Abstract
In Europe, antimicrobial resistance has been monitored since 1998 by the European Antimicrobial Resistance Surveillance System (EARSS). We examined the relationship between penicillin nonsusceptibility of invasive isolates of Streptococcus pneumoniae (an indicator organism) and antibiotic sales. Information was collected on 1998-99 resistance data for invasive isolates of S. pneumoniae to penicillin, based on surveillance data from EARSS and on outpatient sales during 1997 for beta-lactam antibiotics and macrolides. Our results show that in Europe antimicrobial resistance is correlated with use of beta-lactam antibiotics and macrolides.
Key words: antimicrobial resistance, penicillin nonsusceptible S. pneumoniae, antimicrobial use, ecologic study
Antimicrobial resistance is a growing problem worldwide, requiring international approaches. The World Health Organization (WHO) and the European Commission have recognized the importance of studying the emergence and determinants of resistance and the need for strategies for its control (1–3). In European countries, antimicrobial resistance has been monitored in selected bacteria from humans since 1998 through the European Antimicrobial Resistance Surveillance System (EARSS). Funded by the European Commission, EARSS is an international network of national surveillance systems intended to collect comparable and reliable resistance data. The purpose of EARSS is to document variations in antimicrobial resistance over time and place and to provide the basis for and assess the effectiveness of prevention programs and policy decisions.
One of the indicator organisms in EARSS is Streptococcus pneumoniae. It was included for three reasons: it is of major clinical importance for pneumonia, bacterial meningitis, and otitis media; many countries have reported that its resistance to penicillin is increasing; and S. pneumoniae is representative of organisms that are transmitted in the community.
A major risk factor for the development of resistance is thought to be inappropriate use of antimicrobial drugs. Most studies that have investigated the relationship of antimicrobial use and antimicrobial resistance have been undertaken in hospital, multicenter, or country settings (4–7). For infections with penicillin-nonsusceptible S. pneumoniae (PNSP), studies have demonstrated that at the individual level, previous use of beta-lactam antibiotics such as penicillin is an important risk factor (8–10). Studies on carriage of PNSP in children have shown that sulfamethoxazole-trimethoprim (co-trimoxazole) and macrolides such as erythromycin have also been associated with selection of PNSP (11,12). Translated to the population level, sales of beta-lactam antibiotics, co-trimoxazole, or macrolides in a given geographic region may be proportional to microbial resistance to penicillin. If on the European level a relationship between antimicrobial resistance and antimicrobial use could be found (as in the case of S. pneumoniae and resistance to penicillin), efforts to control antimicrobial use and misuse could be stimulated and monitored in Europe.
We used an ecologic study design to examine the correlation between use of relevant antibiotics in the outpatient setting and the proportion of PNSP among invasive isolates of S. pneumoniae in 11 European countries.
Methods
Antimicrobial Resistance Data
The estimated average coverage of the populations of countries participating in EARSS is 52% (range 10% to 90%) (13). Laboratories that participate in EARSS screen invasive S. pneumoniae isolates for oxacillin resistance (14). When an isolate is found to be nonsusceptible, the EARSS protocol requests confirmation as intermediate- or high-level resistance to penicillin by determination of MICs. Laboratories perform microbiologic testing and interpret results according to their own standards. National guidelines in Europe differ; isolates of S. pneumoniae are considered nonsusceptible to penicillin if the MIC is >0.06 (15–18) or >0.12 (19,20) mg/L. For this report, we use nonsusceptibility and intermediate resistance as synonyms; PNSP isolates are either intermediate or fully resistant to penicillin. Only the first invasive isolate per patient per quarter is reported.
To assess the comparability of susceptibility test results, a quality assurance exercise was performed in September 2000 among 482 laboratories from 23 countries participating in EARSS. The concordance (agreement of reported results with intended results) for the detection of penicillin resistance in the three S. pneumoniae control strains was 91% (21). Laboratories sent standardized data to the national EARSS data manager, who checks data contents and ensures conformity with the EARSS data format. In collaboration with WHO, an export module from the laboratory-based software WHONET was developed for EARSS (22). Every quarter, data are forwarded to the central database at the National Institute of Public Health and the Environment (RIVM), Bilthoven, Netherlands, where the project is coordinated.
Antimicrobial Use Data
National outpatient sales data for antibiotics from 1997 were purchased from IMS Health Global Services, London, United Kingdom, for 13 of the 15 member states of the European Union. Corresponding data were obtained from the Danish Medicines Agency for Denmark and from the National Corporation of Swedish Pharmacies for Sweden (23). The IMS data were examined and adjusted according to the Anatomic Therapeutic Classification (ATC) system used by WHO (24). The amount in kilograms for an antimicrobial agent was converted to a number of defined daily doses (DDD). The DDD, which is based on the average daily dose used for the main indication of the drug, is appropriate for comparisons of drug use over time and in different geographic areas. For beta-lactam antibiotics, we combined ATC groups J01C (extended- and narrow-spectrum penicillins) and J01D (cephalosporins); macrolides were classified under code J01F. No data were available for the combination of trimethoprim and sulphonamide.
Nonadherence
We considered nonadherence of patients to the physician’s prescription in individual countries as a possible confounder of antimicrobial resistance. Branthwaite et al. reported nonadherence levels from a population-based survey in seven countries (25). Data from four of the seven countries (Spain, Belgium, the United Kingdom, and Italy) were also captured in EARSS.
Statistical Analysis
We calculated the proportion of PNSP among all invasive S. pneumoniae isolates from each country reported during 1998-99. Because probabilities allow only values between 0 and 1, we modeled the natural logarithm of the odds of PNSP resistance (logodds).
Least-square linear regression analysis was used to assess correlation between antimicrobial use (of beta-lactam antibiotics and macrolides, expressed in DDD per 1,000 population per day) and the logodds of resistance. We correlated nonadherence levels with the logodds of resistance in the same way. We calculated the Spearman coefficient of determination (r-square) and its corresponding p value. For the calculation of the regression lines, we weighted the data points by the inverse of the variance of each data point. We used SAS software (SAS Institute Inc., Release 6.03., Cary, NC).
Results
Antimicrobial Resistance
During 1998-99, 337 laboratories from 11 European Union member states (Belgium, Finland, Germany, Ireland, Italy, Luxembourg, Netherlands, Portugal, Spain, Sweden, and United Kingdom) and one nonmember state (Iceland) reported 4,872 invasive S. pneumoniae isolates to EARSS. The proportion of PNSP among isolates of invasive S. pneumoniae ranged from 1% to 34% (Table) (Figure 1). Southern European countries reported higher rates than northern European countries.
Table. Number of submitting laboratories, number of isolates of Streptococcus pneumoniae, number (#) and percent (%R) of penicillin nonsusceptible S. pneumoniae isolates, logodds of resistance (ln(%R/[1-%R]), and outpatient sales of beta-lactam antibiotics and macrolides.
| Country | No. of labora-tories | No. of S. pneumoniae isolates | Penicillin nonsusceptible S. pneumoniae | Outpatient sales of antibiotics in DDDa/1,000 inhabitants/day | |||
|---|---|---|---|---|---|---|---|
| No. | %R (95% CI) | ln (%R/[1-%R]) | Beta-lactam antibiotics | Macrolides | |||
| Austria | - | - | - | - | - | 6 | 3.7 |
| Belgium | 96 | 940 | 131 | 14 (12-16) | -1.82 | 14 | 4.1 |
| Denmark | - | - | - | - | - | 7 | 2 |
| Finland | 11 | 211 | 8 | 4 (2-8) | -3.18 | 8[REMOVED ADVANCE FIELD] | [REMOVED ADVANCE FIELD]1.9 |
| [REMOVED ADVANCE FIELD]France | [REMOVED ADVANCE FIELD]- | [REMOVED ADVANCE FIELD]- | [REMOVED ADVANCE FIELD]- | [REMOVED ADVANCE FIELD]- | [REMOVED ADVANCE FIELD]- | 24[REMOVED ADVANCE FIELD] | [REMOVED ADVANCE FIELD]6.0 |
| Germany | 15 | 222 | 4 | 2 (1-5) | -3.89 | 5 | 2.5 |
| Iceland | 2 | 54 | 1 | 2 (0-11) | -3.89 | Not available | Not available |
| Ireland | 12 | 157 | 30 | 19 (13-26) | -1.45 | 11 | 2.5 |
| Italy | 46 | 194 | 26 | 13 (9-19) | -1.87 | 15 | 5.1 |
| Luxembourg | 1 | 11 | 2 | 18 (3-52) | -1.52 | 14 | 4.7 |
| Netherlands | 20 | 760 | 8 | 1 (0-2) | -4.6 | 4 | 1.2 |
| Portugal | 12 | 134 | 25 | 19 (13-27) | -1.45 | 16 | 3.7 |
| Spain | 76 | 1,240 | 418 | 34 (31-36) | -0.66 | 21 | 5.9 |
| Sweden | 24 | 706 | 21 | 3 (2-5) | -3.48 | 8 | 1 |
| United Kingdom | 22 | 243 | 21 | 9 (6-13) | -2.31 | 9 | 3.2 |
aDDD = defined daily doses; CI = confidence interval.
Figure 1.
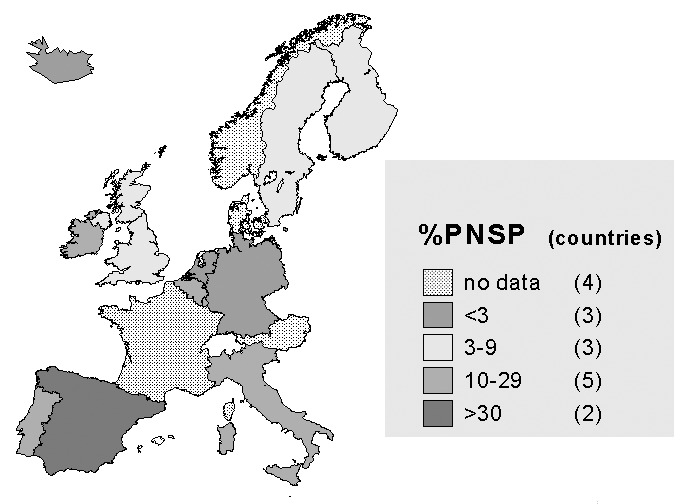
Proportions of invasive isolates of Streptococcus pneumoniae resistant to penicillin (PNSP) among 12 European countries, 1998-99.
Antimicrobial Use
Data on outpatient sales of beta-lactam antibiotics and macrolides were available for 1997 from all 15 European Union member countries. Antimicrobial use varied widely between countries. Sales to outpatients ranged from 3.8 to 23.6 DDD per 1,000 inhabitants per day for beta-lactam antibiotics and from 0.97 to 5.98 DDD for macrolides. The three countries with the highest reported use were France, Spain, and Portugal for beta-lactam antibiotics and France, Spain, and Italy for macrolides; the three countries with the lowest use were the Netherlands, Germany, and Austria for beta-lactam antibiotics and Sweden, the Netherlands, and Finland for macrolides.
Correlation
For 11 countries, information was available for both antimicrobial resistance and antimicrobial use. Linear regression of the correlation of use of beta-lactam antibiotics and the logodds of resistance showed an r-square of 0.80 (p=0.0002) (Figure 2). The equation for the regression is
| logodds of resistance =(-3.94)+(0.16xDDD). |
For the use of macrolides, we calculated an r-square of 0.46. Figure 3 shows the graph for nonadherence to antibiotics and the logodds of resistance. The r-square is 0.8 (p=0.2).
Figure 2.
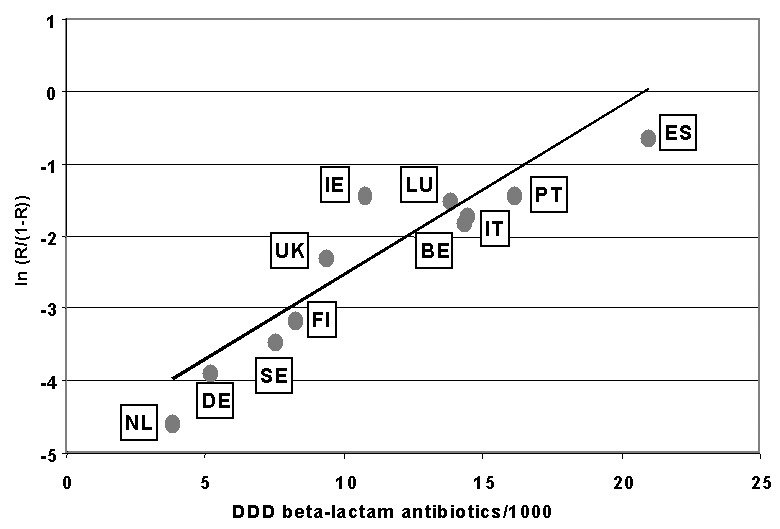
The logodds of resistance to penicillin among invasive isolates of Streptoccus pneumoniae (PNSP; ln(R/[1-R])) is regressed against outpatient sales of beta-lactam antibiotics in 11 European countries; antimicrobial resistance data are from 1998 to 1999 and antibiotic sales data are from 1997. DDD = defined daily dose; BE = Belgium; DE = Germany; FI = Finland; IE = Ireland; IT = Italy; LU = Luxembourg; NL = the Netherlands; PT = Portugal; ES = Spain; Se = Sweden; UK = United Kingdom.
Figure 3.
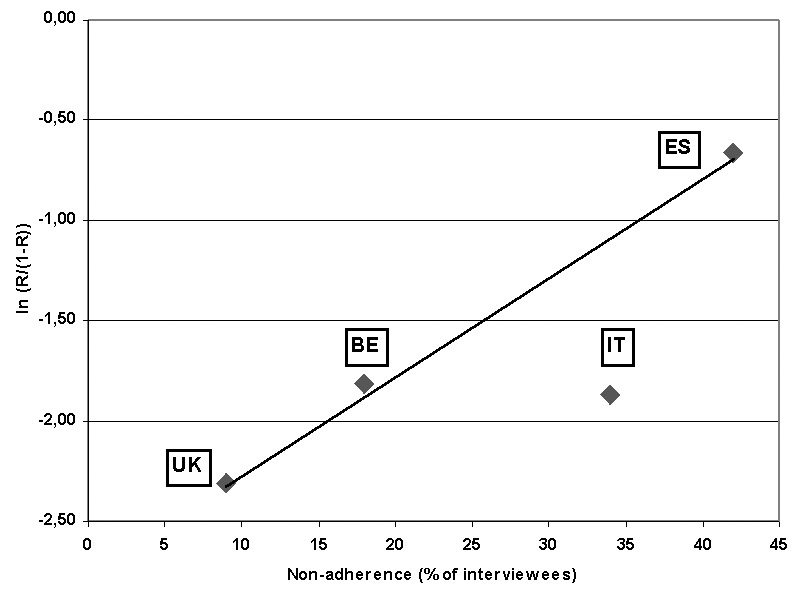
The logodds of resistance of invasive isolates of Streptococcus pneumoniae to penicillin (PNSP; ln(R/(1-R))) is regressed against nonadherence rates to antibiotic therapy in four European countries. Nonadherence rates are from 1993; PNSP data are from 1998-99. UK = United Kingdom; BE = Belgium; IT = Italy; ES = Spain.
Discussion
We present for the first time Europe-wide, country-specific, representative data on antimicrobial resistance collected by EARSS. Using an ecologic study design, we demonstrate through the correlation with data on antimicrobial use one aspect of the usefulness of surveillance for antimicrobial resistance. The results from 11 European countries show a linear relationship between use of beta-lactam antibiotics and macrolides and the proportion of PNSP among all invasive S. pneumoniae isolates.
EARSS data show that resistance for PNSP follows a north-south gradient. Southern European countries have higher proportions of PNSP than countries in northern Europe. A possible reason for this observation could be the difference in antimicrobial use, which also tends to be higher in southern European countries. If use of relevant antibiotics (beta-lactam antibiotics and macrolides) and the logodds of resistance are modeled through linear regression, a strong linear and statistically significant relationship is demonstrated.
Our findings agree with those of Austin et al., who modeled the relationship between antimicrobial use and endemic resistance, based on population genetic methods and epidemiologic observations (26). The correlation in Figure 2 is consistent with the model developed by Austin et al. on theoretical grounds.
We correlate antimicrobial sales data for 1997 with antimicrobial resistance data for 1998 and 1999. Others have observed that after a lag time of 1 or more years, changes in antimicrobial use may be followed by changes in antimicrobial resistance (27,28). Therefore, we believe that it is reasonable to correlate antimicrobial sales data in 1997 with antimicrobial resistance data from 1998-99.
We address several limitations in our study. First, because it is an ecologic study, we can make no inferences on the individual level. Second, resistance rates in some countries (Table) are calculated from a relatively limited number of isolates. However, based on communications with EARSS country representatives, our data are consistent with antimicrobial resistance levels derived from other sources (29). Third, an explanation for the differences in antimicrobial resistance could be sampling bias: clinicians in southern European countries may request blood cultures more frequently than their northern European colleagues, who may sample only in case of empirical treatment failure. Fourth, we have not addressed other, potentially important contributing factors for the development of antimicrobial resistance of organisms that are transmitted in the community, particularly nonadherence and over-the-counter sales of antimicrobial agents. Both these factors are difficult to measure. However, in 1993 nonadherence to prescribed antimicrobial agents was assessed in a survey in six European countries (25). Although the number of data points is limited, Figure 2 suggests a direct relationship between nonadherence rates and logodds of resistance. Thus, if nonadherence is also related to sales of antimicrobial agents, it could potentially confound the relationship between use and resistance. Data on the degree of over-the-counter use among European countries are not widely available; we know of one Spanish and one Greek study reporting an estimate of over-the-counter use (30,31). The influence of these and other parameters on the level of resistance should be quantified and understood. Finally, because children are the main reservoir of carriage of S. pneumoniae, an age-stratified analysis would be desirable, i.e., a correlation of resistance with antimicrobial use among children. However, this analysis would require more detailed use data, for example, of liquid formulations of antibiotics.
At least two studies in northern Europe have demonstrated that PNSP rates can be halted or even reversed when physicians avoid the inappropriate prescription of antimicrobial agents (32,33). Our study is timely because it shows that even at the European level a correlation can be observed between antimicrobial resistance (of S. pneumoniae to penicillin) and antimicrobial use. In several European countries, national action plans for the appropriate use of antimicrobial agents are being planned or implemented; their effectiveness should be monitored through prospective and continuous surveillance of antimicrobial resistance and antimicrobial sales data (34–38).
Acknowledgments
We thank all the dedicated laboratories that contributed data. We specifically thank all the national data managers and representatives of the countries participating in EARSS for their hard work in collecting and processing the data, Karl Kristinsson for highly relevant and constructive comments, John Stelling for help with software development, Nico Nagelkerke for significant statistical help, Marc-Alain Widdowson for thoughtful comments, and José van de Velde for helping to keep EARSS running.
EARSS is funded by the European Commission, DG SANCO (Agreement SI2.123794 [99CVF4-018] European Antimicrobial Resistance Surveillance System [EARSS]). National data for nonhospital antibiotic sales were purchased from IMS Health Global Services with support of the Nepi Foundation, Malmö, Sweden.
Biography
Dr. Bronzwaer is a public health specialist in the Department of Infectious Disease Epidemiology of the National Institute for Public Health and the Environment, Bilthoven, the Netherlands. He helped establish the European Antimicrobial Resistance Surveillance System, for which he now serves as project leader.
Footnotes
Suggested citation: Bronzwaer SLAM, Cars O, Buchholz U, Mölstad S, Goettsch W, Veldhuijzen IK, et al. The Relationship between Antimicrobial Use and Antimicrobial Resistance in Europe. Emerg Infect Dis. [serial on the Internet]. 2002 Mar [date cited]. Available from http://wwwdev.cdc.gov/ncidod/EID/vol8no3/01-0192.htm
References
- 1.World Health Organization. Report on infectious diseases 2000: overcoming antimicrobial resistance. Available at URL: http://www.who.int/infectious-disease-report/index.html Accessed September 23, 2000.
- 2.Centers for Disease Control and Prevention. Preventing emerging infectious diseases. Available at URL: http://www.cdc.gov/ncidod/emergplan/plan98.pdf Accessed September 20, 2000.
- 3.European Community. A strategy against the microbial threat. Official Journal of the European Community. Council Resolution of 8 June 1999 on antibiotic resistance. Official Journal C 195, 13/07/1999 p. 1–3. Available at URL: http://europa.eu.int/eur-lex/en/lif/dat/1999/en_399Y0713_01.html Accessed September 29, 2000.
- 4.Lyytikainen O, Vaara M, Jarviluoma E, Rosenqvist K, Tiittanen L, Valtonen V. Increased resistance among Staphylococcus epidermidis isolates in a large teaching hospital over a 12-year period. Eur J Clin Microbiol Infect Dis. 1996;15:133–8. 10.1007/BF01591486 [DOI] [PubMed] [Google Scholar]
- 5.Fridkin SK, Steward CD, Edwards JR, Pryor ER, McGowan JE Jr, Archibald LK, et al. Surveillance of antimicrobial use and antimicrobial resistance in United States hospitals: project Project Intensive Care Antimicrobial Resistance Epidemiology (ICARE) hospitals, phase 2. Clin Infect Dis. 1999;29:245–52. 10.1086/520193 [DOI] [PubMed] [Google Scholar]
- 6.Mouton RP, Hermans J, Simoons-Smit AM, Hoogkamp-Korstanje JA, Degener JE, van Klingeren B. Correlations between consumption of antibiotics and methicillin resistance in coagulase-negative staphylococci. J Antimicrob Chemother. 1990;26:573–83. 10.1093/jac/26.4.573 [DOI] [PubMed] [Google Scholar]
- 7.Goettsch W, van Pelt W, Nagelkerke N, Hendrix MGR, Buiting AGM, Petit PL, et al. Increasing resistance to fluoroquinolones in Escherichia coli from urinary tract infections in The Netherlands. J Antimicrob Chemother. 2000;46:223–8. 10.1093/jac/46.2.223 [DOI] [PubMed] [Google Scholar]
- 8.Tan TQ, Mason EO Jr, Kaplan SL. Penicillin-resistant systemic pneumococcal infections in children: a retrospective case-control study. Pediatrics. 1993;92:761–7. [PubMed] [Google Scholar]
- 9.Nava JM, Bella F, Garau J, Lite J, Morera MA, Marti C, et al. Predictive factors for invasive disease due to penicillin-resistant Streptococcus pneumoniae: a population-based study. Clin Infect Dis. 1994;19:884–90. [DOI] [PubMed] [Google Scholar]
- 10.Deeks SL, Palacio R, Ruvinsky R, Kertesz DA, Hortal M, Rossi A, et al. Risk factors and course of illness among children with invasive penicillin-resistant Streptococcus pneumoniae. The Streptococcus pneumoniae Working Group. Pediatrics. 1999;103:409–13. 10.1542/peds.103.2.409 [DOI] [PubMed] [Google Scholar]
- 11.Arason VA, Kristinsson KG, Sigurdsson JA, Stefansdottir G, Molstad S, Gudmundsson S. Do antimicrobials increase the carriage rate of penicillin resistant pneumococci in children? Cross sectional prevalence study. BMJ. 1996;313:387–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melander E, Mölstad S, Alsterlund R, Ekdahl K, Jönsson G. Macrolides and broad-spectrum antibiotics are risk-factors for spread of pneumococci with reduced sensitivity to penicillin. Pediatr Infect Dis J. 2000;19:1172–7. 10.1097/00006454-200012000-00011 [DOI] [PubMed] [Google Scholar]
- 13.European antimicrobial resistance surveillance system. Report on feasibility phase, p. 56. Available at URL: http://www.earss.rivm.nl Accessed September 26, 2000.
- 14.Goettsch W, Bronzwaer SLAM, Neeling de AJ, Wale MCJ, Aubry-Damon H, Olsson-Liljequist B, et al. Standardisation and quality assurance for antimicrobial resistance of Streptococcus pneumoniae and Staphylococcus aureus within the European Antimicrobial Resistance Surveillance System (EARSS). Clin Microbiol Infect. 2000;6:59–63. 10.1046/j.1469-0691.2000.00027.x [DOI] [PubMed] [Google Scholar]
- 15.Commissie Richtlijnen Gevoeligheidsbepalingen. Nederlands Tijdschrift voor Medische Microbiologie. 1996;4:5. [Google Scholar]
- 16.The National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing; eight informational supplements. Vol 18, no 1. ISBN 1-56238-337-X. Wayne (PA): The Committee; 1998. [Google Scholar]
- 17.Cars O. Antimicrobial susceptibility testing in Sweden. Available at URL: http://www.ltkronoberg.se/ext/raf/raf.htmhttp://Accessed December 21, 2001.
- 18.Report of the Comité de l’Antibiogramme de la Société Française de Microbiologie. Clin Microbiol Infect. 1996;2(Suppl 1):S1–49. 10.1111/j.1469-0691.1996.tb00868.x [DOI] [PubMed] [Google Scholar]
- 19.British Society for Antimicrobial Chemotherapy. BSAC standardized disc sensitivity testing method. Birmingham (UK): Newsletter of the British Society for Antimicrobial Chemotherapy; 1998. [Google Scholar]
- 20.Deutsches Institut für Normung. Methods for the determination of susceptibility of pathogens (except mycobacteria) to antimicrobial agents. MIC breakpoints of antibacterial agents. Berlin. DIN. 1998;Suppl 1:58940–4. [Google Scholar]
- 21.Buchholz U, Bronzwaer S, Snell J, Courvalin P, Cornaglia G, de Neeling J, et al. Comparability of microbiological susceptibility test results from 23 European countries and Israel: the European Antimicrobial Resistance Surveillance System (EARSS)/NEQAS study. Clin Microbiol Infect. 2001;7(Suppl 1):25. [Google Scholar]
- 22.World Health Organization. Communicable Disease Surveillance and Response. WHONET 5 software. Available at URL: http://www.who.int/emc/WHONET/WHONET.html. Accessed September 28, 2000.
- 23.Cars O, Mölstad S, Melander A. Variation in antibiotic use in the European Union. Lancet. 2001;357:1851–3. 10.1016/S0140-6736(00)04972-2 [DOI] [PubMed] [Google Scholar]
- 24.ATC index with DDDs. Oslo: WHO Collaborating Centre for Drug Statistics Methodology; 1999.
- 25.Branthwaite A, Pechère J-C. Pan-European survey on patients’ attitudes to antibiotics and antibiotic use. J Int Med Res. 1996;24:229–38. [DOI] [PubMed] [Google Scholar]
- 26.Austin DJ, Kristinsson KG, Anderson RM. The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proc Natl Acad Sci U S A. 1999;96:1152–6. 10.1073/pnas.96.3.1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seppala H, Klaukka T, Vuopio-Varkila J, Muotiala A, Helenius H, Lager K, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441–6. 10.1056/NEJM199708143370701 [DOI] [PubMed] [Google Scholar]
- 28.Stephenson J. Icelandic researchers are showing the way to bring down rates of antibiotic-resistant bacteria [news]. JAMA. 1996;275:175. 10.1001/jama.275.3.175 [DOI] [PubMed] [Google Scholar]
- 29.European antimicrobial resistance surveillance system. Annual report EARSS 2000. Available at URL: http://www.earss.rivm.nl Accessed 17 June, 2001.
- 30.González J, Orero A. Consumo de antibióticos en España. Rev Esp Quimioter. 1996;9(Suppl 4):155. [Google Scholar]
- 31.Contopoulos-Ionnides DG, Koliofoti ID, Koutroumpa IC, Giannakakis IA, Ioannides JPA. Pathways for inappropriate dispensing of antibiotics for rhinosinusitis: a randomized trial. Clin Infect Dis. 2001;33:76–82. 10.1086/320888 [DOI] [PubMed] [Google Scholar]
- 32.Mölstad S, Cars O. Major change in the use of antibiotics following a national Programme: Swedish Strategic Programme for the Rational Use of Antimicrobial Agents and Surveillance of Resistance (STRAMA). Scand J Infect Dis. 1999;31:191–5. 10.1080/003655499750006263 [DOI] [PubMed] [Google Scholar]
- 33.Kristinsson KG. Modification of prescribers behaviour: the Icelandic approach. Clin Microbiol Infect. 1999;5(Suppl 4):S43–7. 10.1111/j.1469-0691.1999.tb00856.x [DOI] [PubMed] [Google Scholar]
- 34.Danish Integrated Antimicrobial Resistance Monitoring and Research Programme (DANMAP). Consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark, July 1999. Available at URL: http://www.svs.dk/ Accessed October 27, 2000.
- 35.Institute de Veille Sanitaire, Paris: Propositions pour un plan national d’actions pour la maîtrise de la résistance aux antibiotiques, January 1999 (English version: Proposals for a national action plan to control antibiotic resistance in France July 1999).
- 36.Ministries of Health and Agriculture. Ireland. A draft strategy document for control of Antimicrobial Resistance in Ireland - (SARI), 2000. Available at URL: http://www.ndsc.ie/ Accessed October 27, 2000.
- 37.National Board of Health and Welfare. Sweden. National plan against antibiotic resistance, 2000. Available at URL: http://www.sos.se/ (Swedish). Accessed October 27, 2000.
- 38.United Kingdom Department of Health. UK antimicrobial resistance strategy and action plan. Available at URL: http://www.doh.gov.uk/arbstrat.htm Accessed October 27, 2000.


