Abstract
Hyperthermic stress is known to trigger the loss of unicellular algae from a number of symbiotic cnidarians, a phenomenon commonly referred to as bleaching. Oxidative and nitrosative stress have been suggested to play a major role during the process of bleaching, however the underlying molecular mechanisms are still poorly understood. In animals, the intracellular tripeptide glutathione (GSH) is involved in antioxidant defense, redox homeostasis and intracellular redox signaling. Therefore, we tested the hypothesis that hyperthermal stress-induced bleaching in Aiptasia pallida, a model for symbiotic cnidarians, results in increased levels of GSH synthesis. We report the cDNA sequence and functional analysis of the catalytic subunit of glutamate-cysteine ligase (GCLC), which catalyzes the rate-limiting step in GSH biosynthesis. In a time-series experiment, both GCLC gene expression and total GSH levels increased 4- and 1.5-fold, respectively, in response to hyperthermal stress. These results suggest that hyperthermal stress triggers adaptive increases in intracellular GSH biosynthesis in cnidarians as a protective response to oxidative/ nitrosative stress. Our results show the conserved function of GCLC and GSH across animals while placing a new perspective on the role of GSH in redox signaling during cnidarian bleaching.
Keywords: Aiptasia pallida, cDNA, Cnidarian bleaching, Glutamate-cysteine ligase, Glutathione, Hyperthermal stress, Nitrosative stress, Oxidative stress
1. Introduction
A stable symbiosis between many cnidarians, predominantly corals and anemones, and dinoflagellate algae in the phylum Symbiodinium forms the foundation of the structural and biological complexity of coral reefs. The mutual benefit for the partners in this symbiotic association has been studied extensively (Muscatine, 1990; Trench, 1993; Knowlton and Rohwer, 2003). In return for nutrients, the endosymbiotic algae supply their host with photosynthates and amino acids (Trench, 1979; Muscatine, 1990). However, the photosynthetic activity of the algae also subjects the host to a hyperoxic environment (Dykens and Shick, 1982; Kühl et al., 1995; Richier et al., 2003). Molecular oxygen generated in this process can, in turn, be reduced to form reactive oxygen species (ROS) such as superoxide ( ), hydrogen peroxide (H2O2) or, via Fenton chemistry, hydroxyl radicals (HO·). In order to counteract the cytotoxic effects of ROS, cnidarian hosts and symbiotic algae utilize both enzymatic and non-enzymatic strategies (for a review see Lesser, 2006). For example, the activities of superoxide dismutase (SOD) and catalase have been measured in the sea anemone Anthopleura elegantissima (Dykens and Shick, 1984). Antioxidant defense mechanisms in Symbiodinium spp. include, e.g. ascorbate peroxidase activity (Lesser et al., 1990) and the production of mycosporine-like amino acids (Shick and Dunlap, 2002). A positive correlation between host SOD activity and chlorophyll concentration was used as a proxy for the generation of oxygen in symbiotic anemones (Dykens and Shick, 1982). Similarly, algal SOD and ascorbate peroxidase activities were found increased at higher temperature and/ or UV radiation (Lesser, 1996). Recent advances in the study of antioxidant enzymes from symbiotic cnidarians include the characterization of SOD and catalase isoforms in the symbiotic sea anemone Anemonia viridis (Richier et al., 2003; Merle et al., 2007).
Despite being equipped with such antioxidant defense mechanisms, prolonged hyperthermic and/or UV-stress evoke oxidative stress in these organisms and cause the symbiotic relationship to break down (Dykens et al., 1992; Lesser, 1996; Nii and Muscatine, 1997). This phenomenon is commonly referred to as bleaching, and an ‘oxidative theory of coral bleaching’ has been proposed by (Downs et al., 2002). Current models of bleaching suggest that 1) temperature and/or light stress induce photoinhibition, and 2) a concomitant damage of the reaction center protein Dl of the algal photosystem II results in elevated generation of ROS (Jones et al., 1998; Warner et al., 1999). H2O2 generated by this process could readily diffuse through biological membranes, i.e. from the chloroplast into the cytoplasm of the algae (Asada and Takahashi, 1987), and eventually into the host cell. A correlation between increased oxidative damage products and seasonal coral bleaching lends additional support for an involvement of oxidative stress during natural bleaching events (Downs et al., 2002).
Experimental studies have demonstrated that the hyperthermic stress-induced loss of symbiotic algae in the sea anemone Aiptasia pallida is associated with nitric oxide generation (Perez et al., 2001 ; Perez and Weis, 2006). These results strongly suggest a role of free radicals as a signaling interface between exposure to hyperthermic stress and a cellular response. This response may include necrosis, apoptosis and/or autophagy (Dunn et al., 2004; Dunn et al., 2007a; Dunn et al., 2007b). In mammals, redox signaling pathways are involved in a number of cellular functions such as the regulation of gene expression, cell proliferation and apoptosis (Forman et al., 2003).
The aim of this study was to better understand the intermediate steps that may link the formation of free radicals and signaling events that eventually result in the loss of endosymbiotic algae. Glutathione (γ-glutamylcysteinylglycine; GSH), a non gene-encoded tripeptide, is critically important in maintaining the cellular redox homeostasis. GSH serves antioxidant, cytoprotective and regulatory functions through its linkage to peroxidases, glutathione-S-transferases, and proteins that are regulated by reversible S-glutathionylation, respectively. In many organisms, the up-regulation of genes involved in GSH biosynthesis is an adaptive response to sub-lethal oxidative stress (Dickinson and Forman, 2002). S-glutathionylation in analogy to phosphorylation of proteins may serve as a signaling interface (Fratelli et al., 2005; Ghezzi, 2005). The role of glutathione as both a proxy for the redox state of the cell and potential regulator of proteome activity has not been evaluated in the study of cnidarian bleaching. Whether cnidarians are able to upregulate GSH biosynthesis during hyperthermic stress calls for detailed investigation.
We hypothesized that hyperthermic stress-induced oxidative stress will result in an up-regulation of the catalytic subunit of the glutamatecysteine ligase (GCLC) gene, which catalyzes the rate-limiting step in glutathione biosynthesis. Our results include the molecular cloning and sequence analysis of the GCLC gene of the symbiotic sea anemone A. pallida. The availability of the cDNA sequence allowed us to demonstrate an up-regulation of GCLC gene expression as a response to hyperthermic stress-induced oxidative stress. In addition, complementary measurements of total glutathione levels corroborated the conserved function of GCLC in A. pallida.
2. Materials and methods
2.1. Cloning and sequence analysis of the GCLC (A. pallida) gene
A partial sequence of the GCLC (A. pallida) gene, obtained from an expressed sequence tag (EST) library (Sunagawa et al. in prep), was used as a template to design sequence-specific primers (Table 1), which were used in a combination of 5′ and 3′ rapid amplification of cDNA ends (RACE) reactions using the GeneRacer kit (Invitrogen). One symbiotic A. pallida individual was heat-stressed (33 °C) for 24 h, before total RNA was extracted. Briefly, the freshly sampled anemone was frozen on dry ice and pulverized using a mortar and pestle. The powder was vortexed for 1 min in 1 mL QIAzol lysis reagent (Qiagen). For the remainder of the protocol, we followed the manufacturer's instructions, with the only modification being a second chloroform extraction prior to isopropanol precipitation. Total RNA was cleaned up using the RNeasy Mini Kit (Qiagen) and the integrity analyzed on an Aligent 2100 bioanalyzer.
Table 1.
Oligonucleotide primer sequences used
| Oligonucleotide primer | Sequence (5′→ 3′) |
|---|---|
| Ap_GCLC_PRl | CTCAAAGTCTGTCAATTGGACCT |
| GeneRacer 5′primer | CGACTGGAGCACGAGGACACTGA |
| Ap_GCLC_PFl(rc) | GGTCTGAACTCAACTCTCCATCCAAT |
| GeneRacer 5′ nested primer | GGACACTGACATGGACTGAAGGAGTA |
| M 13 forward primer | GTAAAACGACGGCCAG |
| M 13 reverse primer | CAGGAAACAGCTATGAC |
| Ap_GCLC_Fl | CTTGGATCGCCTCTTTCTTG |
| GeneRacer 3′ primer | GCTGTCAACGATACGCTACGTAACG |
| Ap GCLC F3 | ACGCAAAATTCCAACCACTTTG |
| GeneRacer 3′ nested primer | CGCTACGTAACGGCATGACAGTG |
| Ap_GCLC_PF2 | ATCCAGACTACAAGCAGGACTCG |
| Ap_GCLC_qFl | TGCGCGAAAGGATAAGTTCTACT |
| Ap_GCLC_qRl | CAGACTCACAGCACTCTGAATGG |
High-quality total RNA was subjected to the GeneRacer Kit (Invitrogen) to produce RACE-ready cDNA according to the manufacturer's instructions. 5′ and 3′ RACE products were produced by a modified touchdown and nested polymerase chain reaction (PCR) approach. Briefly, in a first, modified touchdown PCR reaction (94 °C-2 min; 94 °C-30 s, 72 °C-1 min (5×); 94 °C-30 s, 70 °C 1 min (5×); 94 °C-30 s, 62 °C-30 s, 68 °C-2 min (25×), 68 °C-10 min; Platinum Taq DNA Polymerase High Fidelity (Invitrogen)), the gene-specific primer Ap_GCLC_PRl was used in combination with the GeneRacer 5′ primer. 1 µL of this reaction was used in a second, nested PCR (94 °C-2 min; 94 °C-30 s, 68 °C-2 min (20×); 68 °C-10 min) using the gene-specific Ap_GCLC_PFl(rc) primer and the GeneRacer 5′ nested primer. The PCR products were analyzed on a 1% agarose gel and showed 3 bands of ∼1300 to 1500 bp fragment, which were gel excised and cleaned up using the QIAquick Gel Extraction Kit (Qiagen). The 5′RACE products from each band were cloned into the pCR2.1-TOPO vector using the TOPO TA Cloning Kit (Invitrogen) and sequenced using M13 forward and M13 reverse primers. The same cycling conditions as above were applied to generate 3′ RACE products using the gene-specific primer Ap_GCLC_Fl in combination with the GeneRacer 3′ primer. Again, 1 µL of the product served as a template in a nested PCR using the gene-specific Ap_GCLC_F3 and the GeneRacer 3′ nested primer. The resulting product of ∼1500 bp was cloned into the pCR2.1-TOPO vector as described above and sequenced using Ap_GCLC_F3, Ap_GCLC_PF2 and GeneRacer 3′ nested primer as sequencing primers. All RACE products were sequenced at the University of California Berkeley DNA sequencing facility. All primer sequences used in this study are shown in Table 1. Nucleotide and protein sequence analyses were performed using the BioEdit sequence alignment software (Ibis Biosciences).
2.2. Aiptasia culture and thermal stress experiment
A. pallida individuals, originally collected off the Florida coast (purchased through Ward's Natural Science, Rochester, NY, USA), were cultured at 27 °C in artificial seawater (Instant Ocean, 33 ppt), where they were kept on a 12/12-hour light/dark-cycle and fed every other day with freshly hatched brine shrimp. Randomly chosen individuals (approx. 100 ±50 mg wet weight) were transferred to the experimental tank and acclimated for 6 days (27.65 °C±0.12 °C, 33 ppt, 12/12-hour light/ dark-cycle). Temperature was then increased at a rate of 2 °C per hour to 32.13 ±0.10 °C Sample replicates (n = 6) were taken (always after 6 h of light exposure) 1 day prior to (control), and 3 h, 24 h, 72 h and 196 h after reaching the treatment temperature. Sampled individuals were immediately frozen on dry ice and stored at −80 °C before further processing.
2.3. Quantitative polymerase chain reaction (qPCR)
For all qPCR reactions, we used the Power SYBR Green PCR Master Mix (Applied Biosystems) on an Applied Biosystems 7300 Real-Time PCR System. Total RNA was extracted as described above, and digested with DNAse I (1 U/µL; SigmaAldrich) according to manufacturer's instructions to remove contaminating genomic DNA. Subsequently, cDNA was synthesized using the SuperScriptIII First Strand cDNA Synthesis Kit (Invitrogen). For quantitative PCR (qPCR) analysis, an 83 bp long fragment of gclc was selected using the software PrimerExpress (Applied Biosystems). We verified that (a) the primer pair Ap_GCLC_qFl/ Ap_GCLC_qRl spanned over an intron (∼500 bp) region by using genomic DNA from apo-symbiotic anemones (without symbiotic algae) as template DNA, and (b) that the dissociation curve yielded a single peak indicating specific amplification of the target amplicon (data not shown). A standard curve was generated using a dilution-series of total RNA concentrations(50–3.125 ng). Based on these results, we used 25 ng of total RNA in all reactions. Initial experiments using β-actin as a reference gene showed that this gene was unsuitable for a thermal stress experiment due to high variability in gene expression (data not shown). For this reason, we decided to normalize our data to total RNA concentrations, which is a valuable approach if the integrity of the total RNA is quality controlled (Bioanalyzer) and concentrations determined accurately (Huggett et al., 2005). Fold-changes are reported as 2−Δct differences normalized to the control time point.
2.4. Determination of glutathione and total protein amounts
Total GSH, i.e. the sum of GSH and 2 × glutathione disulfide (GSSG) concentrations, were assayed by HPLC according to a modified protocol originally described in Fariss and Reed (1987). To the frozen anemone, 0.55 mL of 10% perchloric acid (HPLC grade, SigmaAldrich) containing 1 mM BPDS (Fluka) and 7.5 nmol of γ-glutamylglutamic acid per 0.45 mL (used as internal standard) was added on ice. The complete anemone was homogenized using a tissue homogenizer for 7 s. The protein was pelleted in a tabletop microcentrifuge for 5 min at 16,000 ×g at 4 °C, and 0.45 mL of the acid-extracts were transferred to new 1.5 mL centrifuge tubes. Total protein concentrations were determined using the BCA Protein Assay Kit (Pierce) after dissolving the protein pellet in 1 mL of 1 N NaOH.
To the acid-extracts, 45 µL of iodoacetic acid (100 mM) were added and the pH adjusted to 8–9 using a KOH (2 M)/KHCO3 (2.4 M) solution. After incubation of 15 min in the dark at room temperature, 0.45 mL of 1% dinitrobenzene (DNB) was added, vortexed and stored at 4 °C overnight. The next morning, 45 µL of L-lysine (1 M) was added to finish the reaction. After incubating the tubes at 4 °C for 2 h, the precipitated salt was removed by centrifugation and the supernatant transferred to 1.5 mL autosampler vials (Shimadzu), before analysis by HPLC as described in (Fariss and Reed, 1987).
2.5. Statistical analysis
Statistical analyses for qPCR and glutathione levels were performed using the software SigmaStat. For the qPCR results, a KruskalWallis one-way ANOVA on ranks was used to test significant differences between time points. Post-hoc tests (Tukey) were performed, and a value of p < 0.05 was considered significant. Glutathione levels were analyzed using one-way ANOVA to test for significant differences between time points. Post-hoc tests were done according to the Fisher's LSD method.
3. Results
3.1. Cloning and sequence analysis
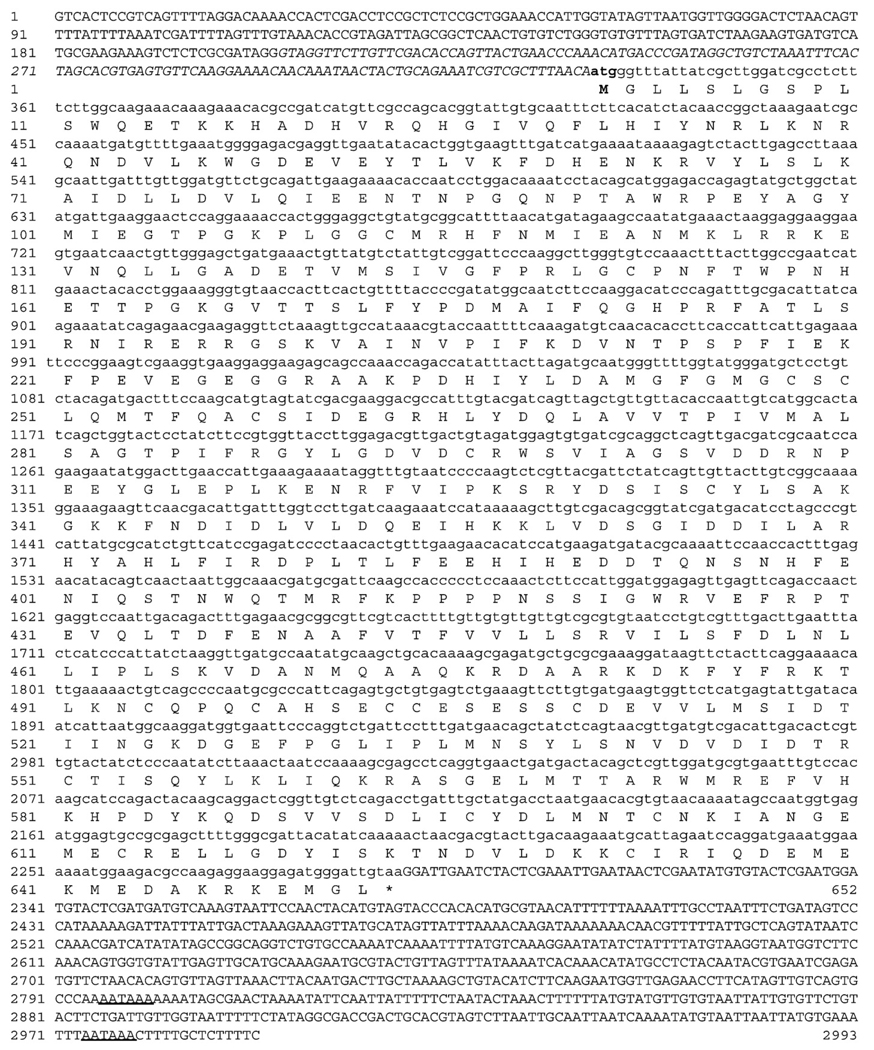
Agarose gel electrophoresis analysis of nested 5′ RACE reactions yielded two strong and a weaker third band, all of which were verified to originate from GCLC (A. pallida) cDNA sequences. Fifteen independent RACE clone sequences resulted in a 330bp long consensus 5′ untranslated region (UTR) upstream of the atg start codon (Fig. 1). In eight sequenced clones, the first 125 bases upstream of the start codon were missing, which is likely to explain the different bands we observed during gel electrophoresis. At this point, it is not possible to determine whether this is a result of alternative splicing, or multiple alleles of the same gene. The putative coding sequence of gclc (A. pallida) comprises 1959 nucleotides encoding a protein of 652 amino acids with a molecular weight of 74.4 kDa (GenBank accession no. EU659817; Fig. 1). The consensus sequence (8× coverage) of the 3′ UTR is 704 bp in length.
Fig. 1.
Cloning of the GCLC gene from the sea anemone Aiptasia pallida. Consensus (n = 15) cDNA sequence with the conceptual translation of the coding sequence are shown in lowercase. 5′ and 3′ untranslated regions (UTR) are in uppercase, and putative poly-adenylation signals (AATAAA) are underlined. Nucleotides in the 5′ UTR shown in italic were present in 8 sequenced clones only. The start codon and first methionine are shown in boldface.
3.2. Hyperthermic stress experiment
In the hyperthermic stress experiment, the temperature was kept constant (27.65°C±0.12) for a period of 160 h before the experimental tanks were heated (heating rate ∼ 1.2°C/h) to the treatment temperature (32.13°C±0.10). Anemones exposed to hyperthermic stress showed initial signs of stress about 24 h after the temperature ramp. Retraction of tentacles was observed, and expelled algal pellets were found in close proximity to the stressed anemones. At the same time, anemones appeared more bleached (paler coloration) than at the beginning of the experiment. This stressed phenotype was continuously observed during the entire period of hyperthermic stress. Although we did not take quantitative measurements, a reduction in body mass became apparent by the end of the experiment (196 h), and complete bleaching, i.e. an absolutely transparent appearance was not observed in any individual.
3.3. Expression of the GCLC (A. pallida) gene and total GSH levels
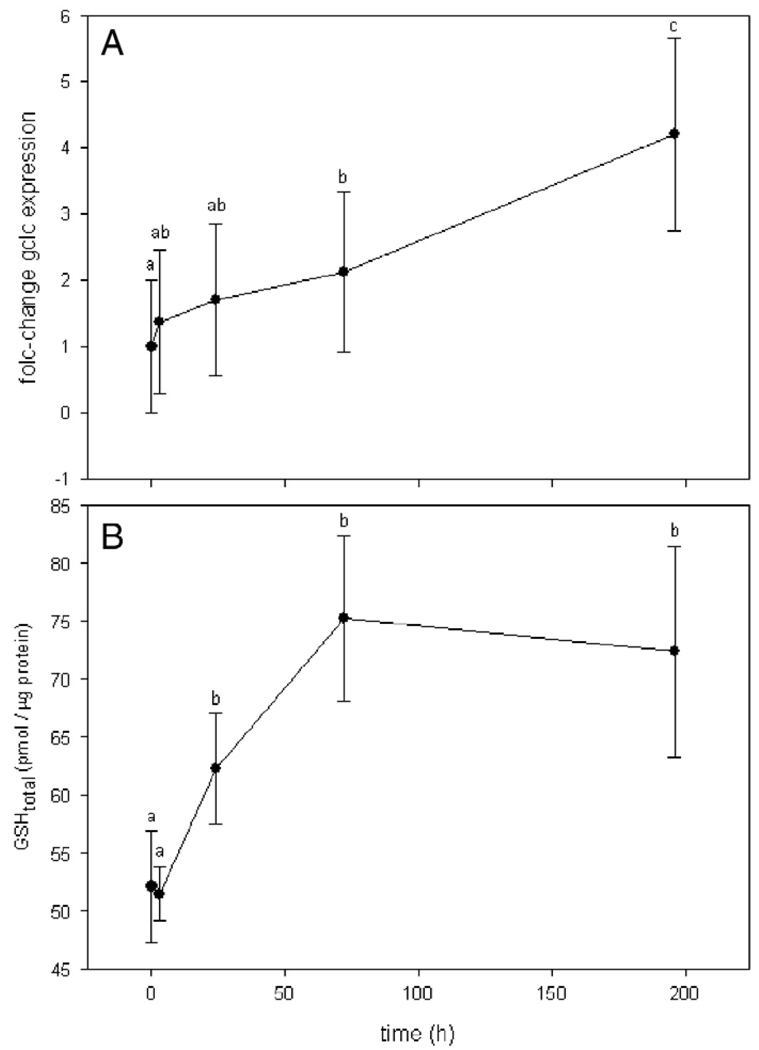
Anemones exposed to hyperthermal stress showed a continuous increase in GCLC gene expression. While the standard error of gene expression was high at each time point, statistical analyses suggested the 2-fold change after 72 h, and the 4-fold change after 196 h to be statistically significant (Fig. 2; ANOVA on ranks: p < 0.001, Tuckey's post hoc test: p < 0.05). A basal glutathione level of approximately 52 pmol/µg protein was determined, which did not change significantly after 3 h of thermal stress exposure (Fig. 2A). A significant increase (p < 0.05) in total glutathione levels was observed after 24 h (p < 0.05). After 72 h, a plateau of about 75 pmol/µg (±7.16) GSH was reached, which did not change significantly until the end of the experiment (Fig. 2B). The expression of the GCLC (A. pallida) gene increased constantly over the course of the experiment, and became statistically significant after 72 h (p < 0.05). Therefore, statistical significance of an increase in GCLC gene expression was lagging behind the increase in GSH.
Fig. 2.
GCLC gene transcript (A) and total glutathione levels (B) in response to hyperthermal stress. (A) Real-time PCR assays showing GCLC gene expression fold-changes in A. pallida individuals (n = 5) relative to 0 h (control) after exposure to thermal stress. Statistical differences between time points are denoted by different letter symbols. (B) Total glutathione levels determined by HPLC in A. pallida individuals (n = 6) after 0, 3, 24, 72 and 196 h of hyperthermal stress conditions. The water temperature was maintained at 27.65°C (±0.12°C) (control) for 160 h before it was increased to 32.13°C (±0.10°C) at time = 0 h (heating rate ∼ 1.2°C/h). Averages and standard errors for each time point are shown. Statistical differences between time points were calculated by ANOVA on ranks and averages with different letters are significantly different at a level of p < 0.05 (Tukey's post hoc test).
4. Discussion
This study describes the molecular cloning and sequencing of the complete cDNA sequence of GCLC in the symbiotic sea anemone A. pallida. We have shown that the GCLC (A. pallida) gene and total glutathione levels are responsive to hyperthermic stress. Based on these results, we suggest that hyperthermic stress indeed generates oxidative/nitrosative stress, and that GSH-related adaptive responses to oxidative/nitrosative stress are functional in cnidarians.
Our approach of obtaining the complete cDNA sequence of GCLC from the non-model organism A. pallida was facilitated by the availability of an expressed sequence tag (EST) data set (> 10,000 ESTs). This outcome represents one example for the potential uses of EST data for A. pallida, which we anticipate to become an invaluable genomic resource in the future (Sunagawa et al. in prep.).
Glutathione (GSH) is the most abundant low-molecular-weight thiol in the cell and involved in a variety of cellular functions (Dickinson and Forman, 2002). As an antioxidant, it acts as an electron donor and effectively detoxifies H2O2 via the activity of glutathione peroxidase. During this process, it becomes oxidized to form glutathione disulfide (GSSG). The reduction GSSG is then catalyzed by glutathione reductase (GR), which requires the availability of NADPH. The GSH/GSSG couple serves as an intracellular redox buffer, and a decrease in their ratio is indicative of oxidative stress. The rate-determining step in GSH biosynthesis is catalyzed by glutamatecysteine ligase (GCL), which is a heterodimer composed of a catalytic (GCLC) and a modulating (GCLM) subunit. Besides its antioxidant function, GSH is involved in a number of detoxification reactions via glutathione-S-transferases. Furthermore, many proteins are regulated, analogous to protein phosphorylation, by S-glutathionylation, which, together with its other functions, highlights the versatility and importance of GSH in cell physiology (Dalle-Donne et al, 2007).
Our data show a substantial increase in both GCLC gene expression (2- to 4-fold) and total GSH levels (∼ 50%) in the symbiotic sea anemone A. pallida as a response to hyperthermal stress (Fig. 2). Earlier studies have documented that heat-stress evokes oxidative and nitrosative stress in symbiotic anemones (Dunn et al., 2002; Perez and Weis 2006; Richier et al., 2006); thus, the increase in GCLC gene expression is likely to reflect the increased demand of intracellular GSH as an adaptive response. The increase in total GSH amounts could be due to a number of reasons, all of which may play important roles during the process of cnidarian bleaching. First, due to its function as an antioxidant, GSH is utilized as a reducing equivalent. Although GSSG can be reduced by GR to replenish the pool of GSH, increased gene expression of GCL is needed to ensure increased demands. The enzyme GCL is feedback inhibited by GSH. Thus, as an adaptive response, an initial depletion of cellular GSH levels will both increase GCL activity as well as GCLC mRNA levels (Dickinson et al., 2002). Second, glutathione-S-transferases (GSTs) are a family of proteins that catalyze the conjugation of GSH to a number of electrophiles, including end products of lipid peroxidation. In mammalian cell lines, the latter reaction has been shown to cause an initial depletion of GSH followed by an increase in gclc expression (Iles and Liu, 2005). Interestingly, our results are in line with data from a study that reported a positive correlation between oxidative damage products (protein carbonyl and lipid peroxides) and GSH levels in corals sampled over the period of one year (Downs et al., 2002). Finally, increased levels of S-glutathionylated proteins may also occur during hyperthermic stress. Under normal conditions, a significant amount of GSH may be bound to proteins as protein mixed disulfide (PSSG) (Sies, 1999), although much of that may be contained in the cisternae of the endoplasmic reticulum where protein folding occurs and GSSG/2GSH is significantly elevated in comparison with the cytosol. Nevertheless, both oxidative or nitrosative stress may increase the formation of PSSG (Dalle-Donne et al, 2007). Strikingly, Perez and Weis (2006) have shown that nitric oxide (NO) is generated during hyperthermal stress exposure in A. pallida, while NO has been proposed as an intracellular regulator of PSSG formation (West et al., 2006). Based on this, we hypothesize that NO-mediated S-glutathionylation of specific target proteins is a regulatory mechanism during the process of cnidarian bleaching. The number of proteins that are modulated by S-glutathionylation has continuously increased (Ghezzi, 2005; Ghezzi et al., 2005; Dalle-Donne et al., 2007) so that some examples will be discussed in the context of bleaching.
4.1. Potential role of S-glutathionylation in cnidarian bleaching
S-glutathionylation of proteins has been shown to regulate a variety of cellular proteins including enzymes, signaling proteins, transcription factors, or heat-shock proteins (Dalle-Donne et al., 2007). Cleavage of caspase 3 induces its proteolytic activity, which is a checkpoint in the apoptotic signaling pathway of the cell. A recent study using human endothelial cells has shown deglutathionylation as a mechanism for caspase 3 activation via glutaredoxin (Grx) (Pan and Berk, 2007). Interestingly, in its reduced state, Grx is an inhibitor of apoptosis signal-regulating kinase 1 (ASK1), so that oxidation of Grx by increased amounts of intracellular GSSG levels would induce the ASK1-mediated apoptotic pathway (Song and Lee, 2003). Taken together, an oxidizing cellular environment and concomitant shift in the GSH/GSSG balance towards the latter would favor both deglutathionylation of caspase 3 and oxidation of Grx converging in an amplification of apoptotic signaling. Our results were limited to the determination of total GSH levels (the GSSG/2GSH ratio was not measured due to the practical problem of organism collection and disruption that results in artifactual oxidation and reduction). Thus, we had no proxy available for the cellular redox status during the stress experiment. Nevertheless, a homolog to caspase 3, acasp (GenBank accession no. DQ218058), has recently been identified in A. pallida (Dunn et al., 2006) and it would therefore be interesting to investigate the potential role of S-glutathionylation of acasp in the context of bleaching. The activity of the signaling kinase MEKK1, an activator of the SAPK/JNK (stress-activated protein kinase/c-Jun N-terminal kinase) pathway, has survival signaling function, which is inhibited by oxidative stress-induced S-glutathionylation. The redox environment of the cell could therefore selectively regulate oxidative stress signaling by balancing MEKK1 versus ASK1 activities to inhibit or promote the induction of apoptosis. Apoptosis has been suggested as an underlying mechanism of cnidarian bleaching (Richier et al., 2006; Dunn et al., 2007b) and therefore, studying the role of glutathione in the context of hyperthermal stress-induced apoptotic signaling pathways would represent an exciting field of future research. Finally, S-glutathionylation of constitutively expressed heat-shock protein 70 has been shown to induce an increase in its chaperone activity (Hoppe et al., 2004), which could be a conserved post-translational heat-shock response in A. pallida.
In addition to the previously reported generation of nitric oxide in the process of bleaching, we demonstrate that hyperthermal stress induces an increase in total GSH levels. Based on these results, we hypothesize that intermediate redox signals are actively involved in the pathway that begins with a physiological malfunction due to heat-stress and eventually results in the loss of symbiotic algae from cnidarian hosts. We propose that redox signaling cascades during hyperthermal stress-induced cnidarian bleaching necessitate further investigation in order to unravel the molecular mechanisms that underlie the loss of symbiotic algae from their animal hosts.
Acknowledgements
We would like to thank Michael DeSalvo for editorial comments. This study was funded by NSF grants BE-GEN 0313708 and IOS 0644438 to MM. HJF is supported by NIH grant ES05511.
References
- Asada K, Takahashi M. Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ, Osmond CB, Arntzen CJ, editors. Topics in Photosynthesis. . Photoinhibition. vol. 9. Amsterdam: Elsevier; 1987. pp. 227–287. [Google Scholar]
- Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. S-glutathionylation in protein redox regulation. Free Radic. Biol. Med. 2007;43:883–898. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Forman HJ. Glutathione in defense and signaling: lessons from a small thiol. Ann. N.Y. Acad. Sci. 2002;973:488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Iles KE, Watanabe N, Iwamoto T, Zhang H, Krzywanski DM, Forman HJ. 4-hydroxynonenal induces glutamate cysteine ligase through JNK in HBE1 cells. Free Radic. Biol. Med. 2002;33:974. doi: 10.1016/s0891-5849(02)00991-7. [DOI] [PubMed] [Google Scholar]
- Downs CA, Fauth JE, Halas JC, Dustan P, Bemiss J, Woodley CM. Oxidative Stress and Seasonal Coral Bleaching. Free Radic. Biol. Med. 2002;33:533–543. doi: 10.1016/s0891-5849(02)00907-3. [DOI] [PubMed] [Google Scholar]
- Dunn SR, Bythell JC, Le Tissier MDA, Burnett WJ, Thomason JC. Programmed cell death and cell necrosis activity during hyperthermic stress-induced bleaching of the symbiotic sea anemone Aiptasia sp. J. Exp. Mar. Biol. Ecol. 2002;272:29–53. [Google Scholar]
- Dunn SR, Phillips WS, Green DR, Weis VM. Knockdown of actin and caspase gene expression by RNA interference in the symbiotic anemone Aiptasiapallida. Biol. Bull. 2007a;212:250–258. doi: 10.2307/25066607. [DOI] [PubMed] [Google Scholar]
- Dunn SR, Schnitzler CE, Weis VM. Apoptosis and autophagy as mechanisms of dinoflagellate symbiont release during cnidarian bleaching: every which way you lose. Proc. Biol. Sci. 2007b;274:3079–3085. doi: 10.1098/rspb.2007.0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn SR, Phillips WS, Spatafora JW, Green DR, Weis VM. Highly conserved caspase and Bcl-2 homologues from the sea anemone Aiptasia pallida: lower metazoans as models for the study of apoptosis evolution. J. Mol. Evol. 2006;63:95–107. doi: 10.1007/s00239-005-0236-7. [DOI] [PubMed] [Google Scholar]
- Dunn SR, Thomason JC, Le Tissier MD, Bythell JC. Heat stress induces different forms of cell death in sea anemones and their endosymbiotic algae depending on temperature and duration. Cell Death Differ. 2004;11:1213–1222. doi: 10.1038/sj.cdd.4401484. [DOI] [PubMed] [Google Scholar]
- Dykens JA, Shick JM. Oxygen production by endosymbiotic algae controls superoxide-dismutase activity in their animal host. Nature. 1982;297:579–580. [Google Scholar]
- Dykens JA, Shick JM. Photobiology of the symbiotic sea anemone, Anthopleura elegantissima, defenses against photodynamic effects, and seasonal photoacclimatization. Biol. Bull. 1984;167:683–697. doi: 10.2307/1541419. [DOI] [PubMed] [Google Scholar]
- Dykens JA, Shick JM, Benoit C, Buettner GR, Winston GW. Oxygen radical production in the sea-anemone Anthopleura elegantissima and its endosymbiotic algae. J. Exp. Biol. 1992;168:219–241. [Google Scholar]
- Fariss MW, Reed DJ. High-performance liquid chromatography of thiols and disulfides: dinitrophenol derivatives. Methods Enzymol. 1987;143:101–109. doi: 10.1016/0076-6879(87)43018-8. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Fukuto J, Torres M. Signal Transduction by Reactive Oxygen and Nitrogen Species: Pathways and Chemical Principles. Dordrecht: Kluwer Academic Publishers; 2003. p. 436. [Google Scholar]
- Fratelli M, Goodwin LO, Orom UA, Lombardi S, Tonelli R, Mengozzi M, Ghezzi P. Gene expression profiling reveals a signaling role of glutathione in redox regulation. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13998–14003. doi: 10.1073/pnas.0504398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi P. Regulation of protein function by glutathionylation. Free Radic. Res. 2005;39:573–580. doi: 10.1080/10715760500072172. [DOI] [PubMed] [Google Scholar]
- Ghezzi P, Bonetto V, Fratelli M. Thiol-disulfide balance: from the concept of oxidative stress to that of redox regulation. Antioxid. Redox Signal. 2005;7:964–972. doi: 10.1089/ars.2005.7.964. [DOI] [PubMed] [Google Scholar]
- Hoppe G, Chai YC, Crabb JW, Sears J. Protein S-glutathionylation in retinal pigment epithelium converts heat shock protein 70 to an active chaperone. Exp. Eye. Res. 2004;78:1085–1092. doi: 10.1016/j.exer.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005;6:279–284. doi: 10.1038/sj.gene.6364190. [DOI] [PubMed] [Google Scholar]
- Iles KE, Liu RM. Mechanisms of glutamate cysteine ligase (GCL) induction by 4-hydroxynonenal. Free Radic. Biol. Med. 2005;38:547–556. doi: 10.1016/j.freeradbiomed.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Jones RJ, Hoegh-Guldberg O, Larkum AWD, Schreiber U. Temperature-induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant. Cell Environ. 1998;21:1219. [Google Scholar]
- Knowlton N, Rohwer F. Multispecies microbial mutualisms on coral reefs: the host as a habitat. Am. Nat. 2003;162:S51–S62. doi: 10.1086/378684. [DOI] [PubMed] [Google Scholar]
- Kühl M, Cohen Y, Dalsgaard T, Jorgensen BB, Revsbech NP. Microenvironment and photosynthesis of zooxanthellae in scleractinian corals studied with microsensors for O2, pH and light. Mar. Ecol. Progr. Ser. 1995;117:159–172. [Google Scholar]
- Lesser MP. Elevated temperatures and ultraviolet radiation cause oxidative stress and inhibit photosynthesis in symbiotic dinoflagellates. Limnol. Oceanogr. 1996;41:271–283. [Google Scholar]
- Lesser MP. Oxidative stress in marine environments: biochemistry and physiological ecology. Annu. Rev. Physiol. 2006;68:253–278. doi: 10.1146/annurev.physiol.68.040104.110001. [DOI] [PubMed] [Google Scholar]
- Lesser M, Stochaj W, Tapley D, Shick J. Bleaching in coral reef anthozoans: Effects of irradiance, ultraviolet radiation and temperature, on the activities of protective enzymes against active oxygen. Coral Reefs. 1990;8:225–232. [Google Scholar]
- Merle PL, Sabourault C, Richier S, Allemand D, Furla P. Catalase characterization and implication in bleaching of a symbiotic sea anemone. Free Radic. Biol. Med. 2007;42:236–246. doi: 10.1016/j.freeradbiomed.2006.10.038. [DOI] [PubMed] [Google Scholar]
- Muscatine L. The role of symbiotic algae in carbon and energy flux in reef corals. In: Dubinsky Z, editor. Ecosystems of the World 25: Coral Reefs. Amsterdam: Elsevier; 1990. pp. 49–74. [Google Scholar]
- Nii CM, Muscatine L. Oxidative stress in the symbiotic sea anemone Aiptasia pulchella (Carlgren, 1943): Contribution of the animal to superoxide ion production at elevated temperature. Biol. Bull. 1997;192:444–456. doi: 10.2307/1542753. [DOI] [PubMed] [Google Scholar]
- Pan S, Berk BC. Glutathiolation regulates tumor necrosis factor-alpha-induced caspase-3 cleavage and apoptosis: key role for glutaredoxin in the death pathway. Circ. Res. 2007;100:213–219. doi: 10.1161/01.RES.0000256089.30318.20. [DOI] [PubMed] [Google Scholar]
- Perez S, Weis V. Nitric oxide and cnidarian bleaching: an eviction notice mediates breakdown of a symbiosis. J. Exp. Biol. 2006;209:2804–2810. doi: 10.1242/jeb.02309. [DOI] [PubMed] [Google Scholar]
- Perez SF, Cook CB, Brooks WR. The role of symbiotic dinoflagellates In the temperature-induced bleaching response of the subtropical sea anemone Aiptasia pallida. J. Exp. Mar. Biol. Ecol. 2001;256:1–14. doi: 10.1016/s0022-0981(00)00282-3. [DOI] [PubMed] [Google Scholar]
- Richier S, Merle PL, Furla P, Pigozzi D, Sola F, Allemand D. Characterization of superoxide dismutases in anoxia- and hyperoxia-tolerant symbiotic cnidarians. Biochim. Biophys. Acta. 2003;1621:84–91. doi: 10.1016/s0304-4165(03)00049-7. [DOI] [PubMed] [Google Scholar]
- Richier S, Sabourault C, Courtiade J, Zucchini N, Allemand D, Furla P. Oxidative stress and apoptotic events during thermal stress in the symbiotic sea anemone, Ammonia viridis. FEBS J. 2006;273:4186–4198. doi: 10.1111/j.1742-4658.2006.05414.x. [DOI] [PubMed] [Google Scholar]
- Shick JM, Dunlap WC. Mycosporine-like amino acids and related Gadusols: biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annu. Rev. Physiol. 2002;64:223–262. doi: 10.1146/annurev.physiol.64.081501.155802. [DOI] [PubMed] [Google Scholar]
- Sies H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- Song JJ, Lee YJ. Differential role of glutaredoxin and thioredoxin in metabolic oxidative stress-induced activation of apoptosis signal-regulating kinase 1. Biochem. J. 2003;373:845–853. doi: 10.1042/BJ20030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trench R. Microalgal-invertebrate symbioses: a review. Endocytobiosis. Cell. Res. 1993;9:135–175. [Google Scholar]
- Trench RK. The cell biology of plant-animal symbiosis. Annu. Rev. Plant. Physiol. 1979;30:485–453. [Google Scholar]
- Warner M, Fitt W, Schmidt G. Damage to photosystem II In symbiotic dinoflagellates: a determinant of coral bleaching. Proc. Natl. Acad. Sci. U. S. A. 1999;96:8007–8012. doi: 10.1073/pnas.96.14.8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MB, Hill BG, Xuan YT, Bhatnagar A. Protein glutathiolation by nitric oxide: an intracellular mechanism regulating redox protein modification. FASEB J. 2006;20:1715–1717. doi: 10.1096/fj.06-5843fje. [DOI] [PubMed] [Google Scholar]