Abstract
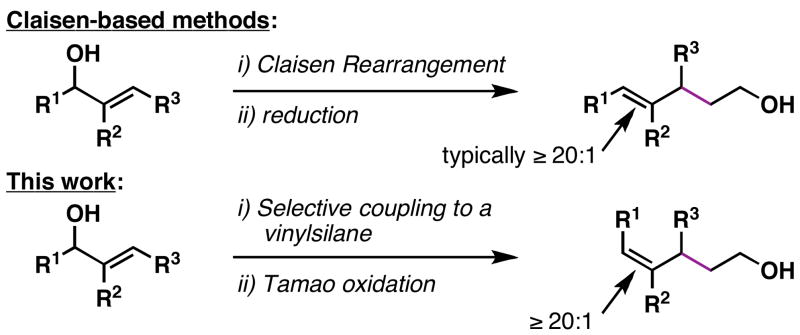
A stereoselective method for the conversion of allylic alcohols to (Z)-trisubstituted alkenes is presented. Overall, the reaction sequence described is stereochemically complimentary to related Claisen rearrangement reactions – processes that typically deliver the stereoisomeric trisubstituted alkene containing products.
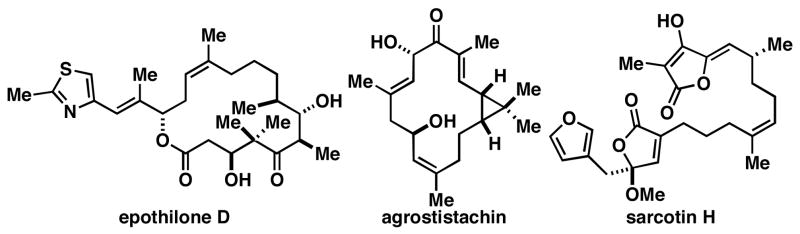
Methods for the synthesis of geometrically defined trisubstituted olefins define a pillar of modern synthetic organic chemistry. From a target-based perspective, these stereodefined structural motifs are ubiquitous in natural products and molecules of biomedical and physical relevance (Figure 1). From a reactivity-based perspective, geometrically defined olefins serve as a foundation for stereoselective synthesis. These factors have driven the invention of a large variety of chemical methods for the convergent synthesis of stereodefined olefins. While many of these methods proceed from carbonyl addition chemistry or alkyne functionalization, the use of allylic alcohol derivatives in sigmatropic processes defines a powerful means to access a subset of stereodefined polysubstituted olefins.1 Of these, Claisen-based methods have been particularly effective at establishing stereodefined (E)-trisubstituted olefins. Here, we describe a metal-mediated reductive cross-coupling reaction that defines a stereochemically complimentary means of converting allylic alcohols to products related to those derived from Claisen rearrangement (Figure 2). While describing a unique stereoselective transformation for complex molecule synthesis, this study also defines a novel reductive cross-coupling reaction between alkenes and allylic alcohols.2, 3
Figure 1.
Natural products possessing stereodefined (Z)-trisubstituted alkenes.
Figure 2.
A stereochemically unique method for the synthesis of stereodefined trisubstituted alkenes from allylic alcohols.
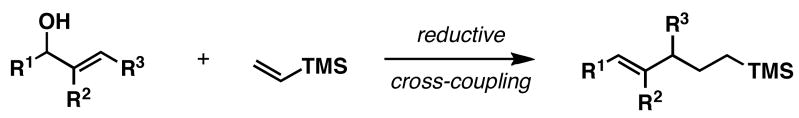
Recently, we demonstrated that allylic alcohols are useful substrates in titanium-mediated reductive cross-coupling reactions with internal alkynes.4 In these reactions, 1,4-dienes result from C–C bond formation between preformed titanium–alkyne complexes and allylic alkoxides. While quite useful for the stereoselective synthesis of substituted 1,4-dienes, we wondered whether a related reductive cross-coupling process could define a stereoselective convergent pathway to isolated di- and trisubstituted olefins. To accomplish such a transformation, we targeted a reductive cross-coupling reaction between allylic alcohols and vinylsilanes.5
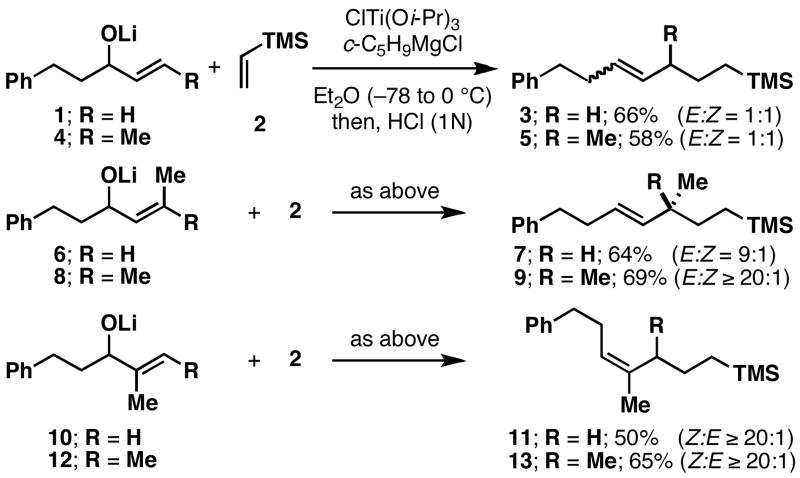
Our initial studies, depicted in Figure 3, provided some hope that the desired stereoselective transformation would be possible. In general, the preformed lithium alkoxide of an allylic alcohol was combined with vinyltrimethylsilane in Et2O, cooled and treated with the combination of ClTi(Oi-Pr)3 and C5H9MgCl (−78 to 0 °C).6 While cross-coupling of allylic alkoxides 1 and 4 with vinyltrimethylsilane (2) provided cross-coupled products 3 and 5 in 58–66% yield, these reactions proceeded without stereoselection (E:Z = 1:1). In contrast, reductive cross-coupling of the (Z)-disubstituted alkene 6 with 2 provided the (E)-alkene 7 in 64% (E:Z = 9:1). Highest levels of (E)-selectivity were observed in the reaction of 8 with 2. This process provided 9 in 69% yield with ≥20:1 selectivity; defining a stereoselective transformation that also establishes a quaternary center.7
Figure 3.
Preliminary study of stereoselection in reductive cross-coupling of allylic alcohols with vinylsilanes.
While the cross-coupling reaction of terminally substituted allylic alcohols (i.e. 6 and 8) delivers stereodefined (E)-disubstituted alkenes, the reaction of allylic alcohols bearing a 1,1-disubstituted olefins proceedes in a stereochemically unique manner. Reductive cross-coupling of 10 with 2 delivers 11 in 50% yield, with ≥ 20:1 selectivity, favoring the formation of the central stereodefined (Z)-trisubstituted alkene. Similarly, the coupling of the trisubstituted allylic alcohol 12 with 2 provides 13 in 65% yield (Z:E ≥ 20:1).
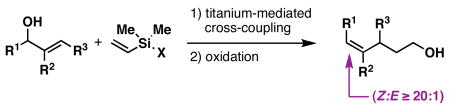
While preliminary studies investigating the coupling of simple acyclic- and cyclic alkenes with vinyltrimethylsilane indicate that this reaction is flexible and stereoselective (Scheme 2 and Table 1, entries 1–3), we searched to identify a coupling partner that would allow for facile oxidation of the C–Si bond resident in the products. The combination of these two reactions, cross-coupling and oxidation, would then define a means to access stereodefined products related to those derived from Claisen rearrangment.1
Table 1.
 | ||||||
|---|---|---|---|---|---|---|
| entry | allylic alcohol | vinylsilane | yield (%) | (Z:E) | dr | product |
| 1a |
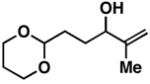 14 |
2 | 62 | ≥ 20:1 | – |
 15 |
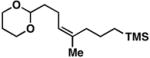
| 2a |
 16 |
2 | 71 | ≥ 20:1 | – |
 17 |
| 3b |
18 |
2 | 64 | – | 5:1 |
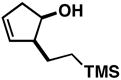 19 |
Reaction conditions: n-BuLi (1 eq), vinylsilane (3 eq), ClTi(Oi-Pr)3 (3 eq), C5H9MgCl (6 eq) (−78 to 0 °C), then HCl (1N).
n-BuLi (2 eq), vinylsilane (3 eq), ClTi(Oi-Pr)3 (3 eq), C5H9MgCl (6 eq) (−78 to 0 °C), then HCl (1N).
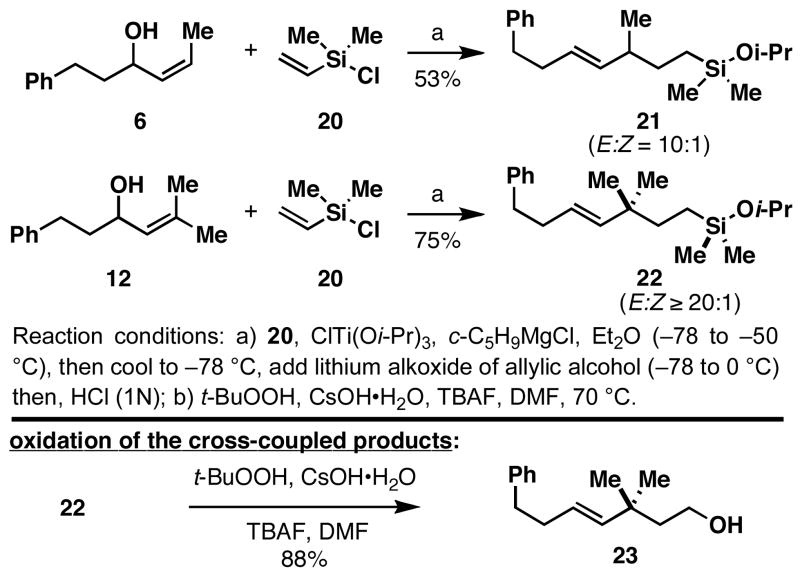
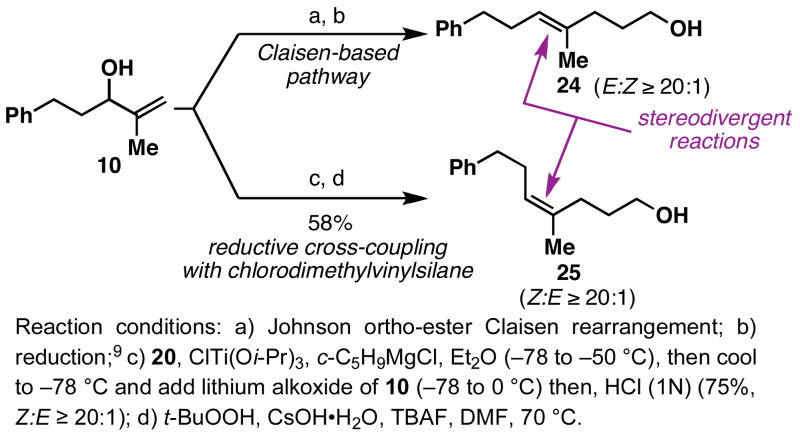
As illustrated in Figure 4, reductive cross-coupling of allylic alcohols 6 and 12 with vinyldimethylchlorosilane5 (20) proceeds in a stereoselective manner, and delivers the corresponding silylethers 21 and 22 in 53% and 75% yield (E:Z =10:1 to ≥ 20:1). Oxidation of the C–Si bond under standard conditions8 then delivers the stereodefined unsaturated primary carbinol (i.e. 22 → 23). While products like 23 could be derived from 12 by the application of well-known Claisen rearrangement-based procedures, the cross-coupling reaction described here has the potential to deliver stereodefined products not readily accessible with these robust [3,3]-sigmatropic rearrangement processes. For example, Claisen rearrangement of 10, followed by carbonyl reduction, provides the (E)-trisubstituted olefin 24 with high levels of stereoselection (E:Z = 20:1).9 In this complimentary process, reductive cross-coupling of 10 with vinyldimethyl-chlorosilane (20), followed by oxidation, provides the isomeric (Z)-trisubstituted olefin 25 in 58% yield (Z:E ≥ 20:1).10
Figure 4.
Cross-coupling reactions with vinyldimethylchlorosilane.
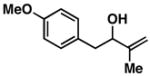
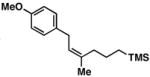
As illustrated in Table 2, this (Z)-selective reductive cross-coupling reaction is useful for the stereoselective functionalization of a variety of allylic alcohols (entries 1–5) Additionally, stereochemically defined products can be prepared from the coupling of mixtures of isomeric allylic alcohols (i.e. entries 6 and 7). Interestingly, coupling of 36 with 20 does not proceed in a similarly stereoconvergent manner, indicating a potential role of the PMB ether in the stereochemical course of this reaction (entry 8).12
Table 2.
 | |||||
|---|---|---|---|---|---|
| entry | allylic alcohol | vinylsilane | yield (%)a | (Z:E) | product |
| 1 |
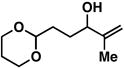 14 |
20 | 51 | ≥ 20:1 |
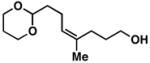 26 |
| 2 |
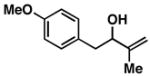 16 |
20 | 58 | ≥ 20:1 |
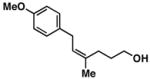 27 |
| 3 |
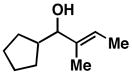 28 |
20 | 69 | ≥ 20:1 |
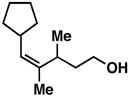 29 |
| 4 |
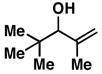 30 |
20 | 56 | ≥ 20:1 |
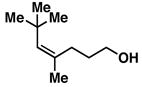 31 |
| 5 |
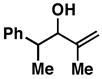 3211 |
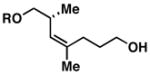
20 | 64 | ≥ 20:1 |
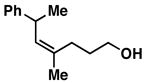 33 |
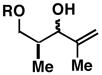
|
|
||||
| 6 | 34; R = TBS11 | 20 | 66 | ≥ 20:1 | 37; R = H |
| 7 | 35; R = TBDPS11 | 20 | 61 | ≥ 20:1 | 37; R = H |
| 8 | 36; R = PMB11 | 20 | 61 | 1.2:112 | 38; R = PMB |
Yield reported is over the two-step process: 1) Reductive cross-coupling (20, ClTi(Oi-Pr)3, c-C5H9MgCl, Et2O (−78 to −50 °C), then cool to −78 °C and add lithium alkoxide of the allylic alcohol (−78 to 0 °C) then, HCl (1N)), 2) oxidation (t-BuOOH, CsOH·H2O, TBAF, DMF, 70 °C).
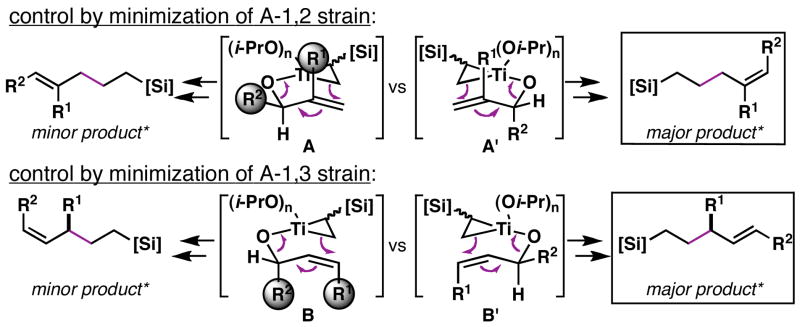
The regio- and stereochemical control observed in this allylic alcohol functionalization process is consistent with the empirical model depicted in Figure 6. In short, pre-association of the allylic alkoxide with a preformed titanacyclopropane (derived from the vinylsilane) produces an intermediate mixed titanate ester capable of rearrangement via formal metallo-[3,3]-rearrangement.4 While the C–C bond formation proceeds with allylic transposition, stereochemical control is thought to derive from minimization of non-bonded steric interactions in a boat-like conformation (i.e. A′ and B′) where the σC–Ti bond is aligned with the πC=C bond.13
Figure 6.
Model of stereoselection.
In sum, we have described a new regio- and stereoselective reductive cross-coupling reaction between allylic alcohols and vinylsilanes. This reaction proceeds with allylic transposition, delivers products with stereodefined di- and trisubstituted olefins, and provides a means to establish allylic tertiary and quaternary carbon centers. In addition to defining a novel olefin functionalization reaction and metal-mediated reductive cross-coupling process,14 this reaction provides a stereochemically unique pathway to functionalized acyclic products not readily accessible with modern [3,3]-sigmatropic rearrangement reactions.1 Future study will explore both the utility of this process in target-oriented synthesis and the interplay between allylic alcohol substitution and selectivity.
Supplementary Material
Experimental procedures and tabulated spectroscopic data for new compounds (PDF) are available free of charge via the Internet at http://pubs.acs.org/paragonplus/submission/jacsat/.
Figure 5.
A stereochemically complimentary process with respect to the Claisen rearrangement.
Acknowledgments
We gratefully acknowledge financial support of this work by the American Cancer Society (RSG-06-117-01), the Arnold and Mabel Beckman Foundation, Boehringer Ingelheim, Eli Lilly & Co. and the National Institutes of Health –NIGMS (GM80266).
References
- 1.For recent reviews, see: Ziegler FE. Chem Rev. 1988;88:1423–1452.Hiersemann M, Nubbemeyer U, editors. The Claisen Rearrangement. Wiley-VCH; Weinheim: 2007. p. 571. See also, Johnson WS, Werthem AL, Bartlett WR, Brocksom TJ, Li TT, Faulkner DJ, Petersen MJ. J Am Chem Soc. 1970;92:741–743.Perrin CL, Faulkner DJ. Tetrahedron Lett. 1969;10:2783–2786.Funk RL, Stallman JB, Wos JA. J Am Chem Soc. 1993;115:8847–8848. For Claisen rearrangement proceeses leading to (Z)-disubstituted olefins, see: Nonoshita K, Banno H, Maruoka K, Yamamoto H. J Am Chem Soc. 1990;112:316–322.Fernández de la Pradilla R, Montero C, Tortosa M. Org Lett. 2002:2372–2376. doi: 10.1021/ol0261130.Takanami T, Hayashi M, Suda K. Tetrahedron Lett. 2005;46:2893–2896. For a rare case of a [3,3]-rearrangement for the stereoselective synthesis of (Z)-trisubstituted olefins, see: Krafft ME, Dasse OA, Jarrett S, Fievre A. J Org Chem. 1995;60:5093–5101. For an example of a (Z)-selective [2,3]-Wittig rearrangement to access related products, see: Still WC, Mitra A. J Am Chem Soc. 1978;100:1927–1928.
- 2.For metal-mediated dimerization of terminal alkenes, see: Isakov VE, Kulinkovich OG. Synlett. 2003:967–970.Lee JC, Sung MJ, Cha JK. Tetrahedron Lett. 2001;42:2059–2061. c) ref 5. For dimerization of, or coupling with, ethylene in route to metallacyclopentanes, see: McDermott JX, Wilson ME, Whitesides GM. J Am Chem Soc. 1976;98:6529–6536.Grubbs RH, Miyashita A. J Chem Soc Chem Comm. 1977:864–865.Cohen SA, Auburn PR, Bercaw JE. J Am Chem Soc. 1983;105:1136–1143.Mashima K, Takaya H. Organometallics. 1985;4:1464–1466.Mashima K, Sakai N, Takaya H. Bull Chem Soc Jpn. 1991;64:2475–2483.Thorn MG, Hill JE, Waratuke SA, Johnson ES, Fanwick PE, Rothwell IP. J Am Chem Soc. 1997;119:8630–8641. For the dimerization of chalcones, see: Schobert R, Maaref R, Dürr S. Synlett. 1995:83–84. For cross-coupling of 1,3-dienes with α-olefins, see: Waratuke SA, Thorn MG, Fanwick PE, Rothwell AP, Rothwell IP. J Am Chem Soc. 1999;121:9111–9119.
- 3.For titanium alkoxide-mediated coupling reactions of allylic alcohols and ethers with Grignard reagents, see: Kulinkovich OG, Epstein OL, Isakov VE, Khmel’nitskaya IA. Synlett. 2001:49–52.Matyushenkov EA, Churikov DG, Sokolov NA, Kulinkovich OG. Russian J Org Chem. 2003;39:478–485. For Cp2ZrCl2-catalyzed carbomagnesiation of allylic alcohols and ethers, see: Hoveyda AH, Xu Z. J Am Chem Soc. 1991;113:5079–5080.Houri AF, Didiuk MT, Xu Z, Horan NR, Hoveyda AH. J Am Chem Soc. 1993;115:6614–6624.Hoveyda AH, Morken JP. Angew Chem Int Ed Engl. 1996;35:1262–1284.
- 4.Kolundzic F, Micalizio GC. J Am Chem Soc. 2007;129:15112–15113. doi: 10.1021/ja075678u. For reductive cross-coupling of allenic alcohols with alkynes via formal metallo-[3,3] rearrangement, see: Shimp HL, Hare A, McLaughlin M, Micalizio GC. Tetrahedron. 2008;64:3437–3445. doi: 10.1016/j.tet.2008.02.015. For reductive cross-coupling of allenic alcohols with aromatic imines via formal metallo-[3,3] rearrangement, see: McLaughlin M, Shimp HL, Navarro R, Micalizio GC. Synlett. 2008:735–738. doi: 10.1055/s-2008-1042808.
- 5.For the first report documenting the ability of vinylsilanes to participate in the ligand exchange reaction with presumed alkoxytitanacyclopropane intermediates, see: Mizojiri R, Urabe H, Sato F. J Org Chem. 2000;65:6217–6222. doi: 10.1021/jo000925x.
- 6.For the use of cycloalkylmagnesium halide reagents to generate presumed alkoxytitanacyclopropane intermediates for intermolecular Kulinkovich cyclopropanation, see: Lee J, Kim H, Cha JK. J Am Chem Soc. 1996;118:4198–4199.
- 7.For reviews on the construction of quaternary carbon centers, see: Martin SF. Tetrahedron. 1980;36:419–460.Corey EJ, Buzman-Perez A. Angew Chem Int Ed. 1998;37:388–401. doi: 10.1002/(SICI)1521-3773(19980302)37:4<388::AID-ANIE388>3.0.CO;2-V.Denissova I, Barriault L. Tetrahedron. 2003;59:10105–10146.
- 8.Smitrovich JH, Woerpel KA. J Org Chem. 1996;61:6044–6046. [Google Scholar]
- 9.Abouabdellah A, Bonnet-Delpon D. Tetrahedron. 1994;50:11921–11932. [Google Scholar]
- 10.Titanium-mediated reductive cross-coupling reactions of allylic alcohols with internal alkynes proceed with a similar sense of stereoselection. See ref 4a for details.
- 11.Derived from addition of 2-propenylmagnesium bromide to the corresponding chiral aldehyde. See Supporting Information for details.
- 12.The stereochemistry of the major/minor isomer was not determined. Future studies will examine the relationship of the relative stereochemistry of 36 on the stereochemical course of the reductive cross-coupling reaction.
- 13.This empirical model does not yet address the number of ligands present on the metal center in the transition state. Others have suggested –ate complexes as reactive intermediates in the Kulinkovich reaction: Kulinkovich OG, Kanonovich DG. Eur J Org Chem. 2007:2121–2132.Kananovich DG, Kulinkovich OG. Tetrahedron. 2008;64:1536–1547. For the proposal of related intermediates in the reductive ethylation of allylic ethers, see: Matyushenkov EA, Churikov DG, Sokolov NA, Kulinkovich OG. Russ J Org Chem. 2003;39:478–485.
- 14.While this reaction has not yet been rendered catalytic in the metal (Ti), the process provides a stereochemically unique transformation of great potential utility in organic synthesis. Like the Claisen rearrangement, these reactions proceed by substrate control, thereby eliminating the necessity to control stereochemistry by reagent- or catalyst-based methods. Finally, due to the low cost of the metal-containing reagents, and benign nature of the byproducts (TiO2 and magnesium (II) salts), the reaction in its current form should be of great utility in organic chemistry.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures and tabulated spectroscopic data for new compounds (PDF) are available free of charge via the Internet at http://pubs.acs.org/paragonplus/submission/jacsat/.