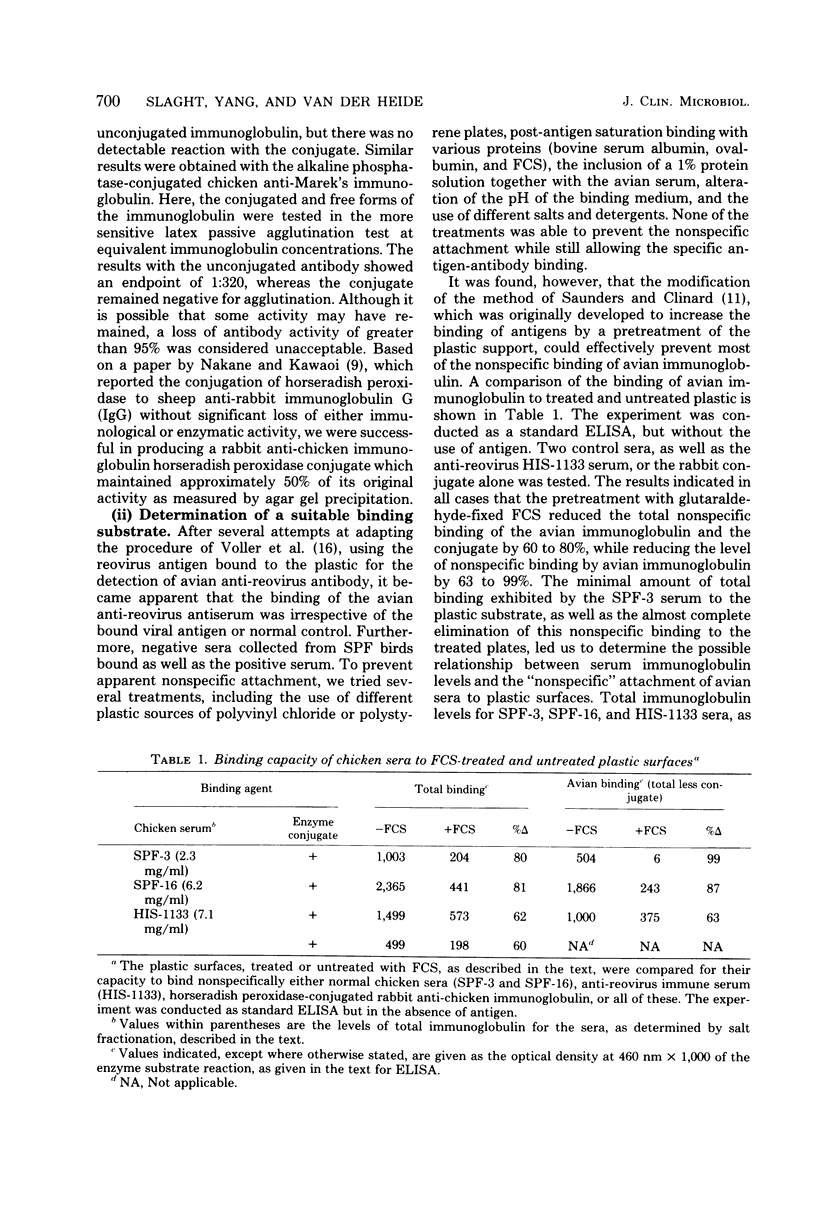
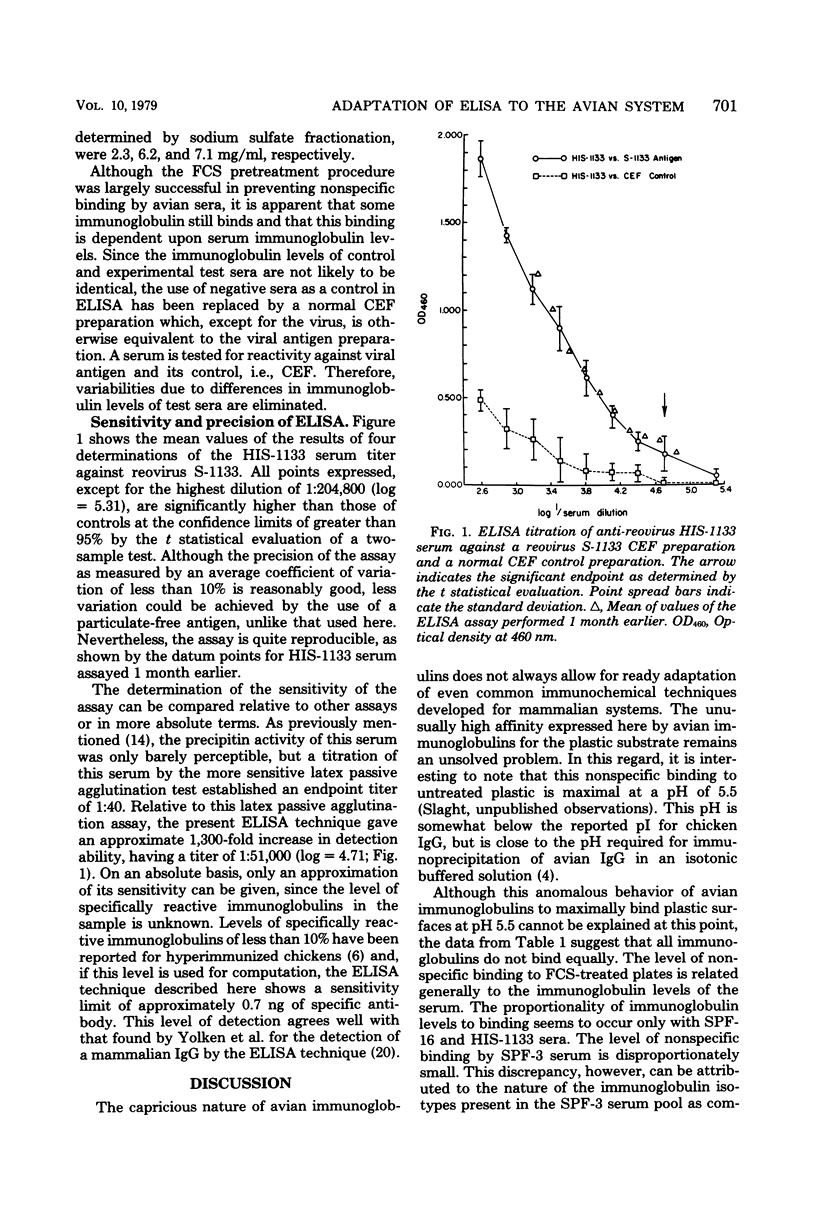
Abstract
A microplate enzyme-linked immunosorbent assay was developed to detect chicken anti-rovirus antibodies. Studies of the parameters which affect the outcome of the assay with avian serum revealed two aspects for a successful assay. First, enzyme-antibody conjugates prepared by the periodate oxidation technique were found to have retained far more immunological activity than conjugates produced by a glutaraldehyde cross-linking. Second, the results indicated an unusually high affinity of chicken immunoglobulin for the microplate plastic which was mostly eliminated by a pretreatment technique with fixed fetal calf serum. The enzyme-linked immunosorbent assay compared favorably with the latex passive agglutination test, yielding a titration endpoint of 1:511,000, or approximately 1,300 times more sensitive than the latex passive agglutination assay. The assay proved not only to be sensitive to less than 1 ng of specific antibody, but also to have low to moderate variance and high reliability.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruins S. C., Ingwer I., Zeckel M. L., White A. C. Parameters affecting the enzyme-linked immunosorbent assay of immunoglobulin G antibody to a rough mutant of Salmonella minnesota. Infect Immun. 1978 Sep;21(3):721–728. doi: 10.1128/iai.21.3.721-728.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Gallagher J. S., Voss E. W., Jr Immune precipitation of purified chicken antibody at low pH. Immunochemistry. 1970 Sep;7(9):771–785. doi: 10.1016/0019-2791(70)90219-3. [DOI] [PubMed] [Google Scholar]
- Hoffmeister M. J., Voss E. W., Jr Binding of epsilon-DNP-L-lysine exceeding two moles by purified chicken IgG anti-DNP antibody. Immunochemistry. 1974 Oct;11(10):641–650. doi: 10.1016/0019-2791(74)90221-3. [DOI] [PubMed] [Google Scholar]
- Horowitz S. A., Cassell G. H. Detection of antibodies to Mycoplasma pulmonis by an enzyme-linked immunosorbent assay. Infect Immun. 1978 Oct;22(1):161–170. doi: 10.1128/iai.22.1.161-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb V. F., Jr, Bernlohr R. W. A new spectrophotometric assay for protein in cell extracts. Anal Biochem. 1977 Oct;82(2):362–371. doi: 10.1016/0003-2697(77)90173-7. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Payment P., Descoteaux J. P. Enzyme-linked immunosorbent assay for the detection of antibodies to pneumonia virus of mice in rat sera. Lab Anim Sci. 1978 Dec;28(6):676–679. [PubMed] [Google Scholar]
- Saunders G. C., Clinard E. H. Rapid micromethod of screening for antibodies to disease agents using the indirect enzyme-labeled antibody test. J Clin Microbiol. 1976 Jun;3(6):604–608. doi: 10.1128/jcm.3.6.604-608.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz H., Doerr H. W., Kampa D., Vogt A. Solid-phase enzyme immunoassay for immunoglobulin M antibodies to cytomegalovirus. J Clin Microbiol. 1977 Jun;5(6):629–634. doi: 10.1128/jcm.5.6.629-634.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurs A. H., Van Weemen B. K. Enzyme-immunoassay. Clin Chim Acta. 1977 Nov 15;81(1):1–40. doi: 10.1016/0009-8981(77)90410-7. [DOI] [PubMed] [Google Scholar]
- Slaght S. S., Yang T. J., van der Heide L., Fredrickson T. N. An enzyme-linked immunosorbent assay (ELISA) for detecting chicken anti-reovirus antibody at high sensitivity. Avian Dis. 1978 Oct-Dec;22(4):802–805. [PubMed] [Google Scholar]
- Van Meter R., Good R. A., Cooper M. D. Ontogeny of circulating immunoglobulin in normal, bursectomized and irradiated chickens. J Immunol. 1969 Feb;102(2):370–374. [PubMed] [Google Scholar]
- Voss E. W., Jr, Watt R. M. Comparison of the microenvironment of chicken and rabbit antibody active sites. Adv Exp Med Biol. 1977;88:391–401. doi: 10.1007/978-1-4613-4169-7_37. [DOI] [PubMed] [Google Scholar]
- Walls K. W., Bullock S. L., English D. K. Use of the enzyme-linked immunosorbent assay (ELISA) and its microadaptation for the serodiagnosis of toxoplasmosis. J Clin Microbiol. 1977 Mar;5(3):273–277. doi: 10.1128/jcm.5.3.273-277.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Barbour B., Wyatt R. G., Kalica A. R., Kapikian A. Z., Chanock R. M. Enzyme-linked immunosorbent assay for identification of rotaviruses from different animal species. Science. 1978 Jul 21;201(4352):259–262. doi: 10.1126/science.208150. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Wyatt R. G., Kim H. W., Kapikian A. Z., Chanock R. M. Immunological response to infection with human reovirus-like agent: measurement of anti-human reovirus-like agent immunoglobulin G and M levels by the method of enzyme-linked immunosorbent assay. Infect Immun. 1978 Feb;19(2):540–546. doi: 10.1128/iai.19.2.540-546.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


