Indigenous to southeast Asia, the plant genus Aglia includes several species that produce a range of cyclopenta[b]tetrahydro-benzofuran-containing metabolites1 including rocaglamide 1, isolated from the roots and stems of Aglia elliptifolia by King.2 King's initial report indicated that 1 showed significant in vivo activity in P388 lymphocytic leukemia-infected mice.2 Since then, rocaglamide and related compounds have shown cytostatic and cytotoxic activity against a variety of human cancer cell lines with IC50 values ranging from 1.0–6.0 ng/mL.3 Stereoselective synthesis of the dense substitution pattern of these targets is a formidable synthetic challenge: the molecules bear five contiguous stereocenters and cis-aryl groups on adjacent carbons. In 27 years of effort, only a handful of completed total syntheses have been reported, evidence of the difficulties associated with the synthesis of rocaglate natural products.4 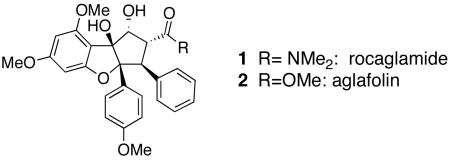
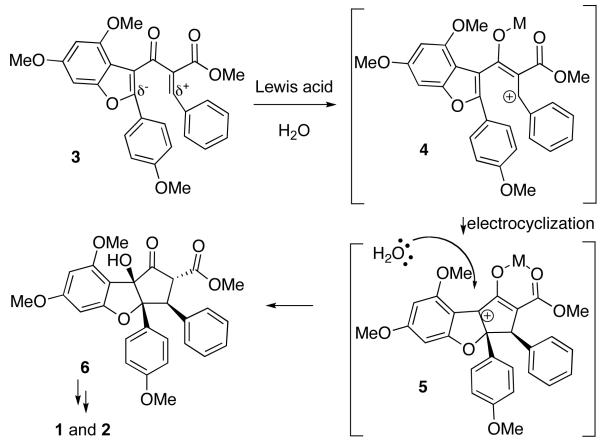
The original plan for the synthesis of rocaglamide focused on the Nazarov cyclization5 of ketone 3, a compound predicted to be reactive due to the juxtaposition of an electon-rich benzofuran and an electron-poor alkylidene β-ketoester.6 Substrate polarization has proven successful in cyclizing a range of heteroaromatic compounds under mild Lewis acid catalysis.7 It was hoped that Lewis acid activation of 3 would generate pentadienyl cation 4, which would undergo conrotatory cyclization to give oxyallyl cation 5 (Scheme 1). It was hoped that the tertiary alcohol could be installed by trapping the cation with water (see 6).8
Scheme 1.
Initial approach: Interrupted Nazarov Cyclization
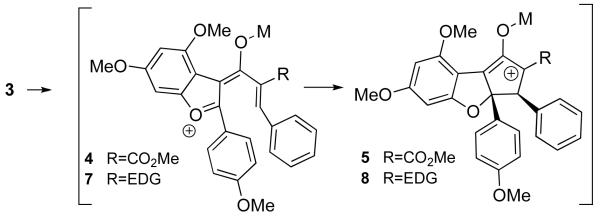
Unfortunately, compound 3 failed to cyclize in the presence of any Lewis acid/trapping agent combination. Only products of hydrolysis were observed. The failure of our original model led to an alternative analysis of the pentadienyl cation, which suggested that intermediate 4 might have significant carbocation character at the 2-position of the benzofuran (Scheme 2).9 Viewed this way, substrate 3 is not favorably polarized for cyclization, because both termini of the pentadienyl cation 4 are electron-deficient.
Scheme 2.
Alternative analysis of pentadienyl cation polarization
If this alternative analysis is correct, installing an electron-donating substituent in place of the ester should reestablish complementary polarization between the reacting termini of the pentadienyl cation (see 7). The oxyallyl cation intermediate (8) is also stabilized in this scenario, which should improve cyclization efficiency.6,10
To test these ideas, we chose to explore the epoxidation of appropriately substituted alkoxyallenes 9. Epoxide opening should give direct access to a pentadienyl cation of type 10 poised for cyclization. Similar transformations have been reported by Goré,11 Corey,12 and Cha,13 and studied by De Lera.14 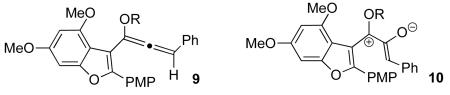
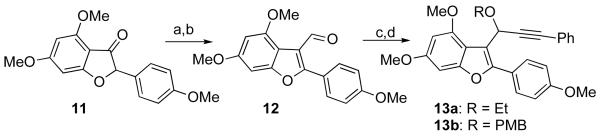
Synthesis began with alkylation of 114b with vinyl magnesium bromide, followed by osmylation and periodate cleavage of the resulting 3-vinyl benzofuran to give aldehyde 12 (Scheme 3). Alkylation with phenylacetylene and protection of the resultant propargyl alcohol with ethyl iodide or p-methoxy benzyl chloride gave propargyl ethers 13a and 13b, respectively.
Scheme 3.
Synthesis of propargyl ethers 13a
Reagents and conditions: (a) CeCl3, vinyl magnesium bromide, then HCl 1M, 65%; (b) (i) OsO4 (4 mol%), NMO (1.2 equiv.), acetone/t-BuOH/H2O; (ii) NaIO4, THF/H2O; (c) phenylacetylene, n-BuLi, THF; (d) KH, EtI, THF, 64% or KH, NaI, PMBCl, THF, 69% (over three steps).
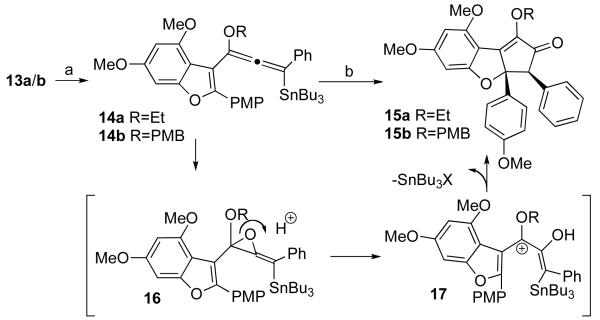
Deprotonation at the propargylic position of 13 with tert-butyllithium gave rise to an allenyl anion, which was trapped with tri-n-butyltin chloride to give stannyl alkoxyallene 14.15 It was not possible to obtain the hydridoalkoxyallene using this protocol: if the allenyl anion was quenched with water, methanol, or imidazole, protonation occurred at the benzofuranylic position exclusively. Treatment of 14 with excess m-CPBA gave 15 (Scheme 4). This novel oxidation / Nazarov cyclization cascade is thought to commence with epoxidation of the allenol ether to generate allene oxide 16. Epoxide opening, facilitated by both the furanyl and ether oxygen atoms and the acidic reaction conditions, unveils pentadienyl cation 17, which cyclizes to form cyclopentenone 15. Cleavage of the tributylstannyl group probably occurs prior to cyclization, but this has not been confirmed. Only one diastereomer was found in the reaction mixture, and its relative configuration was confirmed by X-ray analysis of 15a.
Scheme 4.
Nazarov cyclization initiated by epoxide openinga
Reagents and conditions: (a) t-BuLi, Bu3SnCl, Et2O, −40°C (b) m-CPBA (4 equiv.), DMF, rt, 40–50% over two steps.
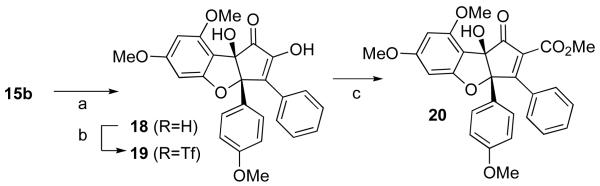
All attempts to functionalize ethyl enol ether 15a failed. The p-methoxybenzyl derivative 15b was prepared to explore the possibility of effecting both enol ether cleavage and installation of the benzylic hydroxyl group under oxidative conditions. Indeed, treatment of 15b with excess DDQ gave diosphenol 18 in excellent yield (Scheme 5). Enol 18 was converted to triflate 19 and then subjected to palladium-mediated carbonylation to install the final C-C linkage (see 20).
Scheme 5.
Completion of the rocaglamide skeleton
Reagents and conditions: (a) DDQ (4 equiv.), DCM, 71% (b) KHMDS, PhNTf2, THF, 0°C, 83% (c) Pd(PPh3)4, CO, MeOH, Hünig's base, THF, 65°C, 79%.
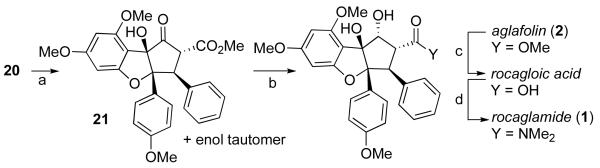
The synthetic work of Trost guided the elaboration of 20 into the natural product 1.4a Hydrogenation of 20 over PtO2 gave 21 as a single diastereomer (Scheme 6). Templated reduction of the ketone afforded the natural product aglafolin (2), and saponification followed by amide formation furnished rocaglamide (1).
Scheme 6.
Completion of the synthesisa
Reagents and conditions: (a) PtO2, H2, EtOH, rt, 65% (b) NaHB(OAc)3, MeCN/AcOH, 56% (c) LiOH, THF/H2O, 82%; (d) Me2NH•HCl, DCC, DMAP, 60%.
The synthetic strategy developed provides natural products aglafolin and rocaglamide can be prepared in 11 and 13 steps, respectively, from known benzofuranone 11,4b and every step is highly diastereoselective. The key transformation is Nazarov cyclization of a pentadienyl cation generated in an unusual way: through peracid oxidation of an allenol ether. Development of an enantioselective approach to the rocaglate natural products using this oxidation / electrocyclization sequence is currently underway in the laboratory.
Supplementary Material
Acknowledgements
The authors thank Dan Canterbury and Wei He (University of Rochester) and Professor P. Magnus (University of Texas, Austin) for helpful discussions. We are also grateful to Dr. W. Brennessel (University of Rochester) for solving X-ray crystallographic structures, and Dr. A. Bergmann (SUNY Buffalo) for carrying out high-resolution mass spectrometry. This work was funded by the NSF (CAREER: CHE-0349045) and the NIH (NIGMS R01 GM079364).
Footnotes
Supporting Information Available: Experimental procedures for the preparation of all compounds, characterization data, X-ray crystal structure data for compounds 15a and 19. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Proksch P, Edrada R, Eble R, Bohnenstengle FI, Nugroho BW. Curr. Org. Chem. 2001;5:923. and references therein. [Google Scholar]
- 2.King ML, Chiang CC, Ling HC, Fujita E, Ochiai M, McPhail AT. J. Chem. Soc., Chem. Commun. 1982:1150. [Google Scholar]
- 3.Zhu JY, Lavrik IN, Mahlknecht U, Giaisi M, Proksch P, Krammer PH, Li-Weber M. Int. J. Cancer. 2007;121:1839. doi: 10.1002/ijc.22883. and references therein. [DOI] [PubMed] [Google Scholar]
- 4.Trost BM, Greenspan PD, Yang BV, Saulnier MG. J. Am. Chem. Soc. 1990;112:9022. (enantioselective synthesis). Taylor RJK, Davey AE, Schaeffer MJ. J. Chem. Soc. Perkin Trans. 1992;1:2657. and references therein. Dobler MR, Bruce I, Cederbaum F, Cooke NG, Diorazio LJ, Hall RG, Irving E. Tetrahedron Lett. 2001;42:8281. Kraus GA, Sy JO. J. Org. Chem. 1989;54:77–83. Porco JA, Jr., Gerard B, Sangji S, O'Leary DJ. J. Am. Chem. Soc. 2006;128:7754. doi: 10.1021/ja062621j. (enantioselective synthesis) and references therein. El Sous M, Khoo ML, Holloway G, Owen D, Scammells PJ, Rizzacasa MA. Angew. Chem., Int. Ed. Engl. 2007;46:7835. doi: 10.1002/anie.200702700. Li HS, Fu B, Wang MA, Li N, Liu WJ, Xie ZQ, Ma YQ, Qin ZH. Eur. J. Org. Chem. 2008:1753.
- 5.(a) Habermas KL, Denmark SE, Jones TK. Org. React. (N.Y.) 1994;45:1. [Google Scholar]; (b) Frontier AJ, Collison C. Tetrahedron. 2005;61:7577. [Google Scholar]; (c) Pellissier H. Tetrahedron. 2005;61:6479. [Google Scholar]; (d) Tius MA. Eur. J. Org. Chem. 2005;11:2193. [Google Scholar]
- 6.(a) He W, Sun X, Frontier AJ. J. Am. Chem. Soc. 2003;125:14278. doi: 10.1021/ja037910b. [DOI] [PubMed] [Google Scholar]; (b) He W, Herrick IR, Atesin TA, Caruana PA, Kellenberger CA, Frontier AJ. J. Am. Chem. Soc. 2008;130:1003. doi: 10.1021/ja077162g. [DOI] [PubMed] [Google Scholar]
- 7.Malona JA, Colbourne JM, Frontier AJ. Org. Lett. 2006;8:5661. doi: 10.1021/ol062403v. [DOI] [PubMed] [Google Scholar]
- 8.Trapping of the intermediate oxyallyl cation with water has been observed in our laboratory (He W, Huang J, Frontier AJ. unpublished results.). For recent reports of trapping with other heteroatom nucleophiles see: Rostami A, Wang Y, Arif AM, McDonald R, West FG. Org. Lett. 2007;9:703. doi: 10.1021/ol070053m. Dhoro F, Kristensen TE, Stockmann V, Yap GPA, Tius MA. J. Am. Chem. Soc. 2007;129:7256. doi: 10.1021/ja0718873. Marx VM, Burnell DJ. Org. Lett. 2009;11:1229. doi: 10.1021/ol900029d.
- 9.Experimental evidence for this mode of reactivity had been reported for a related benzofuran: see Magnus P, Stent MS. Org. Lett. 2005;7:3853. doi: 10.1021/ol0513793. We enjoyed sharing findings with the Magnus group over the course of these studies.
- 10.Denmark SE, Habermas KL, Hite GA. Helv. Chim. Acta. 1988;71:168. [Google Scholar]
- 11.Doutheau A, Goré J, Malacria M. Tetrahedron Lett. 1977;33:2393. [Google Scholar]
- 12.Corey EJ, Ritter K, Yus M, Nájera C. Tetrahedron Lett. 1987;28:3547. [Google Scholar]
- 13.(a) Kim SJ, Cha JK. Tetrahedron Lett. 1988;29:5613. [Google Scholar]; (b) Hess BAJ, Smentek L, Brash AR, Cha JK. J. Am. Chem. Soc. 1999;121:5603. [Google Scholar]
- 14.Lopez CS, Faza ON, York DM, de Lera AR. J. Org. Chem. 2004;69:3635. doi: 10.1021/jo049620z. [DOI] [PubMed] [Google Scholar]
- 15.Landor SR, Landor PD. The Chemistry of Allenes. Academic Press Inc.; New York: 1982. pp. 155–159. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.