Abstract
Male sex is a well-established risk factor for poor neurodevelopmental outcome following premature birth. The mechanisms behind this sex-related difference are unknown. The damage associated with prematurity can be mimicked in rodents by prolonged exposure to sublethal postnatal hypoxia. This chronic hypoxia leads to anatomical changes in mice that strongly resemble the loss of volume, decreased myelination and ventriculomegaly seen in preterm newborns. However, no sex differences have been previously noted in this rodent model. We hypothesized that sex comparisons in hypoxic mice would show sex related differences in brain volume and white matter loss in response to the same degree of hypoxic insult. Mice were placed in chronic sublethal hypoxia from postnatal day 3–11. Cortical, hippocampal, and cerebellar volumes and myelination indices were measured. We found that the male hippocampus, normally larger than the female, undergoes a greater volume loss compared to females (p<0.05). Myelination, generally greater in males, was significantly disrupted by hypoxia in neonatal male forebrain. These results support the use of this rodent model to investigate the basis of sex related susceptibility to brain damage and develop new sex based neuroprotective strategies.
More than 400,000 infants are born prematurely in the United States each year, with 20% of these being very preterm (<32 weeks gestation) (1). Preterm birth can lead to abnormal brain development with subsequent physical, cognitive and behavioral deficits (2,3). Even late preterm birth (32–37 weeks gestation) may have subtle neurological consequences (4). The numbers of children born preterm and the numbers of survivors continues to rise along with the significant costs associated with their poor neurological outcome (5).
Risk factors for these poor neurological outcomes have been identified across multiple studies. Increased risk is independently associated with both bronchopulmonary dysplasia (BPD) and male sex (3,6). The biology that underlies these risk factors is poorly understood. Male susceptibility is of particular interest because it implies that there may be hormonal factors that, if better understood, might provide new avenues in neuroprotective strategies.
Neuroimaging has demonstrated specific reductions in brain volume (age-corrected) in the cortex, hippocampus, and cerebellum plus ventriculomegaly, particularly in male children with history of BPD following preterm birth (6,7). These deficits, particularly in the hippocampus and cerebellum, often correlate with later cognitive deficits (8). While longitudinal studies on human preterm infants suggest factors that correlate with damage, rapid progress requires animal models in which damage can be mimicked, physical and behavioral changes can be assessed, mechanisms can be unraveled and interventions can be stringently tested.
In the past decade, chronic sublethal hypoxia treatment of neonatal rodents has been developed as a model of BPD-related preterm brain damage (9–12). Brain development in newborn rodents during the first two postnatal weeks is similar to that seen in very preterm infants: cortical neurogensis is complete, hippocampal and cerebellar neurogenesis is robust, and synaptogenesis and myelination are beginning (13). Pups treated with sublethal hypoxia during this period have reduced brain volumes and ventriculomegaly, highly reminiscent of that seen in human preterm infants (9–12). In neonatal mice acutely examined at multiple times during a week of sublethal hypoxia, cell loss occurs and synaptogenesis is perturbed (9). Mice that are allowed to recover have a temporary increase in neurogenesis, but neither normal neuronal number nor the appropriate balance of excitatory to inhibitory neurons is regained (14–16). However, no sex differences have previously been reported in any neonatal rodent chronic hypoxia model.
We sought to establish whether there was a detectable sex-linked difference in neurological damage in this rodent hypoxia model. Using unbiased volumetric techniques, we compared specific brain regions in male and female mouse pups undergoing normoxia (21% O2) or hypoxia (10% O2) from postnatal day 3–11, quantified the extent of myelination and determined hippocampal cell proliferation in both sexes.
Materials and Methods
Hypoxia
All experiments complied with approved animal protocols. Hypoxia treatment was carried out as described in (9), with minor modifications. Treatment initiation at P3 with assessment at P11 was chosen to span the period of brain development most closely matched to human preterm infants, to maximize pup survival and to allow comparison to prior reports (9). Sixteen C57BL/6 litters per round of experiments were culled to 4 male and 4 female pups at postnatal day 1 (P1). An additional lactating dam (CD-1 genotype) was introduced to improve pup survival, given the poor maternal behavior of C57BL/6 dams. On P3, half of the litters were placed in a hypoxic chamber. Chamber oxygen levels were reduced to 10% by means of an oxygen controller (Biospherix) that displaces oxygen with nitrogen gas. Control litters were kept in normoxic (21% oxygen) conditions. Animals were maintained in hypoxia or normoxia for 8 days. On P11, brains were collected and fixed in 4% paraformaldehyde for volumetric measurement. Tail clippings were taken to confirm sex via PCR genotyping.
PCR genotyping
DNA was extracted from tail clippings by proteinase K digestion (17). Sex was determined by PCR (18) using specific primers to the smcx and smcy genes [SMCXYF: 5′CCGCTGCCAAATTCTTTGG3′, SMCXYR: 5′TGAAGCTTTTGGCTTTGAG3′].
Brain Sectioning
Parafomaldehyde fixed brains were sunk in 30% sucrose for two days before sagittal sectioning at 50 μm on a sliding microtome. Every 10th section cut throughout one hemisphere was collected onto a slide.
Volumetric Analysis
Sections were Cresyl Violet stained and imaged using a Leica DFC280 camera on a Leica stereoscope. Cortical, hippocampal, and cerebellar volumes were measured using the Cavalieri method as described (19). A point grid was placed over the images and the number of points transecting the region of interest was counted. The estimated volume of each region of interest was calculated using: vol=T*a/p*ΣPi, where T = the distance between sections; a/p = the area associated with each point on the grid; and Pi = the number of points intersecting a brain region of interest. The a/p is calculated by dividing the square of the distance between points on the grid (Δx) by the linear magnification of the images (M). Therefore, a/p = Δx2/M2. Estimated volumes were normalized to control (Fig. 1) or control male values (Fig. 2). The p values were calculated using two-way ANOVA with Bonferroni post-hoc comparison. All measurements were done blind to sex and treatment.
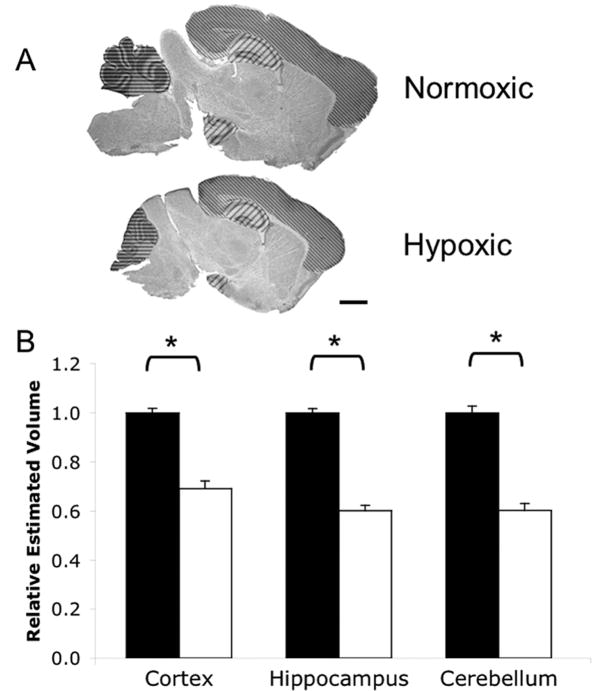
Figure 1. Chronic neonatal hypoxia reduces regional brain volumes.
A) Cresyl Violet staining of normoxic and hypoxic brains of C57BL/6 mice at P11 showing hypoxia-associated size reduction, similar to rat (10). Regions measured are marked. Thin diagonal lines=cortex; thick diagonal lines=hippocampus; horizontal lines=cerebellum. Scale bar=1.0 mm. B) Quantification of cortex, hippocampus, and cerebellum volumes. Black bars=normoxia; white bars=hypoxia. Total N=53 brains, N≥16 per treatment; groups balanced for sex. * p<0.05 vs Normoxic. Error bars=SEM.
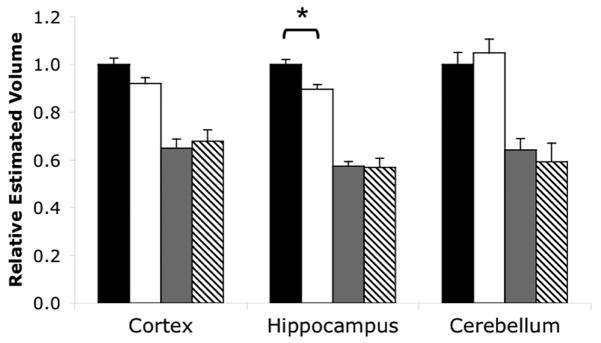
Figure 2. Male hippocampus undergoes greater volume loss compared to female.
Sex differences in regional brain volumes of hypoxic and normoxic mice. Black bars=normoxic males; white bars=normoxic females; gray bars=hypoxic males; hatched bars=hypoxic females. Total N=49 brains N≥9 per group. All hypoxic volumes are significantly smaller (* p<0.05) than the normoxic volumes. Error bars=SEM.
Myelin Staining
Black Gold II (BGII; Histo-Chem, Inc; (20)) was used to reveal myelin. Sections were slide mounted, dried and rehydrated in distilled water for 2 min, placed in 0.3% solution of BGII in 0.9% saline at 65°C for 12 min, rinsed in distilled water for 2 min, placed in 1% sodium thiosulfate for 3 min, washed 3 times in water and finally dehydrated and cleared through ethanol and xylene. Bright field images of the myelin staining were taken using a Leica DFC280 camera on a Leica stereoscope using uniform settings. Unmanipulated images were quantified using Adobe Photoshop Software. For these analyses, the brain was either examined as a whole or subdivided into 5 regions using anatomical markers as guides. Image intensity was inverted, a specific region was selected (lasso tool) and mean pixel intensity of this region was measured (histogram function) to estimate myelin intensity. Statistical analysis was performed using two-way ANOVA with Bonferroni post-hoc comparison for whole brain measurements and three-way ANOVA with Tukey’s correction for multiple comparisons for regional analyses.
Proliferation
Cell proliferation was assayed by phospho-Histone H3 (pHH3) immunohistochemistry. Serially cut sections on slides underwent antigen retrieval. Sections were rehydrated in phosphate buffered saline (0.1M) for 5 min then placed in boiling sodium citrate (10mM) for 45 min. After cooling, sections were washed, blocked in 2% normal goat serum with 0.05% triton X-100 for 40 min and rabbit anti-pHH3 (1:800; Upstate) applied at 4°C overnight. After rinsing, biotinylated anti-rabbit IgG (1:500; Vector Labs) was added for 30 min followed by Vectastain ABC kit and DAB (Vector Labs) per manufacturer’s instructions. Sections were counterstained with Hematoxylin QS (Vector Labs). Immunostaining was visualized on a Nikon E800 microscope. All brown cells within the hippocampus across serially cut brain sections were counted for each animal. Counts were performed blind to treatment and sex. Statistical analysis was performed using two-way ANOVA with Bonferroni post-hoc comparison.
Results
Hypoxia leads to differential reduction of regional brain volumes
Chronic sublethal hypoxia treatment of postnatal mouse pups greatly reduced overall brain size when measured immediately after removal from treatment at postnatal day 11 (Fig. 1A), as anticipated from prior reports (9,10). This reduction in total brain size is accompanied with overall body size reduction: hypoxic mice have a body weight reduction of 57% ± 1.6% compared to controls, while brain weight is only reduced by 33% ± 2.4%. This observation is consistent with previous studies in rats (10). We found no significant difference in the overall hypoxia-induced decrease of body or brain weights when sexes were compared.
To determine whether the growth of different regions of the brain are affected differentially by hypoxia treatment, we measured the volumes of three brain regions – the cortex, hippocampus, and cerebellum – all of which are potentially affected in preterm infants. Using the Cavalieri technique (19), we measured a statistically significant reduction in all regional volumes (Fig. 1B; cortex: p<0.0001, F=78.42, DFn=1, DFd=49; hippocampus: p<0.0001, F=233.6, DFn=1, DFd=49; cerebellum: p<0.0001, F=92.25, DFn=1, DFd=42). Regional volumes were normalized relative to controls in each experimental set to minimize any histological processing related volume changes. A total of 53 mouse brains were measured. Total hippocampal and cerebellar volumes were reduced by 40% each after 8 days of hypoxia (Fig. 1B, middle and right, respectively). Cortical volume was reduced by 31% (Fig. 1B, left).
Hypoxia abolishes normal sex differences observed in the hippocampus
We next compared regional volumes by sex and treatment. Most notably, male hippocampal volume in control pups was 10.5% larger than females (p<0.01, F=4.92, DFn=1, DFd=49; Fig. 2, middle). This sex difference was abolished by hypoxia (Fig. 2, middle). Male mice treated with hypoxia had a 43% overall reduction of hippocampal volume while females had a 36% reduction (difference of the means calculations, p<0.05; (21)). While this difference is small, it was reproduced consistently across multiple, independent experimental runs. Thus, males experience significantly greater hippocampal volume loss due to hypoxia than females.
In other brain regions, there were no significant differences in normal volumes. P11 control males appeared to have larger cortical volumes, but this difference was not significant (p>0.05, F=0.55, DFn=1, DFd=49; Fig. 2, left). Cerebellar volume showed no sex-linked difference (p>0.05, F=2.01e-4, DFn=1, DFd=42; Fig. 2, right). Therefore, despite the striking reductions in cortical and cerebellar volumes under hypoxic conditions, there are no measurable sex-linked decrements in these regions.
Hypoxia reduces total number of proliferating cells, but not proliferation relative to volume in the hippocampus
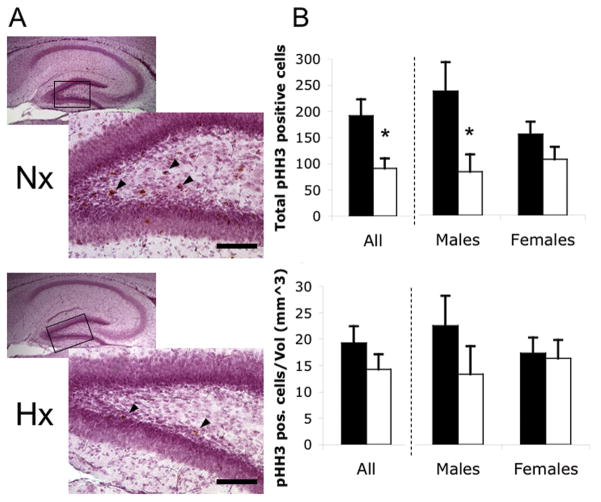
We next examined cell division in normoxic and hypoxic hippocampi. Phospho-Histone H3 (pHH3), a mitosis marker, was used to visualize proliferating cells. Twentythree mouse brains were analyzed. Almost all of the pHH3-positive cells were in the dentate gyrus, the primary hippocampal region of ongoing neurogenesis (Fig. 3A). We found significantly fewer total pHH3-positive cells in hypoxic hippocampus, but only in males (Fig. 3B; p<0.05, F=6.12, DFn=1, DFd=19). However, when adjusted for hippocampal volume, there was no significant change in proliferation per unit volume (Fig. 3C; p=0.29, F=1.14, DFn=1, DFd=19).
Figure 3. Hypoxia reduces absolute but not volume-relative hippocampal progenitor proliferation.
A) PHH3 immunostaining (brown cells, arrowheads) in the dentate gyrus of normoxic (Nx) and hypoxic (Hx) hippocampus. Box shows region of outset. Scale bar=100μm. B) Total numbers of pHH3 positive cells. C) Total numbers of pHH3 positive cells per volume. Black bars=normoxia; white bars=hypoxia. Total N= 23 brains, N≥5 per group. *p<0.05. Error bars=SEM.
Disruption of myelin formation in the brain after hypoxia treatment is sex and region dependent
BGII staining was used to visualize myelin fibers in anatomically matched medial parasagittal sections (Fig. 4A). Myelination is grossly disrupted by sublethal hypoxia (9), consistent with the white matter delays seen in preterm infants (22,23). Thirteen mouse brains were measured. BGII revealed heavily myelinated tracts in hindbrain, midbrain and cerebellum at P11 with lighter myelin staining surrounding the hippocampus and corpus callosum (Fig. 4A). BGII staining was visibly reduced in hypoxic brains, suggesting a disruption in myelination (Fig. 4A).
Figure 4. Chronic neonatal hypoxia produces male forebrain hypo-myelination.
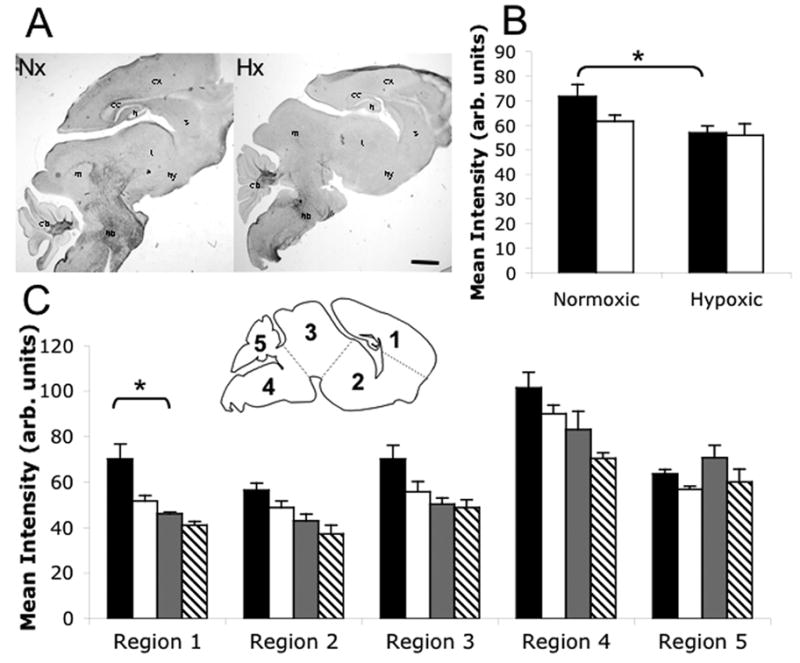
Myelin staining in normoxic and hypoxic brains as visualized with Black Gold II. A) BGII staining; cx=cortex, cc=corpus callosum, h=hippocampus, s=striatum, t=thalamus, hy=hypothalamus, m=midbrain, hb=hindbrain, cb=cerebellum. B) Quantification of myelin staining intensity in whole brains. Black bars=males; white bars=females. C) Quantification of myelin staining intensity in five labeled regions (inset image). Black bars=normoxic males; white bars=normoxic females; gray bars=hypoxic males; hatched bars=hypoxic females. Total N= 13 brains, N≥3 per group. *p<0.05. Scale bar=1mm. Error bars=SEM.
Quantification of BGII staining intensity in whole brain sections demonstrated significant hypo-myelination in hypoxic male brains (Fig. 4B; p<0.05, F=7.26, DFn=1, DFd=9). No statistically significant myelination differences were seen between normoxic and hypoxic females (Fig. 4B), likely due in large part to the tendency of normal P11 female brains to have less myelin than normal males.
Subdividing the brain into 5 regions revealed that the myelination changes were not only sex-dependent, but also regionally distinct. Region 1, containing cortex, hippocampus, and corpus callosum, was the only region where myelination in males showed a statistically significant disruption (Fig. 4C, left; p<0.05, F=4.73, DFn=4, DFd=45). Therefore, forebrain was the main region contributing to the overall decrease in myelination observed in males. Midbrain/hindbrain areas defined as Regions 2–4 showed a similar pattern of reduced myelination, particularly in males, but this was not significant (Fig. 4C, middle). Myelination is not affected in Region 5, cerebellum, which is consistently heavily myelinated by this stage (Fig. 4C, right).
Discussion
The increased male susceptibility to preterm brain damage is poorly understood. Using a hypoxia model that recapitulates the neurological pathology of premature birth (9,10), we have demonstrated significantly greater hippocampal volume loss in male mice compared to females plus associated hypo-myelination. This sex difference was likely not noted previously because hypoxia results in male and female brains that are comparable in size, both globally and regionally. It is only when the regional sex differences in normal brain anatomy are noted that this differential loss is revealed. Our results highlight the importance of examining sex differences in injury response in the context of difference that exist prior to injury.
The neonatal chronic hypoxia model is appealing because it mimics the ongoing low -grade hypoxia associated with the chronic lung disease of prematurity. While many animal models are used to study the effects of prematurity, most suffer from expense, limited genetic tractability or poor imitation of the physiological state of a preterm infant (24). Rodents, which are amenable to genetic manipulation and behavioral testing, do not survive preterm delivery, but are born less mature (13) so many neurological events that are fetal in humans can be manipulated postnatally in mice. The Rice-Vannucci neonatal hypoxic-ischemic model is by far the most common model for studying neuroprotection in the developing brain (25). Additional models of ischemia or excitotoxicity have also been used (26,27). Preterm infants however rarely suffer acute ischemic or excitotoxic events, but rather have ongoing global compromise similar to what occurs in the hypoxia model. Also, in addition to the chronic hypoxia, the mice in our study may have nutritional losses, similar to the impaired nutrition of preterm newborns. Using a foster dam paradigm to control for poor feeding or milk supply in the hypoxic mother, it was previously shown that rat pups exposed to chronic neonatal hypoxia showed poor growth despite the availability of adequate nutrition (10). Affects on pup behavior cannot be ruled out. Our foster dam addition is a variation on the prior work and likely insures adequate nutritional supplies. Thus, the majority of the volume loss is likely secondary to the hypoxia itself, even if malnutrition plays some role. Whether do to hypoxia alone or a mixture of insults, the global neurological damage frequently seen in preterm infants, including grey and white matter loss leading to ex vacuo ventriculomegaly is closely mimicked.
Cortex, hippocampus and cerebellum are not usually considered sexually dimorphic, but sex-linked differences are seen (28,29). To the best of our knowledge, sex-linked volume differences in neonatal brain regions have not been previously reported, but magnetic resonance imaging in adult mice showed a 2.5% sex-linked volume difference, with male brain being larger than female (29). Adult male C57BL/6 mice have a larger posterior hippocampus, while females have a larger anterior hippocampus (29). We measured the entire hippocampal volume, suggesting either that total male hippocampal volume is proportionately larger in young mice or that the posterior difference dominates the measurement. Unlike mice, human volumetric studies have been variable (30). Male brain volumes are generally larger, but no consistent sex-related hippocampal differences have been seen (30). Differences in regional brain volumes may be species specific or may reflect different regional developmental trajectories. Although human anatomical differences may be small, compromised hippocampal function is frequently associated with volume loss in children who were born preterm (31,32). Long-term behavioral studies in both mice and humans are needed to assess the contribution of sex-linked injury to functional hippocampal impairment.
All regions had volume loss with hypoxia, but hippocampal and cerebellar loss was greater than cortical loss, potentially due to developmental differences: cortical neurogenesis is essentially complete in postnatal mice, while the hippocampus and cerebellum are still regions of active neural proliferation. However, unlike hippocampus, no sex volume difference was detected in cerebellum nor did cerebellar myelination change with hypoxia.
Given the differential reductions in hippocampal volumes, we assessed progenitor proliferation in this region. In rats, hypoxia significantly disrupts neural proliferation (33), although reduced neuron size, defects in differentiation and increased apoptosis also result from hypoxic injury (11,33). The role of sex in neurogenesis remains uncertain. For example, estrogen has variable effects on dentate gyrus neurogenesis (34,35). Despite the striking reduction in proliferating cells in hypoxia, there was no statistical decrease in proliferation when normalized for total volume changes. This finding suggests that differential reductions in cell division are not the sole cause of volume loss.
Previous work in this model suggested suppression of glial genes suggesting a role for gliogenesis (9). Adult males have more white matter than females (36,37). This difference is less pronounced in our normal neonatal mice, but appears to be developing by P11. Similar to the difference in hippocampal volume, the decrease in forebrain myelination seen in hypoxic neonatal males may be due to tendency to have a relatively greater normal male baseline. Other brain regions showed smaller decreases in myelin intensity after hypoxia. Differential maturation of glia may underlie these regional differences (38), a hypothesis that requires further investigation with oligodendrocyte lineage markers. Determining whether the hypoxia-induced hypo-myelination in males can be averted or reversed using sex steroids or other agents is critical.
Sexually dimorphic responses to disease or injury have long been recognized. For example, women have a lower risk of stroke and greater recovery, an effect attributed primarily to sex steroids. In vitro evidence shows that estrogen can be neuroprotective in response to hypoxia (39) and oxygen-glucose deprivation (40), while dihydrotestosterone (a “male” hormone) was found to increase muscimol-induced excitotoxic cell death (41). In vivo evidence on sex steroid protection is far more variable, varying with age, insult, brain region and hormone examined (42–44). Sex hormone levels differences in neonatal male and female mice are well established, with males having a neonatal testosterone surge and females an estrogen surge. We have preliminary data that suggests that these hormones are altered differentially in males and females in response to hypoxia treatment (data not shown), but the impact of any alterations is the subject of ongoing investigation. Estrogen and progesterone supplementation has been tried in a small cohort of preterm females (45), but the consequences of this treatment remain uncertain, especially if this approach were to be expanded to males. The complexity and variability underscores the need to assess the effects of these hormones in vivo in a model that appropriately mimics the specific type of damage in question.
Recent studies also suggest sex-specific differences in neuroprotective or apoptotic gene expression (43,44). One intriguing study (46) showed that one could predict a 1/3 lower mortality in females due to hypoxic events if there existed a protective X-linked allele. The identity of the allele that could lower female risk of death remains elusive, but there are hints that there may be differences in the developmental expression of apoptotic pathways (43,44). Using an acute ischemic challenge, male neurons demonstrate greater caspase-independent, apoptosis-inducing factor (AIF) -dependent cell death compared to female neurons (44). The potential roles of differential use of specific apoptotic pathways and the underlying influence of X- and Y-linked gene expression in the setting of chronic hypoxia can now be assessed at a mechanistic level using the multiple genetic tools available in mice.
This study is limited primarily by our ability to detect small, but potentially significant, sex-specific anatomical changes beyond the hippocampus. 3D-MRI analysis of the brains of normal and hypoxia-treated neonatal mice might prove revealing since differences of as small as 2.5% could be detected in adult mice (29). While body weight rapidly recovers following chronic hypoxia, brain volume does not ((11); unpublished observations) despite the increase in cell proliferation in the weeks following hypoxia. Non-invasive measurements of the specific trajectories of grey and white matter damage and recovery after hypoxia in each sex would likely be revealing.
The increased loss of male hippocampal volume following chronic postnatal hypoxia suggests that we will see sex differences in standardized tests of rodent behavior. Determining the link between the anatomical losses and behavioral impact of these changes will be critical in assessing new interventions aimed at ameliorating these losses. Being able to mimic male susceptibility to prematurity-associated neurological damage provides a new way to investigate the biological basis of this damage and to rapidly test new neuroprotective strategies.
Acknowledgments
We thank LR Ment and M Schwartz for their guidance in setting up the hypoxia model system. We thank DL Kimmel for image data quantification and statistical help.
Financial Support: Supported by K08 NS044989, John Merck Fund for Developmental Disabilities and Packard Children’s Health Initiative Funds (A.A.P.); NIH Training Grant T32 MH020016-09 (S.R.M.)
Abbreviations
- BGII
Black Gold II
- pHH3
phospho-Histone H3
References
- 1.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, Munson ML. Centers for Disease Control and Prevention National Center for Health Statistics National Vital Statistics System 2007 Births: final data for 2005. Natl Vital Stat Rep. 56:1–103. [PubMed] [Google Scholar]
- 2.Anderson PJ, Doyle LW. Cognitive and educational deficits in children born extremely preterm. Semin Perinatol. 2008;32:51–58. doi: 10.1053/j.semperi.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 4.Chyi LJ, Lee HC, Hintz SR, Gould JB, Sutcliffe TL. School outcomes of late preterm infants: special needs and challenges for infants born at 32 to 36 weeks gestation. J Pediatr. 2008;153:25–31. doi: 10.1016/j.jpeds.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Petrou S, Henderson J, Bracewell M, Hockley C, Wolke D, Marlow N. Pushing the boundaries of viability: the economic impact of extreme preterm birth. Early Hum Dev. 2006;82:77–84. doi: 10.1016/j.earlhumdev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Thompson DK, Warfield SK, Carlin JB, Pavlovic M, Wang HX, Bear M, Kean MJ, Doyle LW, Egan GF, Inder TE. Perinatal risk factors altering regional brain structure in the preterm infant. Brain. 2007;130:667–677. doi: 10.1093/brain/awl277. [DOI] [PubMed] [Google Scholar]
- 7.Kesler SR, Ment LR, Vohr B, Pajot SK, Schneider KC, Katz KH, Ebbitt TB, Duncan CC, Makuch RW, Reiss AL. Volumetric analysis of regional cerebral development in preterm children. Pediatr Neurol. 2004;31:318–325. doi: 10.1016/j.pediatrneurol.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hart AR, Whitby EW, Griffiths PD, Smith MF. Magnetic resonance imaging and developmental outcome following preterm birth: review of current evidence. Dev Med Child Neurol. 2008;50:655–663. doi: 10.1111/j.1469-8749.2008.03050.x. [DOI] [PubMed] [Google Scholar]
- 9.Curristin SM, Cao A, Stewart WB, Zhang H, Madri JA, Morrow JS, Ment LR. Disrupted synaptic development in the hypoxic newborn brain. Proc Natl Acad Sci USA. 2002;99:15729–15734. doi: 10.1073/pnas.232568799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ment LR, Schwartz M, Makuch RW, Stewart WB. Association of chronic sublethal hypoxia with ventriculomegaly in the developing rat brain. Brain Res Dev Brain Res. 1998;111:197–203. doi: 10.1016/s0165-3806(98)00139-4. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz ML, Vaccarino F, Chacon M, Yan WL, Ment LR, Stewart WB. Chronic neonatal hypoxia leads to long term decreases in the volume and cell number of the rat cerebral cortex. Semin Perinatol. 2004;28:379–388. doi: 10.1053/j.semperi.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Stewart WB, Ment LR, Schwartz M. Chronic postnatal hypoxia increases the numbers of cortical neurons. Brain Res. 1997;760:17–21. doi: 10.1016/s0006-8993(97)00271-0. [DOI] [PubMed] [Google Scholar]
- 13.Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- 14.Fagel DM, Ganat Y, Silbereis J, Ebbitt T, Stewart W, Zhang H, Ment LR, Vaccarino FM. Cortical neurogenesis enhanced by chronic perinatal hypoxia. Exp Neurol. 2006;199:77–91. doi: 10.1016/j.expneurol.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Turner CP, Seli M, Ment L, Stewart W, Yan H, Johansson B, Fredholm BB, Blackburn M, Rivkees SA. A1 adenosine receptors mediate hypoxia-induced ventriculomegaly. Proc Natl Acad Sci USA. 2003;100:11718–11722. doi: 10.1073/pnas.1931975100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fagel DM, Ganat Y, Cheng E, Silbereis J, Ohkubo Y, Ment LR, Vaccarino FM. Fgfr1 is required for cortical regeneration and repair after perinatal hypoxia. J Neurosci. 2009;29:1202–1211. doi: 10.1523/JNEUROSCI.4516-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClive PJ, Sinclair AH. Rapid DNA extraction and PCR-sexing of mouse embryos. Mol Reprod Dev. 2001;60:225–226. doi: 10.1002/mrd.1081. [DOI] [PubMed] [Google Scholar]
- 18.Jimenez A, Fernandez R, Madrid-Bury N, Moreira PN, Borque C, Pintado B, Gutierrez-Adan A. Experimental demonstration that pre- and post-conceptional mechanisms influence sex ratio in mouse embryos. Mol Reprod Dev. 2003;66:162–165. doi: 10.1002/mrd.10345. [DOI] [PubMed] [Google Scholar]
- 19.Howard V, Reed M. Unbiased stereology: three-dimensional measurement in microscopy. Springer; New York: 1998. p. 246. [Google Scholar]
- 20.Schmued L, Bowyer J, Cozart M, Heard D, Binienda Z, Paule M. Introducing Black-Gold II, a highly soluble gold phosphate complex with several unique advantages for the histochemical localization of myelin. Brain Res. 2008;1229:210–217. doi: 10.1016/j.brainres.2008.06.129. [DOI] [PubMed] [Google Scholar]
- 21.Matthews JN, Altman DG. Statistics notes. Interaction 2: Compare effect sizes not P values. BMJ. 1996;313:808. doi: 10.1136/bmj.313.7060.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Constable RT, Ment LR, Vohr BR, Kesler SR, Fulbright RK, Lacadie C, Delancy S, Katz KH, Schneider KC, Schafer RJ, Makuch RW, Reiss AR. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121:306–316. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- 23.Edgin JO, Inder TE, Anderson PJ, Hood KM, Clark CA, Woodward LJ. Executive functioning in preschool children born very preterm: relationship with early white matter pathology. J Int Neuropsychol Soc. 2008;14:90–101. doi: 10.1017/S1355617708080053. [DOI] [PubMed] [Google Scholar]
- 24.Elovitz MA, Mrinalini C. Animal models of preterm birth. Trends Endocrinol Metab. 2004;15:479–487. doi: 10.1016/j.tem.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 26.Derugin N, Ferriero DM, Vexler ZS. Neonatal reversible focal cerebral ischemia: a new model. Neurosci Res. 1998;32:349–353. doi: 10.1016/s0168-0102(98)00096-0. [DOI] [PubMed] [Google Scholar]
- 27.Nunez JL, Alt JJ, McCarthy MM. A new model for prenatal brain damage. I. GABAA receptor activation induces cell death in developing rat hippocampus. Exp Neurol. 2003;181:258–269. doi: 10.3201/eid0906.030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 1999;19:4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spring S, Lerch JP, Henkelman RM. Sexual dimorphism revealed in the structure of the mouse brain using three-dimensional magnetic resonance imaging. Neuroimage. 2007;35:1424–1433. doi: 10.1016/j.neuroimage.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beauchamp MH, Thompson DK, Howard K, Doyle LW, Egan GF, Inder TE, Anderson PJ. Preterm infant hippocampal volumes correlate with later working memory deficits. Brain. 2008;131:2986–2994. doi: 10.1093/brain/awn227. [DOI] [PubMed] [Google Scholar]
- 32.Isaacs EB, Lucas A, Chong WK, Wood SJ, Johnson CL, Marshall C, Vargha-Khadem F, Gadian DG. Hippocampal volume and everyday memory in children of very low birth weight. Pediatr Res. 2000;47:713–720. doi: 10.1203/00006450-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Robinson S, Petelenz K, Li Q, Cohen ML, Dechant A, Tabrizi N, Bucek M, Lust D, Miller RH. Developmental changes induced by graded prenatal systemic hypoxic-ischemic insults in rats. Neurobiol Dis. 2005;18:568–581. doi: 10.1016/j.nbd.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 34.Galea LA. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2008;57:332–341. doi: 10.1016/j.brainresrev.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Lagace DC, Fischer SJ, Eisch AJ. Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus. 2007;17:175–180. doi: 10.1002/hipo.20265. [DOI] [PubMed] [Google Scholar]
- 36.De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- 37.Yang S, Li C, Zhang W, Wang W, Tang Y. Sex differences in the white matter and myelinated nerve fibers of Long-Evans rats. Brain Res. 2008;1216:16–23. doi: 10.1016/j.brainres.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 38.Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93:F153–F161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heyer A, Hasselblatt M, von Ahsen N, Hafner H, Siren AL, Ehrenreich H. In vitro gender differences in neuronal survival on hypoxia and in 17beta-estradiol-mediated neuroprotection. J Cereb Blood Flow Metab. 2005;25:427–430. doi: 10.1038/sj.jcbfm.9600056. [DOI] [PubMed] [Google Scholar]
- 40.Liu M, Hurn PD, Roselli CE, Alkayed NJ. Role of P450 aromatase in sex-specific astrocytic cell death. J Cereb Blood Flow Metab. 2007;27:135–141. doi: 10.1038/sj.jcbfm.9600331. [DOI] [PubMed] [Google Scholar]
- 41.Nunez JL, McCarthy MM. Androgens predispose males to GABAA-mediated excitotoxicity in the developing hippocampus. Exp Neurol. 2008;210:699–708. doi: 10.1016/j.expneurol.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner CK. Progesterone receptors and neural development: a gap between bench and bedside? Endocrinology. 2008;149:2743–2749. doi: 10.1210/en.2008-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nunez JL, McCarthy MM. Sex differences and hormonal effects in a model of preterm infant brain injury. Ann N Y Acad Sci. 2003;1008:281–284. doi: 10.1196/annals.1301.032. [DOI] [PubMed] [Google Scholar]
- 44.Renolleau S, Fau S, Charriaut-Marlangue C. Gender-related differences in apoptotic pathways after neonatal cerebral ischemia. Neuroscientist. 2008;14:46–52. doi: 10.1177/1073858407308889. [DOI] [PubMed] [Google Scholar]
- 45.Trotter A, Pohlandt F. The replacement of oestradiol and progesterone in very premature infants. Ann Med. 2000;32:608–614. doi: 10.3109/07853890009002031. [DOI] [PubMed] [Google Scholar]
- 46.Mage DT, Donner M. Female resistance to hypoxia: does it explain the sex difference in mortality rates? J Womens Health (Larchmt) 2006;15:786–794. doi: 10.1089/jwh.2006.15.786. [DOI] [PubMed] [Google Scholar]