Abstract
(−)-Epigallocatechin-3-gallate [(−)-EGCG], the most abundant polyphenolic catechin in green tea, showed chemoprevention and anticancer activities. (−)-EGCG was reported to bind to the C-terminal domain of heat shock protein 90 (Hsp90). The purpose of this study is to investigate (−)-EGCG as a novel Hsp90 inhibitor to impair Hsp90 super-chaperone complex for simultaneous down-regulation of oncogenic proteins in pancreatic cancer cells. MTS assay showed that (−)-EGCG exhibited anti-proliferative activity against pancreatic cancer cell line Mia Paca-2 in vitro with IC50 below 50 μM. (−)-EGCG increased caspase-3 activity up to 3-fold in a time- and concentration-dependent manner. Western Blotting analysis demonstrated that (−)-EGCG induced down-regulation of oncogenic Hsp90 client proteins by approximately 70%~95%, including Akt, Cdk4, Raf-1, Her-2, and pERK. Co-immunoprecipitation showed that (−)-EGCG decreased the association of co-chaperones p23 and Hsc70 with Hsp90 by more than 50%, while it had little effect on the ATP binding to Hsp90. Proteolytic fingerprinting assay confirmed direct binding between (−)-EGCG and the Hsp90 C-terminal domain. These data suggest that the binding of (−)-EGCG to Hsp90 impairs the association of Hsp90 with its co-chaperones, thereby inducing degradation of Hsp90 client proteins, resulting anti-proliferating effects in pancreatic cancer cells.
Keywords: (−)-EGCG, Hsp90, co-chaperone, ATP, pancreatic cancer cell
Introduction
Pancreatic cancer is the fourth leading cause of cancer death in the United States 1. The overall 5-year survival rate after diagnosis for pancreatic cancer patients is less than 5% 2. Major therapeutic targets for pancreatic cancer include K-ras pathway downstream signaling (e.g., Raf-MEK ERK pathway), epidermal growth factor receptor (EGFR), ErbB-2 (Her-2), phosphoinositide 3-OH kinase (PI3K)/Akt, and p53 mutant 3–6. Due to the complexity of the disease, targeting multiple oncogenic pathways would be beneficial for chemoprevention of pancreatic cancer.
Heat shock protein 90 (Hsp90), a highly abundant molecular chaperone in the stress response, assists maturation of more than 200 proteins, which include transmembrane tyrosine kinases (Her-2, EGFR), metastable signaling proteins (Akt, K-ras, Raf-1), mutated signaling proteins (p53, v-Src), chimeric signaling proteins (Bcr-Abl), cell cycle regulators (Cdk4, Cdk6), and steroid receptors (androgen, estrogen, and progesterone receptors) 7–10, 11. Many of these client proteins are mutated and/or over-expressed in pancreatic cancer 12, 13. Based on crystal structure, Hsp90 protein consists of three highly conserved domains: an N-terminal ATP-binding domain, a middle domain and a C-terminal dimerization domain 14. The N-terminus of Hsp90 contains a specific ATP binding pocket, which has been well characterized 6, 15. Benzoquinone ansamycins, such as geldanamycin (GA) 16 and its derivatives 17-allylamino-17-desmethoxygeldanamycin (17-AAG) 16, 17, competitively block ATP binding to this N-terminal ATP binding site on Hsp90 18, 19, resulting in ubiquitination and proteasomal degradation of client proteins.
Hsp90 requires an array of co-chaperones to assemble a super-chaperone complex for its function. These co-chaperones, including Cdc37, Hsc70, Hsp40, Hop, Hip, p23, pp5, and immunophilins, bind to and release from the complex at various stages to accomplish the folding and maturation of Hsp90 client proteins 18. A newly synthesized client protein binds to Hsc70/Hsp40 complex, and then associates with the “open” state Hsp90 via the bridging co-chaperone Hop, which interacts simultaneously with Hsp90 and Hsc70 12. Upon ATP binding, Hsp90 then binds to p23 and immunophilins, converting the intermediate chaperone complex into a mature complex 18. Upon ATP hydrolysis, the correctly-folded client protein is released from Hsp90 22.
Recently, the C-terminal domain of Hsp90 has been shown to possess a second ATP binding site 23. Novobiocin, a coumarin antibiotic isolated from Streptomyces species, binds Hsp90 at the C-terminal ATP binding site 23. This binding induced an alteration in Hsp90 conformation 23, 24, interfering Hsp90/Hsc70 and Hsp90/p23 interactions 24. An allosteric regulation is suggested between the C-terminal and N-terminal domains of Hsp90 such that the interaction of ligands with one site might affect the occupancy of the other site 23, 25.
Green tea is one of the most widely consumed beverages in the world. Epidemiological studies suggest an association between green tea consumption and cancer prevention effects 26. The various polyphenolic catechins contained in green tea are thought to contribute to its chemoprevention against certain types of cancer. In particular, several studies indicate that (−)-epigallocatechin-3-gallate [(−)-EGCG], the most abundant catechin in green tea, is a potent chemoprevention and anticancer component 27. However, the underlying mechanism of (−)-EGCG for its chemoprevention is not well defined. In 2005, Palermo et al. reported that (−)-EGCG could inhibit the transcriptional activity of aryl hydrocarbon receptor (AhR) through a mechanism involving direct binding to the C-terminal region of Hsp90. It remains unclear whether (−)-EGCG could inhibit Hsp90 function through direct binding and how (−)-EGCG affect the chaperone function through this binding. The purpose of this study is to investigate (−)-EGCG as a novel Hsp90 inhibitor to impair Hsp90 super-chaperone complex for inhibiting its chaperoning function, which simultaneously down-regulates oncogenic proteins in pancreatic cancer cell line Mia Paca-2.
Materials and Methods
Drugs and Antibodies
(−)-EGCG was purchased from Calbiochem (EMD Biosciences, Inc., San Diego, CA), and dissolved in DMSO as a stock solution. The following antibodies were used for immunoblotting: Akt, phospho-ERK1/2 (Thr202/Tyr204), ERK1/2 (p44/42 MAPK) (Cell Signaling, Beverly, MA), Hop (Assay Designs, Inc., Ann Arbor, MI), p23 (Abcam, Cambridge, MA), Cdk4, Cdc37, Hsp90, Hsp70, Hsc70, Her-2, Raf-1, β-actin (Santa Cruz Biotechnology, Santa Cruz, CA). Purified Hsp90β N-terminus (N-Hsp90β) (amino acids 1-246) was a gift from Dr. Dan Bolon (University of Massachusetts Medical School).
MTS Assay
Human pancreatic cancer cells, Mia Paca-2, were seeded in 96-well microplates at a density of 3,000 to 5,000 cells per well. Cells were treated with increasing concentrations of (−)-EGCG as indicated, and after 24 hr incubation cell viability was assessed by MTS assay (Promega, Madison, WI) according to the manufacturer’s instruction. The number of living cells in the culture is directly proportional to the absorbance at 490 nm by a formazan product bioreduced from MTS by living cells. The anti-proliferative effect of (−)-EGCG was also tested on pancreatic cancer cell lines (Panc-1, BxPC-3, and AsPC-1) with similar results, and thus only one cell line (Mia Paca-2) was used for the following mechanistic studies.
Caspase-3 Fluorometric Assay
Mia Paca-2 cells were treated with (−)-EGCG and collected at different time points as indicated. The following Caspase-3 activity assay was based on the manufacturer’s instruction of Caspase-3/CPP32 Fluorometric Assay Kit (Biovision Research Products, Mountain View, CA). Cellular protein was extracted with the supplied lysis buffer, followed by determination of protein concentration using BCA Protein Assay Reagents (Pierce, Rockford, IL). The cleavage of DEVD-AFC, a substrate of caspase-3, was quantified by using a fluorescence microtiter plate reader with a 400 nm excitation filter and a 505 nm emission filter. Results are reported as arbitrary fluorescence units (AFU) normalized to milligram of cellular protein.
Protein Expression and Purification
The expression plasmids pET15b-hHsp90β, pET28a(+)-hHsp90β (530-724) for human full-length Hsp90β and Hsp90β C-terminus (C-Hsp90β) were kindly provided by Dr. Thomas Ratajczak (University of Western Australia, Australia). The plasmids were transformed into E. coli strain Rosetta 2(DE3) (EMD Biosciences, Inc., San Diego, CA) following the protocol provided by manufacturer. Primary cultures of transformed cells were grown overnight, pelleted by centrifugation, resuspended in fresh culture medium, and grown for 1–2 hr(s) at 37°C until OD600 reached 0.6. Protein expression was induced by 0.2 mM IPTG (isopropyl-beta-D-thiogalactopyranoside) (GE Healthcare, Piscataway, NJ) for 2 hrs. His-tagged proteins were purified by affinity chromatography through mixed with HisPurTM Cobalt Resin (Pierce, Rockford, IL), followed by dialysis against PBS. The purity was assessed by SDS-PAGE, and the concentration was determined by BCA assay (Pierce, Rockford, IL). Proteins were stored at −70°C after adding glycerol to 10%.
Western Blotting Analysis
The procedure for Western blotting analysis was briefly described below. After treated with (−)-EGCG for the indicated time periods, Mia Paca-2 cells were washed twice with ice-cold PBS, collected in RIPA lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 5 mM EDTA, 1 mM Na3VO4, pH 7.5) supplemented with a protease inhibitor mixture (Sigma-Aldrich, St. Louis, MO), and incubated on ice for 20 min. Afterwards cell lysate was centrifuged at 14,000 x rpm for 10 min, and the supernatant was recovered. Protein concentration was determined with BCA Protein Assay Reagents (Pierce, Rockford, IL). Equal amounts of total protein were subject to SDS-PAGE, transferred to PVDF membrane (BioRad, Richmond, CA), and then probed with appropriate antibodies.
ATP-Sepharose Binding Assay
Hsp90 Protein (200 μg) was extracted from treated Mia Paca-2 cells and incubated with 25 μl pre-equilibrated γ-phosphate-linked ATP-Sepharose (Jena Bioscience GmbH, Jena, Germany) in 200 μl incubation buffer (10 mM Tris-HCl, 50 mM KCl, 5 mM MgCl2, 2 mM DTT, 0.01% NP-40, pH 7.5) overnight at 4 °C. The beads were washed four times and bead-bound proteins were subsequently analyzed by SDS-PAGE. For ATP binding assay with purified protein, 5 μg protein was pre-incubated with (−)-EGCG on ice in 200 μl incubation buffer (10 mM Tris-HCl, 50 mM KCl, 5 mM MgCl2, 2 mM DTT, 0.01% NP-40, pH 7.5) for 1 hr. Following incubation, ATP-Sepharose was added and further incubated at 37 °C for 30 min with frequent agitation. The beads were washed and bound proteins were subject to Western blotting.
Co-immunoprecipitation
Mia Paca-2 cells were treated with (−)-EGCG for the indicated time period, and then harvested. Cells were lysed in 20 mM Tris-HCl (pH 7.4), 25 mM NaCl, 2 mM DTT, 20 mM Na2MoO4, 0.1% NP-40, and protease inhibitors. After centrifugation, supernatant was recovered and protein concentrations were determined with BCA Protein Assay Reagents (Pierce, Rockford, IL). Protein (500 μg) was first incubated with H9010 antibody (Axxora, San Diego, CA) followed by addition of protein A/G agarose (Santa Cruz Biotechnology, Santa Cruz, CA). The bound proteins were resolved by SDS-PAGE and analyzed by Western blotting.
Trypsinolytic Fingerprinting Assay
The experiment was performed similarly to previously described 24. Purified human N-Hsp90β (1-246) and C-Hsp90β (530-724) (0.5 μg) was incubated with DMSO, (−)-EGCG or other compounds in assay buffer (10 mM Tris-HCl, 50 mM KCl, 5 mM MgCl2, 0.1 mM EDTA, pH 7.4) on ice for 1 hr. The samples were digested on ice with different concentrations of trypsin for 6 min. The reactions were terminated by adding SDS sample buffer followed by boiling for 3–5 min. The digested products from N-Hsp90β and C-Hsp90β were analyzed by Western blotting with Hsp90 (N-17) antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and Hsp90 (AC88) antibody (Assay Designs, Inc., Ann Arbor, MI), respectively.
Statistical Analysis
Statistical analysis was performed using student t-test. Data are presented as mean ± SD (n = 3, p < 0.01).
Results
(−)-EGCG Inhibits Cell Growth and Induces Apoptosis in Pancreatic Cancer Cells (Mia Paca-2)
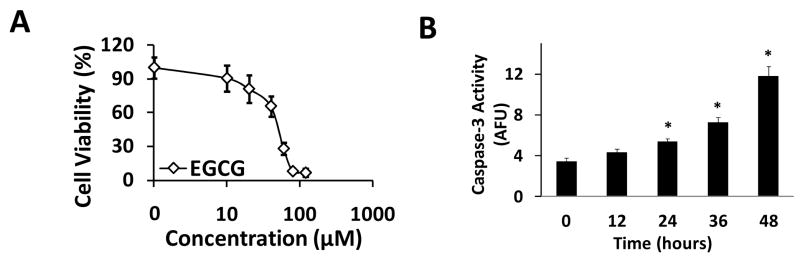
First, we selected a human pancreatic cancer cell line, Mia Paca-2, to evaluate the therapeutic potential of (−)-EGCG. As shown in Figure 1A, (−)-EGCG exhibited a dose-dependent inhibitory effect on Mia Paca-2 with IC50 less than 50 μM. One of the primary events in apoptosis is activation of caspase-3 29. Caspase-3 activity assay showed that (−)-EGCG induced activation of caspase-3 in a time- and dose-dependent manner (Fig. 1B). Markedly, more than a 3-fold increase in caspase-3 activity was observed in Mia Paca-2 cells after incubated with 60 μM (−)-EGCG for 48 hrs in comparison with untreated cells.
Figure 1.
(−)-EGCG inhibited pancreatic cancer proliferation and increased caspase-3 activity. (a) Mia Paca-2 cells growing in log phase were treated with increasing concentrations of (−)-EGCG for 48 hrs. The anti-proliferation effect of (−)-EGCG was measured by MTS assay. (b) Mia Paca-2 cells were treated with (−)-EGCG (60 μM/24 hrs) and collected at the indicated time. Cell lysates were prepared for caspase-3 activity assay. Results are expressed as arbitrary fluorescent units (AFU) normalized to milligram of cytosolic protein. Data are presented as mean ± SD (n = 3).
(−)-EGCG Decreases Cellular Levels of Hsp90 Client Proteins
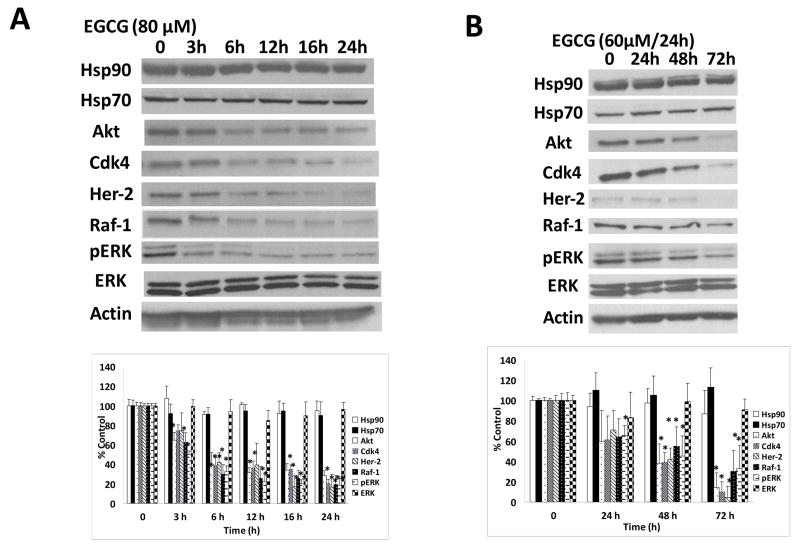
Since the inhibition of Hsp90 will result in simultaneous down-regulation of multiple oncogenic proteins, we examined whether (−)-EGCG could decrease the levels of cancer-associated Hsp90 client proteins in pancreatic cancer cells. Mia Paca-2 cells were treated with either 80 μM (−)-EGCG for 0–24 hrs or 60 μM (−)-EGCG every 24 hrs up to 72 hrs. As shown in Figure 2A and 2B, (−)-EGCG caused a progressive decline in the protein levels of Her-2, Akt, Cdk4, Raf-1, and pERK in a time- and dose-dependent manner. Akt, Raf-1 and pERK were down-regulated by 35%~50% as early as 3 hrs after 80 μM (−)-EGCG incubation (Fig. 2A). All five client proteins were decreased by approximately 70%–80% upon 24 hr incubation with 80 μM (−)-EGCG (Fig. 2A). Moreover, although a 48 hr treatment of Mia Paca-2 cells with 60 μM (−)-EGCG only moderately decreased the cellular levels of Akt, Raf-1, Her-2, Cdk4, and pERK, an additional 24 hr treatment with another dose of 60 μM (−)-EGCG was able to completely abrogate the endogenous levels of Akt, Cdk4, and Her-2 and down-regulate Raf-1 and pERK by about 70% (Fig. 2B). In contrast to ansamycin inhibitors of Hsp90 (e.g., GA, 17-AAG), (−)-EGCG did not induce the protein level of Hsp70 (Fig. 2B).
Figure 2.
Effect of (−)-EGCG on Hsp90 client proteins. (a) Mia Paca-2 cells were treated with 80 μM (−)-EGCG for different time periods. (−)-EGCG induced a time-dependent degradation of Hsp90 client proteins. (b) Cells were treated with (−)-EGCG at a dose of 60 μM/24 hrs. Data are normalized to actin and presented as mean ± SD (n = 3). * P < 0.01 vs. Control.
(−)-EGCG Impairs the Association of Co-chaperones p23 and Hsc70 with Hsp90 in Pancreatic Cancer Cells (Mia Paca-2)
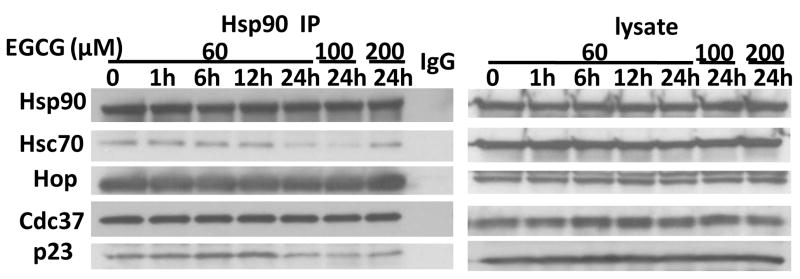
We further characterized the effect of (−)-EGCG on the association between co-chaperones and Hsp90. After incubation with different concentrations of (−)-EGCG for various time periods, Mia Paca-2 cells were harvested for extraction of total cellular protein. Hsp90 was immunoprecipitated using its antibody, and the amounts of Hsc70, Hop, Cdc37, and p23 were detected by Western blotting in the precipitated Hsp90 complexes. The result showed that 24 hr treatment with 60 μM (−)-EGCG significantly suppressed the interaction of Hsc70 and p23 with Hsp90 by approximately 60% and 55%, respectively, while this treatment had little effect on the amount of Cdc37 or Hop in the Hsp90 complex (Fig. 3). Higher concentrations of (−)-EGCG further reduced Hsc70/Hsp90 and p23/Hsp90 associations (Fig. 3).
Figure 3.
Influence of (−)-EGCG on Hsp90/co-chaperones association. Cells were treated with 60, 100, or 200 μM of (−)-EGCG. (−)-EGCG treatment decreased the amount of p23 and Hsc70 bound with Hsp90, but showed no effect on other co-chaperones.
(−)-EGCG Directly Binds the C-terminal Region of Hsp90
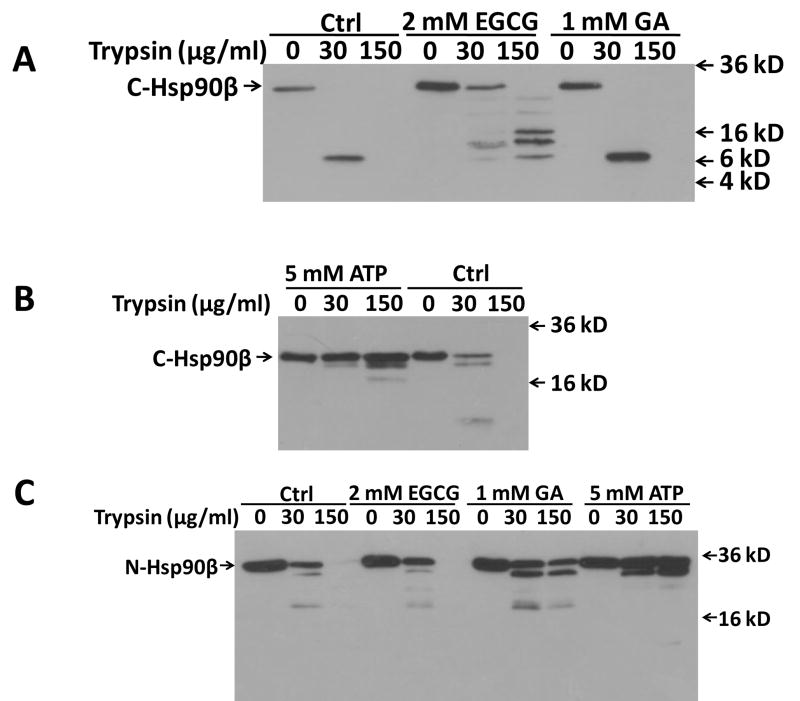
In order to examine how (−)-EGCG impairs the association of p23 and Hsc70 with Hsp90, we utilized proteolytic fingerprinting assay to investigate the region that is involved in the interaction between (−)-EGCG and Hsp90. In the absence of (−)-EGCG, the C-terminal region of Hsp90β (C-Hsp90β, amino acids 530-724) was highly sensitive to proteolytic enzyme digestion. While 30 μg/ml of trypsin yielded a single band close to 6 kD, 150 μg/ml of the enzyme was able to completely hydrolyze the C-Hsp90β (Fig. 4A, lane 1–3). Consistent with the fact that GA does not interact directly with the C-terminal domain of Hsp90β, GA had no effect on the trypsinolytic fingerprint of C-Hsp90β in comparison to control (Fig. 4A, lane 7–9). However, incubation of (−)-EGCG with Hsp90 blocked the trypsin hydrolysis of Hsp90 and produced a band representing complete C-Hsp90β at a lower concentration of trypsin (Fig. 4A, lane 4 & 5). (−)-EGCG protected C-terminus from cleavage by a higher concentration of trypsin (Fig. 4A, lane 4 & 6). As a positive control, binding of ATP to the C-Hsp90β allowed very limited trypsin digestion to occur (Fig. 4B). In addition, the trypsinolytic patterns were different between (−)-EGCG- and ATP-protected C-Hsp90β. This suggests that the binding site of (−)-EGCG on C-Hsp90β may be different from the C-terminal ATP binding site.
Figure 4.
(−)-EGCG bound to the C-terminus but not N-terminus of Hsp90. (a)(b) Purified recombinant C-Hsp90β (530-724) protein was incubated with DMSO, 2 mM (−)-EGCG, 1 mM GA, or 5 mM ATP (10 mM phosphocreatine, 7 units of creatine phosphokinase). After incubation, each sample was digested on ice for 6 min with the indicated concentrations of trypsin. Hsp90 (AC88) antibody, which detects C-terminus epitope of Hsp90, was used for immunoblotting. (c) Purified N-Hsp90β (1-246) protein was incubated with DMSO, 2 mM (−)-EGCG, 1 mM GA, or 5 mM ATP, followed by the same procedure of trypsin digestion. Hsp90 (N-17) antibody was used for immunoblotting.
In contrast, (−)-EGCG did not change the trypsinolytic fingerprint of N-Hsp90β, either with the cleavage by 30 or 150 μg/ml trypsin compared to control (Fig. 4C, lane 1–6). As expected, Geldanamycin (GA) remarkably protected the enzyme digestion by interacting with the N-terminal nucleotide binding pocket, producing a digest pattern similar to ATP-bound N-Hsp90β (Fig. 4C, lane 7–12).
Next, we examined the effect of (−)-EGCG on ATP binding capacity of cellular Hsp90, recombinant full-length Hsp90β, and purified C-Hsp90β by utilizing ATP-sepharose pull-down assay. The results showed that (−)-EGCG treatment had little effect on the ATP binding to endogenous Hsp90 in pancreatic cancer cells (Fig. 5A). (−)-EGCG did not block the ATP binding to either recombinant full-length Hsp90β or C-Hsp90β (Fig. 5B & Fig. 5C).
Figure 5.
Effect of ()-EGCG on ATP binding to Hsp90. (a) Mia Paca-2 cells were treated with indicated concentrations of (−)-EGCG for 24 hrs. Cell lysates were incubated with γ-phosphate-linked ATP-Sepharose. The ATP-bound Hsp90 was detected by Western blotting. (b) Purified recombinant full-length Hsp90β protein was incubated with ATP-Sepharose and the bound Hsp90 was detected by Western blotting. (c) Purified C-Hsp90β (530-724) protein was pulled down by ATP-Sepharose.
Discussion
In recent years, many studies have shown chemopreventive and chemotherapeutic effect of green tea against skin, lung, breast, colon, liver, stomach, and prostate cancers 30. Numerous studies have suggested that (−)-EGCG, the most abundant catechin in green tea, is the primary component for these activities 32. (−)-EGCG induces apoptosis and cell cycle arrest in cancer cells without affecting normal cells 30, 33. The majority in vitro studies have revealed that (−)-EGCG inhibited NF-κB activity, MAPK pathway, activator protein-1 (AP-1) activity, and EGFR-mediated downstream signaling pathways 34. Clinical trials further verified the cancer preventive effect of (−)-EGCG 27, 35. The purpose of the current study is to reveal a new chemopreventive mechanism of (−)-EGCG against pancreatic cancer cells.
Recently, Hsp90 has emerged as a target in cancer therapeutics based on the Hsp90 super-chaperone complex status in cancer cells. First, Hsp90 is involved in the maturation and stabilization of a wide range of oncogenic client proteins that are crucial for oncogenesis and malignant progression 11, 37. Second, Hsp90 comprises as much as 4–6% of total protein in tumor cells, in contrast with the 1–2% within normal cells 38. Finally, Hsp90 predominantly exists as multi-chaperone complex with high affinity for ATP and drug, whereas in normal cells most Hsp90 is present in an uncomplexed state 38. hence, cancer cells are dependent on Hsp90 function for their survival and proliferation 38.
The first class of Hsp90 inhibitors, represented by GA, competitively binds to the N-terminal ATP pocket of Hsp90, thus restraining Hsp90 in its ADP-bound conformation and preventing the subsequent “clamping” of Hsp90 around a client protein 19, 20, 39, resulting in proteasome-dependent degradation of the client. Another type of Hsp90 inhibitor, novobiocin, interacts with Hsp90 at the C-terminal ATP binding site with relatively weak activity 23. Inhibition of Hsp90 by novobiocin was able to induce similar cellular responses as N-terminal inhibitors, i.e., destabilization of a range of Hsp90 client proteins such as Her-2, Raf-1 and p53 mutant 23, 40. In addition, novobiocin interferes with Hsp90/Hsc70 and Hsp90/p23 association 23, 24. Furthermore, it was suggested that an allosteric regulation may correlate the C-terminal domain of Hsp90 with the N-terminus, where the interaction of ligands with one site might affect the occupancy of the other site 23, 25, 41.
(−)-EGCG was reported to bind the C-terminus of Hsp90 at the region of amino acids 538-728 on Hsp90; this interaction region was discovered by using affinity chromatography with immobilized (−)-EGCG-sepharose and various purified fragments and truncation mutants of Hsp90 28. Because of the similar binding region of novobiocin and (−)-EGCG on Hsp90, in the current study we aim to investigate whether (−)-EGCG: (1) impairs Hsp90 association with its co-chaperones, (2) interferes with Hsp90 chaperoning function, and (3) exerts inhibitory effect on pancreatic cancer cells. Indeed, the data suggest that binding of (−)-EGCG to Hsp90 impairs the association of Hsp90 with its co-chaperones (Hsc70 and p23), thereby inducing degradation of Hsp90 client proteins, resulting in anti-proliferating effects in pancreatic cancer cells.
Identification of the (−)-EGCG binding site on Hsp90 is the key to understand its effects on Hsp90 function. Proteolytic fingerprinting assay revealed that (−)-EGCG directly bound the purified Hsp90β C-terminus but not N-terminus. Both ATP and (−)-EGCG could prevent C-Hsp90β from trypsin cleavage, although they exhibited different protection patterns. These data suggest that (−)-EGCG may bind to Hsp90 C-terminus differently from ATP binding. A very recent study by using mass spectrometry and chemical detection methods discovered that (−)-EGCG could form covalent adducts with the thiol group of cysteine residues in proteins through autoxidation 42. Based on the amino acid sequence search (NP 005339), there are three cysteine residues within C-terminal fragment of human Hsp90β (residues 530-724), Cys572, Cys597, and Cys598, all of which do not fall into the C-terminal ATP binding region (residues 663-676). The possible reactions between (−)-EGCG and Hsp90β C-terminus need to be further investigated.
To further validate these assays, we used geldanamycin (GA) as a negative control. Since GA competes with ATP binding to N-terminal pocket of Hsp90 rather than C-terminus, the trypsin digestion of C-terminus of Hsp90 was not affected by GA. On the contrary, ATP and GA shielded the N-terminus of Hsp90β from trypsin cleavage with a similar pattern and high efficiency, while (−)-EGCG did not alter the proteolytic fingerprint of Hsp90N-terminus. These data suggest that (−)-EGCG directly binds to the C-terminal domain of Hsp90β, specifically within the region of amino acids 530-724, which is supported by the previous study using immobilized (−)-EGCG affinity chromatography 28.
In the current study, co-immunoprecipitation of endogenous Hsp90 from cell lysates demonstrated that (−)-EGCG impaired the association of Hsc70 and p23 with Hsp90. This effect of (−)-EGCG is very similar to that of novobiocin on Hsp90/co-chaperone association as previously reported 23, 24, which provides the biologic significance of (−)-EGCG binding to Hsp90 for its chemoprevention efficacy. Considering the similarity between (−)-EGCG and novobiocn in Hsp90 binding, the influence of (−)-EGCG on association of Hsp90 with the co-chaperones may be expected. First, according to Marcu et al. 23, the association region of Hsc70 on Hsp90 overlaps with the C-terminal dimerization domain and contains the novobiocin binding site 43. In addition, both N- and C-terminal regions of Hsp90 are necessary for interaction with the yeast homolog of p23, SBA1 44. Finally, Allan et al. 40 suggests that modulation within the Hsp90 C-terminus by novobiocin could significantly impact other regions in Hsp90, probably through allosteric effects 40. This is consistent with the evidence that an allosteric regulation may influence the conformation of the C-terminus and N-terminus of Hsp90, where the interaction of ligands with one site might affect the occupancy of the other site 23, 25, 40, 41. Therefore, (−)-EGCG is likely to alter the conformation and/or occupy the necessary residues of Hsp90 by directly binding to its C-terminal domain, subsequently leading to the impairment of Hsp90/Hsc70 and Hsp90/p23 interactions.
In order to assess whether (−)-EGCG affects ATP binding activity of Hsp90, we applied ATP-sepharose binding assay. However, (−)-EGCG treated Mia Paca-2 cells did not show altered ATP binding to Hsp90. ATP-sepharose pull-down assay with recombinant full-length Hsp90 and C-Hsp90β further confirmed this finding. During the preparation of this manuscript, we located a very recent manuscript by Yin et al. 45, which reported that (−)-EGCG inhibited the ATP binding to purified Hsp90 and Hsp90 C-terminus. Presumably, experimental conditions may contribute to this discrenpancy. In ATP-sepharose binding assay, sodium molybdate (Na2MoO4) is a common constituent in the incubation buffer, because molybdate can “freeze” Hsp90 complex in the presence of ATP. However, we observed that mixing colorless (−)-EGCG and sodium molybdate together immediately appeared brown, which indicated a reaction occurring between these two compounds. Thus, the incubation buffer of the ATP binding assay used in the current study did not contain sodium molybdate.
As a result of direct binding to Hsp90 and interference with co-chaperone association to Hsp90, (−)-EGCG exhibited a simultaneous down-regulation of oncogenic Hsp90 client proteins in Mia Paca-2 cells. Consequently, the cell growth was inhibited and apoptosis was dramatically induced. Unlike ansamycin inhibitors of Hsp90 (e.g., GA, 17-AAG), (−)-EGCG did not significantly induce the increase of Hsp70 even after a prolonged treatment. This is in contrast to GA, since binding of ansamycin drugs usually induces a heat shock response through the release, activation, nuclear localization and trimerization of heat shock factor-1 (HSF-1) 46. HSF1 binds to heat shock elements (HSE) to trigger the expression of some stress-responsive proteins such as Hsp70 46, 47. This up-regulation of Hsp70 is believed to compromise the Hsp90-targeted drug efficacy by inhibiting apoptosis signaling 46, 47.
In summary, the data presented in this manuscript suggest that (−)-EGCG, a novel Hsp90 inhibitor, impairs the association of Hsp90/Hsc70 and Hsp90/p23 by directly binding to the C-terminal region of Hsp90, inhibits Hsp90 chaperoning function, and simultaneously degrades multiple cancer-related Hsp90 client proteins. This finding provides a new mechanism for chemoprevention efficacy of (−)-EGCG.
Acknowledgments
This study is partially supported by NIH funding RO1 CA 120023, UM cancer center research grant (Munn), UM cancer center core grant to DS. We thank Dr. Dan Bolon (University of Massachusetts Medical School) and Dr. Thomas Ratajczak (University of Western Australia, Australia) for the generous gifts of the purified N-Hsp90β and expression plasmids, pET15b-hHsp90β and pET28a(+)-hHsp90β (530-724), respectively.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Ghaneh P, Costello E, Neoptolemos JP. Biology and management of pancreatic cancer. Gut. 2007;56(8):1134–52. doi: 10.1136/gut.2006.103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korc M, Chandrasekar B, Yamanaka Y, Friess H, Buchier M, Beger HG. Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increases in the levels of epidermal growth factor and transforming growth factor alpha. J Clin Invest. 1992;90(4):1352–60. doi: 10.1172/JCI116001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamanaka Y, Friess H, Kobrin MS, Buchler M, Kunz J, Beger HG, Korc M. Overexpression of HER2/neu oncogene in human pancreatic carcinoma. Hum Pathol. 1993;24(10):1127–34. doi: 10.1016/0046-8177(93)90194-l. [DOI] [PubMed] [Google Scholar]
- 5.Schlieman MG, Fahy BN, Ramsamooj R, Beckett L, Bold RJ. Incidence, mechanism and prognostic value of activated AKT in pancreas cancer. Br J Cancer. 2003;89(11):2110–5. doi: 10.1038/sj.bjc.6601396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90(1):65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 7.Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem. 2005;280(39):33097–100. doi: 10.1074/jbc.R500010200. [DOI] [PubMed] [Google Scholar]
- 8.Welch WJ. The role of heat-shock proteins as molecular chaperones. Curr Opin Cell Biol. 1991;3(6):1033–8. doi: 10.1016/0955-0674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- 9.McClellan AJ, Xia Y, Deutschbauer AM, Davis RW, Gerstein M, Frydman J. Diverse cellular functions of the Hsp90 molecular chaperone uncovered using systems approaches. Cell. 2007;131(1):121–35. doi: 10.1016/j.cell.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 10.Falsone SF, Gesslbauer B, Tirk F, Piccinini AM, Kungl AJ. A proteomic snapshot of the human heat shock protein 90 interactome. FEBS Lett. 2005;579(28):6350–4. doi: 10.1016/j.febslet.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Kamal A, Boehm MF, Burrows FJ. Therapeutic and diagnostic implications of Hsp90 activation. Trends Mol Med. 2004;10(6):283–90. doi: 10.1016/j.molmed.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearl LH, Prodromou C, Workman P. The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem J. 2008;410(3):439–53. doi: 10.1042/BJ20071640. [DOI] [PubMed] [Google Scholar]
- 13.Didelot C, Lanneau D, Brunet M, Joly AL, De Thonel A, Chiosis G, Garrido C. Anti-cancer therapeutic approaches based on intracellular and extracellular heat shock proteins. Curr Med Chem. 2007;14(27):2839–47. doi: 10.2174/092986707782360079. [DOI] [PubMed] [Google Scholar]
- 14.Ali MM, Roe SM, Vaughan CK, Meyer P, Panaretou B, Piper PW, Prodromou C, Pearl LH. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature. 2006;440(7087):1013–7. doi: 10.1038/nature04716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheibel T, Neuhofen S, Weikl T, Mayr C, Reinstein J, Vogel PD, Buchner J. ATP-binding properties of human Hsp90. J Biol Chem. 1997;272(30):18608–13. doi: 10.1074/jbc.272.30.18608. [DOI] [PubMed] [Google Scholar]
- 16.Workman P. Altered states: selectively drugging the Hsp90 cancer chaperone. Trends Mol Med. 2004;10(2):47–51. doi: 10.1016/j.molmed.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Solit DB, Ivy SP, Kopil C, Sikorski R, Morris MJ, Slovin SF, Kelly WK, DeLaCruz A, Curley T, Heller G, Larson S, Schwartz L, Egorin MJ, Rosen N, Scher HI. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. Clin Cancer Res. 2007;13(6):1775–82. doi: 10.1158/1078-0432.CCR-06-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neckers L. Development of small molecule Hsp90 Inhibitors: utilizing both forward and reverse chemical genomics for drug identification. Current Medicinal Chemistry. 2003;10(9):733–739. doi: 10.2174/0929867033457818. [DOI] [PubMed] [Google Scholar]
- 19.Roe SM, Prodromou C, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem. 1999;42(2):260–6. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 20.Neckers L. Chaperoning oncogenes: Hsp90 as a target of geldanamycin. Handb Exp Pharmacol. 2006;(172):259–77. doi: 10.1007/3-540-29717-0_11. [DOI] [PubMed] [Google Scholar]
- 21.Prodromou C, Siligardi G, O’Brien R, Woolfson DN, Regan L, Panaretou B, Ladbury JE, Piper PW, Pearl LH. Regulation of Hsp90 ATPase activity by tetratricopeptide repeat (TPR)-domain co-chaperones. EMBO J. 1999;18(3):754–62. doi: 10.1093/emboj/18.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terasawa K, Minami M, Minami Y. Constantly updated knowledge of Hsp90. J Biochem. 2005;137(4):443–7. doi: 10.1093/jb/mvi056. [DOI] [PubMed] [Google Scholar]
- 23.Marcu MG, Chadli A, Bouhouche I, Catelli M, Neckers LM. The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J Biol Chem. 2000;275(47):37181–6. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- 24.Yun BG, Huang W, Leach N, Hartson SD, Matts RL. Novobiocin induces a distinct conformation of Hsp90 and alters Hsp90-cochaperone-client interactions. Biochemistry. 2004;43(25):8217–29. doi: 10.1021/bi0497998. [DOI] [PubMed] [Google Scholar]
- 25.Marcu MG, Schulte TW, Neckers L. Novobiocin and related coumarins and depletion of heat shock protein 90-dependent signaling proteins. J Natl Cancer Inst. 2000;92(3):242–8. doi: 10.1093/jnci/92.3.242. [DOI] [PubMed] [Google Scholar]
- 26.Landis-Piwowar KR, Huo C, Chen D, Milacic V, Shi G, Chan TH, Dou QP. A novel prodrug of the green tea polyphenol (−)-epigallocatechin-3-gallate as a potential anticancer agent. Cancer Res. 2007;67(9):4303–10. doi: 10.1158/0008-5472.CAN-06-4699. [DOI] [PubMed] [Google Scholar]
- 27.Fujiki H. Two stages of cancer prevention with green tea. J Cancer Res Clin Oncol. 1999;125(11):589–97. doi: 10.1007/s004320050321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palermo CM, Westlake CA, Gasiewicz TA. Epigallocatechin gallate inhibits aryl hydrocarbon receptor gene transcription through an indirect mechanism involving binding to a 90 kDa heat shock protein. Biochemistry. 2005;44(13):5041–52. doi: 10.1021/bi047433p. [DOI] [PubMed] [Google Scholar]
- 29.Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66(5):2500–5. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 30.Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- 31.Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64(23):8715–22. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- 32.Nagle DG, Ferreira D, Zhou YD. Epigallocatechin-3-gallate (EGCG): chemical and biomedical perspectives. Phytochemistry. 2006;67(17):1849–55. doi: 10.1016/j.phytochem.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst. 1997;89(24):1881–6. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu M, Deguchi A, Lim JT, Moriwaki H, Kopelovich L, Weinstein IB. (−)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin Cancer Res. 2005;11(7):2735–46. doi: 10.1158/1078-0432.CCR-04-2014. [DOI] [PubMed] [Google Scholar]
- 35.Jatoi A, Ellison N, Burch PA, Sloan JA, Dakhil SR, Novotny P, Tan W, Fitch TR, Rowland KM, Young CY, Flynn PJ. A phase II trial of green tea in the treatment of patients with androgen independent metastatic prostate carcinoma. Cancer. 2003;97(6):1442–6. doi: 10.1002/cncr.11200. [DOI] [PubMed] [Google Scholar]
- 36.Solit DB, Chiosis G. Development and application of Hsp90 inhibitors. Drug Discov Today. 2008;13(1–2):38–43. doi: 10.1016/j.drudis.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Powers MV, Workman P. Inhibitors of the heat shock response: biology and pharmacology. FEBS Lett. 2007;581(19):3758–69. doi: 10.1016/j.febslet.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 38.Chiosis G, Neckers L. Tumor selectivity of Hsp90 inhibitors: the explanation remains elusive. ACS Chem Biol. 2006;1(5):279–84. doi: 10.1021/cb600224w. [DOI] [PubMed] [Google Scholar]
- 39.Blagg BS, Kerr TD. Hsp90 inhibitors: small molecules that transform the Hsp90 protein folding machinery into a catalyst for protein degradation. Med Res Rev. 2006;26(3):310–38. doi: 10.1002/med.20052. [DOI] [PubMed] [Google Scholar]
- 40.Allan RK, Mok D, Ward BK, Ratajczak T. Modulation of chaperone function and cochaperone interaction by novobiocin in the C-terminal domain of Hsp90: evidence that coumarin antibiotics disrupt Hsp90 dimerization. J Biol Chem. 2006;281(11):7161–71. doi: 10.1074/jbc.M512406200. [DOI] [PubMed] [Google Scholar]
- 41.Garnier C, Lafitte D, Tsvetkov PO, Barbier P, Leclerc-Devin J, Millot JM, Briand C, Makarov AA, Catelli MG, Peyrot V. Binding of ATP to heat shock protein 90: evidence for an ATP-binding site in the C-terminal domain. J Biol Chem. 2002;277(14):12208–14. doi: 10.1074/jbc.M111874200. [DOI] [PubMed] [Google Scholar]
- 42.Ishii T, Mori T, Tanaka T, Mizuno D, Yamaji R, Kumazawa S, Nakayama T, Akagawa M. Covalent modification of proteins by green tea polyphenol (−)-epigallocatechin-3-gallate through autoxidation. Free Radic Biol Med. 2008;45(10):1384–94. doi: 10.1016/j.freeradbiomed.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 43.Young JC, Obermann WM, Hartl FU. Specific binding of tetratricopeptide repeat proteins to the C-terminal 12-kDa domain of hsp90. J Biol Chem. 1998;273(29):18007–10. doi: 10.1074/jbc.273.29.18007. [DOI] [PubMed] [Google Scholar]
- 44.Fang Y, Fliss AE, Rao J, Caplan AJ. SBA1 encodes a yeast hsp90 cochaperone that is homologous to vertebrate p23 proteins. Mol Cell Biol. 1998;18(7):3727–34. doi: 10.1128/mcb.18.7.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin Z, Henry EC, Gasiewicz TA. (−)-Epigallocatechin-3-gallate is a novel Hsp90 inhibitor. Biochemistry. 2009;48(2):336–45. doi: 10.1021/bi801637q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaur J, Ralhan R. Induction of apoptosis by abrogation of HSP70 expression in human oral cancer cells. Int J Cancer. 2000;85(1):1–5. doi: 10.1002/(sici)1097-0215(20000101)85:1<1::aid-ijc1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 47.Schmitt E, Maingret L, Puig PE, Rerole AL, Ghiringhelli F, Hammann A, Solary E, Kroemer G, Garrido C. Heat shock protein 70 neutralization exerts potent antitumor effects in animal models of colon cancer and melanoma. Cancer Res. 2006;66(8):4191–7. doi: 10.1158/0008-5472.CAN-05-3778. [DOI] [PubMed] [Google Scholar]