochnick M, Capparelli E, et al. Reduced lopinavir exposure during pregnancy. AIDS. 2006;20:1931–1939. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- 11.Mathias CV, Mathias CFV, Simoes MJ, et al. Safety of nelfinavir use during pregnancy. An experimental approach in rats. Clin Exp Obst Gyn. 2005;32:163–165. [PubMed] [Google Scholar]
- 12.Timmermans S, Tempelman C, Godfried MH, et al. Nelfinavir and nevirapine side effects during pregnancy. AIDS. 2005;19:795–799. doi: 10.1097/01.aids.0000168973.59466.14. [DOI] [PubMed] [Google Scholar]
- 13.Pfizer, Inc. Dear Healthcare Professional Letter. New York, NY: Pfizer, Inc; [accessed 23 July 2008]. Available at: www.pfizer.com/files/products/viracept_letter_9_10_2007.pdf. [Google Scholar]
- 14.Marzolini C, Rudin C, Decosterd LA, et al. Transplacental passage of protease inhibitors at delivery. AIDS. 2002;16:889–893. doi: 10.1097/00002030-200204120-00008. [DOI] [PubMed] [Google Scholar]
- 15.Mirochnick M, Dorenbaum A, Holland D, et al. Concentrations of protease inhibitors in cord blood after in utero exposure. Pediatr Infect Dis J. 2002;21:835–838. doi: 10.1097/00006454-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 16.European Collaborative Study. Time to undetectable viral load after highly active antiretroviral therapy initiation among HIV-infected pregnancy women. Clin Infect Dis. 2007;44:1647–1656. doi: 10.1086/518284. [DOI] [PubMed] [Google Scholar]
- 17.Read JS, Cahn P, Losso M, et al. Management of human immunodeficiency virus-infected pregnant women at Latin American and Caribbean sites. Obstet Gynecol. 2007;109:1358–1367. doi: 10.1097/01.AOG.0000265211.76196.ac. [DOI] [PubMed] [Google Scholar]
- 18.Joao E, Calvet G, Sidi L, et al. Virologic control and infant outcomes among pregnant women exposed to different ART regimens during pregnancy. 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. February 2007; [Abstract 757] [Google Scholar]