Abstract
Background
Androgen ablation (AA) causes apoptosis of normal and neoplastic prostate cells. It is a standard treatment for advanced prostate cancer. Androgen ablation-mediated immunological effects include bone marrow hyperplasia, thymic regeneration, T and B cell lymphopoeisis and restoration of age-related peripheral T cell dysfunction. Androgens also regulate the transcription of several cytokines. Dendritic cells (DC) are the most potent antigen presenting cells that can activate antigen-specific naïve T cells. Despite myriad clinical trials involving DC-based prostate cancer immunotherapies, the effects of AA on DC function remain largely uncharacterized. Therefore, we investigated the effects of AA on DC and whether it could improve the efficacy of prostate cancer immunotherapy.
Methods
Cytokine expression changes due to AA were quantified by multiplex ELISA. Flow cytometry was used to assess AA-mediated effects on DC maturation and expression of costimulatory markers. Mixed leukocyte reactions and cell-mediated lysis assays elucidated the role of androgens in DC function. The effect of AA on the efficacy of vaccination against a prostate tumor-associated antigen was tested using Elispot assays.
Results
Androgen ablation increased dendritic cell maturation and costimulatory marker expression, but had no effect on DC costimulatory function. However, DC isolated from castrated mice increased the expression of key cytokines by antigen-experienced T cells while decreasing their expression in naïve cells. Finally, androgen ablation improved immune responses to vaccination only when applied after immunization.
Conclusion
Androgen ablation causes differential effects of DC on primary and secondary T cell responses, thus augmenting vaccine immunogenicity only when applied after immunization.
Keywords: Hormonal therapy, Prostate cancer, Immunotherapy
Introduction
In 1941, Huggins and Hodges reported that androgen withdrawal led to regression of prostate cancer and the alleviation of patient pain (1,2). This demonstrated the androgen dependence of normal prostate and prostate cancer cells for survival. Since then, androgen ablation (AA) therapy has become an important part in managing the disease and it is the standard line of care for patients with metastatic prostate cancer. Prior to 2005, androgen ablation therapy was the only treatment proven to prolong survival in prostate cancer patients (3,4). However, androgen ablation remains a palliative approach because it does not eliminate all prostate cancer cells. While initially effective at reducing tumor burden, most patients eventually develop disease refractory to androgen withdrawal. Until recently, no single agent or combination therapy has been shown to prolong survival in prostate cancer patients who have failed hormonal therapy. Even chemotherapy has played only a palliative role in the treatment of prostate cancer until 2005 when two docetaxel-based randomized clinical trials demonstrated 20% and 24% improvement in survival benefit compared to mitoxanthrone and prednisone in hormone refractory prostate cancer patients (5,6). However, chemotherapy has significant adverse effects such as granulocytopenia, anemia, nausea, thrombocytopenia, vomiting, diarrhea and thrombosis that may not be tolerable for patients of advanced age. There exists a clear need to find new therapeutic alternatives for patients with hormone refractory prostate cancer that have failed chemotherapy or are ineligible for chemotherapy. Immunotherapy is emerging as an attractive strategy with fewer side effects compared to treatments such as surgery, radiation and chemotherapy. The adverse effects reported from dendritic cell-based prostate cancer immunotherapy trials (7–9) were mild and transient compared to the permanent side-effects of surgery or radiation (10). The side effects of AA are reversible upon cessation of treatment. The immune system is subject to suppression by androgens (11–13) and it is of great interest to see whether immunotherapy can be carried out following androgen ablation when the immunosuppression is alleviated.
Gene expression profiling has also demonstrated that a variety of immune inflammatory response genes are regulated by androgens (14,15). Testosterone has been found to increase production of immunosuppressive interleukin 10 (IL-10) by CD4+T-helper (Th) cells (13) but the anti-tumoral interferon pathway is also activated by testosterones (14). Therefore, the immune effects of androgen ablation may be complex. Since most patients with advanced prostate cancer will undergo some form of androgen ablation therapy, it is important to determine how androgen ablation will affect the efficacy of cancer vaccines.
Castration of mice stimulates B and T lymphopoiesis and thymic and bone marrow hyperplasia (16). A recent report demonstrated that androgen ablation leads to the activation of thymic regeneration in both mice and men (17). The thymic regeneration led to increased thymic emigration of T cells that restored the defects in peripheral T cell function observed in aged mice (17). The splenic enlargement observed post androgen ablation was largely due to expansion of the B-cell population but the spleens did contain more activated T cells than sham-castrated mice (18). This finding was corroborated by Roden et al. who found that T cell proliferation following non-specific stimulation using anti-CD28 and anti-CD3 antibody treatment was augmented following androgen ablation (19). Such findings have spurred investigators in the field of prostate cancer research to investigate the possibility that androgen ablation could have a synergistic effect on prostate cancer immunotherapy. In the androgen ablation setting where testosterone-mediated immune suppression is reduced and prostate antigens are released due to the apoptosis of androgen-dependent prostate cells or prostate cancer cells, the naïve thymic emigrants can potentially be activated through vaccination to induce auto-immune attack of prostate antigen-expressing cells.
Dendritic cells are specialized antigen presenting cells that migrate through the periphery to capture antigens, process them into polypeptides, migrate into lymphoid organs and present these antigens to lymphocytes. Depending on the level of inflammatory stimulation during antigen encounter, the DC can prime or tolerize lymphocytes to the antigen. Inflammatory stimuli cause the DC to mature and upregulate expression of costimulatory molecules, making them the most potent antigen presenting cells (APC) in the immune system (20,21). While B cells (22,23) and macrophages (24) have been demonstrated to prime naïve T cells, DC are the most potent APC for the induction of primary responses in naïve T cells (20). DC-based vaccines, alone or in combination with other therapies are being widely tested in clinical trials for prostate cancer immunotherapy (7–9,25–27). AA leads to infiltration of T cells, macrophages and DC into prostate tissues in prostate cancer patients (28). While it is known that AA augments T cell level and responses (19), its effects on DC has not been investigated.
Prostate stem cell antigen (PSCA) is a cell surface protein related to the Ly-6/Thy-1 family of glycosylphosphatidyl-inositol (GPI)-anchored antigens. PSCA is expressed in normal prostate and bladder and is up-regulated in a large proportion of localized and metastatic prostate cancers (29,30). PSCA is an especially attractive target for immunotherapy of advanced prostate cancer since its expression increases with high Gleason score, advanced stage and invasion (31–34). In this study we demonstrate that effective anti-PSCA immune responses can be generated even though it is a self-antigen and we analyzed the effects of androgen ablation on a PSCA-targeted vaccination strategy.
Methods and materials
Mice
Eight to ten weeks or seven to nine months old C57BL/6, and DBA/2 mice were purchased from Taconic (Hudson, NY). Eight to ten weeks C57BL/6bm1 mice were purchased from Jackson laboratory (Bar harbor, ME).
Castration and sham surgery
Surgery was performed on mice anesthetized with intraperitoneal injection of ketamine (50–80 mg/kg) and xylazine (5–10 mg/kg). Using sterile techniques, an anterior/posterior incision was made longitudinally in the scrotum. The testes were pushed out and hemostatic clamps were used to clamp the vessels and the vas deferens. After a few minutes, the testes were resected and the clamps removed. The wound was closed by compression. Buprenex (0.01–0.05 mg/kg) was given as an analgesic subcutaneously. Sham-castrated mice followed the same procedure, except the vessels and vas deferens were not clamped or resected. Mice were sacrificed three weeks post surgery for FACS analysis of dendritic cells or mixed lymphocyte reaction.
Multiplex cytokine assay of serum
Blood was collected from tail snips from C57BL/6 mice a day post surgery in Microtainer (Becton Dickinson, NJ) serum gel collection tubes. Blood was allowed to clot for 30 min and spun for 2 min at 13 000 × g. Serum was then collected from the top of the tube and stored at −80°c until use. A 23-plex mouse or a Th1/Th2 cytokine assay (Bio-Rad Laboratories Inc., Hercules, CA) was carried out as per manufacturer’s instructions. Briefly, a 96-well Multiscreen Resist Vacuum Manifold filter plate (Millipore, Billerica, MA) was pre-wetted with Bio-plex assay buffer, and multiplex beads were added to the wells. Multiplex beads were washed twice with Bio-plex wash buffer and 50 μl of reconstituted standards or diluted serum samples were added to the wells. Serum samples were diluted 1:3 with mouse serum diluent (Bio-Rad Laboratories Inc.). The filter plate was incubated with gentle shaking at 300 rpm for 30 min at room temperature and washed three times with Bio-plex wash buffer. Bio-plex detection antibody was then added to the wells and incubated with shaking at 300 rpm for 30 min room temperature. The filter plate was washed three times with Bio-plex wash buffer and 50 μl Streptavidin-PE was added to each well and incubated with shaking at 10 min in the dark. The filter plate was washed three times with Bio-plex wash buffer and resuspended in 125 μl of Bio-plex assay buffer and the beads detected using the Bio-plex HTF system (Bio-Rad Laboratories Inc.) and results analyzed using the Bio-plex Manager software (Bio-Rad Laboratories Inc.).
Dendritic cell isolation using Optiprep
The axilary, inguinal, brachial and superficial cervical lymph nodes or the spleens were harvested from C57BL/6 mice and dissociated in 0.5 mg/ml Collagenase (Sigma-Aldrich, St. Louis, MO) in 5% FBS RPMI supplemented with 10 mM HEPES (Gibco-Invitrogen, Carlsbad, CA) for 1 hr at 37 °C, shaking at 25000 rpm. The supernatant was collected and cells were harvested by spinning at 12000 rpm for 5 min. The cell suspension was resuspended in 3 ml 5% FBS Hanks buffered salt solution (HBSS) with 5 μg/ml of DnaseI (Sigma-Aldrich). 1 ml of OptiPrep (Axis-shield, Oslo, Norway) was added and the suspension resuspended thoroughly. 5 ml of 11.5% OptiPrep (diluted in Solution C) was layered on top of the cell suspension and then another 3 ml HBSS was layered on top of the 11.5% OptiPrep. The suspension was spun at 600 xg, 15 min with no brakes. The DC floating at the interface of the HBSS and 11.5% OptiPrep were collected and washed with 5% FBS-HBSS before use. Solution C was a diluent made of 0.88% (w/v) NaCl, 1 mM EDTA, 0.5 % (w/v) BSA, 10 mM Hepes-NaOH, pH 7.4.
CD4+ CD62L+ T cell isolation by MACS
CD4+ CD62L+ T cells were isolated using a CD4+ CD62L+ T cell isolation kit (Miltenyi Biotech, Auburn, CA) per the manufacturer’s instructions. Cell suspensions derived from spleens were counted resuspended in 400 μl of MACS buffer (PBS pH 7.2, 0.5% BSA and 2 mM EDTA) per 108 cells. 100 μl of CD4+ T Cell Biotin-Antibody Cocktail was added per 108 total cells and incubated for 10 min on ice. 300 μl of buffer and 200μl of Anti-Biotin microbeads were then added per 108 total cells and incubated on ice for another 15 min. Cells were washed by adding 10 ml of buffer and centrifugation at 300 × g for 10 min at 4 – 8°C. Cells were resuspended at 500 μl of MACS buffer for per 1×108 cells and depletion carried out with autoMACS Separator using the Depletes program. Cells were washed and resuspended in 800 μl of MACS and 200 μl of CD62L microbeads added. The mixture was incubated for 15 min on ice, washed, and then resuspended at 500 μl of MACS buffer for per 1×108 cells. Cells were positively selected with autoMACS Separator using the Posseld2 program. The positive fraction was collected as naïve CD4+ CD62L+ T cells and the negative fraction was collected as antigen-experienced CD4+ CD62L− T cells.
Splenocyte isolation by Lympholyte-M gradient
Spleens from the appropriate strain of mice were harvested and mashed with the base of a syringe. The cells were passed through a 70 μm cell strainer and collected by centrifugation at 12000 rpm for 5 min. The cells were treated with ammonium chloride/potassium (ACK) lysing buffer (0.15 mM NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA, pH 7.2) to lyse the red blood cells. The cells were suspended in 5 ml of 5% FBS-HBSS pH 7.4, layered on top of 5 ml of Lympholyte-M (Cedarlane laboratories, Burlington, NC) and centrifuged at 15000 xg for 20 min without brakes. The lymphocytes at the interface were collected and washed twice with 5% FBS-HBSS pH 7.4 before use.
Flow cytometry
Cells were stained with PE-conjugated anti-CD8 (53–6.7), PE-conjugated anti-CD4 (L3T4), APC-conjugated anti-CD3e (145-2C11), PE-CY7-conjugated anti-CD11c (HL3), PE-conjugated anti-CD40 (3/23), PE-conjugated anti-CD80 (16-10A1), PE-conjugated anti-CD83 (Michel-19), PE-conjugated anti-CD86 (GL1) or FITC-conjugated anti-H2-IAb (AF6-120.1) and gated based on lymphocyte or dendritic cell population according to the logarithmic forward and side scatter patterns. All antibodies were purchased from BD-Pharmingen Flow cytometry was carried out using a Cytomics FC500 (Beckman Coulter, Fullerton, CA) and analyzed with CXP analysis software (Beckman Coulter).
Mixed lymphocyte reaction cytokine assay
DC isolated from the axilary, inguinal, brachial and superficial cervical lymph nodes from castrated or sham-castrated C57BL/6 mice using the OptiPrep discontinuous gradient were irradiated at 3000 rad and cocultured with naïve CD4+ CD62L+ T cells or antigen-experienced CD4+ CD62L− T cells isolated from DBA/2 mice by MACS at a stimulator: responder ratio of 1:5 for 5 days in 20% dextran-treated charcoal-stripped FCS-IMDM. The cells were washed after 5 days and re-plated at 5×104 cells per well and nonspecifically stimulated with 50 ng/ml PMA (Sigma-Aldrich) and 1 μg/ml calcium Ionophore (Sigma-Aldrich) for 5 hrs. The cells were spun down by gentle centrifugation and 100 μl of supernatant collected, stored at −80 °C until multiplex cytokine analysis.
Mixed lymphocyte reaction proliferation assay
DC isolated from the lymph nodes of castrated or sham-castrated C57BL/6 mice using the OptiPrep discontinuous gradient were irradiated at 3000 rads and cocultured with lymphocytes isolated from DBA/2 mice using Lympholyte-M at a stimulator: responder ratio of 1:10 for 6 days in 20% dextran-treated charcoal-stripped FBS-IMDM in 96-well round-bottom plates. 1 μCi of 3H-Thymidine was added to each well on the 5th day and pulsed for 15 hrs. The cells were harvested using the Packard Filtermate harvester (Perkin-Elmer, Boston, MA) and left to dry overnight. 25 μl of Microscint20 (Perkin-Elmer) was added and 3H-Thymidine incorporation measured using the Packard Topcount NXT microplate scintillation and luminescence counter (Perkin-Elmer).
MLR cell-mediated lysis
DC isolated from the lymph nodes of castrated or sham-castrated C57BL/6 mice using the OptiPrep discontinuous gradient were irradiated at 3000 rad and cocultured with lymphocytes isolated from C57BL/6bm1 mice using Lympholyte-M at a stimulator: responder ratio of 1:4 for 6 days in 20% dextran-treated charcoal-stripped FCS-IMDM. On the 4th day, one C57BL/6 mouse and one C57BL/6bm1 mouse were sacrificed and their spleens harvested and the splenocytes treated with ACK lysis buffer to remove red blood cells. 50×106 splenocytes were incubated with 3 μg/ml of Concanavalin A (ConA) at 5×106 cells/ml to prepare ConA blast cells as targets for the cell mediated lysis assay. On the 6th day, the ConA blasts were collected, washed and 1.2×106 cells were labeled with 100 μCi of 51Chromium for 1 hr at 37 °C. The radioactive targets were mixed with YAC-1 cells that served as cold targets at a ratio of 1:30 and plated out on 96-well V-bottom plates. YAC-1 cells are susceptible to natural killer cells lysis and reduce the amount of non-specific lysis. The stimulators were collected, washed and added to the targets at various effector: target ratios. After 5 hrs, 50 μl of supernatant was collected onto 96-well LumaPlates (Perkin-Elmer) and left to dry overnight. 51Chromium incorporation was measured using the Topcount NXT microplate scintillation and luminescence counter (Packard). Spontaneous release was determined by measuring chromium release in the supernatants of target cells cultured in the absence of effectors. Maximum release was determined by lysis of target cells with 1% TritonX-100. The percentage specific lysis was calculated using the following formula: Specific lysis (%) = (Experimental release-spontaneous release/Maximal release-spontaneous release) × 100%
DNA-gold preparation for gene gun vaccination
Competent TOP10 Escherichia coli cells were transformed with pcDNA3-PSCA or the empty vector, pcDNA3, using heat shock and plated on ampicillin (100 μl/ml)/LB plates at 37 °C overnight. Colonies were randomly selected and grown in LB media containing 10% ampicillin. Colonies containing plasmid sequences verified by DNA sequencing were grown in LB media containing 10% ampicillin. DNA was prepared using EndoFree Maxi Prep kits (Qiagen, Valencia, CA). DNA-coated gold particles were prepared as follows: 2.5 mg of 1.0 μm gold microcarrier (Bio-Rad) were resuspended in 100 μl of 0.05 M spermidine (Sigma-Aldrich) and sonicated. 50 μg of pcDNA3-PSCA or the empty vector control, pcDNA3 were added to the microcarrier and this mixture was precipitated by the addition of 1.0 M CaCl2 (100 μl) for 10 min. The precipitate was washed three times with brand new absolute ethanol and resuspended in 3 ml of 0.1 mg/ml polyvinylpyrrolidone (Bio-rad) dissolved in absolute ethanol. The DNA-coated gold was then loaded into the tubing and allowed to settle for 3 min. Ethanol was gently drawn out and the tubing was dried by flowing nitrogen gas at a rate of 0.3 L/min. The tubing was cut into 0.5 inch pieces, placed into cartridges and stored at 4 °C until use.
VRP preparation
VRP were generously supplied by Alphavax, Inc. (Research Triangle Park, NC). The procedures used for making viral replicon particles (VRP), based on a two-helper system are described in detail elsewhere (35). In brief, RNA transcripts for the replicon and two helper RNAs, encoding for the capsid and glycoproteins, were transcribed in-vitro from linearized plasmids using a T7 RiboMax kit (Promega, Madison, WI) as per manufacturer’s instructions. RNA was purified using RNeasy purification columns (Qiagen) as per manufacturer’s instructions. Vero cells (1×108) suspended in PBS were combined with 30 μg of replicon and each helper RNA in 0.4 cm electroporation cuvettes and electroporated using a Bio-Rad Gene Pulser (Bio-Rad). The cells and RNA were pulsed four times with the electroporator set at 590 volts and 25 μFarads. Electroporated cell suspensions were seeded into individual roller bottles containing 150 ml of OptiPro medium (Invitrogen) supplemented with antibiotics and incubated at 37 °C in 5% CO2 for 16–24 hrs. VRP were harvested and the titers of the VRP determined by immunofluorescence assay using goat anti-V22 nsP2 specific polyclonal antiserum as the primary antibody and donkey anti-goat Alex Fluor 488 (Invitrogen) as the secondary antibody on methanol fixed cells using a Nikon Eclipse TE300 fluorescence microscope. The VRP were tested for the presence of contaminating replication competent Venezuelan equine encephalitis virus (VEE) using two blind passages of Vero cells. Briefly, 1×108 VRP were used to infect Vero cells in 75 cm2 flasks (MOI=0.5) for 1 hr. The VRP inoculum was removed, the cell monolayers were washed with PBS and 35 ml of fresh media was added to each flask. The flasks were then incubated at 37 °C for 24 hrs. After incubation, the first pass media was collected and used to inoculate fresh 75 cm2 flasks of Vero cells for 1 hr. The first pass media was then removed, 35 ml of fresh media added to each flask and the flasks incubated for an additional 72 hrs at 37 °C. After this second pass of media, the flasks were inspected for the presence of cytopathic effects. The absence of cytopathic effects in second pass flasks was deemed to indicate the absence of replication competent VEE. VRP were purified by affinity chromatography using HiTrap Heparin HP columns (Amersham Biosciences, Piscataway, NJ), resuspended in an isotonic PBS with 1% mouse serum (formulation buffer) and stored at −80 °C until use.
Immunization
Groups of four male C57BL/6 mice were vaccinated before or after undergoing orchiectomy or sham surgery. Vaccination was carried out three weeks after surgery. DNA-gold particles were delivered to a shaved are of the abdomen using a helium-driven gene gun (Bio-Rad) with a discharge pressure of 400 psi. Each mouse received 2 μg of pcDNA3-mPSCA DNA vaccine or the pcDNA3 empty vector as control. Two weeks after gene gun vaccination, the mice were boosted intramuscularly with 106 infectious units (IU) of mPSCA-VRP or GFP-VRP as controls.
IFN-γ Elispot
Multiscreen-HTS IP plates (Millipore) were pre-wet with 15 μl of 35% ethanol and rinsed twice with 200 μl sterile PBS. IFN-γ capture antibody (Pharmingen, R4-6A2) was coated onto the plates at 5 μg/ml overnight at 4 °C. Capture antibody was discarded the next day by flicking and the plates washed with 0.5% PBS-TWEEN-20 (PBST) and then twice with 200 μl PBS. The plates were blocked with 10% IMDM for 1 hr at 37 °C. Splenocytes from vaccinated or sham-castrated control mice were cultured for 24 hrs in 10% IMDM containing 5 μg/ml IL-2 and 1 μg/ml PSCA83–91 (NITCCYSDL) (36) or E749–57 (RAHYNIVTF) (37) as an irrelevant peptide control. The lymphocytes were then washed and replated at 2-fold serial dilutions in the Multiscreen-HTS IP plates with the appropriate peptide. After 24 hrs incubation at 37 °C, the cells were discarded and washed five times with 0.5% PBST and five times with water. 100 μl of 5 μg/ml of biotinylated IFN-γ (Pharmingen, XMG1.2) was added to the wells and incubated at room temperature for 2 hours. Biotinylated anti-IFN-γ was discarded by flicking and the plates washed six times with 0.05% PBST. 100 μl of 1 μg/ml horseradish peroxidase-conjugated strepavidin in 0.05% PBST and 1% BSA was added to the wells and incubated for 1 hr at room temperature. The plates were washed three times with 0.05% PBST and three times with PBS. The substrate, 3-amino-9-ethylcarbazole (AEC) was dissolved in dimethylformide (DMF) and diluted in 0.1 M NaAc buffer pH 5.0. 5 μL of H2O2 was added per 10 ml of AEC substrate solution just prior to adding substrate to the wells. Substrate was allowed to develop for 15 min to and then the plates were washed and allowed to dry. The filters were punched out and the spots read with the Axioplan 2 microscope and the Zeiss KS ELISPOT program (Both from Carl Zeiss Vision, Germany). The spots were counted, averaged and subtracted from the background spots counted in the wells stimulated with the irrelevant E7 peptide.
Statistical analysis
Graphs and statistical analysis was carried out using the Prism software (Graphpad, San Diego, CA). A two-tailed t-test at 95% confidence interval was used.
Results
Androgen ablation affects serum cytokine and chemokine levels
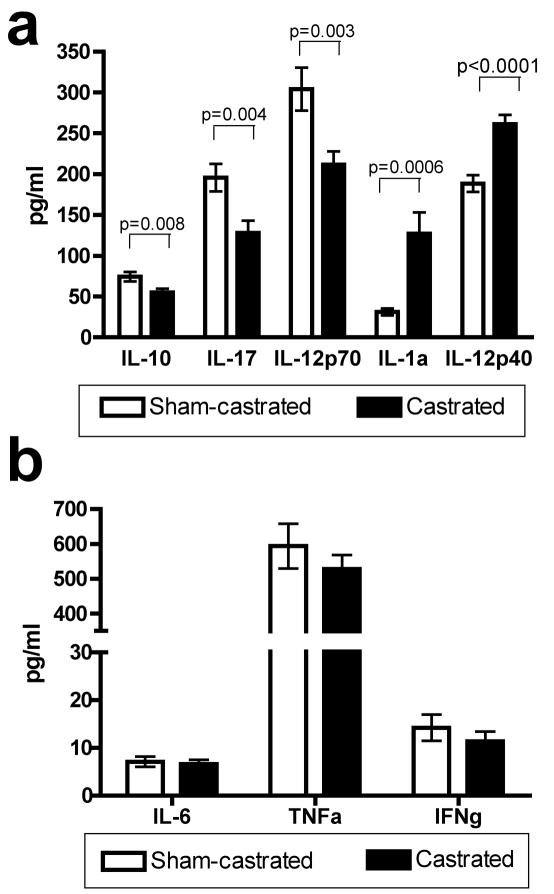
A multiplex cytokine assay was carried out on the serum from castrated and sham-castrated mice three weeks post surgery in order to analyze the changes (Fig. 1a) in cytokine milieu resulting from AA and determine if the resulting changes would favor the generation of cell-mediated responses. Three weeks post surgery, the immunosuppressive IL-10 that has been found to be induced by testosterone was decreased from 74.2 pg/ml to 54.9 pg/ml in castrated mice (p=0.008). However, the proinflammatory IL-17 and bioactive IL-12p70 were also statistically significantly reduced in castrated mice compared to sham-castrated controls (p=0.004 and p=0.003 respectively). IL-1a and IL-12p40 were significantly increased in the serum of castrated mice compared to sham-castrated controls (p<0.0001 and p=0.0006 respectively). Levels of other cytokines such as IL-6, TNFα and IFNγ were not statistically different in castrated mice compared to sham-castrated mice (Fig. 1b).
Fig. 1.
Effects of androgen ablation on levels of serum cytokines and chemokines. Serum was collected from sham-castrated (n=11) and castrated (n=13) C57BL/6 mice three weeks post surgery and a multiplex cytokine assay was carried out to determine whether there were differences in serum cytokine/chemokine levels in castrated compared to sham-castrated mice. A: IL-10, IL-17 and IL-12p70 were statistically significantly reduced in the serum of castrated mice compared to sham-castrated controls. IL-1a and IL-12p40 were statistically significantly increased in the serum of castrated mice compared to sham-castrated controls. B: Serum levels of IL-6, TNFα and IFNγ were not statistically significantly different between castrated mice and sham-castrated controls. Figures are representative of three independent experiments. Data were analyzed using two-tailed t-tests. Abbreviation: ELISA; enzyme-linked immunosorbent assay.
Androgen ablation induces dendritic cell maturation and increases their expression of costimulatory markers
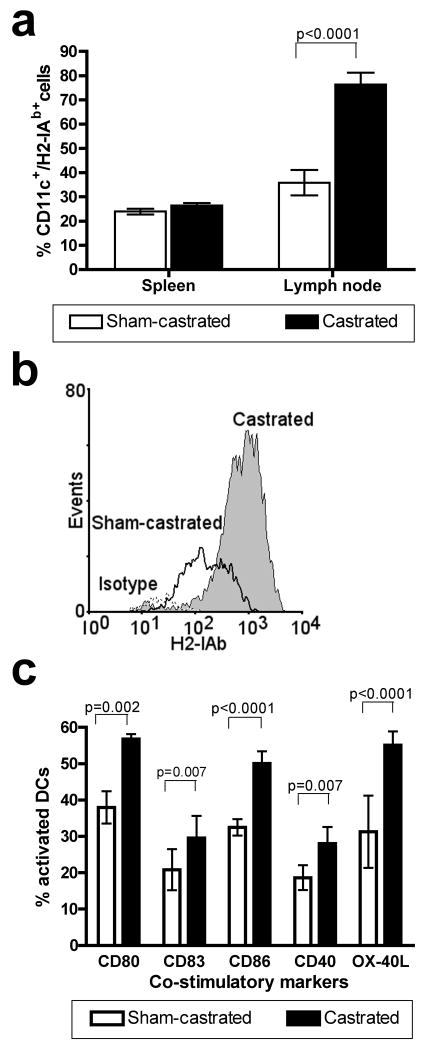
Spleen and lymph node cell suspensions from castrated or sham-castrated eight to ten weeks old C57BL/6 mice were analyzed three weeks post surgery for DC numbers and levels of costimulatory molecules expression. The cells were gated based on CD11c expression and assessed for H2-IAb and CD80, CD83, CD86, CD40 and OX40-L staining. The percentage of CD11c and H2-IAb cells in the spleens of castrated and sham-castrated mice was not significantly different (Fig. 2a) but there was a significant increase in the percentage of CD11c+/H2-IAb hi cells in the lymph nodes (p<0.001). The CD11c+/H2-IAb hi cells had at least a ten-fold increase in H2-IAb staining intensity compared to H2-IAb lo cells (Fig. 2b) and probably represent matured DC within the lymph nodes. Next, the DC were stained for costimulatory markers such as CD80, CD83, CD86, CD40 and OX-40L and the matured DC population analyzed. There were increased numbers of matured DC expressing costimulatory markers in the lymph nodes of castrated mice compared to sham-castrated mice (Fig. 2c).
Fig. 2.
Ex vivo analysis of dendritic cells isolated from androgen ablated mice. DC were isolated from the spleen and lymph nodes of castrated (n=4) or sham-castrated (n=4) C57BL/6 mice and analyzed by flow cytometry. A: There was a statistically significant increase in CD11c and H2-IAb double positive cells in the lymph nodes of castrated mice (p<0.001). B: Histogram overlay plot showing the increase in H2-IAbHi lymphocytes isolated from castrated mice (black line), sham-castrated control mice (filled gray histogram) and to unstained cells (broken line). C: Percentages of cells expressing CD80, CD83, CD86, CD40 and OX-40L in the H2-IabHi lymphocytes isolated from castrated mice were statistically significantly higher than in those isolated from sham-castrated mice. Figures are representative of five independent experiments. Data were analyzed using two-tailed t-tests. Abbreviation: DC; dendritic cell.
Dendritic cells isolated from androgen ablated mice have differential effects on cytokine expression by antigen experienced versus naïve T cells
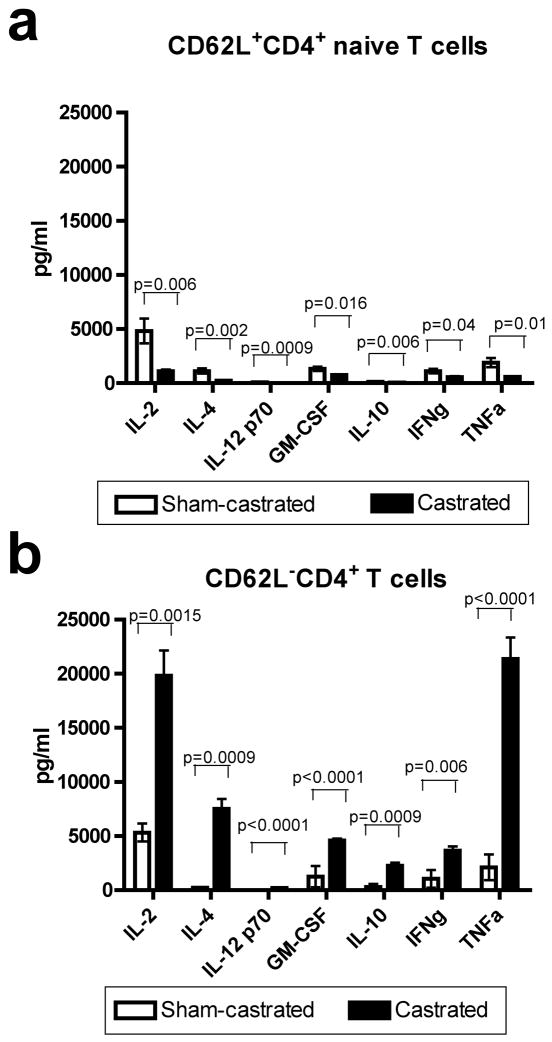
Next, a mixed leukocyte reaction was carried out to determine if costimulation from DC isolated from castrated mice would skew the immune response towards a Th1 or Th2 response. Therefore we tested the costimulatory function of DC from castrated and sham-castrated mice on naïve or antigen-experienced T-helper cells in an allogeneic mixed leukocyte reaction. DC from castrated and sham-castrated mice were co-cultured with MACS-purified naïve CD62L+CD4+ T cells (Fig. 3a) or antigen-experienced CD62L−CD4+ T cells (Fig. 3b) in an allogeneic mixed leukocyte reaction and the supernatants of the cultures were tested for cytokine production using a Th1/Th2 multiplex cytokine assay kit to determine whether castration resulted in DC that might skew the naïve T-helper response towards the Th2 phenotype. DC from castrated mice did not skew the naïve CD4+ T cells towards either a Th1 or Th2 phenotype (Fig. 3a). However, less IL-2, IL-4, IL-12, IL-10, GM-CSF, IFNγ and TNFα was secreted from the naïve CD4+ T cells when cocultured with DC from castrated mice. The reduction in cytokine production from naïve T cells cocultured with DC from castrated mice was generally small (but statistically significant) for most cytokines tested. In marked contrast, antigen-experienced CD4+ T cells secreted much higher levels of these cytokines when cocultured with DC from castrated mice as compared to those cocultured with DC from sham-castrated controls (Fig. 3b). It should be noted that cytokine expression levels in naïve and antigen-experienced T cells was approximately equal when they were cocultured with DC from sham-castrated controls.
Fig. 3.
Dendritic cells isolated from castrated mice have differential effects on naïve (CD62L− and antigen-experienced (CD62L+) CD4+ T cells. Irradiated DC isolated from sham-castrated (n=4) or castrated (n=6) C57BL/6 mice were cocultured with MACS-purified naïve or antigen-experienced CD4+ T cells from DBA/2 mice at a stimulator to responder ratio of 1:5 for 5 days. The cells were washed and re-plated to yield 5×104 cells/well and non-specifically stimulated with PMA and calcium ionophore for 5 hours. The supernatant was collected and a Th1/Th2 multiplex cytokine assay carried out to determine if the DC skewed the Th1/Th2 phenotype of the naïve or antigen-experienced CD4+ T cells. A: Coculture of naïve CD62L+ CD4+ T cells DC isolated from castrated mice resulted in reduced cytokine expression. B: Coculture of antigen-experienced CD62L− CD4+ T cells DC isolated from castrated mice resulted in increased cytokine expression. Figures are representative of two independent experiments. Data were analyzed using two-tailed t-tests. Abbreviations: DC; dendritic cell, MACS; magnetic-activated cell separation, PMA; phorbol 12-myristate 13-acetate.
Androgen ablation improves immune responses only when applied after vaccination
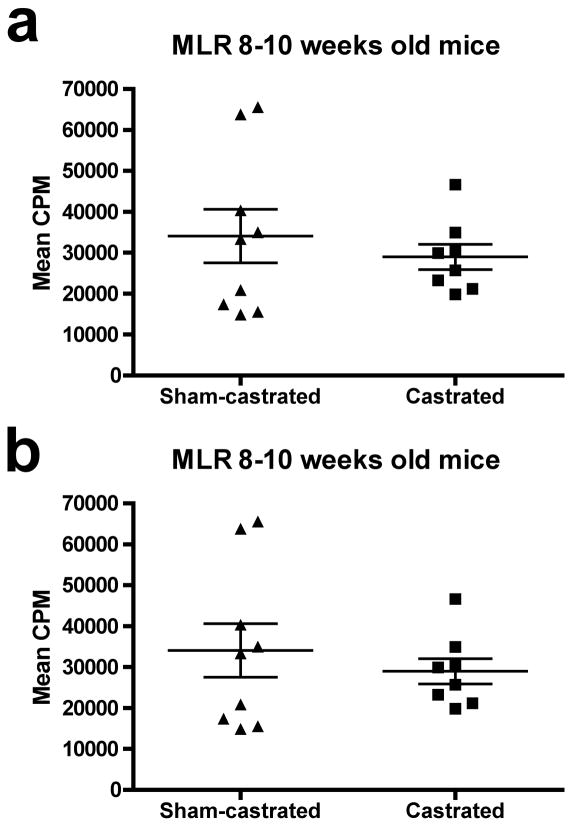
To determine whether the increase in DC costimulation results in functional improvements in T cell responses, DC from castrated and sham-castrated mice were used in allogeneic mixed leukocyte reactions and a vaccination strategy was tested in combination with androgen ablation. It was shown (Figure 4a) that irradiated DC from castrated or sham-castrated mice did not differ in their ability to support an alloreactive T cell response using unfractionated T cells cultures composed of both naïve and antigen-experienced T cells. To increase the probability of detecting age-related differences in the immune response, the allo-MLR was also carried out using older, seven to nine months old C57BL/6 mice (Figure 4b). Again, there was no difference in the proliferation response of the allo-reactive T cells. These data show that there was no functional improvement in the costimulatory ability of the DC post AA in either young or middle-aged mice.
Fig. 4.
Androgen ablation does not improve the costimulatory function of dendritic cells. DC were isolated from the lymph nodes of sham-castrated or castrated young or middle-aged C57BL/6 mice. The DC were irradiated and used as stimulators in a mixed leukocyte reaction with the lymphocytes isolated from eight to ten weeks old DBA/2 mice at a 1:10 stimulator to responder ratio. Scatter dot plots show lymphocyte proliferation for each individual mouse. A: Lymphocyte proliferation in MLR assays carried out using DC isolated from mice castrated (n=8) at eight to ten weeks as stimulators was not statistically significantly different from lymphocyte proliferation in MLR assays in which DC isolated from age-matched sham-castrated mice (n=9) were used as stimulators (p = 0.24). B: Lymphocyte proliferation in MLR assays carried out using DC isolated from mice castrated (n=8) at seven to nine months as stimulators was not statistically significantly different from lymphocyte proliferation in MLR assays in which DC isolated from age-matched sham-castrated (n=7) mice were used as stimulators (p = 0.07). The mean lymphocyte proliferation of each group is shown as a horizontal bar. Error bars represent standard error of mean lymphocyte proliferation counts. Figures are representative of three and two experiments, respectively. Abbreviations: DC; dendritic cell, MLR; mixed leukocyte reaction.
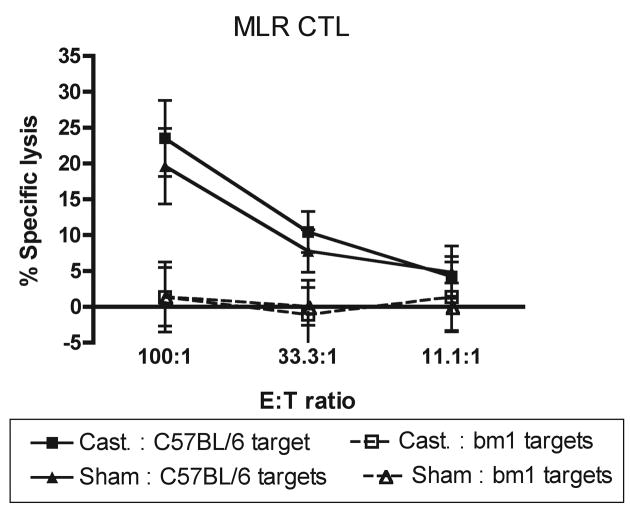
The H-2K and H-2D regions of the major histocompatibilty complex serve as strong transplantation antigens in mice (38). Kb locus mutations that consist of one to three amino acid substitutions in the Kb glycoprotein lead to positive MLR cell-mediated lympholysis reactions in vitro when the lymphocytes from two different Kb mutants are co-cultured (39). DC from castrated or sham-castrated mice did not differ in their ability to support the cytotoxic T lymphocyte killing of C57BL/6bm1 targets following an MLR reaction between C57BL/6 mice and C57BL/6bm1 (Fig. 5).
Fig. 5.
Androgen ablation does not improve costimulation of cytotoxic T lymphocytes by dendritic cells. Cell-mediated lysis assays were carried out after a 6 day mixed leukocyte reaction. DC isolated from the lymph nodes of either castrated or sham-castrated C57BL/6 mice were used as stimulator cells in cocultures with lymphocytes isolated from C57BL/6bm1 mice at a responder to stimulator ratio of 1:4. C57BL/6 ConA blasts were used as specific targets and C57BL/6bm1 ConA blasts were used to show non-specific killing. Points represent the mean percent specific lysis for each group. Error bars represent the standard error of the mean percent specific lysis for each group. There was no statistically significant difference in the mean specific lysis mediated by T cells cocultured with DC isolated from castrated mice compared to those cocultured with DC from sham-castrated mice. Figure is representative of three independent experiments (n = 4 mice per group). Abbreviations: ConA; concanavalin A, CTL: cytotoxic T lymphocyte, DC; dendritic cell, MLR; mixed leukocyte reaction.
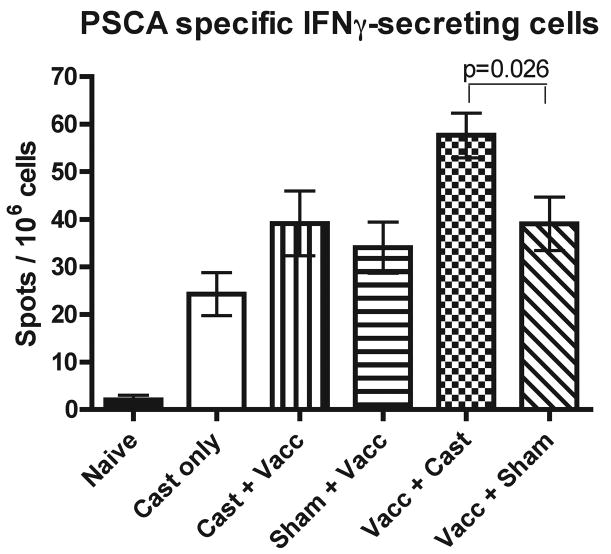
In order to definitively demonstrate the effect of AA on prostate cancer immunotherapy when carried out either before or after vaccination, mice were subjected to combined castration and vaccination protocols. For vaccination, a heterologous prime-boost vaccination strategy targeting the murine prostate stem cell antigen (mPSCA) was used (Fig. 6). The numbers of PSCA-specific interferon-γ (IFNγ) secreting cells was tested in order to determine if the timing of vaccination relative to AA would affect the vaccine efficacy. Castration alone was able to induce a PSCA-specific response (Cast only versus Naïve in Fig. 6). This suggests that castration led to apoptosis of androgen-dependent prostate cells that expressed PSCA, resulting in the development of a PSCA-specific response. When vaccination was carried out prior to castration, mice that underwent androgen ablation had slightly increased numbers of PSCA-specific IFNγ-secreting cells compared to vaccinated mice that were sham-castrated (Vacc+Cast versus Vacc+Sham). Although this increase was statistically significant (p=0.026), the increase in PSCA-specific IFNγ-secreting cells was modest and similar in magnitude to that observed between the castrated only group and naïve mice (Cast only versus Naïve). Therefore, this may simply be an additive effect. Interestingly, when the treatment order was reversed and castration was carried out before vaccination, androgen ablation did not lead to an increase in PSCA-specific IFNγ-secreting cells when compared to animals that underwent sham-surgery and received vaccination (Cast+Vacc versus Sham+Vacc). The castration-induced reaction against PSCA was not detected when androgen ablation was carried out prior to vaccination (Cast+Vacc versus Cast only).
Fig. 6.
Androgen ablation additively increases the immune response elicited by vaccination only if castration is performed after immunization. Groups of eight to ten weeks old C57BL/6 mice were vaccinated with DNA encoding murine PSCA by gene gun and boosted two weeks later with VRP encoding murine PSCA. Vaccinated mice were divided into groups which either underwent androgen ablation by castration three weeks before or after vaccination. Additional PSCA vaccinated mice underwent sham-castration either three weeks before or after vaccination. Elispot assays were carried out on splenocytes isolated from mice in each treatment group. Bars represent the mean number of spots (representing PSCA-specific IFNγ-secreting cells) per million cells for each treatment group. Error bars represent the standard error of the mean number of IFNγ-producing cells. When vaccination was performed after castration, the mean number of IFNγ-secreting cells was not statistically significantly different between castrated and sham-castrated mice. When castration was performed after vaccination, the mean number of IFNγ-secreting cells was statistically significantly different between castrated and sham-castrated mice (p = 0.026). Figure shown is compiled from three experiments (n = 6 mice per group). Abbreviations: PSCA; prostate stem cell antigen, VRP; Venezuelan equine encephalitis virus replicon particles.
Discussion
Human cancers are heterogenic and genetically unstable. In an effort to improve clinical outcomes, current therapies are being combined in order to treat cancer. Androgen ablation is a standard line of treatment for advanced prostate cancer and is being accessed in clinical trials in combination with immunotherapy (40–42). Androgens have been found to transcriptionally regulate a variety of immune response genes (13–15), therefore the effects of androgen ablation on the immune response is complicated and requires closer investigation. In order to avoid the complex effects of tumors on the immune systems of host animals, we investigated the immunological effects of androgen ablation in tumor-free mice. We first confirmed that the cytokine milieu three weeks post androgen ablation was not skewed towards a Th1 or Th2 phenotype (data not shown). However, IL-10 production by CD4+ T cells has been found to be activated by testosterone (13). In the serum of castrated mice, we demonstrated that IL-10 levels were reduced by almost 24% in castrated mice compared to sham-castrated mice (p=0.0005). This reduction in an immunosuppressive environment could be advantageous for vaccination. In addition, proinflammatory IL-1a levels were increased (p=0.0006). In addition, IL-17 levels were reduced (p=0.004) in castrated mice compared to sham-castrated mice. Though IL-17 is a proinflammatory cytokine, prostate tumors express IL-17 receptors that promote angiogenesis when activated (43). Therefore a reduction in IL-17 levels may be advantageous if the beneficial proinflammatory effects this cytokine mediates are outweighed by the negative effects of increased angiogenesis in prostate tumors. There were no statistically significant changes in IL-6, IFNγ or TNFα levels between castrated and sham-castrated mice (Fig 1b). IL-12 promotes the differentiation of naïve T cells into Th1 and is therefore important for cell-mediated antitumor immunity (44). IL-12 is composed of two subunits, p35 and p40. The bioactive IL-12 (p70) is composed of the covalently bound p40 and p35. IL-12 p40 can exist as a homodimer that can have antagonistic activity towards IL-12 function (45,46) and was earlier identified as a gene that was negatively regulated by androgens (14). As expected, its levels were significantly increased following androgen ablation (p=0.0001). Reduced levels of bioactive IL-12 and increased levels of the antagonistic IL-12 p40 homodimer in castrated animals could compromise the vaccine response. Therefore, despite the reduction in IL-10 levels in castrated mice, these data suggested that it might not be advantageous to vaccinate post androgen ablation.
Dendritic cells are the most potent activators of the immune system. Many current immunotherapy clinical trials for prostate cancer involve the reinfusion of autologous DC either transfected or loaded with prostate cancer associated antigens (9). In prostate cancer patients, AA resulted in T cell, macrophage and DC infiltration of the prostate tissue (28). Though androgen ablation is a standard line of treatment for patients with advanced prostate cancer, the effects of AA on DC or vaccine approaches have not been well studied. Ex vivo analysis of freshly isolated DC from the spleens and lymph nodes of castrated and sham-castrated mice showed that androgen ablation led to an increase in DC numbers in the lymph node (Fig. 2a). A mixed population of cells from both the draining and non-draining lymph nodes of each mouse was used for these experiments due to the limited numbers of cells that could be harvested from individual animals. There was also an increase in the percentage of MHC IIhi DC that expressed CD80, CD83, CD86, CD40 and OX-40L. CD80, CD86 and CD40 all provide important costimulation to T cells. In addition, OX40-OX40L interaction has been shown to be important for the clonal expansion and generation of CD4+ T effector memory cells (47,48). Roden et al (19) have reported that androgen ablation leads to increases in T cell proliferation in response to allogeneic reaction and in response to OVA-specific vaccination. We were unable to demonstrate that costimulation of T cells from normal mice by DC from castrated mice led to increases in proliferation in a mixed leukocyte reaction. However, we did detect an overall increase in cytokine production when antigen-experienced CD4+ T cells were cocultured with DC from castrated mice compared to CD4+ T cells that were cocultured with DC isolated from sham-castrated mice. The increase in T cell proliferation observed in castrated mice reported by Roden et al could be due to a direct effect of androgen ablation on T cells and not due to an increase in DC-dependent costimulation.
Although a greater percentage of DC from castrated mice expressed costimulatory markers, and were able to substantially increase the production of cytokines (most notably IL-2) by antigen-experienced T cells, we found that androgen ablation-induced activation of DC did not lead to either augmented proliferation or cytotoxic responses of T cells from intact mice (Fig. 4 and Fig 5). This apparent discrepancy in our data may stem from the fact that mixed populations of T cells (naïve and antigen-experienced) were used in the experiments represented in Fig. 4 and Fig 5. It is conceivable that there are simply too few antigen-experienced T cells within these mixed populations to result in a measurable change in their proliferation overall or cytotoxic activity, despite the significantly increased IL-2 production of the antigen-experienced T cells. Future studies will repeat these experiments using separated populations of naïve and antigen-experienced T cells in order to test whether this hypothesis is correct. The data presented in Fig. 4 and Fig. 5 may also indicate that the DC isolated from castrated mice may only be partially matured. Many of the prostate cancer clinical trials involve re-infusion of autologous DC transfected to express prostate cancer-associated antigens (7,49). Studies have shown that the initial maturation status of DC used for these vaccination strategies does not impact the outcome (50). Therefore there is unlikely to be any advantage in harvesting DC from patients post androgen ablation therapy for such vaccination strategies.
It has been widely suggested that immunotherapy for cancer would be an ideal adjuvant treatment when carried in conjunction with conventional therapies because the cancer vaccine can activate “sentinel” lymphocytes that circulate around the body to seek out and destroy small pockets of tumor cells after the initial debulking of the tumor by the other treatment modalities. For example, androgen ablation leads to the massive apoptosis of androgen-dependent prostate cells, induces immune infiltration into the prostate and increases immune responses towards a prostate-restricted antigen even in a model without vaccination (51). It has been demonstrated that in vitro activated prostate-specific antigen-targeting T cells can home to the prostate tissue. However, this immune infiltration was not sufficient to induce prostate tissue destruction (52). As a result, efforts have been made to combine androgen ablation with a therapeutic cancer vaccine in an effort to improve the antitumor immune response. Arlen et al published findings from a clinical trial designed to compare PSA vaccination against antiandrogen treatment in nonmetastatic hormone refractory prostate cancer (41). Patients from the clinical trial were allowed to cross over from one treatment arm to the other upon rising PSA levels. Although the clinical trial was not designed to compare whether vaccination should be carried out before or after antiandrogen treatment, the crossover group that received vaccination prior to antiandrogen therapy were compared to the crossover group that was vaccinated after the initiation of antiandrogen treatment. The median time to treatment failure in the group that was vaccinated then subjected to androgen ablation therapy was 25.9 months from initiation of treatment versus 15.9 months in the group that was vaccinated after androgen ablation.
To further investigate this phenomenon, we used a PSCA-targeted heterologous prime-boost vaccination strategy to test whether the timing of AA relative to vaccination would alter immune responses in mice (Fig. 6). Androgen ablation alone was able to induce a slight immune response against PSCA, demonstrating that apoptosis of androgen-dependent cells led to release of PSCA antigens and induction of PSCA-specific response. Therefore, combining androgen ablation with our vaccination strategy would mean that T cells received three strong stimulatory events (DNA priming, VRP boosting and the release of antigen due to androgen ablation-mediated apoptosis of prostate and prostate cancer cells). Considering this, there was concern that T cells in vaccinated and androgen ablated mice would undergo activation induced cell death (AICD) (53–55). To determine how best to avoid this, we performed preliminary studies and determined that a three week gap between vaccination and androgen ablation was sufficient to prevent T cells undergoing AICD upon restimulation in vitro (data not shown). The mice that were vaccinated prior to castration had modestly increased numbers of PSCA-specific IFNγ-secreting cells compared to sham-castrated mice (58 cells per million cells versus 39 cells per million cells, respectively, p=0.026). The numbers of PSCA-specific IFNγ-secreting cells induced by castration alone was 24 cells per million cells. However, this additive effect was not observed when vaccination was carried out after castration, consistent with the observations of Arlen et al. In addition, no effect was observed when vaccination and castration were carried out simultaneously (data not shown). Interestingly, similar results have been obtained by our laboratory with the same DNA prime/VRP boost heterologous vaccination strategy when using mSTEAP as the target antigen (unpublished data). An explanation for this phenomenon may be the differential effect of androgen ablation-matured DC on primary versus secondary T cell responses (Fig. 3). Naïve CD62L+CD4+ T cells co-cultured with DC from castrated mice secreted slightly (but statistically significantly) lower amounts of cytokines than the sham-castrated controls (Fig. 3a). In contrast, antigen-experienced CD62L−CD4+ T cells cocultured with DC from castrated mice secreted vastly higher levels of cytokines than the sham-castrated controls (Fig. 3b). Taken together, our data indicate that vaccination carried out prior to castration provides a pool of antigen-experienced T cells that are capable of responding to the androgen ablation-matured DCs generated by castration. When castration precedes vaccination, the resident naïve T cells apparently cannot be activated by the androgen ablation-matured DC. It is likely that these cells are either no longer present or functional by the time subsequent vaccination has produced antigen-experienced T cells in our protocol. Overall, an improved immune response was observed when castration followed vaccination, though the improvement was modest and is additive rather than synergistic. Nevertheless, our data argue for applying therapeutic prostate cancer vaccines prior to androgen ablation, which is in marked contrast to the current paradigm of administering such vaccines only after hormone therapy has failed and the patient has developed hormone-refractory prostate cancer (56).
Immunotherapy strategies have also been successfully combined with radiotherapy (57,58) in prostate cancer, which has been shown to synergize with antitumor vaccination strategies possibly by increasing the tumor’s susceptibility to immune recognition and eradication. Some chemotherapy drugs such as docetaxel have also been shown not to have an inhibitory effect on vaccination (40). It has been demonstrated that chemotherapy and radiation therapy can have synergistic effects on immunotherapy by reducing immunosuppressive factors such as regulatory T cells or by increasing homeostatic proliferation (59–61). It is possible that generating a population of preprimed T cells specific for prostate tumor-associated antigens prior to applying these therapies would be advantageous, as is the case with androgen ablation. Prostate cancer is an ideal candidate for therapeutic vaccination at the earliest stage of disease, since many patients undergo a period of “active surveillance”, previously known as “watchful waiting”, in which they are simply monitored for signs of disease progression before any treatment is administered. Based on our findings, we propose that vaccination should be carried out before androgen ablation therapy is applied to the patient, perhaps during this period of watchful waiting. This approach may yield two treatment benefits; the primary effect of the therapeutic vaccine on the patient’s early tumor burden, and the secondary effect of enhancing the benefit of subsequent androgen ablation. This prostate cancer treatment paradigm would put the “active” into “active surveillance”.
Conclusions
Androgen ablation increased dendritic cell maturation and costimulatory marker expression, but had no effect on DC costimulatory function. However, DC isolated from castrated mice had differential effects on primary and secondary T cell responses. As a result, androgen ablation was capable of augmenting the immunogenicity of a vaccine directed against a prostate tumor-associated antigen, but only when it was applied after immunization.
Acknowledgments
This study was supported by DAMD grant 17-02-1-0244. Andrew Gray received support from NIH Training Grant T32GM067587 and DAMD grant PC073417. W. Martin Kast holds the Walter A. Richter Cancer Research Chair.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Huggins C, Stevens RE, Jr, Hodges CV. Studies of Prostate Cancer and the Effects of Castration on Advanced Cancer of the Prostate. Archives of surgery. 1941;43:209. [Google Scholar]
- 2.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22(4):232–240. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 3.Bolla M, Gonzalez D, Warde P, Dubois JB, Mirimanoff R-O, Storme G, Bernier J, Kuten A, Sternberg C, Gil T, Collette L, Pierart M. Improved Survival in Patients with Locally Advanced Prostate Cancer Treated with Radiotherapy and Goserelin. N Engl J Med. 1997;337(5):295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 4.Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate Hormonal Therapy Compared with Observation after Radical Prostatectomy and Pelvic Lymphadenectomy in Men with Node-Positive Prostate Cancer. N Engl J Med. 1999;341(24):1781–1788. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- 5.Berthold DR, Sternberg CN, Tannock IF. Management of advanced prostate cancer after first-line chemotherapy. J Clin Oncol. 2005;23(32):8247–8252. doi: 10.1200/JCO.2005.03.1435. [DOI] [PubMed] [Google Scholar]
- 6.Petrylak DP. Docetaxel-based chemotherapy trials in androgen-independent prostate cancer: first demonstration of a survival benefit. Curr Oncol Rep. 2005;7(3):205–206. doi: 10.1007/s11912-005-0074-1. [DOI] [PubMed] [Google Scholar]
- 7.Mu LJ, Kyte JA, Kvalheim G, Aamdal S, Dueland S, Hauser M, Hammerstad H, Waehre H, Raabe N, Gaudernack G. Immunotherapy with allotumour mRNA-transfected dendritic cells in androgen-resistant prostate cancer patients. Br J Cancer. 2005;93(7):749–756. doi: 10.1038/sj.bjc.6602761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schellhammer PF, Hershberg RM. Immunotherapy with autologous antigen presenting cells for the treatment of androgen independent prostate cancer. World J Urol. 2005;23(1):47–49. doi: 10.1007/s00345-004-0475-z. [DOI] [PubMed] [Google Scholar]
- 9.Ragde H, Cavanagh WA, Tjoa BA. Dendritic cell based vaccines: progress in immunotherapy studies for prostate cancer. J Urol. 2004;172(6 Pt 2):2532–2538. doi: 10.1097/01.ju.0000144211.51111.e4. [DOI] [PubMed] [Google Scholar]
- 10.Scholz M. PCRInsights. 2003. Newly diagnosed prostate cancer: Evaluating the options Part 2 of 3; pp. 1–11. [Google Scholar]
- 11.Posma E, Moes H, Heineman MJ, Faas MM. The effect of testosterone on cytokine production in the specific and non-specific immune response. Am J Reprod Immunol. 2004;52(4):237–243. doi: 10.1111/j.1600-0897.2004.00216.x. [DOI] [PubMed] [Google Scholar]
- 12.Cutolo M, Seriolo B, Villaggio B, Pizzorni C, Craviotto C, Sulli A. Androgens and estrogens modulate the immune and inflammatory responses in rheumatoid arthritis. Ann N Y Acad Sci. 2002;966:131–142. doi: 10.1111/j.1749-6632.2002.tb04210.x. [DOI] [PubMed] [Google Scholar]
- 13.Liva SM, Voskuhl RR. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol. 2001;167(4):2060–2067. doi: 10.4049/jimmunol.167.4.2060. [DOI] [PubMed] [Google Scholar]
- 14.Asirvatham AJ, Schmidt M, Gao B, Chaudhary J. Androgens Regulate the Immune/Inflammatory Response and Cell Survival Pathways in Rat Ventral Prostate Epithelial Cells. Endocrinology. 2006;147(1):257–271. doi: 10.1210/en.2005-0942. [DOI] [PubMed] [Google Scholar]
- 15.Desai KV, Michalowska AM, Kondaiah P, Ward JM, Shih JH, Green JE. Gene expression profiling identifies a unique androgen-mediated inflammatory/immune signature and a PTEN (phosphatase and tensin homolog deleted on chromosome 10)-mediated apoptotic response specific to the rat ventral prostate. Mol Endocrinol. 2004;18(12):2895–2907. doi: 10.1210/me.2004-0033. [DOI] [PubMed] [Google Scholar]
- 16.Grossman CJ. Interactions between the gonadal steroids and the immune system. Science. 1985;227(4684):257–261. doi: 10.1126/science.3871252. [DOI] [PubMed] [Google Scholar]
- 17.Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, Blazar BR, Millar JL, Malin MA, Chidgey AP, Boyd RL. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175(4):2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 18.Viselli SM, Stanziale S, Shults K, Kovacs WJ, Olsen NJ. Castration alters peripheral immune function in normal male mice. Immunology. 1995;84(2):337–342. [PMC free article] [PubMed] [Google Scholar]
- 19.Roden AC, Moser MT, Tri SD, Mercader M, Kuntz SM, Dong H, Hurwitz AA, McKean DJ, Celis E, Leibovich BC, Allison JP, Kwon ED. Augmentation of T cell levels and responses induced by androgen deprivation. J Immunol. 2004;173(10):6098–6108. doi: 10.4049/jimmunol.173.10.6098. [DOI] [PubMed] [Google Scholar]
- 20.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 21.Guery JC, Adorini L. Dendritic cells are the most efficient in presenting endogenous naturally processed self-epitopes to class II-restricted T cells. J Immunol. 1995;154(2):536–544. [PubMed] [Google Scholar]
- 22.Rodriguez-Pinto D, Moreno J. B cells can prime naïve CD4+ T cells in vivo in the absence of other professional antigen-presenting cells in a CD154-CD40-dependent manner. Eur J Immunol. 2005;35(4):1097–1105. doi: 10.1002/eji.200425732. [DOI] [PubMed] [Google Scholar]
- 23.Ahmadi T, Flies A, Efebera Y, Sherr DH. CD40 Ligand-activated, antigen-specific B cells are comparable to mature dendritic cells in presenting protein antigens and major histocompatibility complex class I- and class II-binding peptides. Immunology. 2008;124(1):129–140. doi: 10.1111/j.1365-2567.2007.02749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pozzi LA, Maciaszek JW, Rock KL. Both dendritic cells and macrophages can stimulate naïve CD8 T cells in vivo to proliferate, develop effector function, and differentiate into memory cells. J Immunol. 2005;175(4):2071–2081. doi: 10.4049/jimmunol.175.4.2071. [DOI] [PubMed] [Google Scholar]
- 25.Brian I, Rini VW, Fong Lawrence, Conry Shauna, Hershberg Robert M, Smal Eric J. Combination immunotherapy with prostatic acid phosphatase pulsed antigen-presenting cells (provenge) plus bevacizumab in patients with serologic progression of prostate cancer after definitive local therapy. Cancer. 2006;107(1):67–74. doi: 10.1002/cncr.21956. [DOI] [PubMed] [Google Scholar]
- 26.Murphy GP, Tjoa BA, Simmons SJ, Ragde H, Rogers M, Elgamal A, Kenny GM, Troychak MJ, Salgaller ML, Boynton AL. Phase II prostate cancer vaccine trial: report of a study involving 37 patients with disease recurrence following primary treatment. Prostate. 1999;39(1):54–59. doi: 10.1002/(sici)1097-0045(19990401)39:1<54::aid-pros9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 27.Murphy GP, Tjoa BA, Simmons SJ, Rogers MK, Kenny GM, Jarisch J. Higher-dose and less frequent dendritic cell infusions with PSMA peptides in hormone-refractory metastatic prostate cancer patients. Prostate. 2000;43(1):59–62. doi: 10.1002/(sici)1097-0045(20000401)43:1<59::aid-pros8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 28.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, Ellis TM, Wojcik EM, Yang D, Flanigan RC, Waters WB, Kast WM, Kwon ED. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98(25):14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang D, Holt GE, Velders MP, Kwon ED, Kast WM. Murine six-transmembrane epithelial antigen of the prostate, prostate stem cell antigen, and prostate-specific membrane antigen: prostate-specific cell-surface antigens highly expressed in prostate cancer of transgenic adenocarcinoma mouse prostate mice. Cancer Res. 2001;61(15):5857–5860. [PubMed] [Google Scholar]
- 30.Zhigang Z, Wenlv S. Prostate stem cell antigen (PSCA) expression in human prostate cancer tissues: implications for prostate carcinogenesis and progression of prostate cancer. Jpn J Clin Oncol. 2006;36(2):121. doi: 10.1093/jjco/hyi253. [DOI] [PubMed] [Google Scholar]
- 31.Reiter RE, Gu Z, Watabe T, Thomas G, Szigeti K, Davis E, Wahl M, Nisitani S, Yamashiro J, Le Beau MM, Loda M, Witte ON. Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc Natl Acad Sci U S A. 1998;95(4):1735–1740. doi: 10.1073/pnas.95.4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu Z, Thomas G, Yamashiro J, Shintaku IP, Dorey F, Raitano A, Witte ON, Said JW, Loda M, Reiter RE. Prostate stem cell antigen (PSCA) expression increases with high gleason score, advanced stage and bone metastasis in prostate cancer. Oncogene. 2000;19(10):1288–1296. doi: 10.1038/sj.onc.1203426. [DOI] [PubMed] [Google Scholar]
- 33.Watabe T, Lin M, Ide H, Donjacour AA, Cunha GR, Witte ON, Reiter RE. Growth, regeneration, and tumorigenesis of the prostate activates the PSCA promoter. Proc Natl Acad Sci U S A. 2002;99(1):401–406. doi: 10.1073/pnas.012574899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han KR, Seligson DB, Liu X, Horvath S, Shintaku PI, Thomas GV, Said JW, Reiter RE. Prostate stem cell antigen expression is associated with gleason score, seminal vesicle invasion and capsular invasion in prostate cancer. J Urol. 2004;171(3):1117–1121. doi: 10.1097/01.ju.0000109982.60619.93. [DOI] [PubMed] [Google Scholar]
- 35.Pushko P, Parker M, Ludwig GV, Davis NL, Johnston RE, Smith JF. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology. 1997;239(2):389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Hernandez Mde L, Gray A, Hubby B, Klinger OT, Kast WM. Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cncer in the absence of autoimmunity. Cancer research. 2008;68(3) doi: 10.1158/0008-5472.CAN-07-0445. [DOI] [PubMed]
- 37.Feltkamp MC, Smits HL, Vierboom MP, Minnaar RP, de Jongh BM, Drijfhout JW, ter Schegget J, Melief CJ, Kast WM. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23(9):2242–2249. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 38.Melief CJ, Stukart MJ, de Waal LP, Kast WM, Melvold RW. Specificity and regulation of cytotoxic T-lymphocyte responses analyzed with H-2 mutants. Transplant Proc. 1983;15(4):2086–2089. [PubMed] [Google Scholar]
- 39.Kast WM, Van Twuyver E, Mooijaart RJ, Verveld M, Kamphuis AG, Melief CJ, De Waal LP. Mechanism of skin allograft enhancement across an H-2 class I mutant difference. Evidence for involvement of veto cells. Eur J Immunol. 1988;18(12):2105–2108. doi: 10.1002/eji.1830181238. [DOI] [PubMed] [Google Scholar]
- 40.Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, Beetham P, Tsang KY, Grosenbach DW, Feldman J, Steinberg SM, Jones E, Chen C, Marte J, Schlom J, Dahut W. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12(4):1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arlen PM, Gulley JL, Todd N, Lieberman R, Steinberg SM, Morin S, Bastian A, Marte J, Tsang KY, Beetham P, Grosenbach DW, Schlom J, Dahut W. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J Urol. 2005;174(2):539–546. doi: 10.1097/01.ju.0000165159.33772.5b. [DOI] [PubMed] [Google Scholar]
- 42.Arlen PM, Madan RA, Hodge JW, Schlom J, Gulley JL. Combining Vaccines with Conventional Therapies for Cancer. Update Cancer Ther. 2007;2(1):33–39. doi: 10.1016/j.uct.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 45.Presky DH, Minetti LJ, Gillessen S, Wilkinson VL, Wu CY, Gubler U, Chizzonite R, Gately MK. Analysis of the multiple interactions between IL-12 and the high affinity IL-12 receptor complex. J Immunol. 1998;160(5):2174–2179. [PubMed] [Google Scholar]
- 46.Heinzel FP, Hujer AM, Ahmed FN, Rerko RM. In vivo production and function of IL-12 p40 homodimers. J Immunol. 1997;158(9):4381–4388. [PubMed] [Google Scholar]
- 47.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165(6):3043–3050. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 48.De Smedt T, Smith J, Baum P, Fanslow W, Butz E, Maliszewski C. Ox40 costimulation enhances the development of T cell responses induced by dendritic cells in vivo. J Immunol. 2002;168(2):661–670. doi: 10.4049/jimmunol.168.2.661. [DOI] [PubMed] [Google Scholar]
- 49.Kyte JA, Gaudernack G. Immuno-gene therapy of cancer with tumour-mRNA transfected dendritic cells. Cancer Immunol Immunother. 2006;55(11):1432–1442. doi: 10.1007/s00262-006-0161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michiels A, Tuyaerts S, Bonehill A, Corthals J, Breckpot K, Heirman C, Van Meirvenne S, Dullaers M, Allard S, Brasseur F, van der Bruggen P, Thielemans K. Electroporation of immature and mature dendritic cells: implications for dendritic cell-based vaccines. Gene Ther. 2005;12(9):772–782. doi: 10.1038/sj.gt.3302471. [DOI] [PubMed] [Google Scholar]
- 51.Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, Hipkiss EL, Flies DB, Kennedy EP, Long M, McGary PW, Coryell L, Nelson WG, Pardoll DM, Adler AJ. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7(3):239–249. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lees JR, Charbonneau B, Hayball JD, Diener K, Brown M, Matusik R, Cohen MB, Ratliff TL. T-cell recognition of a prostate specific antigen is not sufficient to induce prostate tissue destruction. Prostate. 2006;66(6):578–590. doi: 10.1002/pros.20307. [DOI] [PubMed] [Google Scholar]
- 53.Brenner D, Krammer PH, Arnold R. Concepts of activated T cell death. Crit Rev Oncol Hematol. 2008;66(1):52–64. doi: 10.1016/j.critrevonc.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 54.Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 55.Mittal A, Papa S, Franzoso G, Sen R. NF-kappaB-dependent regulation of the timing of activation-induced cell death of T lymphocytes. J Immunol. 2006;176(4):2183–2189. doi: 10.4049/jimmunol.176.4.2183. [DOI] [PubMed] [Google Scholar]
- 56.Gray A, Raff AB, Chiriva-Internati M, Chen SY, Kast WM. A paradigm shift in therapeutic vaccination of cancer patients: the need to apply therapeutic vaccination strategies in the preventive setting. Immunol Rev. 2008;222:316–327. doi: 10.1111/j.1600-065X.2008.00605.x. [DOI] [PubMed] [Google Scholar]
- 57.Sharp HJ, Wansley EK, Garnett CT, Chakraborty M, Camphausen K, Schlom J, Hodge JW. Synergistic antitumor activity of immune strategies combined with radiation. Front Biosci. 2007;12:4900–4910. doi: 10.2741/2436. [DOI] [PubMed] [Google Scholar]
- 58.Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, Tsang KY, Yokokawa J, Hodge JW, Menard C, Camphausen K, Coleman CN, Sullivan F, Steinberg SM, Schlom J, Dahut W. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11(9):3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 59.Gelbard A, Garnett CT, Abrams SI, Patel V, Gutkind JS, Palena C, Tsang KY, Schlom J, Hodge JW. Combination chemotherapy and radiation of human squamous cell carcinoma of the head and neck augments CTL-mediated lysis. Clin Cancer Res. 2006;12(6):1897–1905. doi: 10.1158/1078-0432.CCR-05-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Emens LA, Jaffee EM. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res. 2005;65(18):8059–8064. doi: 10.1158/0008-5472.CAN-05-1797. [DOI] [PubMed] [Google Scholar]
- 61.Nowak AK, Lake RA, Robinson BW. Combined chemoimmunotherapy of solid tumours: improving vaccines? Adv Drug Deliv Rev. 2006;58(8):975–990. doi: 10.1016/j.addr.2006.04.002. [DOI] [PubMed] [Google Scholar]