Abstract
In this review, we begin with a historical accounting of the evolution of the concept of mild cognitive dysfunction including nomenclature and criteria from Kral to Petersen. A critical analysis of the main elements relating to assessment and diagnosis of mild cognitive dysfunction are described. Methodological limitations in design, measurement, and characterization, especially as they relate to older African Americans, are identified. Data from a 15-year longitudinal study of community-dwelling, African Americans in Indianapolis indicate 23% prevalence of all-cause mild cognitive dysfunction with approximately 25% progressing to dementia in 2 years and another 25% reverting to normal in the same interval. Factors contributing to this longitudinal variability in outcome are reviewed including the role of medical health factors. We close with suggestions for next steps in the epidemiological research of mild cognitive impairment.
Keywords: cognition, memory, mild cognitive impairment, dementia, aging, epidemiology
INTRODUCTION
Improved understanding of the pathogenesis of dementia brings renewed hope that scientists might soon discover disease-modifying treatments for this disorder. Initial evidence suggests that such treatments would be most effectively employed in the preclinical phase of dementia1 since the pathologic processes underlying dementia may predate clinical symptoms by many years.2 Early identification of mild cognitive dysfunction will be critical to any programs directed toward prevention and treatment of dementing illnesses; however, there is substantial inaccuracy in the diagnosis of dementia and these mistakes in diagnosis are associated with important mistakes in treatment.3 Calls for even earlier diagnosis and treatment further complicate this situation because the natural history of mild cognitive dysfunction is unclear.
This paper attempts to provide a historical accounting of the evolution of the concept of mild cognitive dysfunction including nomenclature and criteria and a discussion of the areas of overlap and divergence between the different concepts. Following that, we describe the main elements relating to measurement and diagnosis including the place of subjective complaint and psychometric assessment. Next, the epidemiology (prevalence, incidence, and risk factors) of mild cognitive dysfunction is reviewed with an emphasis on population studies and presentation of data from our 15-year longitudinal study of community-dwelling, African Americans in Indianapolis.4–6 Hopefully, this information will help to summarize current understanding of mild cognitive dysfunction and provide direction for future research.
NOMENCLATURE: APPROACHES TO CLASSIFICATION
A wide variety of labels have been applied to the intermediate state between normalcy and dementia. The first approaches were interview-based and did not rely on psychometric testing. Most current approaches include information from cognitive testing in the diagnostic process though there are differences in the tests included and threshold for impairment (see Table 1).
Table 1.
Methodological characteristics of several approaches to mild cognitive dysfunction.
| Name | Source | Cognitive Domain of Interest | Information Sources | Diagnostic Process | Psychometric Threshold Defining Impairment and Type Normative Group |
|---|---|---|---|---|---|
| Malignant Senescent Forgetfulness | Kral7 | Memory | MD | Clinical | Not applicable, no testing |
| Benign Senescent Forgetfulness | Kral7 | Memory | MD | Clinical | Not applicable, no testing |
| Age- Associated Memory Impairment | NIMH13 | Memory | S, T | Psychometric | 1 SD below young adult norms |
| Questionable Dementia/C DR 0.5 | Memory Aging Project8,9 | Memory | MD, I | Clinical | Not applicable, no testing |
| Aging- Associated Cognitive Decline | WHO21 | All | S/I, T | Psychometric | 1 SD below age- and education-adjusted norms |
| Mild Cognitive Impairment | Mayo Clinic22,23 | Memory | MD, S/I, T | Both | 1.5 SD below age- and education-adjusted norms |
| Mild Cognitive Impairment (expanded) | Mayo Clinic27,28 | All | MD, S/I, T | Both | 1.5 SD below age- and education-adjusted norms |
| Cognitive Impairment No Dementia | CSHA29,3 0 | All | MD, I, T | Both | Age-adjusted norms used. Unclear if education-adjusted norms used. Psychometric threshold defining impairment not specified. |
| Cognitive Impairment No Dementia | ISHA6 | All | MD, I, T | Both | 1.5 SD below age- and education-adjusted norms |
Notes: MD = physician examination, S = self-report, I = informant interview, T = testing, SD = standard deviation, NIMH = National Institute of Mental Health, WHO = World Health Organization, CSHA = Canadian Study of Health and Aging, ISHA = Indianapolis Study of Health and Aging.
Interview-based Approaches
Malignant senescent forgetfulness (MSF) was first described by Kral7 in 1962 to characterize a subgroup of older patients who had difficulty recalling recent events and who ultimately became globally demented in the span of a few years. Kral distinguished MSF from benign senescent forgetfulness (BSF) which was characterized by occasional and incomplete forgetfulness that did not have a progressive quality and which was not qualitatively different than normal aging. The diagnosis was based on clinical bedside examination of the severity and depth of the memory dysfunction. No standardized procedures or explicit diagnostic criteria were enumerated. MSF is the forerunner of all clinic-based approaches to mild cognitive dysfunction that attempt to refer to a clinically pathological entity.
In 1982, Hughes and colleagues,8 and later Morris and colleagues,9–12 described a scale for establishing cognitive and functional status of older adults termed the Clinical Dementia Rating (CDR). Based on detailed interviews with the patient and an informant, a clinician rates impairment in each of six cognitive categories (Memory, Orientation, Judgment and Problem Solving, Community Affairs, Home and Hobbies, and Personal Care). The individual category ratings are combined into an overall or global CDR. In this schema, CDR 0 = no dementia (normal range function), CDR 0.5 = questionable dementia, and CDR 1–3 = dementia. The CDR 0.5 stage includes patients with isolated, clinically important memory loss comparable to Kral’s MSF.
Age-Associated Memory Impairment (AAMI)
In 1986, an NIMH work group laid out specific research criteria to operationally define memory loss that occurs in the elderly, called age-associated memory impairment (AAMI).13 The criteria call for a subjective complaint of memory loss that is gradual and confirmed by a score on a memory test that is at least 1 SD below the mean for young adults and that occurs in the context of normal intellect and no dementia or neurologic disease. Given the well-documented age-related changes in memory and cognition, 14–20 the use of young adults as a comparison group significantly limits the clinical relevance of AAMI to abnormal cognitive aging. The distinction between normal aging and AAMI is absent or at least unclear.
Aging-Associated Cognitive Decline (AACD)
This designation was first described in 1994 by the International Psychogeriatric Association in collaboration with the WHO21 as means of identifying memory or other cognitive losses that may include the prodrome of dementia as well as other stable conditions associated with aging. A key distinguishing feature is that cognitive loss is judged relative to age- and education-matched peers – not young adults (as in AAMI). Note also that the losses in any cognitive domain (e.g., language, abstraction, visuospatial skill) are assessed. The criteria for AACD require subjective report of cognitive decline (either from the subject or an informant), of at least 6 months duration, that is confirmed by a score at least 1 SD below age- and education-matched peers and occurs in the absence of known neurologic or psychiatric disease. There is no requirement for a clinical examination. The psychometric threshold defining impairment is rather liberal. By definition, performances 1 SD below the mean will include 16% of any sample. This lack of specificity served to limit the clinical relevance of AACD.
Mild Cognitive Impairment (MCI)
In 1995, Petersen and colleagues at the Mayo Clinic used the term mild cognitive impairment (MCI) to describe older adults with relatively isolated memory loss that is normatively rare among matched peers.22–24 The criteria for MCI require subjective memory complaint (by the patient or an informant), impaired memory function for age (more than 1.5 SD below the mean), preserved general cognition (MMSE > 24/30), intact activities of daily living, and no dementia on examination. Most studies on MCI have used the criteria as part of a clinical diagnosis process, although it has been adapted to an algorithm format in some large-scale studies.25,26
The concept of MCI has been expanded recently and now allows for the classification of patients with deficits outside the memory domain and those that have multiple cognitive deficits.27,28 This phenotypic subtyping approach is based on the number and nature of the cognitive domains affected. The original designation is now called single-domain amnestic MCI to indicate the isolated and memory-dominant nature of the deficit. In addition, there are several single domain non-amnestic MCI forms where the deficit might involve linguistic, visuospatial, or executive ability. The possibility of a single patient having multiple deficits is also considered. In the case when memory is one of the two or more domains involved, it is called multi-domain amnestic MCI. When memory is not involved, it is called multi-domain non-amnestic MCI. The revised MCI approach allows for the possibility that there may be more than one cause of MCI but does not require an etiology to be identified.
Cognitive Impairment No Dementia (CIND)
In 1997, researchers in the Canadian Study of Health and Aging (CSHA) were the first to coin the term cognitive impairment, no dementia (CIND).29,30 The intent was to capture, in their large, population-based study of predominantly white older adults, persons with clinically significant impairment on cognitive tests who did not meet criteria for dementia and who were also not normal. The CSHA utilized a large battery of neuropsychological tests, age-adjusted norms for interpretation and made clinical diagnoses using a consensus conference format (as opposed to an algorithm).
Our own epidemiological work focuses on community-dwelling, elderly, African Americans living in Indianapolis, the Indianapolis Study of Health and Aging (ISHA). The ISHA is a two-stage study with over 2000 subjects and several years of longitudinal follow-up.4,5 Our methods closely parallel those of the CSHA; however, we have been explicit in presenting the diagnostic criteria for CIND in our study as follows: 1) informant-reported or clinician-detected clinically significant decline in cognition; or 2) cognitive test score(s) below approximately the 7th percentile of age- and education-adjusted norms; and 3) normal range function in daily living tasks.6,31
For both the CSHA29 and the ISHA,6 CIND subtypes are identified according to presumed etiology based on medical history and examination findings. In this approach, prodromal AD is defined by progressive, prominent, and medically unexplained memory impairment. Similarly, post-stroke, alcoholism and substance abuse, medical illnesses, depression, and other causes (e.g., neoplasm) can be distinguished.
Summary
Currently the CDR, MCI, and CIND approaches dominate the clinical and epidemiological research on mild cognitive dysfunction. Of the three, only the CDR approach does not use psychometric testing to inform the classification process and it tends to be rather closely oriented to memory loss (or at least has less explicit assessment of non-memory cognitive domains). These limitations do diminish the utility of the CDR to an extent.
On the other hand, there has been a convergence in concept and methodology between MCI and CIND over the past several years. MCI and CIND both allow fully for the possibility that non-memory cognitive dysfunction may be the sole or primary presenting feature and that memory loss is frequently associated with deficits in other cognitive domains in subjects with mild cognitive dysfunction.32. Both systems utilize formal psychometric tests of cognitive ability, have shared threshold for impairment, and incorporate informant, clinician, and psychometric data in a clinical diagnostic process as opposed to an algorithm. At this point, MCI and CIND classification schemes will identify substantially the same range of older adults. These systems do differ in the approach to subtyping (MCI - phenotypic and CIND - etiologic) and this variation may facilitate research on outcomes like time to dementia, response to treatment, and correlation with neuropathology. This type of research would help to establish the clinical general relevance of mild cognitive dysfunction as a condition and any advantage of one approach over the other.
MEASUREMENT AND DIAGNOSIS
Just as the different research contexts (clinic-based versus population-based) shape the approach to diagnostic nosology reviewed above, so to the methods related to assessment and diagnosis flow out of the different demands and practical needs of each situation creating variability in approach and outcome. Continued cross-disciplinary exchange is crucial to progress in definition and assessment in this area.
Subjective Cognitive Complaint
There is diversity of opinion on the utility of subjective complaint in the criteria for cognitive dysfunction. The criteria for AAMI require complaint from the subject while AACD is satisfied by a complaint from either the subject or an informant. Subjective sense of memory loss is not required but can satisfy the “complaint” criteria for MCI (along with informant-reported memory loss or physician-detected memory loss). CIND does not require a self-report of memory loss from the subject which is an adaptation born of the fact that knowledgeable informants are frequently unavailable in population-based studies. Some studies indicate clear limitations in the validity of self-report including the fact that it tends not to be well correlated with psychometric memory performance but is highly correlated with depression.33 On the other hand, other studies suggest that self-report of memory loss may represent the leading edge of mild cognitive impairment - even before cognitive tests capture impairment34 and that self-report may have more predictive validity among the well-educated subjects and those with incipient memory loss.35,36 Self-report of memory loss has complex determinants. Studies relying on self-report in the diagnostic criteria require careful interpretation.
Informant Interview
The informant perspective is one of the fundamental aspects of the CDR,9 MCI,22 and CIND approaches.6 Although informant-report of ability loss is not immune to bias,37 it does correspond to psychometric performance,37 is superior to subject self-report,38 and has been shown to have value in predicting incident dementia.38,39 Documentation of cognitive and functional status via a knowledgeable informant is a critical aspect of the differential diagnosis of age-related cognitive disorders.
Neuropsychological Examination
Objective, psychometric assessment of cognition is integral to most approaches to mild cognitive dysfunction. While a standard battery has not been endorsed, most studies attempt to assess major cognitive domains including: attention, memory, language, visuospatial, and executive function.6,23,29,40,41 Standardized assessments of mood are usually included as well. Where subjects are few (e.g., registries, research centers) the assessment tends to be very detailed with multiple tests of a given domain resulting in administration times of many hours. Where the number of subjects to be seen is high, as in epidemiological studies, total assessment time needs to be shorter and single tests of a domain may be utilized. There is no standard neuropsychological battery for MCI or CIND. There is also no agreement on the number of tests per domain that should be included in an assessment nor the number of tests within a domain that must be failed to be considered impaired. There is agreement that only relatively low scores define impairment with −1.5 SD and 7th percentile (each criterion refers to approximately the same raw score in a normal distribution) and that interpretation of raw scores requires use of reference samples representative of the target population in terms of age, education, and ethnicity.42,43
Functional Competence (IADL)
Self-report of functional competence is generally accurate in normal subjects but questionable in incipient dementia patients.38 Performance-based assessments have the advantage of objective measurement. However, they still suffer from limited assessment of behaviors and use of non-naturalistic props and context. Dementia research and clinical practice have historically relied on informant-based reporting or ratings in characterizing the daily functioning of patients, but this may be a weakness for mild cognitive dysfunction where the earliest changes in daily function may be represented by subjective difficulty in completing a task rather than frank inability to perform a task. In addition, there is a clear need for field-wide consensus on a specified set of tasks, response options, scoring convention, and a cut-score that constitutes impairment in daily function. There is no such standard at this time which clearly hinders further advance in the field.
Clinician Examination
A clinical examination with the history of the present illness, mental status examination, and physical and neurological exams is an integral part of the differential diagnosis and subtyping mild cognitive impairment. A comprehensive assessment is time consuming and, when done by a physician, expensive. In the context of research studies, non-physician clinical staff, after appropriate training and implementation of structured interview methods, can gather the key elements of the clinical examination reliably, validly, and cost effectively with the interpretation of the clinical data, diagnoses, and subtyping reserved for the physician and multidisciplinary care team.
Special Issues in the Assessment of African American
A critical requirement is that appropriate norms be used when interpreting test scores of any patient or subject. Inattention to this procedure can result in overestimated rates of cognitive impairment43,44 and poor diagnostic specificity.45 Norms should be derived from a pool of community-dwelling persons who function independently and who live in the same community as the target sample under study. Several normative resources for elderly African Americans exist including studies based on the global screening tests,46,47 CERAD test battery,43,48 or traditional clinical neuropsychological tests.42,44,49–55 Age and education are well known to affect cognitive test performance. Racial disparities in education are an issue particularly for older African Americans. Awareness of this has lead to innovative studies probing quality of education, reading ability, and degree of acculturation as factors that impact performance.42,56–60 The practical means of addressing these factors has not been settled on, but regression-based approaches could allow for an automated and granular accounting of the independent influences of gender, age, education, quality of education, reading ability, and acculturation on test performance.
In older subjects who have no or low literacy, changes to the form of the assessment need to be considered, particularly consideration toward replacing tests of constructional ability involving drawing geometric figures with tests of spatial processing and construction that do not rely on drawing.61 More work needs to be done to determine the magnitude of the effect of ethnicity matched and mismatched examiner-examinee dyads during test administration on performance in subjects over age 65 years. In addition, systematic studies of ethnic differences in informant report of functional status are needed.
Summary
Approaches to measurement are driven by the context (e.g., clinic-based research vs. epidemiological survey). Subjective complaint as a criterion has historical roots in clinical medicine but may have limited utility at least in regard to self-report of cognitive status. For that reason, the informant perspective and cognitive testing are mainstays for the assessment of mild cognitive. A thoughtful approach to interpretation of cognitive test scores is required as these are generated from within a cultural context - factors beyond age and years of education completed need to be carefully considered. The use of well designed local norms will generally address these concerns. The most important remaining gap in method of assessment is the lack of a gold standard measure for quantifying functional competence (IADL). In order to advance, the field needs a single metric and common cut score identifying impairment. Ideally, the measure of functional competence would be a self-administered questionnaire completed by an informant with a parallel self-report version. To be most useful, the measure would need to assess all aspects of daily function, recognize gender roles and cultural influences, and not be overly memory-centric in its thrust (recognizing that there are multiple pathways to cognitive dysfunction beyond Alzheimer disease).
EPIDEMIOLOGY OF MILD COGNITIVE DYSFUNCTION
Epidemiological studies of mild cognitive dysfunction are critical to establishing the dimensions of the condition and its natural history. As will be seen, many factors including evolving definitions, variable methodology, and diverse samples combine to produce a wide range of results.
Prevalence and Incidence
The prevalence of cognitive impairment short of dementia is a function of the criteria, assessment and diagnostic methodology, and sample. In five large-scale epidemiological studies the prevalence of CIND has ranged from 11–23%.6,29,62–64 The study with the lowest prevalence figure used a two-stage design but did not sample for false negatives which creates an underestimate of actual cases.62 The prevalence of amnestic MCI and questionable dementia range from 3–27%.6,25,29,40,41,63–70 Studies with the highest rates tended to use very old subjects,67 a broad definition where one or more of the MCI diagnostic criteria were expanded or dropped,6,40,67 or the CDR may have included a significant number of mild dementia cases.70
Using weighted logistic regression controlling for age and the probability of selection into the clinical assessment to determine overall and age-standardized CIND prevalence rates, the ISHA found approximately 23% of elderly, community-dwelling African Americans to meet criteria for CIND. The most common subtype was prodromal AD which had a community prevalence of 12%.6 The ISHA prodromal AD subtype corresponds roughly to a combination of single domain amnestic and multi-domain amnestic MCI. The community prevalence of the other CIND subtypes in the ISHA was: medical illness 4%, stroke 3%, alcohol abuse 1%, and other/indeterminate 2%. Increasing age was associated with higher prevalence of CIND (as is also the case with dementia). Importantly however, CIND is much more common than dementia especially in the younger age groups (up to 7 times more common among those aged 65 to 74 years, see Table 2).
Table 2.
Overall and age-specific prevalence rates for CIND, Dementia, and Alzheimer disease in Indianapolis Study of Health and Aging.4,6
| Age Group | CIND | Dementia | AD |
|---|---|---|---|
| 65 – 74 years | 19.4 | 2.6 | 1.6 |
| 75 – 84 years | 27.2 | 11.4 | 8.0 |
| 85 + years | 30.2 | 32.4 | 28.9 |
| Overall Rate | 22.9 | 8.2 | 6.2 |
Notes: CIND = cognitive impairment no dementia, D = dementia, AD = Alzheimer disease.
Our estimate of the prevalence of CIND (23%) is greater than the 17% rate reported in the CSHA.29 A general diagnostic bias seems an unlikely explanation for the difference because the rates for stroke- and alcohol-related CIND are quite comparable between the studies and the prevalence rates for dementia and Alzheimer disease are almost identical.4,71 Most of the difference in overall rates probably relates the CIND subtype of prodromal AD (12% in ISHA vs. 5% in CSHA). The Canadian group did not describe the cutoff point that they used to interpret psychometric test scores. If they used a more conservative cutoff point, it would produce a lower prevalence rate for circumscribed memory impairment. Alternatively, the higher rates of medical comorbidity and poor cardiovascular health among the African American subjects in the ISHA could have contributed to the excess of CIND cases seen there.
Our prevalence rates are actually quite comparable to those reported in a retrospective study of MCI in a mixed, racial-ethnic group consisting of non-Hispanic whites, non-Hispanic African Americans, and Hispanics in Northern Manhattan, New York. Among all subjects, MCI had a prevalence of 28% and memory-related MCI had a prevalence of 11% (amnestic MCI 5% and multi-domain amnestic MCI 6%).72 Race and ethnicity did not affect rates in that study.
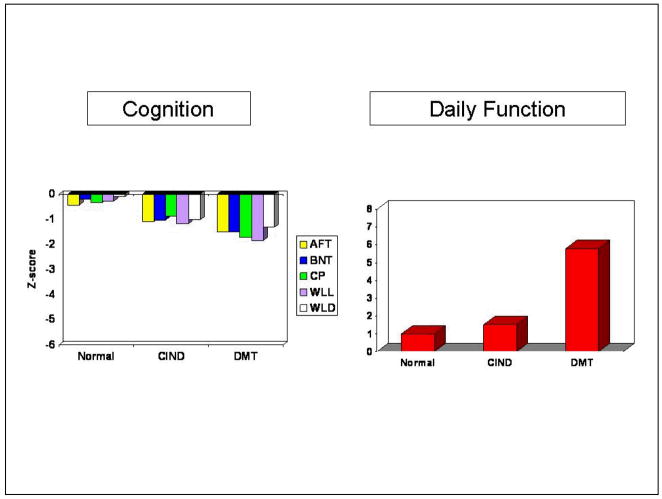
The cognitive and functional characteristics of community-dwelling older African Americans with diagnosed Normal cognition, CIND, and Dementia in the ISHA are presented in Figure 2. The cognitive tests have been standardized to z-scores by indexing individual scores to the mean and standard deviation of a normative reference sample.43 As can be seen, the CIND group mean cognitive performances are intermediate between Normal and Demented groups on each test. In contrast, the right panel of Figure 2 shows the CIND group to be well functioning on instrumental and basic activities of daily living (higher scores on the Blessed scale indicate more dependence in daily function).6
Figure 2.
Cognitive test z-score and daily function characteristics of community-dwelling, clinically assessed subjects in the Indianapolis Study of Health and Aging (AFT = Animal Fluency Test, BNT = Boston Naming Test, CP = Constructional Praxis, WLL = Word List Learning, WLD = Word List Delay, DMT = dementia, CIND = Cognitive impairment no dementia).
Longitudinal Stability
Community- and population-based studies indicate that CIND patients develop dementia at a very high rates, from 13–48% over 12–60 months follow-up intervals;6,39,40,73–75 however, one study with a short interval of follow-up and an algorithm-based approach to diagnosis reported no conversion to dementia after 12 months.25 Interestingly, many of these studies have found some degree of “revert” to normal in patients initially classified as CIND. Studies with consensus-based, clinical diagnosis report reversion rates in the range of 13–25% 6,39 while studies with algorithm-based diagnosis and shorter follow-up intervals had higher rates of revert to normal, up to 93% for MCI25 and 47–52% for CIND/AACD.74,75 In the ISHA, the rate of conversion to dementia and reversion to normal were fairly steady regardless of whether the subject was identified at the prevalence wave or one of two subsequent incidence waves. After about two and half years of follow-up, just under 1/3 convert to dementia; just over 1/4 revert to normal in the same interval (see Table 3).
Table 3.
Longitudinal outcome of CIND cases at follow-up in Indianapolis Study of Health and Aging.6
| Follow-up Diagnosis | ||||
|---|---|---|---|---|
| Initial CIND Diagnosis | No. seen at F/U | Normal | CIND | Dementia |
| Prevalence (n=106) | 67 | 25% | 49% | 25% |
| 2-year Incidence (n=26) | 13 | 46% | 23% | 31% |
| 5-year Incidence2 (n=61) | 21 | 24% | 29% | 48% |
| Total | 101 | 28% | 42% | 31% |
Notes: CIND = cognitive impairment no dementia.
In the ISHA study, higher rates of conversion to dementia were seen in CIND subtypes of stroke (43%) and prodromal AD (34%, see Table 4). Rates of reversion to normal were higher in the other/indeterminate (40%) and alcohol abuse (33%) subtypes.
Table 4.
Longitudinal outcome of CIND in Indianapolis Study of Health and Aging as a function of etiologic subtype at baseline.
| Diagnosis at 2 yr Follow-up | ||
|---|---|---|
| Baseline CIND Subtype | Revert to Normal | Convert to Dementia |
| Prodromal AD | 25% | 34% |
| Post stroke | 14% | 43% |
| Medical or Neurologic illness | 14% | 29% |
| Alcohol abuse | 33% | - |
| Other | 40% | 10% |
Notes: AD = Alzheimer disease.
We examined the effect of CIND criterion on rates of reversion and conversion in the ISHA. Among CIND subjects at baseline, those who met the informant report of decline criterion (a ‘yes’ response to queries about any evidence of mental decline, memory decline, or language decline) showed a slightly lower rate of reversion to normal (see Table 5). Subjects that met the CIND criterion of cognitive test score below the 7th percentile reverted to normal at a rate of 24%, while subjects that met adapted Petersen criteria for MCI reverted to normal at a rate of 35%.
Table 5.
Effect of CIND criterion on longitudinal outcome in Indianapolis Study of Health and Aging.
| Follow-up Diagnosis (n = 92) | |||
|---|---|---|---|
| Baseline | Normal | CIND | Dementia |
| Informant report | 19% | 51% | 30% |
| Cognitive test | 24% | 44% | 33% |
| Petersen amnestic MCI | 35% | 29% | 35% |
Note: Cases are collapsed across three waves (Prevalence, 2-year incidence, and 5-year incidence). CIND = cognitive impairment no dementia, MCI = mild cognitive impairment.
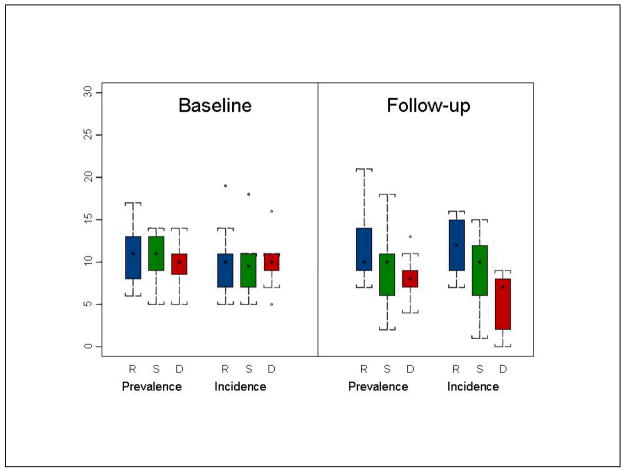
Since psychometric test performance loads into the diagnostic criteria of CIND and MCI, a factor to consider in the revert-to-normal phenomena is statistical regression to the mean. We examined this possibility by plotting scores from the Word List Learning test (sum of the three learning trials) in the CIND subjects at baseline and follow-up as a function of outcome status at baseline: revert-to-normal, stable CIND, or progress-to-dementia; prevalent and incident cases were plotted separately to see if this factor had any independent effect. As can be seen in Figure 3, the revert group’s WLL scores were relatively stable to slightly improved at follow-up. This group may be a reservoir for some low functioning normals and potentially temporarily medically compromised persons. On the other hand, the group that ultimately went on to develop dementia clearly declined. While this does not rule out statistical regression to the mean as a factor in revert-to-normal, it does suggest that there are dynamic changes in cognitive test performance in both directions over time and that there are challenges in interpreting WLL performance at the lower end of the distribution of scores.
Figure 3.
Mean Word List Learning score in CIND subjects by occasion by final diagnosis in Indianapolis Study of Health and Aging (R = revert-to-normal, S = stable CIND, D = convert-to-dementia).
Summary
The epidemiological studies suggest that mild cognitive dysfunction is a very common condition with multiple causes and presentations and variable outcome. The longitudinal data suggest that about a one third of these subjects will go on to become demented within two and half years indicating that mild cognitive dysfunction is definitely associated with clinical morbidity. It also appears that some subjects with MCI and CIND may be in a dynamic state in the sense that they appear to be improved at a later time point. The basis for this is unclear but longitudinal studies tracking acute and chronic medical conditions in this group may help to untangle the cause. The low retention, lack of detailed medical health documentation, and limited assessment of psychiatric status are weaknesses in current studies.
FACTORS INFLUENCING MILD COGNITIVE DYSFUNCTION
Risk Factors for Cognitive Dysfunction
The role of cardiovascular risk factors in causing or contributing to cognitive decline is an area of intense research interest. Increased blood pressure has been associated with cognitive impairment and decline in some76–78 but not all studies79,80 of older adults. Recently, obesity and hypertension were found to be independently related to cognitive decline.81 Long-term use of antihypertensives reduced the risk of cognitive impairment in African Americans.82 Diabetes is associated with amnestic MCI83 and cognitive decline.84–86 High LDL has been associated with cognitive impairment and reductions in LDL with better cognitive functioning87 while diets high in saturated or trans-unsaturated fat have been linked to cognitive decline.88 However, negative studies on the role of cholesterol have also been reported89 These data suggest that cardiovascular health factors may play a role in the development and progression of cognitive dysfunction and that longitudinal investigation of the effects of these comorbidities on MCI and CIND outcomes would be fruitful.
In addition to cardiovascular disease, other conditions may decrease oxygen delivery to the brain (e.g., COPD) or result in deleterious effects on the central nervous system (e.g., medication toxicities). Older adults are frequently prescribed medications with anticholinergic side effects.90,91 These factors may contribute to cognitive dysfunction via mechanisms not directly analogous to cardiovascular disease and may contribute to variability in cognitive function even if they do not produce progressive cognitive decline. Part of this may be due to exacerbation of chronic conditions or transient effects of medications with anticholinergic side effects.
Risk Factors for Progression from Mild Cognitive Dysfunction to Dementia
Understanding the factors that affect longitudinal stability is important. As disease-modifying, possibly risky or high cost treatments, become available, it will be critical to be able to distinguish among MCI and CIND patients who will decline from those that will not and to focus treatment efforts on the former. Among MCI patients, those with multi-domain amnestic subtype are at highest risk to convert to dementia than those with the single-domain amenstic form.92,93 The conversion and reversion rates as a function of CIND etiologic subtyping in the ISHA (Table 4 above) suggest that prodromal AD and stroke subtypes of CIND may be associated with greater likelihood of conversion to dementia, while alcohol-based impairment and other-cause subtypes may be more likely to revert to normal.6 The CSHA study found that informant-reported memory loss and informant-reported incipient IADL decline at baseline were associated with conversion to dementia, highlighting the need for careful structured assessment of informants as to function and symptoms.39
Summary
While limited by small sample sizes and some inconsistent results, it appears that further study of cardiovascular health and medical status more generally may be fruitful in determining the range of modifiable risk factors in the development of MCI and CIND and later progression to dementia.
CONCLUSIONS AND NEXT STEPS
Mild cognitive dysfunction is common among community-dwelling elders, heterogeneous in cause, and variable in outcome. Broadly defined, mild cognitive impairment short of dementia may affect up to a quarter or more of persons over age 65 years, making it up to 3 times more frequent than dementia. The public health implications of this ubiquitous condition have yet to be fully explored in terms of care burden and economic impact.
Reported prevalence rates are quite variable due to a host of methodological factors including variability in: sampling frame, single- vs. two-stage design (with or without correction for false negatives), number and type of cognitive tests used, the threshold for defining psychometric impairment, the use of age- and education-adjusted “local” norms, the time frame for follow-up, the stringency of the diagnostic criteria, and the method for making diagnoses (algorithm versus clinical diagnosis).
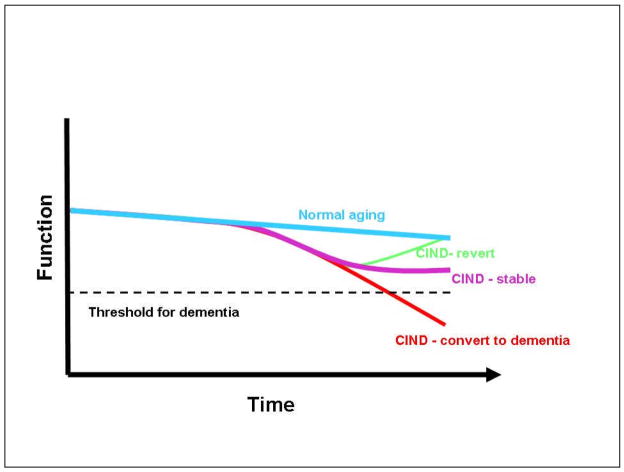
The variability in longitudinal outcomes in mild cognitive dysfunction suggests a complex picture of cognitive aging (Figure 4), one that includes multiple trajectories with some persons maintaining good function over the long term (normal aging), others declining to dementia fairly directly, and others with changeable status at the borders between normalcy and dementia. The heterogeneity in outcome suggests that a fair bit of caution must be exercised when communicating the implications of this diagnosis to patients and other health care providers. As markers are identified that are associated with outcomes (reversion or conversion), it will be possible to offer more specific prognoses. Variability in outcome may be lower in certain etiologic subtypes of CIND6 and among MCI subjects with multiple impairments.92,93
Figure 4.
Trajectories of abnormal cognitive aging in Cognitive Impairment No dementia (CIND).
Relatively low rates of retention over follow-up intervals plague most of the epidemiological studies in this area and may contribute to apparent variability. The subjects most likely to be lost to follow-up are those who experience decline and for that reason move either to be closer to relatives or into nursing homes. Rates of conversion to dementia may be underestimated as a result. Low retention will also introduce bias into risk factor analyses. Retention can be improved by increasing the frequency of contact from 12 to 24 months, the typical interval, to 6 months.
There is near unanimity of opinion and practice that results from neuropsychological tests should be interpreted using local norms and that this information needs be used in the diagnostic process of mild cognitive dysfunction. There is also good agreement that the main domains of cognition that need to be assessed relate to memory, language, visuospatial skill, and executive function. What has yet to be settled is the set of tests to be used and the threshold of performance defining impairment. For most research programs, it is likely that two tests per domain will be sufficient to provide reliability and consistency. These can then be combined to form a composite and a single cut threshold applied to each domain. The 7th percentile of local norms and 1.5 SD below the mean are practically equivalent in the raw score identified in normal distributions. Requiring a performance with this level of rarity provides a reasonable balance between sensitivity and specificity.
A major area of the assessment that needs standardization is the approach to determining functional competence. A welter of rating scales and approaches are used with little operational agreement on the daily tasks to be measured, the response options available, the scoring system to be used, the source of information (informant vs. directly from the subject), or the threshold that defines impairment. The field needs a standard assessment tool for of functional competence (with both informant and patient forms) and a commonly agreed upon cut score denoting impairment.
The diagnostic process itself would benefit from explicit guidance on how discrepancies in information are handled, for instance when an informant reports a cognitive deficit but the testing does not (or vice versa). Beyond that, some studies use an algorithm-based diagnosis which appears to be associated with higher rates of diagnostic instability (particularly in the direction of revert to normal). There is also concern that rigid application of sometimes arbitrary cut-off scores may produce spurious findings. A consensus conference approach grounded in criteria but also allowing for the exercise of clinical judgment seems to produce more solid and reproducible findings.
Finally, there is need for prospective assessment of cardiovascular health factors in progression from mild cognitive dysfunction to dementia and better understanding of factors associated with reversion to normal. There is also a critical need to integrate reliable, detailed medical information into risk factor analyses. Many older adults suffer from acute and chronic conditions whose clinical manifestations, exacerbations, and treatments may affect performance on cognitive testing. The ability to map years of premorbid and current medical conditions and treatments onto trajectories of cognitive impairment would provide valuable insights into the factors affecting conversion to dementia and reversion to normal.
We appear to be at a critical juncture where disease modifying treatments for dementia may be at hand. Interventions that could achieve even modest reductions in the rate at which mild cognitive dysfunction converts to dementia would have major public health benefits by avoiding use of unnecessary and expensive drugs in people without underlying AD. Accurate information on the risk of conversion-to-dementia will improve management of the underlying illness, management of comorbid conditions, and planning for long-term concerns. Heterogeneity in presentation and outcomes of mild cognitive dysfunction reflect, to some degree, variability in the condition itself and the forces that trigger and maintain it. Recognition of the possibility of non-AD contributions to cognitive impairment and dementia increase the range of factors that present themselves as targets for early intervention.
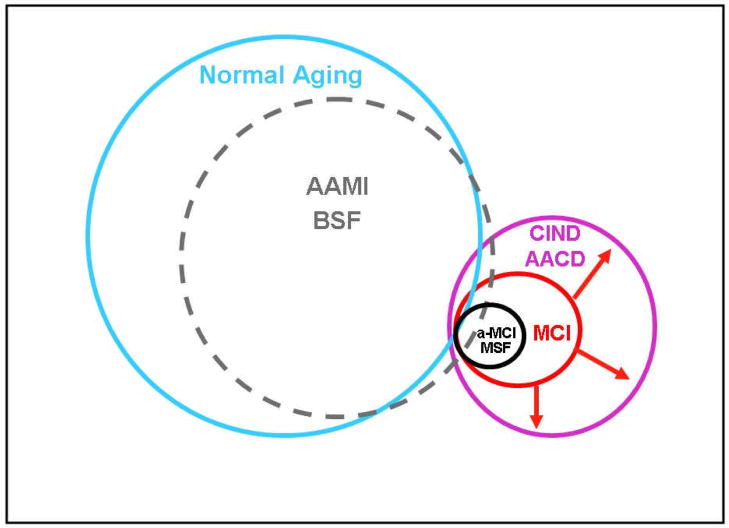
Figure 1.
Relationship Among Cognitive Impairment Entities (AAMI = Age-Associated Memory Impairment, BSF = Benign Senescent Forgetfulness, CIND = Cognitive Impairment No Dementia, AACD = Aging-Associated Cognitive Decline, MCI = Mild Cognitive Impairment, a-MCI = amnestic MCI, MSF = Malignant Senescent Forgetfulness). AAMI/BSF have indistinct borders with Normal Aging and this is reflected in the porous outline of the circle separating AAMI/BSF from Normal Aging. The large size of the circles containing these non-clinical entities reflects the large proportion of the general population contained within. The circles representing CIND/AACD and MCI/MSF are smaller reflecting the relative rarity of these clinical disorders compared to the general population of basically cognitively healthy older adults. The enclosure of MCI and a-MCI/MSF within CIND/AACD indicates that these are subsets within CIND/AACD. The expansion of the MCI concept 27 to include non-amnestic and multi-domain forms is represented by the outward pointing arrows extending the disorder to be equivalent in scope to CIND.
Acknowledgments
Supported by grants from the National Institutes of Health to Indiana University School of Medicine, R01 AG026096, R01 AG09956 and P30 AG10133. We gratefully acknowledge the assistance of Ms. Lyndsi Moser, Ms. Millicent Pettaway, and Mr. William Malone.
Reference List
- 1.DeKosky ST, Marek K. Looking backward to move forward: early detection of neurodegenerative disorders. Science. 2003;302:830–834. doi: 10.1126/science.1090349. [DOI] [PubMed] [Google Scholar]
- 2.Riley KP, Snowdon DA, Markesbery WR. Alzheimer’s neurofibrillary pathology and the spectrum of cognitive function: Findings from the Nun Study. Ann Neurol. 2002;51:567–577. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- 3.Callahan CM, Hendrie HC, Tierney WM. Documentation and evaluation of cognitive impairment in elderly primary care patients. Annals of Internal Medicine. 1995;122:422–429. doi: 10.7326/0003-4819-122-6-199503150-00004. [DOI] [PubMed] [Google Scholar]
- 4.Hendrie HC, Osuntokun BO, Hall KS, et al. Prevalence of Alzheimer’s disease and dementia in two communities: Nigerian Africans and African Americans. Am J Psychiatry. 1995;152:1485–1492. doi: 10.1176/ajp.152.10.1485. [DOI] [PubMed] [Google Scholar]
- 5.Hendrie HC, Ogunniyi A, Hall KS, et al. Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. JAMA. 2001;285:739–747. doi: 10.1001/jama.285.6.739. [DOI] [PubMed] [Google Scholar]
- 6.Unverzagt FW, Gao S, Baiyewu O, et al. Prevalence of cognitive impairment: Data from the Indianapolis Study of Health and Aging. Neurology. 2001;57:1655–1662. doi: 10.1212/wnl.57.9.1655. [DOI] [PubMed] [Google Scholar]
- 7.Kral VA. Senescent forgetfulness: Benign and malignant. Canadian Medical Association Journal. 1962;86:257–260. [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 9.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 10.Morris JC, McKeel DW, Storandt M, et al. Very mild Alzheimer’s disease: Informant-based clinical, psychometric, and pathologic distinction from normal aging. Neurology. 1991;41:469–478. doi: 10.1212/wnl.41.4.469. [DOI] [PubMed] [Google Scholar]
- 11.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 12.Rubin EH, Morris JC, Grant EA, Vendegna T. Very mild senile dementia of the Alzheimer’s type. I. Clinical assessment. Arch Neurol. 1989;46:379–382. doi: 10.1001/archneur.1989.00520400033016. [DOI] [PubMed] [Google Scholar]
- 13.Crook T, Bartus RT, Ferris SH, Whitehouse P, Cohen GD, Gershon S. Age-associated memory impairment: Proposed diagnostic criteria and measures of clinical change-Report of a National Institute of Mental Health Work Group. Developmental Neuropsychology. 1986;2:261–276. [Google Scholar]
- 14.Ivnik RJ, Malec JF, Tangalos EG, Petersen RC, Kokmen E, Kurland LT. Mayo’s Older Americans Normative Studies: WMS-R norms for ages 56 to 94. The Clinical Neuropsychologist. 1992;6:49–82. [Google Scholar]
- 15.Ivnik RJ, Malec JF, Tangalos EG, Petersen RC, Kokmen E, Kurland LT. Mayo’s Older Americans Normative Studies: Updated AVLT norms for ages 56 to 97. The Clinical Neuropsychologist. 1992;6:83–104. [Google Scholar]
- 16.Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC. Neuropsychological tests’ norms above age 55: COWAT, BNT, MAE Token, WRAT-R Reading, AMNART, STROOP, TMT, and JLO. The Clinical Neuropsychologist. 1996;10:262–278. [Google Scholar]
- 17.Ivnik RJ, Malec JF, Tangalos EG, Petersen RC, Kokmen E, Kurland LT. The Auditory-Verbal Learning Test (AVLT): Norms for ages 55 years and older. Psychological Assessment. 1990;2:304–312. [Google Scholar]
- 18.Ivnik RJ, Malec JF, Smith GE, et al. Mayo’s Older Americans Normative Studies: WAIS-R norms for ages 56 to 97. The Clinical Neuropsychologist. 1992;6(Supplement):1–30. [Google Scholar]
- 19.Wechsler D. Wechsler Memory Scale-Revised manual. San Antonio: The Psychological Corporation; 1987. [Google Scholar]
- 20.Wechsler D. WAIS-III Administration and Scoring Manual. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 21.Levy R. Report: Aging-Associated Cognitive Decline. International Psychogeriatrics. 1994;6:63–68. [PubMed] [Google Scholar]
- 22.Petersen RC, Smith GE, Ivnik RJ, et al. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. JAMA. 1995;273:1274–1278. [PubMed] [Google Scholar]
- 23.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 24.Smith GE, Petersen RC, Parisi JE, et al. Definition, course, and outcome of Mild Cognitive Impairment. Aging, Neuropsychology, and Cognition. 1996;3:131–147. [Google Scholar]
- 25.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- 26.Fisk JD, Merry HR, Rockwood K. Variations in case definition affect prevalence but not outcomes of mild cognitive impairment. Neurology. 2003;61:1179–1184. doi: 10.1212/01.wnl.0000089238.07771.c7. [DOI] [PubMed] [Google Scholar]
- 27.Petersen R. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 28.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 29.Graham JE, Rockwood K, Beattie LB, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349:1793–1796. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- 30.Ebly EM, Hogan DB, Parhad IM. Cognitive impairment in the nondemented elderly: Results from the Canadian Study of Health and Aging. Arch Neurol. 1995;52:612–619. doi: 10.1001/archneur.1995.00540300086018. [DOI] [PubMed] [Google Scholar]
- 31.Baiyewu O, Unverzagt FW, Ogunniyi A, et al. Cognitive impairment in community-dwelling older Nigerians: clinical correlates and stability of diagnosis. European Journal of Neurology. 2002;9:573–580. doi: 10.1046/j.1468-1331.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 32.Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer’s disease: A meta-analysis. Neuropsychology. 2005;19:520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- 33.O’Connor DW, Pollitt PA, Roth M, Brook PB, Reiss BB. Memory complaints and impairment in normal, depressed, and demented elderly persons identified in a community survey. Arch Gen Psychiatry. 1990;47:224–227. doi: 10.1001/archpsyc.1990.01810150024005. [DOI] [PubMed] [Google Scholar]
- 34.Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmand B, Jonker C, Geerlings MI, Lindeboom J. Subjective memory complaints in the elderly: depressive symptoms and future dementia. Br J Psychiatry. 1997;171:373–376. doi: 10.1192/bjp.171.4.373. [DOI] [PubMed] [Google Scholar]
- 36.Schofield PW, Jacobs D, Marder K, Sano M, Stern Y. The validity of new memory complaints in the elderly. Arch Neurol. 1997;54:756–759. doi: 10.1001/archneur.1997.00550180064014. [DOI] [PubMed] [Google Scholar]
- 37.Kemp NM, Brodaty H, Pond D, Luscombe G. Diagnosing dementia in primary care: the accuracy of informant reports. Alzheimer Disease & Associated Disorders. 2002;16:171–176. doi: 10.1097/00002093-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Tabert MH, Albert SM, Borukhova-Milov L, et al. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. 2002;58:758–764. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- 39.Tuokko H, Frerichs R, Graham J, et al. Five-year follow-up of cognitive impairment with no dementia. Archives of Neurology. 2003;60:577–582. doi: 10.1001/archneur.60.4.577. [DOI] [PubMed] [Google Scholar]
- 40.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 41.Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 42.Manly JJ, Jacobs DM, Sano M, et al. Cognitive test performance among nondemented elderly African Americans and whites. Neurology. 1998;50:1238–1245. doi: 10.1212/wnl.50.5.1238. [DOI] [PubMed] [Google Scholar]
- 43.Unverzagt FW, Hall KS, Torke AM, et al. Effects of age, education, and gender on CERAD neuropsychological test performance in an African American sample. The Clinical Neuropsychologist. 1996;10:180–190. [Google Scholar]
- 44.Marcopulos BA, Mclain CA, Giuliano AJ. Cognitive impairment or inadequate norms? A study of healthy, rural, older adults with limited education. Clinical Neuropsychologist. 1997;11:111–131. [Google Scholar]
- 45.Fillenbaum G, Heyman A, Williams K, Prosnitz B, Burchett B. Sensitivity and Specificity of Standardized Screens of Cognitive Impairment and Dementia Among Elderly Black-And-White Community Residents. Journal of Clinical Epidemiology. 1990;43:651–660. doi: 10.1016/0895-4356(90)90035-n. [DOI] [PubMed] [Google Scholar]
- 46.Murden RA, McRae TD, Kaner S, Bucknam ME. Mini-Mental State Exam scores vary with education in Blacks and Whites. J Am Geriatr Soc. 1991;39:149–155. doi: 10.1111/j.1532-5415.1991.tb01617.x. [DOI] [PubMed] [Google Scholar]
- 47.Brown LM, Schinka JA, Mortimer JA, Graves AB. 3MS normative data for elderly African Americans. J Clin Exp Neuropsychol. 2003;25:234–241. doi: 10.1076/jcen.25.2.234.13643. [DOI] [PubMed] [Google Scholar]
- 48.Fillenbaum GG, Heyman A, Huber MS, Ganguli M, Unverzagt FW. Performance of elderly African American and White community residents on the CERAD Neuropsychological Battery. Journal of the International Neuropsychological Society. 2001;7:502–509. doi: 10.1017/s1355617701744062. [DOI] [PubMed] [Google Scholar]
- 49.Lucas JA, Ivnik RJ, Smith GE, et al. A brief report on WAIS-R normative data collection in Mayo’s Older African Americans Normative Studies. Clinical Neuropsychologist. 2005;19:184–188. doi: 10.1080/13854040590945283. [DOI] [PubMed] [Google Scholar]
- 50.Lucas JA, Ivnik RJ, Smith GE, et al. Mayo’s Older African Americans Normative Studies: WMS-R norms for African American elders. Clinical Neuropsychologist. 2005;19:189–213. doi: 10.1080/13854040590945292. [DOI] [PubMed] [Google Scholar]
- 51.Lucas JA, Ivnik RJ, Smith GE, et al. Mayo’s Older African Americans Normative Studies: Norms for Boston Naming Test, Controlled Oral Word Association, Category Fluency, Animal Naming, Token Test, WRAT-3 Reading, Trail Making Test, Stroop Test, and Judgment of Line Orientation. Clinical Neuropsychologist. 2005;19:243–269. doi: 10.1080/13854040590945337. [DOI] [PubMed] [Google Scholar]
- 52.Ferman TJ, Lucas JA, Ivnik RJ, et al. Mayo’s Older African American Normative Studies: Auditory Verbal Learning Test norms for African American elders. Clinical Neuropsychologist. 2005;19:214–228. doi: 10.1080/13854040590945300. [DOI] [PubMed] [Google Scholar]
- 53.Ross TP, Lichtenberg PA. Expanded normative data for the Boston Naming Test for use with urban, elderly medical patients. Clinical Neuropsychologist. 1998;12:475–481. doi: 10.1076/1385-4046(200005)14:2;1-Z;FT149. [DOI] [PubMed] [Google Scholar]
- 54.Marcopulos BA, Mclain CA. Are our norms “normal”? A 4-year follow-up study of a biracial sample of rural elders with low education. Clinical Neuropsychologist. 2003;17:19–33. doi: 10.1076/clin.17.1.19.15630. [DOI] [PubMed] [Google Scholar]
- 55.Friedman MA, Schinka JA, Mortimer JA, Graves AB. Hopkins Verbal Learning Test -Revised: Norms for elderly African Americans. Clinical Neuropsychologist. 2002;16:356–372. doi: 10.1076/clin.16.3.356.13857. [DOI] [PubMed] [Google Scholar]
- 56.Johnson AS, Flicker LJ, Lichtenberg PA. Reading ability mediates the relationship between education and executive function tasks. Journal of the International Neuropsychological Society. 2006;12:64–71. doi: 10.1017/S1355617706060073. [DOI] [PubMed] [Google Scholar]
- 57.Manly JJ, Byrd DA, Touradji P, Stern Y. Acculturation, reading level, and neuropsychological test performance among African American elders. Applied Neuropsychology. 2004;11:37–46. doi: 10.1207/s15324826an1101_5. [DOI] [PubMed] [Google Scholar]
- 58.Manly JJ, Jacobs DM, Touradji P, Small SA, Stern Y. Reading level attenuates differences in neuropsychological test performance between African American and White elders. Journal of the International Neuropsychological Society. 2002;8:341–348. doi: 10.1017/s1355617702813157. [DOI] [PubMed] [Google Scholar]
- 59.Byrd DA, Sanchez D, Manly JJ. Neuropsychological test performance among Caribbean-born and US-born African American elderly: The role of age, education and reading level. J Clin Exp Neuropsychol. 2005;27:1056–1069. doi: 10.1080/13803390490919353. [DOI] [PubMed] [Google Scholar]
- 60.Manly JJ, Jacobs DM, Sano M, et al. Effect of literacy on neuropsychological test performance in nondemented, education-matched elders. Journal of the International Neuropsychological Society. 1999;5:191–202. doi: 10.1017/s135561779953302x. [DOI] [PubMed] [Google Scholar]
- 61.Baiyewu O, Unverzagt FW, Lane KA, et al. The Stick Design test: A new measure of visuoconstructional ability. Journal of the International Neuropsychological Society. 2005;11:598–605. doi: 10.1017/S135561770505071X. [DOI] [PubMed] [Google Scholar]
- 62.DiCarlo A, Baldereschi M, Amaducci L, et al. Cognitive impairment without dementia in older people: Prevalence, vascular risk factors, impact on disability. The Italian Longitudinal Study on Aging. J Am Geriatr Soc. 2000;48:775–782. doi: 10.1111/j.1532-5415.2000.tb04752.x. [DOI] [PubMed] [Google Scholar]
- 63.Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 64.Prencipe M, Santini M, Casini AR, Pezzella FR, Scaldaferri N, Culasso F. Prevalence of non-dementing cognitive disturbances and their association with vascular risk factors in an elderly population. Journal of Neurology. 2003;250:907–912. doi: 10.1007/s00415-003-1094-0. [DOI] [PubMed] [Google Scholar]
- 65.Busse A, Bischkopf J, Riedel-Heller SG, Angermeyer MC. Mild cognitive impairment: prevalence and incidence according to different diagnostic criteria. Results of the Leipzig Longitudinal Study of the Aged (LEILA75+) Br J Psychiatry. 2003;182:449–454. [PubMed] [Google Scholar]
- 66.Hanninen T, Hallikainen M, Tuomainen S, Vanhanen M, Soininen H. Prevalence of mild cognitive impairment: a population-based study in elderly subjects. Acta Neurologica Scandinavica. 2002;106:148–154. doi: 10.1034/j.1600-0404.2002.01225.x. [DOI] [PubMed] [Google Scholar]
- 67.Boeve B, McCormick J, Smith G, et al. Mild cognitive impairment in the oldest old. Neurology. 2003;60:477–480. doi: 10.1212/wnl.60.3.477. [DOI] [PubMed] [Google Scholar]
- 68.Ikeda M, Shigenobu K. The prevalence of mild cognitive impairment (MCI) among the community-dwelling elderly: findings from the 2nd Nakayama study. Seishin Shinkeigaku Zasshi - Psychiatria et Neurologia Japonica. 2003;105:381–386. [PubMed] [Google Scholar]
- 69.Pfeffer RI, Afifi AA, Chance JM. Prevalence of Alzheimer’s disease in a retirement community. American Journal of Epidemiology. 1987;125:420–436. doi: 10.1093/oxfordjournals.aje.a114548. [DOI] [PubMed] [Google Scholar]
- 70.Prencipe M, Casini AR, Ferretti C, Lattanzio MT, Fiorelli M, Culasso F. Prevalence of dementia in an elderly rural population: effects of age, sex, and education. Journal of Neurology, Neurosurgery & Psychiatry. 1996;60:628–633. doi: 10.1136/jnnp.60.6.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Canadian Study of Health and Aging Working Group. Canadian Study of Health and Aging: Study methods and prevalence of dementia. Canadian Medical Association Journal. 1994;150:899–913. [PMC free article] [PubMed] [Google Scholar]
- 72.Manly JJ, Bell-McGinty S, Tang MX, Schupf N, Stern Y, Mayeux R. Implementing Diagnostic Criteria and Estimating Frequency of Mild Cognitive Impairment in an Urban Community. Arch Neurol. 2005;62:1739–1746. doi: 10.1001/archneur.62.11.1739. [DOI] [PubMed] [Google Scholar]
- 73.O’Connor DW, Pollitt PA, Hyde JB, Fellowes JL, Miller ND, Roth M. A follow-up study of dementia diagnosed in the community using the Cambridge Mental Disorders of the Elderly Examination. Acta Psychiatrica Scandanavia. 1990;81:78–82. doi: 10.1111/j.1600-0447.1990.tb06453.x. [DOI] [PubMed] [Google Scholar]
- 74.Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59:1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- 75.Busse A, Bischkopf J, Riedel-Heller SG, Angermeyer MC Leipzig Longitudinal Study of the Aged LEILA. Mild cognitive impairment: prevalence and predictive validity according to current approaches. Acta Neurologica Scandinavica. 2003;108:71–81. doi: 10.1034/j.1600-0404.2003.00118.x. [DOI] [PubMed] [Google Scholar]
- 76.Elias MF, Wolf PA, D’Agostino RB, Cobb J, White LR. Untreated blood pressure level is inversely related to cognitive functioning: the Framingham Study. American Journal of Epidemiology. 1993;138:353–364. doi: 10.1093/oxfordjournals.aje.a116868. [DOI] [PubMed] [Google Scholar]
- 77.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA. 1995;274:1846–1851. [PubMed] [Google Scholar]
- 78.Guo Z, Fratiglioni L, Winblad B, Viitanen M. Blood pressure and performance on the Mini-Mental State Examination in the very old. Cross-sectional and longitudinal data from the Kungsholmen Project. American Journal of Epidemiology. 1997;145:1106–1113. doi: 10.1093/oxfordjournals.aje.a009073. [DOI] [PubMed] [Google Scholar]
- 79.Pandav R, Dodge HH, DeKosky ST, Ganguli M. Blood pressure and cognitive impairment in India and the United States: a cross-national epidemiological study. Arch Neurol. 2003;60:1123–1128. doi: 10.1001/archneur.60.8.1123. [DOI] [PubMed] [Google Scholar]
- 80.Hebert LE, Scherr PA, Bennett DA, et al. Blood pressure and late-life cognitive function change: a biracial longitudinal population study. Neurology. 2004;62:2021–2024. doi: 10.1212/01.wnl.0000129258.93137.4b. [DOI] [PubMed] [Google Scholar]
- 81.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. International Journal of Obesity & Related Metabolic Disorders. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 82.Murray M, Lane K, Gao S, et al. Preservation of Cognitive Function With Antihypertensive Medications: A Longitudinal Analysis of a Community-Based Sample of African Americans. Arch Intern Med. 2002;162:2090–2096. doi: 10.1001/archinte.162.18.2090. [DOI] [PubMed] [Google Scholar]
- 83.Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol. 2007;64:570–575. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 84.Gregg EW, Yaffe K, Cauley JA, et al. Is diabetes associated with cognitive impairment and cognitive decline among older women? Study of Osteoporotic Fractures Research Group. Arch Intern Med. 2000;160:174–180. doi: 10.1001/archinte.160.2.174. [DOI] [PubMed] [Google Scholar]
- 85.Grodstein F, Chen J, Wilson R, Manson J. Type 2 Diabetes and Cognitive Function in Community-Dwelling Elderly Women. Diabetes Care. 2001;24:1060–1065. doi: 10.2337/diacare.24.6.1060. [DOI] [PubMed] [Google Scholar]
- 86.Logroscino G, Kang J, Grodstein F. Prospective study of type 2 diabetes and cognitive decline in women aged 70–81 years. BMJ. 2004;328:548–553. doi: 10.1136/bmj.37977.495729.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yaffe K, Barrett-Connor E, Lin F, Grady D. Serum Lipoprotein Levels, Statin Use, and Cognitive Function in Older Women. Arch Neurol. 2002;59:378–384. doi: 10.1001/archneur.59.3.378. [DOI] [PubMed] [Google Scholar]
- 88.Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Dietary fat intake and 6-year cognitive change in an older biracial community population. Neurology. 2004;62:1573–1579. doi: 10.1212/01.wnl.0000123250.82849.b6. [DOI] [PubMed] [Google Scholar]
- 89.Li G, Shofer J, Kukull W, et al. Serum cholesterol and risk of Alzheimer disease: A community-based cohort study. Neurology. 2005;65:1045–1050. doi: 10.1212/01.wnl.0000178989.87072.11. [DOI] [PubMed] [Google Scholar]
- 90.Basu R, Dodge H, Stoehr GP, Ganguli M. Sedative-hypnotic use of diphenhydramine in a rural, older adult, community-based cohort: effects on cognition. Am J Geriatr Psychiatry. 2003;11:205–213. [PubMed] [Google Scholar]
- 91.Mulsant BH, Pollock BG, Kirshner M, Shen C, Dodge H, Ganguli M. Serum anticholinergic activity in a community-based sample of older adults: relationship with cognitive performance. Arch Gen Psychiatry. 2003;60:198–203. doi: 10.1001/archpsyc.60.2.198. [DOI] [PubMed] [Google Scholar]
- 92.Bozoki A, Giordani B, Heidebrink JL, Berent S, Foster NL. Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch Neurol. 2001;58:411–416. doi: 10.1001/archneur.58.3.411. [DOI] [PubMed] [Google Scholar]
- 93.Tabert MH, Manly JJ, Liu XH, et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006;63:916–924. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]