Abstract
Receptor interacting protein 140 (RIP140) undergoes extensive posttranslational modifications (PTMs), including phosphorylation, acetylation, arginine methylation, and pyridoxylation. PTMs affect its sub-cellular distribution, protein-protein interaction, and biological activity in adipocyte differentiation. Arginine methylation on Arg240, Arg650, and Arg948 suppresses the repressive activity of RIP140. Here we find that endogenous RIP140 in differentiated 3T3-L1 cells is also modified by lysine methylation. Three lysine residues, Lys591, Lys653, and Lys757 are mapped as potential methylation sites by mass spectrometry. Site-directed mutagenesis study shows that lysine methylation enhances its gene repressive activity. Mutation of lysine methylation sites enhances arginine methylation, while mutation on arginine methylation sites has little effect on its lysine methylation, suggesting a relationship between lysine methylation and arginine methylation. Kinetic analysis of PTMs of endogenous RIP140 in differentiated 3T3-L1 cells demonstrates sequential modifications on RIP140, initiated from constitutive lysine methylation, followed by increased arginine methylation later in differentiation. This study reveals a potential hierarchy of modifications, at least for lysine and arginine methylation, which bi-directionally regulate the functionality of a non-histone protein.
Keywords: Mass spectrometry, Receptor interacting protein 140, Post-translational modification, Lysine methylation, Adipocytes differentiation
Introduction
Signal transduction pathways can utilize posttranslational modification (PTM) of protein to translate the extra-cellular signals into effects on gene expression.1–3 Various types of PTMs are known, such as phosphorylation of serine, threonine, and tyrosine residues, ubiquitylation, sumoylation, acetylation, methylation, pyridoxylation of lysine residue, and methylation of arginine residue, etc. Protein arginine methylation and protein lysine methylation for core histones have been extensively studied in light of their roles in chromatin remodeling and transcriptional regulation.4 A number of methyltransferases for histone modification have been found, including arginine methyltransferases PRMT1, PRMT2, PRMT3, CARM1 (PRMT4), PRMT5 and PRMT6, and lysine methyltransferases SET1, SET2, SET9, G9a, and SUV39H1, etc.4,5 These enzymes can be recruited to chromatin to methylate histones for transcriptional regulation,6 and can also methylate other components of transcription complexes such as nuclear receptors and transcription factors.4 In terms of biological effects, arginine methylation can regulate sub-cellular localization and protein-protein interactions of many non-histone proteins.7 Lysine methylation of non-histone proteins is also increasingly known, such as those occurred on p53 and TAF10.8,9 Recent report for trimethylation of retinoic acid receptor alpha (RAR alpha) and automethylation of histone lysine methyltransferase G9a have presented additional examples of lysine methylation for non-histone proteins,10,11 suggesting potential lysine methylation of more non-histone proteins that remain to be explored.
Receptor interacting protein 140 (RIP140) is a nuclear co-repressor for almost all nuclear receptors and many transcription factors and exerts effects in hormone receptor signaling. RIP140-null mice are lean and resistant to diet-induced obesity, and exhibit defects in female reproduction.12 Using LC-ESI-MS/MS, we have identified extensive PTM of RIP140, including twelve phosphorylation sites,13,14 eight acetylation sites,15 three arginine methylation sites,16 and a pyridoxylation site.17 Most of these modifications play roles in regulating the property and activity of RIP140. In this study, we find three additional methylated residues, on Lys591, Lys653, and Lys757. This study focuses on this newly found modification that also plays a regulatory role in the gene-repressive activity of RIP140.
We have first confirmed lysine methylation on the endogenous RIP140 in mammalian cells, and determined the functional role for lysine methylation in modulating RIP140’s gene-repressive activity. Mutating methylatable lysine residues each into an alanine (negative mutation) reduces the repressive activity of RIP140, suggesting a positive role for lysine methylation in enhancing its gene-repressive activity, which is opposite to that of arginine methylation as reported previously.16 Further, negative mutation of lysine methylation enhances arginine methylation, but negative mutation of arginine methylation has no effect on lysine methylation. Kinetic study of endogenous RIP140 modification in differentiated 3T3-L1 cells reveals constitutive lysine methylation on RIP140. However, arginine methylated RIP140 is detectable only in later stages of differentiation. We now report the results demonstrating modulation of the function of a non-histone protein through protein lysine methylation and protein arginine methylation.
Materials and methods
Plasmids
Full-length Gal4BD-RIP140, Gal4V16-HDAC3, GFP fused RIP140 were constructed as described.14,18 Mouse wild type G9a,19 and Suv39H1,20 constructs were obtained as generous gift, respectively from Dr. Michael Stallcup (University of Southern California, Los Angeles, CA) and Dr. Janet Stavnezer (University of Massachusetts, Worcester, MA)
Expression, purification and mass spectrometry of mouse RIP140
Mouse RIP140 having a 6xHis-epitope was expressed in Sf21 using Baculovirus and purified by affinity chromatography for mass spectrometry analysis as described.13 Purified His-tagged RIP140 was resolved on SDS-PAGE and subjected to in-gel trypsin digestion prior to analysis by LC-ESI-MS/MS. The LC system was online with QSTAR Pulsar quadrupole time of flight (TOF) mass spectrometer (MS) (Applied Biosystems, Inc., Foster City, California), which was equipped with the Protana’s nano-electrospray source. An electrospray voltage of 2250 V was applied distal to the analytical column. The TOF region acceleration voltage was 4 kV and the injection pulse repetition rate was 6.0 kHz. The [M + 3H]3+ monoisotopic peak at m/z 586.9830 and [M + 2H]2+ monoisotopic peak at m/z 879.9705 from human renin substrate tetradecapeptide (Sigma-Aldrich, St. Louis, MO) were used for external calibration. As peptides were eluted from the column they were focused into the mass spectrometer. The information-dependent acquisition (IDA) was used to acquire MS/MS data with experiments designed such that the three most abundant peptides were subjected to collision-induced dissociation, using argon as the collision gas in every 15 seconds. Collision energies were varied as a function of the m/z and the charge state of each peptide. To avoid continued MS/MS of peptides that had already undergone collision induced dissociation, a dynamic exclusion was incorporated for a further 45 s. IDA mode settings included continuous cycles of three full scan TOF MS from 400–550 m/z, 550–750 m/z and 750–1200 m/z (1.5 seconds) plus three product ion scans from 50–4000 m/z (3 seconds each). Precursor m/z values were selected from a peak list automatically generated (default) by Analyst QS software v1.0 (ABI) from the TOF MS scans during acquisition, starting with the most intense ion. Peptide mass software MS-digest from the ProteinProspector (http://prospector.ucsf.edu) available online was used to generate a theoretical tryptic digest of RIP140 by considering arginine, and lysine containing peptides to account for methylation. From the LC-MS data, the molecular mass of the detected peptides were calculated using the LC-MS reconstruct feature of Analyst QS. Experimentally measured peptide masses were compared with the theoretical digest.
Protein sequence database search and manual verification
To confirm the sequence of the peptides and the sites of modification, MS/MS spectra were examined. All MS/MS spectra generated from IDA experiments were subjected to MS/MS Ion search using MASCOT version 2.2 against the National Center for Biotechnology Information non-redundant (NCBI-nr) protein sequence database (version 20080410 with 6,417,748 entries) specifying the taxonomy to Mus musculus with 139,127 entries. Enzyme specificity was set to trypsin and two missed cleavages were allowed. The database searches were performed with the fixed modification for carbamidomethylation of cysteine and with variable modifications for methylation of lysine and arginine, and oxidation of methionine. Mass tolerance for precursor peptides and MS/MS fragment ions was ± 1.2 Da and ± 0.6 Da, respectively.
Threshold for accepting individual MS/MS spectra was set to a Mascot score of 40. Searched peptide sequence with an expectation value (Mascot search) of less than 0.05 indicated identity, which generally showed a MASCOT score greater than 40 against the NCBI-nr database (M. musculus). All MS/MS spectra with a Mascot score >20 were manually examined to confirm that they had sufficient y- and b-ions for peptide identification as well as for unambiguous assignment of the methylated site. All methylated peptides in database search fell into Mascot scores between 23 and 71. The Mascot search engine uses +14 Da, + 28 Da, and + 42 Da mass shift for the assignment of mono-, di- and tri-methylation sites, respectively. We also searched for 14 Da, + 28 Da, and + 42 Da mass shift in our manual validation of methylated sites using the Bioanalyst software v1.0 (Applied Biosystems/MDS Sciex, USA). Peaks with a minimum height of 3% relative to the base peak were considered and a 100 ppm tolerance was used to establish matches with the theoretical b and y ions which were predicted with Bioanalyst software v1.0 (Applied Biosystems/MDS Sciex, USA)
Site-directed mutagenesis
Site directed mutagenesis on methylated lysine residue of full-length RIP140 was performed using Quick Change XL site-directed mutagenesis kit (Stratagene). Replacement of lysine residues with alanine (A) were made by employing mutagenic primers. The mutagenic primers employed to generate mutations (uppercase letters) were: K591A: sense: 5’-agt act ccc gct tcc GCG ctc acg aac acc gcg -3’ and anti-sense: 5’-cgc ggt gtt cgt gag CGC gga agc ggg agt act-3’; K653A: sense: 5’-gag cag aga ccc tgc GCA cag ctg tta agt gga-3’ and anti-sense: 5’-tcc act taa cag ctg TGC gca ggg tct ctg ctc-3’; and K757A: sense: 5’-ctc atg gtg aag att GCA tcc gag cct tgt gac-3’ and anti-sense: 5’-gtc aca agg ctc gga TGC aat ctt cac cat gag-3’. The positive clones were verified by DNA sequencing.
Cell culture, transfection, and reporter assay
COS-1 cells were maintained in DMEM supplemented with 10% FBS. Transient-transfection was performed using Lipofectamine™-2000 (Invitrogen). Reporter assay was conducted in 24-well plates in COS-1 cells using 0.1 µ g of Gal4BD-RIP140 or control plasmid (Gal4BD empty vector), Gal4-tk-luciferase (0.5µg) reporter and a CMV-lacZ as an internal control (0.05 µg) per well. 12 h post-transfection, the medium was replaced with a fresh medium containing FBS and treated with methylase inhibitor (adenosine 2,3-dialdehyde, 10 µM) for 12 h. Mammalian two hybrid test was conducted in COS-1 cells on a Gal4 reporter using Gal4BD-fused RIP140 and Gal4VP16 fused HDAC3. 36 h post-transfection total cell extracts were prepared by freeze-thaw and tested for luciferase and lacZ activities. The fold-relative luciferase activity was calculated by normalizing RLU (relative luciferase unit) activity of the experimental groups to the RLU activity of the empty vector control group. 3T3-L1 cells were maintained and differentiated to adipocytes by a differentiation mixture containing insulin (170 nM), isobutylmethylxanthine (IBMX, 250 µM), thyroid hormone (2 nM), and dexamethasone (2 µM) as described.21
Metabolic labeling, IP, and western blot
In vivo methylation on endogenous RIP140 in differentiated 3T3-L1 cells and ectopically expressed RIP140 protein in COS-1 cells was conducted by metabolic labeling using tritium labeled S-adenosyl methionine (3H-SAM, 25 µCi/mL). Briefly, the confluent cells were washed with methionine free DMEM (Gibco) twice and 3H-SAM (Sigma) was added directly to the medium and incubated for 4 hour. The cells were washed twice with PBS and harvested. The cell lysates were prepared in a co-immunoprecipitation (Co-IP) buffer (100 mM Tris-HCl, 150 mM NaCl, 10% glycerol, 0.1% NP-40, pH 8.0) by freeze–thaw cycles for immunoprecipitation experiment and autoradiography. To detect the methylation status of RIP140 in mammalian cells, immunoprecipitation (IP) was conducted for endogenous, and GFP-fused exogenous, RIP140 using anti-RIP140 (in-house) and for Gal4BD-RIP140 with anti-Gal4 (Santa Cruz) antibodies followed by detection with an anti-methylated lysine antibody (Stressgen Bioreagents) or anti-methylated arginine antibody (Abcam) on the western blot. We have verified the specificity of these commercial pan-methyl lysine and arginine antibodies in detecting RIP140 modified by lysine and arginine methylation, respectively using RIP140 expressed in both mammalian cells and bacteria.
RESULTS
Liquid chromatography-tandem mass spectrometry of RIP140
Prior studies have validated the expression of recombinant proteins in insect cells as an efficient way to generate large quantities of low abundant protein for biochemical study .22,23 While stoichiometry may vary, the sites of modification on proteins purified from insect cells can usually be found on proteins expressed in mammalian cells.23,24 Purification of the recombinant RIP140 protein has yielded over a 95% homogeneity and the tryptic peptides of purified protein were analyzed by LC-ESI-MS/MS to identify the sites of modification as described.13 We recorded three independent full-scan ion chromatograms in the ranges of 400–550 m/z, 550–750 m/z, and 750–1200 m/z. The information-dependent acquisition (IDA) was used to acquire MS/MS data which were searched with a MASCOT search using the parameters described in the experimental section. The search result confirmed the identification of the protein (Supplementary Table 1) with a significant sequence coverage to over 60% of the total protein as reported before,13 and revealed 6 tryptic methylated peptides (Table 1 and Supplementary Table 2, online). Among these, three methylated peptides were found to have only lysine methylation. Two showed only arginine methylation and one showed both arginine and lysine methylation. Importantly, LC-ESI-MS/MS analyses of purified RIP140 expressed in E. coli under identical settings (e.g., purification, sample preparation, mass analyses, and database search, etc.) revealed no methylated peptide, suggesting that RIP140 modification requires a eukaryotic cellular environment. Previously, we have reported the mapping of three arginine methylation by mass spectrometry.16 Here, we have focused on the sites of lysine methylation.
Table 1. LC-ESI-MS profile of the methylated tryptic peptide of RIP140.
Tryptic digests of RIP140 protein were subjected to LC-ESI-MS/MS. Three independent full-scan ion chromatograms from 400–550 m/z, 550–750 m/z, and 750–1200 m/z were recorded in an information-dependent acquisition (IDA) mode to acquire the MS/MS data. The IDA data was searched online at MASCOT (http://www.matrixscience.com) MS/MS data search in the NCBI data bank. The MS/MS data was analyzed manually to confirm the sequence of the modified and the unmodified forms of the same peptide identified by the data bank search. The full scan chromatograms were analyzed to assign the charged state, retention time, and intensities of the peptides. The bold character indicates the site of modification.
| Sequence 1) (score)2) | m/z (z); Mass (Da)3); RT (min)4) | ΔMass | |
|---|---|---|---|
| unmodified | modified | (Da) | |
| 230-VMSEPLSCAAR-240 (-/44) | Not detected | 625.79(2), 1249.57, 32.41 | 14 |
| 578-WNSYPPYACSTPASK-591 (62/38) | 783.95 (2), 1564.69, 35.61 | 790.36 (2), 1578.71, 35.73 | 14 |
| 631-LLQNLAQCGLQSSGEEQRPCK-653 (44/71) | 857.40 (3), 2569.24, 39.09 | 862.08(3), 2583.24, 39.12 | 14 |
| 631-LLQNLAQCGLQSSGEEQRPCK-653 (33/23) | 866.74 (3), 2597.25, 39.45 | 28 | |
| 756-IKSEPCDDFQTHNTNLPLNHDAK-758 (50/38) | 539.85 (5), 898.75 (3), | 903.41 (3), 677.82 (4), | 14 |
| 674.31 (4), 2693.23, 35.61 | 2707.24, 35.67 | ||
| 939-QLLLSENCVR-948 (56/55) | 616.32 (2), 1230.63, 38.63 | 623.33 (2), 1244.64, 31.95 | 14 |
Cysteine was modified by idoacetamide; methionine was oxidized; bold letters indicate methylated residue;
MASCOT scores for peptides (unmodified/modified) are in the parentheses;
Precursor ion mass in Da;
RT, retention time (underlined numbers) in minute
Searched peptide sequence with a MASCOT score greater than 40 indicates positive identity (p < 0.05). All lysine methylated peptides fell into a Mascot score between 38 and 71, except a di-methylated peptide showing a Mascot score of 23 (Table 1). Further, to eliminate false positive identification of any methylated lysine residue, other type of methylation including N-methylation (Arg/Asn/Gln), O-methylation (Ser/Thr/Tyr) and carboxymethylation (Cys) were also analyzed during manual inspection of MS/MS spectra. We have found that a MASCOT threshold ion score showing a 95% probability (p < 0.05) of positive identity of a particular peptide could be used to accurately assign the peptide sequence, but sometimes it failed to accurately assign the sites of modification. Therefore, manual validation considering all possibilities of modification by methylation, as stated above, could enhance the accuracy of the assignment for lysine methylation.
Mapping of lysine methylation sites on RIP140
The MS/MS data of the methylated peptides were analyzed manually. In order to identify the methylated peptides in the total ion chromatogram (TIC), 14 Da, 28 Da and 42 Da positive mass shifts were taken into consideration for mono-, di- and tri- methylation, respectively. The y and b ions containing the methyl group, immonium ions for modified amino acids, as well as other signature ions for modification by lysine methylation were taken into account to confirm the sites of modification.
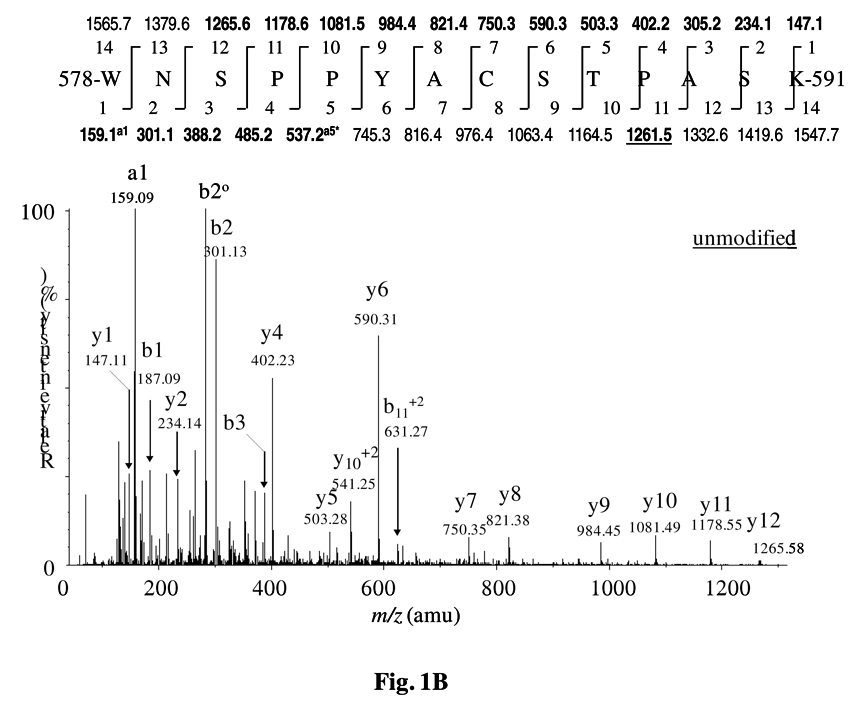
A 14.02 unit mass difference was found between the mass of the doubly charged precursor ions of the modified peptide at m/z 790.36 (mass 1578.71 Da) and the unmodified peptide at m/z 783.95 (mass 1564.69 Da) spanning 578–591 aa (Table 1), indicating a mono-methylation site on the peptide. The MS/MS spectrum of the modified peptide (Figure 1A and Supplementary Figure 1) demonstrated a series of y-ions, particularly y5 at m/z 517.30 and y7 through y12 ions, each showing a 14 unit mass shift as compared to that of the unmodified peptide (Figure 1B). This suggested that the methylated site might be located in the internal sequence between y1 and y5. The doubly charged b11 ion at m/z 631.27 corresponded to that of the unmodified peptide (Figure 1B), which preferentially ruled out the possibility of any O-methylation at Thr587. This eventually narrowed down the location of the modified site in the internal sequence between y1 and y3, and suggested the location of the methylated site at either Ser590 or Lys591. The intense y3* ion at m/z 302.17 generated from the loss of ammonia could not exclude the possibility of O-methylation for Ser590. However, the low intensity y1* ion at m/z 144.10 (not marked) and the immonium ion at m/z 115 for methylated lysine, and the typical marker ions at m/z 84 and m/z 98 for mono-methylated lysine appeared in the MS/MS spectrum,25 which signified Lys591 methylation.
Figure 1.
Identification of Lys591 methylation site on mouse RIP140 by LC-ESI-MS/MS. (A) The modified peptide (578–591 aa) showed a precursor ion at m/z 790.36 (z = 2). (B) The unmodified peptide (578–591 aa) displayed a precursor ion at m/z 783.36 (z = 2). The mass of the precursor ion of the modified peptide showed a + 14.02 Da shift as compared to that of the unmodified peptide, suggesting a covalent modification by a single mono-methylation. The MS/MS of modified peptide (m/z 790.36) y3* ion at m/z 302.17, along with y5 through y12 ions consecutively at m/z517.30, m/z 604.30, m/z 764.40, m/z 835.40, m/z 998.46, m/z 1095.51, m/z 1192.57, and m/z 1279.72 showed a +14 Da mass shift as compared to the corresponding ions in the unmodified peptide. This suggested the modification of Lys591 by mono-methylation. The underlined mass value over the spectrum indicates that the ion originally appeared as a doubly charged ion.
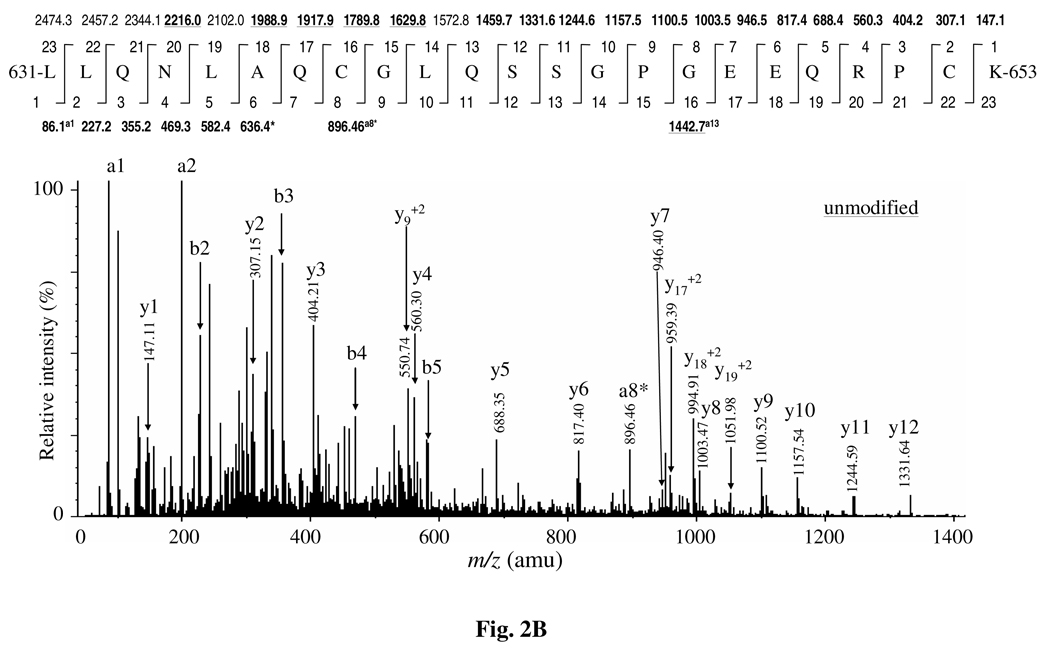
Two triply charged precursor ions of the modified peptide spanning 631–653 aa appeared at m/z 862.08 (precursor molecular mass 2583.24 Da) and m/z 866.74 (precursor molecular mass 2597.25 Da) at 39.12 and 39.45 minutes, respectively, in the full scan ion chromatogram set for 750–1200 m/z. The precursor mass of these two ions for the modified peptide showed 14 Da and 28 Da shifts as compared to the unmodified version of the peptide appeared at m/z 857.40 as a triply charged ion (mass 2569.24) at 39.09 minute, attributing to mono- and di- methylated versions of the peptide. In the MS/MS spectrum of the mono-methylated version of the peptide (Figure 2A and Supplementary Figure 2), the intense y4 and y5 ions at m/z 574.31 and m/z 702.37, each showed a 14 Da mass shift as compared to the unmodified version of the peptide (Figure 2B). This suggested the methylated site to be located between y1 and y4. However, the MS/MS spectrum showed two species of methylated y ions, defined as yK and yR for methylated lysine and methylated arginine, respectively. This indicated that there were two species of mono-methylated peptides co-eluted in the TIC. In one species, the y1, y2 and y3 ions corresponded to the native peptide (Figure 2B), strongly suggesting the methylated site at Arg650. The spectrum also showed a low intense immonium ion (IK) at m/z 115, accounting for the methylated lysine. In addition, the y2 and y3 ions, defined as y2K and y3K ions for the methylated lysine, appeared at m/z 321.16 and m/z 418.21, respectively, each exhibiting a 14 Da shift as compared to the corresponding ion peaks for Arg650 methylated peptide. This confirmed methylation of Lys653.
Figure 2.
Assignment of Lys653 mono-methylation by MS/MS analysis. (A) The precursor ion of the modified peptide (631–653 aa) appeared at m/z 646.819 (z = 4) and m/z 862.093 (z = 3). (B), The MS/MS spectrum of the precursor ion at m/z (857.42 (z = 3) of the unmodified peptide (631–653 aa). (C), The mass of the precursor ion of the di-methylated peptide appeared at m/z 866.34 (z = 3). The MS/MS spectrum of the mono-methylated peptide (A) showed two species of y-ions, one for the methylation of lysine residue define as ‘yK’ and the second for arginine methylation designated as ‘yR’. The spectrum showed intense y2K and y3K ions at m/z 321.16 and m/z 418.21, respectively. Each exhibited a + 14.02 Da shift as compared to the unmodified peptide (B). Further, the spectrum (B) also showed y1 through y3 ions (defined as y1R, y2R and y3R) at m/z 147.11, m/z 307.15 and m/z 404.21, corresponding to those of the unmodified peptide. This suggested that some species of the mono-methylated peptide lacked lysine methylation at Lys653. However, it showed intense y ions from y4 through y11 (defined as y4K/R, y5K/R and so on) and exhibited a +14 Da mass shift, suggesting Arg650 methylation. The MS/MS spectra of the di-methylated peptide (C) showed same pairs of y ions from y1 through y11 as shown in the mono-methylated peptide spectrum (B). In addition, the spectrum showed doubly charged y17 through y20 ions, each demonstrating a + 28 Da mass shift. This suggested the presence of a second methylation site between y11 and y17. The b1 through b5 ions were identical to the unmodified peptide. However, doubly charged a7 ion at m/z 384.24 and singly charged a8* ion (due to the loss of NH3) at m/z 910.48 contained a single methyl group (+ 14 Da shift). This confirmed that the second mono-methylation site was located at Gln637 in this di-methylated version of the peptide. The doubly charged ions are indicated by underlined mass values over the peptide sequence.
The MS/MS spectrum of the di-methylated version of the triply charged precursor ion of peptide (631–653 aa) at m/z 866.74 (mass 2597.25 Da) (Figure 2C) showed y1, y2, y3 and y4 ions identical to those for Arg650 and Lys653 methylation in the mono-methylated peptide (Figure 2A). This further substantiated independent mono-methylation of Arg650 (reported previously, see ref. 16) as well as Lys653 as shown above (Figure 2A and Supplementary Figure 2) in the di-methylated version of the peptide. Although there was no additional lysine or arginine residue to be methylated in the peptide, we continued analyzing the b ions as well as y ions beyond y4 ion in order to assign the second methylation site in this di-methylated version of the peptide. The b5 ion at m/z 582.40 corresponded to that of the native peptide, while the several y ions beyond y4, in particular, y5 through y7 and y9 through y11 clearly continued to show a 14 Da mass shift for mono-methylation. This suggested that the second methylated site might be located between b5 and y11. A series of doubly charged y-ions, particularly y17 through y20, each showed a 28 Da mass shift for di-methylation. This narrowed down the second methylated site to a position between y11 and y17. The singly charged a1 ion and b2 through b5 ions corresponded to that of the unmodified peptide. The intense doubly charged a7 ion at m/z 384.24 and singly charged a8* ion at m/z 910.48 due to the neutral loss of ammonia, showed a 14 Da mass shift for a mono-methylated site. This suggested that Gln637 could also be methylated along with Lys653 or with Arg650 in the di-methylated version of the peptide. Together, these data accounted for both mono- and di- methylated versions of the peptide (631–653 aa), and confirmed mono-methylation of Lys653.
The precursor ion of the modified peptide spanning 756–778 aa appeared at m/z 903.41 as a triply charged ion and at m/z 677.82 as a quartet charged ion (precursor mol. mass 2704.24 Da). The precursor ion mass of the modified peptide showed a 14 Da mass shift as compared to that of the precursor ion of the unmodified peptide appeared as a triply charged ion at m/z 898.75 and a quadruple charged ion at m/z 674.31 (mass 2693.23 Da) (Table 1). This suggested the modification of the peptide by mono-methylation (Figure 3A and Supplementary Figure 3). The singly charged y1, y9, y10 and y11 were identical to those of the unmodified peptide (Figure 3B and Supplementary Figure 3). This excluded possible modification of Lys778 as well as other sites located in the sequence between y1 and y11. However, a series of b ions, particularly b6, b7, and b8 ions, which appeared at m/z 729.36, m/z 844.38 and m/z 959.41, respectively, each contained an intact methyl group. This suggested that mono-methylated site was likely to be located between b1 and b6. The intense b3° and b4° ions from the neutral loss of H2O appeared at m/z 325.22 and m/z 454.27, respectively, corresponding to the mono-methylated peptide mass. The a1 ion was identical to that of the native peptide. In addition, a low intense a2* ion at m/z 211.18 due to the loss of ammonia from a2 ion, and immonium ion at m/z 115 for mono-methyl lysine were also shown in the MS/MS spectrum. This suggested the location of the mono-methylated site at Lys757.
Figure 3.
Mapping of Lys757 methylation by MS/MS analysis. (A) The precursor ion of the modified peptide (756–778 aa) appeared at m/z 903.429 (z = 3) and m/z 677.818 (z = 4). (B) The MS/MS spectrum of the precursor ion at m/z 898.7459 (z = 3) and 674.3176 (z = 4) of the unmodified peptide (756–778 aa). The mass of the precursor ion of the modified peptide showed a + 14.02 Da shift as compared to the unmodified peptide, suggesting a covalent modification by a single mono-methylation. The MS/MS of the modified peptide (A) showed a series of consecutive singly charged y ions, in particularly y1 through y7 ions, which were identical to the unmodified peptide (B). However, it showed a1 and a2 ions at m/z 86.09 and m/z 211.18, and singly charged b6, b7, and b8 ions at m/z 729.36, m/z 844.38, and m/z 959.41. Each showed a +14 Da mass shift as compared to the unmodified peptide. Thus, Lys757 mono-methylation was confirmed. The underlined mass value over the spectrum indicates that the ion originally appeared as a doubly charged ion.
Protein lysine methylation of RIP140 in vivo
Metabolic labeling experiment using 3H-SAM (tritiated S-adenosyl methionine) as methyl donors was conducted 2 days after induction of differentiation of 3T3-L1 cells, which expressed a detectable level of endogenous RIP140. The cell lysate was immunoprecipitated (IP) with anti-RIP140 antibody and resolved on SDS-PAGE (Figure 4A). Metabolic labeling, as revealed by autoradiography, showed that the endogenous RIP140 in differentiated 3T3-L1 cells was methylated. Further, IP followed by detection with a methylated lysine-specific antibody confirmed that endogenous RIP140 could be modified by lysine methylation. We also confirmed lysine methylation of ectopically expressed GFP-tagged RIP140 expressed in COS-1 cells by IP with anti-RIP140 antibody followed by detection with the methylated lysine-specific antibody as shown in Figure 4B. Finally, the level of lysine methylation of exogenously expressed RIP140 could be reduced by the addition of a methyltransferase inhibitor (MTI), adenosine 2, 3-dialdehyde, an adenosine analog and S-adenosylmethionine-dependent methyltransferase inhibitor.26 This supports the specificity of the antibody for detecting lysine methylation of RIP140.
Figure 4.
In vivo lysine methylation of RIP140. (A) Metabolic labeling of endogenous RIP140 in differentiated 3T3-L1 cells using 3H-SAM (top) followed by immunoprecipitation (IP) with anti-RIP140 antibody confirmed RIP140 methylation. IP with anti-RIP140 or control IgG and western blot (WB) with anti-methylated lysine specific antibody demonstrated that endogenous RIP140 was modified by lysine methylation (middle). (B) Similar IP experiment and WB detection with the methylated lysine specific antibody on ectopically expressed GFP-tagged RIP140 in COS-1 cells supported that exogenous RIP140 could also be lysine methylated in mammalian cells. The level of lysine methylation was reduced in the presence of a general methyltransferase inhibitor (adenosine 2,3-dialdehyde), supporting the specificity in the detection of lysine methylation. (C) Detection of lysine and arginine methylation of endogenous RIP140 in 3T3-L1 differentiation. IP experiment with anti-RIP140 antibody and WB detection by the methylated lysine- and the methylated arginine-specific antibodies revealed that lysine methylation of endogenous RIP140 appeared immediately, while arginine methylation occurred only in later stages of 3T3-L1 differentiation. NS indicates non-specific bands. All immunoblot experiments were repeated at least twice and were reproducible.
Previously we reported arginine methylation of endogenous RIP140 in differentiating 3T3-L1 cells, which was able to modulate RIP140’s activity in fat accumulation.16 To decipher any potential relationship between arginine methylation and lysine methylation of RIP140, we first conducted a kinetic study of these two forms of methylation occurring on the endogenous RIP140 in 3T3-L1 adipocyte differentiation (Figure 4C). Endogenous RIP140 was precipitated (IP with anti-RIP140), and detected with the methylated arginine, or methylated lysine-specific antibody. The result showed a lack of detected arginine-methylated RIP140 in early phases and increasingly detectable arginine methylation of RIP140 over time (top panel). Interestingly, a constitutive, basal level of lysine-methylated RIP140 was readily detectable from day 0 (second panel), which was gradually elevated along the course of differentiation, coincided with a significant increase in RIP140 expression level (bottom panel). This kinetic study suggested a possible relationship between lysine methylation and arginine methylation of RIP140 in differentiating cultures (see below). It is important to note that we have verified the specificity of these commercial pan-methyl lysine and arginine antibodies in detecting RIP140 modified by lysine and arginine methylation, respectively using RIP140 expressed in both mammalian cells and bacteria. Both antibodies detected only RIP140 expressed in mammalian cells (as shown in Figure 4), but not the RIP140 expressed in bacteria (data not shown). This supports the specificity of these pan-methylated antibodies in detecting methylated lysine or methylated arginine residues on RIP140.
The effects of lysine mono-methylation on RIP140 repressive activity
We first examined the impact of mono-methylation at Lys591, Lys653 and Lys757 on the trans-repressive activity of RIP140 using a classical Gal4 reporter system where RIP140 (wild type or various mutants) was fused to the Gal4 DNA binding domain (Figure 5A). As expected, the Gal4-fused wild type RIP140 drastically reduced the reporter activity, confirming its trans-repressive activity in this reporter system. Three individual single negative mutants (lysine to alanine), at K591, K653, and K757, however, all exhibited a lower trans-repressive activity of RIP140 (reporter activity returning to the control or even a higher level), suggesting that these methylatable lysine residues are important for gene repressive activity of RIP140. Incorporation of double mutation (Di-K/A: K591A/K653A) or triple mutation (Tri-K/A: K591A/K653A/K757A) similarly reduced the trans-repressive activity (Figure 5B). The status of lysine methylation for the triple Lys/Ala mutant (K591A/K653A/K757A) was compared to that of the wild type protein using the methylated lysine specific antibody as well as in metabolic labeling using 3H-SAM as shown on the lower panel of Figure 5B. Gal4-RIP140 was precipitated with anti-Gal4, followed by western blot with the methylated lysine-specific antibody (IB: α-meK) and autoradiography for the detection of lysine methylation and total methylation status, respectively. The input protein was monitored using anti-RIP140. In comparing the total methylation level of RIP140 by metabolic labeling (Figure 5B, bottom, middle) between the wild type and the mutant RIP140, it appeared to be complicated by the potential interdependency between lysine methylation and arginine methylation on RIP140 (see Figure 6). However, the immunoblot with methylated lysine antibody confirmed that the triple Lys/Ala mutant, while remained lysine methylatable, exhibited a much reduced level of lysine methylation. Since the triple mutation failed to completely block lysine methylation, it is possible that certain sites of methylation at very low levels escaped the MS/MS analysis, or they might not occur efficiently in insect cells. According to the manufacturer, the antibody against methylated lysine cross-reacted with all versions of mono-, di-, and tri- methylated lysine. Therefore, it is possible that certain di- or tri-methylated lysine may also be present in RIP140 expressed in mammalian cells, but not in RIP140 expressed insect cells or it may exist at a very low level that was not detected by mass analysis.
Figure 5.
Role of lysine methylation on RIP140 repressive activity. (A) Trans-activation assay using Gal4-RIP140-full length on a Gal4BD-Luc reporter was conducted in COS-1 cells. Single mutation of methylated Lys591, Lys653 and Lys757 (K591A, K653A, and K757A) each reduced the repressive activity of RIP140, suggesting a positive role for lysine methylation in the repressive function of RIP140. (B) Double (K91A/K563A) or triple (K591A/K653A/K757A) mutation also abrogated its repressive activity (top). IP with anti-RIP140 antibody and WB detection with the methylated lysine-specific antibody demonstrated that mutation of all methylatable lysine residues reduced the level of lysine methylation (bottom). (C) Interaction of RIP140 with HDAC3 detected in a mammalian two hybrid assay in COS-1 cells. Single mutation at Lys591, Lys653 and Lys757 (K591A, K653A, and K757A) each reduced the level of Gal4-RIP140 interaction with AD-HDAC3. All reporter assays were repeated at least three times. Data are presented as mean ± SD. IP experiment was repeated twice and was reproducible.
Figure 6.
Relationship between arginine- and lysine- methylation of RIP140. (A) Constitutive negative triple arginine mutation (R240A/R650A/R948A) did not affect the status of lysine methylation on GFP-tagged RIP140 in COS-1 cells. The experiment was carried out by IP with anti-RIP140 antibody and WB detection with the methylated lysine-specific antibody. (B) Mutation of the methylatable lysine residue enhanced the overall arginine methylation status of RIP140. The basal level (without PRMT1 supplementation) of arginine methylation on the wild type RIP140 in COS-1 cells was very low. The experiment was carried out by IP using anti-Gal4 antibody and WB detection with the methylated arginine-specific antibody on Gal4-fused RIP140 expressed in COS-1 cells. IP experiments were repeated twice and were reproducible.
The effect of lysine methylation on RIP140 interaction with HDAC3
RIP140 is known to exert its co-repressive activity by, partially, recruiting HDACs or by interaction with another co-repressor CtBP through its central domain.27–30 We then assessed the effect of lysine methylation on its interaction with HDAC3 using a mammalian two-hybrid interaction test (Figure 5C). The wild type RIP140 interacted efficiently with HDAC, but all three single mutants, as well as the triple-Lys/Ala mutant (data not shown), lost the ability to interact with HDAC3. This suggested that lysine methylation on RIP140 could enhance its interaction with HDAC3.
Relationship between protein-arginine methylation and protein lysine methylation of RIP140
Previously we have shown that increased arginine methylation of RIP140 by co-expressing PRMT1 reduced its gene repressive activity because arginine methylated RIP140 could be better exported out of the nucleus.16 Here we found that mutation of methylatable lysine into alanine (to reduce lysine methylation) also rendered RIP140 less repressive. Therefore, we suspected some form of interdependency between lysine methylation and arginine methylation of RIP140. We first examined if the negative triple mutant of arginine methylation, where all three methylatable arginine residues were each mutated to an alanine (shown as Arg/Ala), could be methylated on lysine, and if the negative mutants of lysine methylation could be methylated on arginine (Figure 6). As shown in panel A, the wild type RIP140 (middle lane) could be effectively lysine methylated as detected by methylated lysine-specific antibody. The triple negative arginine methylation mutant (right lane) remained as effectively methylated as the wild type did. Thus, failure in arginine methylation did not affect lysine methylation (Figure 6A). On the contrary, as shown in panel B, despite the low level of arginine methylation on the wild type RIP140 in COS-1 without supplemental PRMTs, all the negative mutants of lysine methylation, K591A, K653A and K757A, were more effectively arginine-methylated (panel B, three right lanes), assessed with the methylated arginine-specific antibody. These results, together with the constitutive lysine methylation of RIP140 in the differentiating culture (Figure 4C), suggested that lysine methylation occurred readily or constitutively, which is probably required for its effective gene repressive activity in the early differentiating culture (Figure 5B). On the contrary, arginine methylation was occurred later (Figure 4C), probably because it required the induction of PRMT1 in more matured or differentiated cultures (data not shown). Further, it could also be regulated by changes in lysine methylation (see Discussion).
DISCUSSION
Rapid identification of proteins and their modifications by mass spectrometry has provided important information to address biological problems in the post-genomic area.31 Proteins can be modified extensively, such as phosphorylation, acetylation, methylation, and glycosylation, etc. We have carried out systematic identification of PTM sites on RIP140, a co-regulator for many transcription factors, by LC-ESI-MS/MS, using RIP140 purified from insect cells as the material source. Previously, we have reported eleven phosphorylation sites (Ser104, Thr202, Thr207, Ser358, Ser380, Ser488, Ser519, Ser531, Ser543, Ser672, and Ser1003),13,14 eight acetylation sites (Lys111, Lys158, Lys287, Lys311, Lys482, Lys529, Lys607, and Lys932),15 three protein arginine methylation sites (Arg240, Arg650, and Arg948),16 and a pyridoxylation site on Lys613.17 This current study reports three newly identified sites of lysine methylation (Lys591, Lys653, and Lys757). We have reported that Ser/Thr phosphorylation and lysine acetylation of RIP140 are interdependent.32 Phosphorylation was found to facilitate lysine acetylation for RIP140. It is interesting to note that there were no overlapping lysine residues for lysine acetylation and lysine methylation. Further, both lysine methylation and lysine acetylation on RIP140 could positively modulate the repressive activity of RIP140. We have also shown the interdependency of PKC-mediated phosphorylation and arginine methylation of RIP140.33 However, it remains to be determined whether modulation of RIP140 activity by lysine methylation and lysine acetylation is independent or interdependent. In this study, we have also reported a Gln methylation site on Gln637 on RIP140. Gln methylation has been well known for prokaryotic cells, but Gln methylation of protein for mammalian cells has only been reported recently.34 Since this study focused on the role of lysine and arginine methylation of RIP140, the functional role for Gln methylation of RIP140 needs be investigated. It also needs to be further confirmed whether Gln methylation of RIP140 occurs in mammalian cells, or it might be specfic to RIP140 expressed in insect cells, which is beyond the scope of the current study.
Lysine methylation on endogenous RIP140 in differentiated 3T3-L1 cells is validated in metabolic labeling and detection by the methylated lysine specific antibody. The functional role of lysine methylation is determined using a site-directed mutagenesis approach, and found to enhance its gene repressive activity by, at least partially, regulating its HDAC-interacting property. More interestingly, endogenous RIP140 is constitutively lysine-methylated in differentiating 3T3-L1 cells that usually are differentiated into adipocytes, but its arginine methylation is significantly elevated only in more advanced, or differentiated cultures (Figure 4). Further, negative mutation of lysine methylation facilitates arginine methylation to some extent, whereas negative mutation of arginine methylation does not seem to affect lysine-methylation (Figure 6). These results suggest that lysine methylation could enhance the co-repressive activity of RIP140 independently or in combination with a lack of arginine methylation, or it might simply repress arginine methylation in early differentiating cultures. It can also be speculated that arginine methylated RIP140 might be less effective in terms of gene repression in advanced differentiated cultures because repression of these gene is no longer critical in these more mature adipocytes. This coincides with the arginine methylation-stimulated export of RIP140 to the cytoplasm during later stages of differentiation as we reported previously.16 Together, these results reveal a potential hierarchy of PTMs on RIP140 in differentiated 3T3-L1 cells, initiated from constitutive lysine methylation, followed by increased arginine methylation during later stages of differentiation. Incidentally, PRMTs are also induced in more differentiated adipocyte cultures as we have detected earlier31, consistent with the delayed arginine methylation on RIP140 as shown in Figure 4. This study presents the first example of a non-histone protein regulated by lysine and arginine modifications that can differentially regulate the function of the protein.
Lysine methylation of core histone proteins is widely demonstrated and has an important functional role in gene regulation. However, much less is known about lysine methylation of non-histone proteins. Only recently, transcription factors such as p53 and TAF10 were found to be modified by lysine mono-methylation,8,9 which affected their biological activities. Histone lysine methylase G9a was also shown to auto-catalyze its own lysine methylation.11 For histone proteins, arginine methylation widely occurs and, as lysine methylation, also plays an important role in gene regulation.35,36 This current study demonstrates not only lysine methylation of a nuclear co-repressor, but also, by incorporating previously discovered arginine methylation information, uncovers a possible relationship between lysine methylation and arginine methylation on a non-histone protein. Interestingly, in the 3T3-L1 adipocyte differentiating model, RIP140 activity is bi-directionally regulated by these two forms of modification, with lysine methylation as an enhancing signal and arginine methylation as a repressive signal. This coincides with the required gene repressive activity of RIP140 in early differentiated 3T3-L1 cells where lysine methylation readily occurs. Once the culture is more differentiated, arginine methylation is increased (Figure 4) to facilitate the export of RIP140 to the cytoplasm as shown previously.16
While we have confirmed that arginine methylation of RIP140 can be mediated by the action of several protein arginine methyltransferases (PRMTs), we were unable to unambiguously identify specific lysine methylase responsible for effective RIP140’s lysine methylation. Several enzymes that methylate specific lysine residues of histone tails, the histone lysine methyltransferases (HKMTs), have been isolated and characterized.5 Among these known members, SET9 (also known as SET7) is a protein lysine methyltransferase (PKMT) with a specific histone methyltransferase (HMTase) activity for H3-K4, and it can mono- and di-methylate these substrates. SET7/9 also methylates p53 tumor suppressor, and recognizes a conserved K/R-S/T/A motif preceding the lysine substrate, with a propensity to bind aspartates and asparagines on the C-terminal side of the lysine target.37 The sequences spanning the modified Lys591 (PPYACSTPASKLTNTVPSHL), Lys653 (QSSGPGEEQRPCKQLLSGNP) and Lys757 (AANEQILMVKIKSEPCDDFQ) on RIP140 do not resemble the consensus target for SET7/9. Therefore, RIP140 is likely to be methylated by other PKMTs. Proteins containing a SET domain constitute a family, and are classified into at least four groups on the basis of structural or sequence similarities. Among these members, the SUV39H1 protein — a mammalian homologue of Drosophila position-effect variegation modifier Su(var)3–9 — has H3-K9 methyltransferase activity. SET1 and SET2 show a H3-K4 and a H3-K36 methyltransferase activity, respectively. PR-SET7 targets H4-K20; whereas G9a functions as a 'dual' methyltransferase, catalyzing reactions for histone H3-K9 and H3-K27.38 Recenty some non-histone substrates for G9a have been reported.39 To explore the PKMT responsible for lysine methylation of RIP140, we have tested SUV39H1 and G9a on RIP140. It appeared that G9a could marginally enhance the level of lysine methylation of RIP140 (by ~ 2 fold), and SUV39H1 exerted little effect (Data not shown). We have also confirmed that both enzymes could barely affect the repressive activity of RIP140. Therefore, it remains to be determined as to the specific methylase for lysine methylation of RIP140.
Supplementary Material
The supplementary information regarding database search for MS/MS spectra, protein and identity are included in the Supplementary Tables. The zoomed MS/MS spectra are included in Supplementary Figures. The supplementary information are available free of charge online at http://pubs.acs.org
Acknowledgments
This work was supported, in part by Philip Morris USA Inc. and by NIH grants DK54733, DK60521, K02-DA13926 and DA11190 to L.-N. Wei. The authors wish to thank Dr. LeeAnn Higgins at the Mass Spectrometry Consortium for the Life Sciences, University of Minnesota, for recording the mass spectra and cordial assistance in database search. We also thank Dr. Michael Stallcup and Dr. Janet Stavnezer for the generous gifts of the lysine methyltransferases constructs.
Abbreviations
- RIP140
receptor interacting protein 140
- HDAC3
histone deacetylase 3
- CtBP
carboxy-terminal binding protein
- PKMT
protein lysine methyltransferase
- HKMT
histone lysine methyltransferase
- PRMT
protein arginine methyltransferase
- IBMX
isobutylmethylxanthine
REFRENCES
- 1.Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 2.Pawson T. Protein modules and signalling networks. Nature. 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 3.Hunter T. Signaling—2000 and beyond. Cell. 2000;100:113–127. doi: 10.1016/s0092-8674(00)81688-8. [DOI] [PubMed] [Google Scholar]
- 4.Lee DY, Teyssier C, Strahl BD, Stallcup MR. Role of protein methylation in regulation of transcription. Endocr. Rev. 2005;26:147–170. doi: 10.1210/er.2004-0008. [DOI] [PubMed] [Google Scholar]
- 5.Kouzarides T. Histone methylation in transcriptional control. Curr. Opin. Genet. Dev. 2002;12:198–209. doi: 10.1016/s0959-437x(02)00287-3. [DOI] [PubMed] [Google Scholar]
- 6.Rice JC, Briggs SD, Ueberheide B, Barber CM, Shabanowitz J, Hunt DF, Shinkai Y, Allis CD. Histone methyltransferases direct different degrees of methylation to define distinct chromatin domains. Mol. Cell. 2003;12:1591–1598. doi: 10.1016/s1097-2765(03)00479-9. [DOI] [PubMed] [Google Scholar]
- 7.McBride AE, Silver PA. State of the arg: protein methylation at arginine comes of age. Cell. 2001;106:5–8. doi: 10.1016/s0092-8674(01)00423-8. [DOI] [PubMed] [Google Scholar]
- 8.Chuikov S, Kurash JK, Wilson JR, Xiao B, Justin N, Ivanov GS, McKinney K, Tempst P, Prives C, Gamblin SJ, Barlev NA, Reinberg D. Regulation of p53 activity through lysine methylation. Nature. 2004;432:353–360. doi: 10.1038/nature03117. [DOI] [PubMed] [Google Scholar]
- 9.Kouskouti A, Scheer E, Staub A, Tora L, Talianidis I. Gene-specific modulation of TAF10 function by SET9-mediated methylation. Mol. Cell. 2004;14:175–182. doi: 10.1016/s1097-2765(04)00182-0. [DOI] [PubMed] [Google Scholar]
- 10.Huq MD, Tsai NP, Khan SA, Wei LN. Lysine trimethylation of retinoic acid receptor-alpha: a novel means to regulate receptor function. Mol. Cell. Proteomics. 2007;6:677–688. doi: 10.1074/mcp.M600223-MCP200. [DOI] [PubMed] [Google Scholar]
- 11.Chin HG, Estève PO, Pradhan M, Benner J, Patnaik D, Carey MF, Pradhan S. Automethylation of G9a and its implication in wider substrate specificity and HP1 binding. Nucleic Acids Res. 2007;35:7313–7323. doi: 10.1093/nar/gkm726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leonardsson G, Steel JH, Christian M, Pocock V, Milligan S, Bell J, So PW, Medina-Gomez G, Vidal-Puig A, White R, Parker MG. Nuclear receptor corepressor RIP140 regulates fat accumulation. Proc. Natl. Acad. Sci. USA. 2004;101:8437–8442. doi: 10.1073/pnas.0401013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huq MD, Khan SA, Park SW, Wei LN. Mapping of phosphorylation sites of nuclear co-repressor receptor interacting protein 140 by liquid chromatography-tandem mass spectroscopy. Proteomics. 2005;5:2157–2166. doi: 10.1002/pmic.200401090. [DOI] [PubMed] [Google Scholar]
- 14.Gupta P, Huq MD, Khan SA, Tsai NP, Wei LN. Regulation of co-repressive activity of and HDAC recruitment to RIP140 by site-specific phosphorylation. Mol. Cell. Proteomics. 2005;4:1776–1784. doi: 10.1074/mcp.M500236-MCP200. [DOI] [PubMed] [Google Scholar]
- 15.Huq MD, Wei LN. Post-translational modification of nuclear co-repressor receptor-interacting protein 140 by acetylation. Mol. Cell. Proteomics. 2005;4:975–983. doi: 10.1074/mcp.M500015-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Mostaqul Huq MD, Gupta P, Tsai NP, White R, Parker MG, Wei LN. Suppression of receptor interacting protein 140 repressive activity by protein arginine methylation. EMBO J. 2006;25:5094–5104. doi: 10.1038/sj.emboj.7601389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huq MD, Tsai NP, Lin YP, Higgins L, Wei LN. Vitamin B6 conjugation to nuclear corepressor RIP140 and its role in gene regulation. Nat. Chem. Biol. 2007;3:161–165. doi: 10.1038/nchembio861. [DOI] [PubMed] [Google Scholar]
- 18.Lee CH, Chinpaisal C, Wei LN. Cloning and characterization of mouse RIP140, a corepressor for nuclear orphan receptor TR2. Mol. Cell. Biol. 1998;18:6745–6755. doi: 10.1128/mcb.18.11.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee DY, Northrop JP, Kuo MH, Stallcup MR. Histone H3 lysine 9 methyltransferase G9a is a transcriptional coactivator for nuclear receptors. J. Biol. Chem. 2006;281:8476–8485. doi: 10.1074/jbc.M511093200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley SP, Kaminski DA, Peters AH, Jenuwein T, Stavnezer J. The histone methyltransferase Suv39h1 increases class switch recombination specifically to IgA. J. Immunol. 2006;177:1179–1188. doi: 10.4049/jimmunol.177.2.1179. [DOI] [PubMed] [Google Scholar]
- 21.Christian M, Kiskinis E, Debevec D, Leonardsson G, White R, Parker MG. RIP140-targeted repression of gene expression in adipocytes. Mol. Cell. Biol. 2005;25:9383–9391. doi: 10.1128/MCB.25.21.9383-9391.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahmus ME, Hart GW. RNA polymerase II is a glycoprotein. Modification of the COOH-terminal domain by O-GlcNAc. J. Biol. Chem. 1993;268:10416–10424. [PubMed] [Google Scholar]
- 23.Cheng X, Hart GW. Glycosylation of the murine estrogen receptor-α. J. Steroid Biochem. Mol. Biol. 2000;75:147–148. doi: 10.1016/s0960-0760(00)00167-9. [DOI] [PubMed] [Google Scholar]
- 24.Wu RC, Qin J, Yi P, Wong J, Tsai SY, Tsai MJ, O’Malley BW. Selective phosphorylations of the SRC-3/AIB1 coactivator integrate genomic responses to multiple cellular signaling pathways. Mol. Cell. 2004;15:937–949. doi: 10.1016/j.molcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Zhang K, Tang H, Huang L, Blankenship JW, Jones PR, Xiang F, Yau PM, Burlingame AL. Identification of acetylation and methylation sites of histone H3 from chicken erythrocytes by high-accuracy matrix-assisted laser desorption ionization-time-of-flight, matrix-assisted laser desorption ionization-postsource decay, and nanoelectrospray ionization tandem mass spectrometry. Anal. Biochem. 2002;306:259–269. doi: 10.1006/abio.2002.5719. [DOI] [PubMed] [Google Scholar]
- 26.Dasgupta A, Jung KJ, Jeong SJ, Brady JN. Inhibition of methyltransferases results in induction of g2/m checkpoint and programmed cell death in human T-lymphotropic virus type 1-transformed cells. J. Virol. 2008;82:49–59. doi: 10.1128/JVI.01497-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei LN, Hu X, Chandra D, Seto E, Farooqui M. Receptor-interacting protein 140 directly recruits histone deacetylases for gene silencing. J. Biol. Chem. 2000;275:40782–40787. doi: 10.1074/jbc.M004821200. [DOI] [PubMed] [Google Scholar]
- 28.Hu X, Chen Y, Farooqui M, Thomas MC, Chiang CM, Wei LN. Suppressive effect of receptor-interacting protein 140 on coregulator binding to retinoic acid receptor complexes, histone-modifying enzyme activity, and gene activation. J. Biol. Chem. 2004;279:319–325. doi: 10.1074/jbc.M307621200. [DOI] [PubMed] [Google Scholar]
- 29.Vo N, Fjeld C, Goodman RH. Acetylation of nuclear hormone receptor-interacting protein RIP140 regulates binding of the transcriptional corepressor CtBP. Mol. Cell. Biol. 2001;21:6181–6188. doi: 10.1128/MCB.21.18.6181-6188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christian M, Tullet JM, Parker MG. Characterization of four autonomous repression domains in the corepressor receptor interacting protein 140. J. Biol. Chem. 2004;279:15645–15651. doi: 10.1074/jbc.M313906200. [DOI] [PubMed] [Google Scholar]
- 31.Haynes PA, Gygi SP, Figeys D, Aebersold R. Proteome analysis: biological assay or data archive? Electrophoresis. 1998;19:1862–1871. doi: 10.1002/elps.1150191104. [DOI] [PubMed] [Google Scholar]
- 32.Ho PC, Gupta P, Tsui YC, Ha SG, Huq M, Wei LN. Modulation of lysine acetylation-stimulated repressive activity by Erk2-mediated phosphorylation of RIP140 in adipocyte differentiation. Cell Signal. 2008;20:1911–1919. doi: 10.1016/j.cellsig.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta P, Ho PC, Huq MDM, Khan AA, Pei NP, Wei LN. PKCe stimulated arginine methylation of RIP140 for its nuclear-cytoplasmic export in adipocyte differentiation. PLoS ONE. 2008;3:e2658. doi: 10.1371/journal.pone.0002658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishizawa T, Nozaki Y, Ueda T, Takeuchi N. The human mitochondrial translation release factor HMRF1L is methylated in the GGQ motif by the methyltransferase HMPrmC. Biochem Biophys Res Commun. 2008;373:99–103. doi: 10.1016/j.bbrc.2008.05.176. [DOI] [PubMed] [Google Scholar]
- 35.Kirmizis A, Santos-Rosa H, Penkett CJ, Singer MA, Vermeulen M, Mann M, Bähler J, Green RD, Kouzarides T. Arginine methylation at histone H3R2 controls deposition of H3K4 trimethylation. Nature. 2007;449:928–932. doi: 10.1038/nature06160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guccione E, Bassi C, Casadio F, Martinato F, Cesaroni M, Schuchlautz H, Lüscher B, Amati B. Methylation of histone H3R2 by PRMT6 and H3K4 by an MLL complex are mutually exclusive. Nature. 2007;449:933–937. doi: 10.1038/nature06166. [DOI] [PubMed] [Google Scholar]
- 37.Couture JF, Collazo E, Hauk G, Trievel RC. Structural basis for the methylation site specificity of SET7/9. Nat. Struct. Mol. Biol. 2006;13:140–146. doi: 10.1038/nsmb1045. [DOI] [PubMed] [Google Scholar]
- 38.Kouskouti A, Scheer E, Staub A, Tora L, Talianidis I. Gene-specific modulation of TAF10 function by SET9-mediated methylation. Mol. Cell. 2004;14:175–182. doi: 10.1016/s1097-2765(04)00182-0. [DOI] [PubMed] [Google Scholar]
- 39.Rathert P, Dhayalan A, Murakami M, Zhang X, Tamas R, Jurkowska R, Komatsu Y, Shinkai Y, Cheng X, Jeltsch A. Protein lysine methyltransferase G9a acts on non-histone targets. Nat. Chem. Biol. 2008;4:344–346. doi: 10.1038/nchembio.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplementary information regarding database search for MS/MS spectra, protein and identity are included in the Supplementary Tables. The zoomed MS/MS spectra are included in Supplementary Figures. The supplementary information are available free of charge online at http://pubs.acs.org