Abstract
Mutations in parkin, PTEN-induced kinase 1 (PINK1) and DJ-1 can all cause autosomal recessive forms of Parkinson disease. Recent data suggest that these recessive parkinsonism-associated genes converge within a single pathogenic pathway whose dysfunction leads to the loss of substantia nigra pars compacta neurons. The major common functional effects of all three genes relate to mitochondrial and oxidative damage, with a possible additional involvement of the ubiquitin proteasome system. This review highlights the role of the mitochondrial kinase, PINK1, in protection against mitochondrial dysfunction and how this might relate to loss of substantia nigra neurons in recessive parkinsonism.
Keywords: parkinsonism, parkin, Drp1, fission, fusion, oxidative stress
Introduction
Defects in mitochondrial metabolism are implicated in many common diseases of aging. Of these, Parkinson disease (PD) is a common neurodegenerative disorder that is characterized in part by the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc). Although mitochondrial defects have been proposed to be part of the pathophysiology of many age-related neurodegenerative disorders, there are several distinct and complementary lines of evidence suggesting links between mitochondria and PD. These ideas have been re-emphasized in the last few years by the discovery of mutations in genes that are directly associated with mitochondrial function where the phenotype includes loss of SNpc neurons, as seen in PD.
One piece of supportive data for the hypothesis that mitochondria may be important in PD is that the mitochondrial complex I inhibitors, rotenone and MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), lead to death of dopaminergic cells in the SNpc (Betarbet et al., 2000, Langston et al., 1983, Langston et al., 1984, Liou et al., 1996, Ricaurte et al., 1986). Therefore, damage to mitochondria, specifically to complex I is sufficient to produce a phenotype that mimics the cell loss in PD.
One specific link between PD and mitochondria is that complex I activity of the electron transport chain is diminished in the substantia nigra of PD patients (Schapira et al., 1989). Inhibition of electron transport produces damaging reactive oxygen species (ROS) and limits the capacity of mitochondria to generate ATP, which has profound effects on the cell. This type of continuous oxidative insult induces cell death, which in many model systems includes cytochrome c release via the mitochondrial permeability transition pore and features typical of apoptosis (Tsujimoto and Shimizu, 2007).
Oxidative damage secondary to ROS generation has been proposed to be associated with the pathogenesis of PD (Dexter et al., 1989, Shimura-Miura et al., 1999, Sofic et al., 1992, Yoritaka et al., 1996, Youdim et al., 1989). ROS are capable of damaging many macromomolecules including nuclear and, especially, mitochondrial DNA (mtDNA), which is not histone bound. Mammalian mitochondrial DNA is a circular molecule of 16.6 kb, present in several copies per mitochondria which encode 13 subunits of respiratory chain complexes I, III–V, which are essential components of oxidative phosphorylation (OXPHOS). MtDNA also encodes the 12S and 16S rRNA genes and the 22 tRNA genes required for mitochondrial protein synthesis. The remaining mitochondrial OXPHOS proteins, the metabolic enzymes, the DNA and RNA polymerases, the ribosomal proteins and the mtDNA regulatory factors are all encoded by nuclear genes, synthesized in the cytosol and then imported into the mitochondria (Wallace, 1997). A supportive piece of evidence for a mitochondrial pathophysiology of PD is the observation that mtDNA abnormalities are found in patients with PD (Rana et al., 2000, Swerdlow et al., 1998, Swerdlow et al., 1996). Furthermore, although mtDNA deletions are rarely found in nigral neurons in young subjects, they are found increasingly in the same neuronal groups in older subjects (Bender et al., 2006, Kraytsberg et al., 2006).
However, decreased complex I activity in PD patients is also seen in peripheral tissues of PD patients (Krige et al., 1992, Parker et al., 1989, Wallace et al., 1992, Blake et al., 1997), and it is therefore unclear why neurons in general, and SNpc neurons in particular, would be susceptible to loss of complex I activity. Furthermore, consistent mtDNA mutations have not been identified in either familial or sporadic forms of parkinsonism (Tan et al., 2000). One possible interpretation of this data is that mtDNA mutations are secondary to the pathological process of PD and are not causal for the disease. The age-related loss of mtDNA integrity and biochemical mitochondrial function may be a precipitating event for neuronal damage that is fundamentally driven by other events that are yet to be described.
These several lines of evidence are largely derived from studies of sporadic PD, which accounts for the majority of all cases and is not familial in nature. However, there are rare inherited diseases that partially overlap with the clinical syndrome of PD. These have been given designations of PARK1-14 in OMIM (http://www.ncbi.nlm.nih.gov/omim/) and are discussed elsewhere. This review will focus on autosomal recessively inherited early onset disease where parkinsonism is a major clinical feature.
It is important at this point to distinguish parkinsonism from PD. Parkinsonism is a clinically defined syndrome that describes predominantly motor problems (tremor, slowness of movement, rigidity and instable upright posture) deriving from loss of SNpc neurons. Parkinson disease, at least in the way the term is currently used, is a clinico-pathological diagnosis that requires the presence of protein deposition as Lewy bodies superimposed on a picture of neuronal loss. Importantly, PD is not ‘simply’ a nigral disease and includes both non-dopaminergic and non-motor symptoms. Recessive parkinsonism tends to be earlier onset, have a milder course, respond better to symptomatic treatment and have fewer non-motor complications such as dementia compared to sporadic PD. This distinction between PD and parkinsonism is partly an issue of terminology but it is important here because if these are different diseases (ie having a different underlying cause), we have to be careful about extrapolating from one to the other in relation to mitochondrial mechanisms.
There are three genes for autosomal recessive parkinsonism: parkin, DJ-1 and PINK1. Several recent lines of data suggest that recessive parkinsonism-associated genes converge within a single pathogenic pathway whose dysfunction leads to the loss of SNpc neurons. The major common functional effects of all three genes relate to mitochondria and oxidative damage, with a possible additional involvement of the ubiquitin proteasome system (UPS) (for a recent review on the UPS and neuronal dysfunction, see Tai and Schuman, 2008) (Tai and Schuman, 2008). This review will focus specifically on the role of the mitochondrial kinase, PINK1, in protection against mitochondrial dysfunction and how this might relate to loss of SNpc neurons in recessive parkinsonism.
PINK1 and mitochondrial dysfunction
The PINK1 (phosphatase and tensin homolog (PTEN)-induced kinase 1) gene consists of eight exons, encoding a 581-amino acid protein with a predicted molecular mass of 62.8 kilodaltons. PINK1 mRNA is expressed ubiquitously, but the highest expression levels are found in the heart, skeletal muscle, and testes and intermediate levels of expression are found in the liver, kidney pancreas and brain (Unoki and Nakamura, 2001, Taymans et al., 2006). In the brain, expression is primarily neuronal in the hippocampus, substantia nigra and cerebellar Purkinje cells (Blackinton et al., 2007).
The PINK1 protein has a central domain with homology to serine/threonine kinases (Valente et al., 2004a) and exhibits autophosphorylation activity in vitro (Nakajima et al., 2003, Beilina et al., 2005, Silvestri et al., 2005). An N-terminal mitochondrial-targeting signal (MTS) is sufficient for mitochondrial import of PINK1 (Muqit et al., 2006, Silvestri et al., 2005) and the protein can be found the outer mitochondrial membrane (OMM) (Gandhi et al., 2006, Weihofen et al., 2009), the mitochondrial intermembrane space (IMS) (Plun-Favreau et al., 2007, Pridgeon et al., 2007, Silvestri et al., 2005) and the inner mitochondrial membrane (IMM) (Gandhi et al., 2006, Lin and Kang, 2008, Muqit et al., 2006, Pridgeon et al., 2007, Silvestri et al., 2005). However, PINK1 also localizes to the cytosol (Beilina et al., 2005, Haque et al., 2008, Lin and Kang, 2008, Takatori et al., 2008, Weihofen et al., 2008, Weihofen et al., 2009, Zhou et al., 2008). Recent work suggests that the topology of PINK1, including the endogenous protein, relies on the presence of a transmembrane domain located after the MTS to insert the N-terminal tail into the outer mitochondrial membrane with the kinase domain facing the cytoplasm (Zhou et al., 2008). This suggested orientation would imply that physiological PINK1 substrates are localized in the outer mitochondrial membrane or possibly in the cytosol at the mitochondrial surface. The cytoplasmic pool of PINK1 found in overexpression models may represent excess protein that is shed from the mitochondrial surface; it is of interest that this pool is rapidly degraded by the proteasome (Lin and Kang, 2008, Takatori et al., 2008).
The first reported PINK1 mutations, G309D and the truncation mutant W437X, were identified in patients of Spanish and Italian origin, respectively (Valente et al., 2004a). Over 20 pathological mutations have now been identified in PINK1 varying from point mutations, truncations and whole gene heterozygous deletions (Klein et al., 2006, Marongiu et al., 2007, Zadikoff et al., 2006), which between them account for between 1–9% of cases with early onset parkinsonism (Healy et al., 2004, Li et al., 2005, Rohe et al., 2004, Tan et al., 2006, Tan et al., 2005, Valente et al., 2004b).
Because they are recessively inherited, pathogenic mutations are predicted to inactivate the protein, leading to a loss of function. Some mutations cause loss of function in very simple way. For example, L347P is less stable than wild type PINK1 (Beilina et al., 2005, Moriwaki et al., 2008), probably due to increased protein turnover by the proteasome (Moriwaki et al., 2008). Other pathogenic mutations such as G309D are stable have decreased in vitro kinase activity (Beilina et al., 2005), measured using autophosphorylation as a convenient assay.
However, it is difficult to say whether mutations outside the kinase domain affect kinase activity or whether they work in some other fashion. PINK1 C-terminal truncations identified produce clinical phenotypes to missense PINK1 mutations (Rohe et al., 2004). The C-terminal regions of many protein kinases contain functional motifs that control catalytic activity of the kinase domain and/or bind regulatory proteins and substrates (Jeffrey et al., 1995, Niefind et al., 1998, Nolen et al., 2001). Deletion of the C-terminal region of PINK1 results in decreased kinase activity when expressed in baculovirus-infected insect cells (Sim et al., 2006) but enhanced kinase activity when recombinant PINK1 was expressed in E. coli (Silvestri et al., 2005). Again, these studies measured autophosphorylation activity and so an open question is whether these types of assays capture the true effects on mutations under more physiological conditions.
Two putative mitochondrial substrates for PINK1, tumor necrosis factor type 1 receptor associated protein 1 (TRAP1) (Pridgeon et al., 2007) and the serine protease Omi/HtrA2 (high temperature requirement protein A2) (Plun-Favreau et al., 2007), have been identified. TRAP1 has been reported to be a direct substrate for PINK1, based on in vitro assays using phospho-serine specific antibodies but the site of modification is not known. TRAP1 has been shown to localize primarily in the mitochondrial matrix, but it has also been found in the IMS (Pridgeon et al., 2007) and at extramitochondrial sites (Cechetto et al., 2000). Omi/HtrA2 is phosphorylated at Ser142 by p38γ and this is modified by the presence or absence of PINK1. Whether Omi/HtrA2 is therefore a direct PINK1 substrate is unclear and it is possible that differences in cell viability resulting from PINK1 inactivation (see below) might indirectly affect Omi/HtrA2 through other kinases, including p38. Omi/HtrA2 is released from the intermembrane space of the mitochondria during apoptosis to the cytosol where it interacts with the inhibitor of apoptosis protein (IAP) (Strauss et al., 2005). If the model of PINK1 topology proposed by Zhou et al. is correct, then neither TRAP1 nor Omi/HtrA2 would be substrates of PINK1 under physiological conditions, as such substrates would need to be present on the cytoplasmic edge of the mitochondria. However, it is not clear how much endogenous PINK1 is present in different mitochondrial locations under basal conditions and under conditions of cellular toxicity, where PINK1 has been shown to have a functional role.
Such a neuroprotective effect has been demonstrated in cell culture models under various forms of cellular stress. PINK1 has been shown to protect against cell death induced by proteasome inhibition (Muqit et al., 2006, Valente et al., 2004a, Wang et al., 2007) and oxidative damage with rotenone (Deng et al., 2005) and MPP+, the active metabolite of the complex I inhibitor MPTP (Deng et al., 2005, Haque et al., 2008, Tang et al., 2006). PINK1 has also been shown to protect against MPTP toxicity in vivo in a kinase-dependent fashion (Haque et al., 2008). Interestingly, the MTS is not required for the protective function of overexpressed PINK1 in vivo against complex I inhibition (Haque et al., 2008), which supports the idea that the functional part of the molecule is exposed to the cytoplasmic surface (Zhou et al., 2008). Presumably, high levels of cytoplasmic PINK1 can substitute for endogenous protein in this context by phosphorylating substrates at the mitochondrial surface or in the cytoplasm in the vicinity of mitochondria. Loss of function mutations (Hoepken et al., 2008, Hoepken et al., 2007, Pridgeon et al., 2007, Valente et al., 2004a) or PINK1 silencing (Clark et al., 2006, Gautier et al., 2008, Wang et al., 2006, Wood-Kaczmar et al., 2008) enhances susceptibility to cell death mediated by oxidative damage.
Prolonged ROS exposure can cause mitochondrial dysfunction, in part because proteins with iron sulfur clusters that are involved in oxidative phosphorylation and the electron transport chain are sensitive to oxidative stress (Gardner and Fridovich, 1991). PINK1 silencing also results in mitochondrial respiratory dysfunction. PINK1 knockout mice exhibit impaired striatal mitochondrial respiration and decreased activity of aconitase (Gautier et al., 2008), an iron sulfur cluster-containing enzyme involved in oxidative phosphorylation. This loss of mitochondrial aconitase activity is an intracellular indicator of superoxide generation and oxidative damage. In addition, the impaired mitochondrial respiration in these mice can be exacerbated by exposure of isolated mitochondria to heat shock or H202, or by letting the animals age (Gautier et al., 2008). Similarly, analysis of fibroblasts obtained from a patient carrying the homozygous W437X PINK1 nonsense mutation showed a lower mitochondrial respiratory activity (Piccoli et al., 2008). ATPase activity was lower in W437X patient fibroblasts when cultured with galactose, which causes cells to utilize oxidative phosphorylation instead of relying on glycolytic ATP, which suggests a basal oxidative phosphorylation deficit in these cells (Piccoli et al., 2008). Complex I respiratory defects were also found in primary fibroblasts isolated from patients homozygous for G309D PINK1 (Piccoli et al., 2008).
In addition to oxidative stress, loss of mitochondrial membrane potential (Δψm) can cause mitochondrial dysfunction. Mitochondrial membrane potential results from the charge imbalance across the inner mitochondrial membrane as the respiratory chain builds up the proton gradient that is required for oxidative phosphorylation. One mechanism resulting in the decrease of Δψm results from the permeabilization of the inner mitochondrial membrane by oxidative stress or Ca2+ overload. Inner mitochondrial membrane permeabilization results in increased mitochondrial matrix volume, reduced matrix electron density and the disorganization of the cristae—the internal compartments formed by the inner membrane of a mitochondrion (Nieminen et al., 1997, Petronilli et al., 1999). Mitochondrial membrane potential is maintained with wild type PINK1 overexpression and decreased by the presence of the G309D mutation after treatment with the proteasome inhibitor MG-132 (Valente et al., 2004a), heterozygous PINK1 mutations (Abou-Sleiman et al., 2006) or PINK1 silencing (Exner et al., 2007). Parkin, another recessive parkinsonism gene can also affect Δψm (Mortiboys et al., 2008) and decrease mitochondrial swelling once parkin has translocated to the outer mitochondrial membrane (Darios et al., 2003). Recently it has been shown that when parkin is overexpressed it is selectively recruited to mitochondria with low membrane potential (Narendra et al., 2008). Therefore, whether by direct or indirect mechanisms, PINK1 and parkin influence the ability of mitochondria to maintain Δψm, particularly under conditions such as oxidative stress or a shift to galactose as a respiratory substrate to stress the cell.
In combination, the results above show that PINK1 is a mitochondrial kinase that promotes cell survival, particularly under conditions of oxidative/metabolic stress. Although the precise physiological substrate(s) of PINK1 are not resolved, two have been proposed and it is clear that the kinase activity is important. Given a likely functional pool of PINK1 at the cytoplasmic face of the organelle, the protein is positioned to potentially play a role in many aspects of mitochondrial function. The remainder of this review will focus on one facet of mitochondria, namely the regulation of morphology, which is a regulated and dynamic process in many cells and tissues.
PINK1 and mitochondrial dynamics
The combinatorial consequences of mitochondrial dysfunction and oxidative stress, both of which are modified by PINK1, are known to influence mitochondrial morphology. Free radical generation by the respiratory chain (Jezek and Hlavata, 2005) and reactive oxygen species (Pletjushkina et al., 2006) modify mitochondrial morphology (Benard and Rossignol, 2008), leading to rapid mitochondrial fragmentation (Yu et al., 2006).
Three groups concurrently reported abnormal mitochondrial phenotypes, and a genetic interaction between PINK1 and parkin, in Drosophila models (Clark et al., 2006, Park et al., 2006, Yang et al., 2006). The phenotype associated with PINK1 knockout in Drosophila was found to be similar to that of parkin knockout, with altered mitochondrial morphology consisting of fragmentation and disintegration with loss of cristae, flight defects attributable to the degeneration of flight-wing muscle. Such flies also have an increased sensitivity to oxidative stress (Greene et al., 2003, Pesah et al., 2004) and male sterility due to ineffective spermatid maturation (Clark et al., 2006, Park et al., 2006). Transgenic expression of parkin could rescue both the PINK1-null phenotypes of male sterility and mitochondrial abnormalities (Clark et al., 2006, Park et al., 2006). However, PINK1 transgenic flies could not ameliorate the parkin-null phenotype, suggesting that PINK1 lies upstream of parkin in a common genetic pathway. Also, the phenotype of double parkin/PINK1 null flies resembled that of single mutants, suggesting that PINK1 and parkin do not act in parallel (Clark et al., 2006, Park et al., 2006).
A similar PINK1/parkin relationship may hold in mammalian cells where fragmented and truncated mitochondrial phenotypes associated with the loss of PINK1 could be rescued by overexpression of parkin (Exner et al., 2007). Additionally, altered cristae were observed in PINK1 deficient cells, suggesting one similarity with the Drosophila models (Exner et al., 2007). In contrast, no gross mitochondrial morphological defects were found in PINK1 knockout mice (Kitada et al., 2007), even though functional effects were seen (Gautier et al., 2008). This suggests that mitochondrial morphology changes can result from loss of PINK1 function but may possibly be secondary to other changes. It would therefore seem important to understand the mechanism(s) involved in morphological changes in mitochondria in PINK1 deficient cells or tissues to understand the precise role of PINK1 in this process.
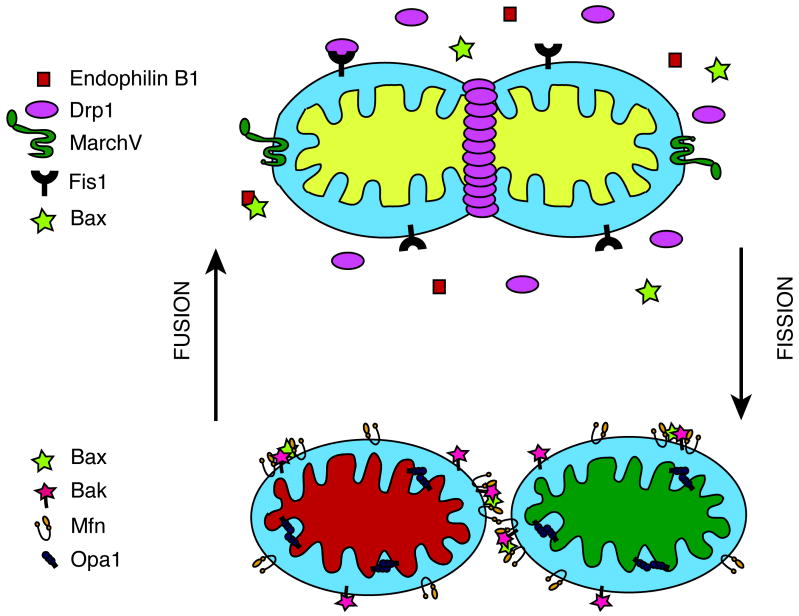
Mitochondria are dynamic organelles that continually undergo the opposing processes of fusion and fission to maintain a distinct morphology. The balance between fission and antagonizing fusion events regulate mitochondrial morphology, which includes controlling the shape, number and length of mitochondria. The shape of the mitochondria corresponds to the metabolic status (Rossignol et al., 2004) and the health of the cell (Youle and Karbowski, 2005). The process of fusion is necessary for maintaining mitochondrial function, including mitochondrial inner membrane potential, respiration, and genomic content (Chen et al., 2003). Fission events mediate apoptosis by regulating the release of pro-apoptotic factors from the intermembrane space to the cytosol. Proteins involved in mitochondrial dynamics originally identified in yeast are highly conserved in mammals. The fission mediators include Drp1 (Dnm1 in yeast) and Fis1, while the mediators of fusion are Mitofusins 1 and 2 (Mfn) (Fzo1 in yeast) and Opa1 (Mgm1 in yeast) (Westermann, 2008). However, additional proteins have been found in mammals that do not appear in yeast, such as Bax (Karbowski et al., 2006) and Endophilin B1 (Karbowski et al., 2004), which also influence mitochondrial dynamics. Yeast also have mitochondrial dynamics players that have not been identified in mammals (Coonrod et al., 2007). Therefore, although many aspects of mitochondrial dynamics are highly conserved from single cell organisms to humans, there are species differences, especially in some of the modulatory machinery for dynamics. Figure 1 illustrates the localization of mammalian proteins involved in mitochondrial dynamics.
Figure 1. Mitochondrial fission and fusion machinery.
A schematic of the localization of the proteins involved in mitochondrial dynamics is illustrated. Mitochondrial fission divides a mitochondrion into two daughter units by the coordinated actions of Drp1, Endophilin B1, Fis1 and MarchV. Tethering of two mitochondria results in mitochondrial fusion of the membranes mediated by Mfn and Opa1. Bcl-2 family proteins, Bax and Bak are involved primarily in the induction of apoptosis, which influences mitochondrial dynamics.
In mammalian cells, the large GTPase, dynamin-related protein 1 (Drp1) mediates division of the mitochondrial network (Hoppins et al., 2007, Okamoto and Shaw, 2005). Drp1 belongs to the conserved dynamin large GTPase superfamily that controls membrane tubulation and fission (Praefcke and McMahon, 2004). Dynamin, a Drp1 homolog, is assembled in the cytosol and forms spirals around endosomes to mediate fission from the plasma membrane. Helices of dynamin mediate lipid tubule scission by constricting and twisting the anchored tubules upon GTP cleavage (Danino et al., 2004, Roux et al., 2006). Presumably acting like dynamin, Drp1 oligomerizes into ring-like structures at division sites on the outer mitochondrial membrane to initiate fission in a GTP-dependent manner (Ingerman et al., 2005). However, unlike dynamin, Drp1 contains a carboxy-terminal GTPase effector domain (GED), which facilitates intra- and intermolecular interactions that regulate GTPase activity (Chang and Blackstone, 2007, Zhu et al., 2004). Dominant negative mutations that block Drp1 GTPase activity result in an elongated mitochondrial phenotype through the inhibition of GTP-mediated fission (Otsuga et al., 1998, Smirnova et al., 2001).
Soluble Drp1 is mostly cytosolic, but can be recruited to the mitochondria to form punctate foci at sites of mitochondrial fission (Smirnova et al., 2001, Wasiak et al., 2007). Both actin filaments and microtubules are integral in the recruitment of Drp1 to mitochondria (De Vos et al., 2005, Varadi et al., 2004). Drp1 recruitment to the outer mitochondrial membrane occurs via a transient interaction with Fis1 (Yoon et al., 2003, Yu et al., 2005), a tetratricopeptide domain protein that contains a C-terminal transmembrane domain localized to the outer mitochondrial membrane and an N-terminus, which protrudes into the cytosol (Yoon et al., 2003, Dohm et al., 2004, Suzuki et al., 2003). It has been suggested that Fis1 acts as a limiting factor in mitochondrial fission, supporting the notion that Fis1 acts as a receptor for Drp1 on the outer mitochondrial membrane (Yoon et al., 2003). However, Drp1 can assemble on mitochondria in the absence of Fis1 (Lee et al., 2004, Stojanovski et al., 2004), leaving the mechanism of Drp1 localization to mitochondria unclear.
Three large GTPases from the dynamin family, Mitofusin 1 (Mfn1), Mitofusin 2 (Mfn2) and Optic atrophy protein 1 (Opa1), are conserved from yeast to mammals and mediate mitochondrial fusion. It has been demonstrated that Mfn1 and Mfn2 regulate fusion of the outer mitochondrial membrane while Opa1 is responsible for fusion of the inner mitochondrial membrane (Meeusen et al., 2006, Meeusen et al., 2004). Although both Opa1 and Mfn1/2 are necessary for mitochondrial fusion, no direct interactions between the two proteins have been observed in mammalian cells. However, Mfn1 was reported to be required for Opa1-dependent inner mitochondrial membrane fusion (Cipolat et al., 2004).
Mitofusins 1 and 2 localize to the outer mitochondrial membrane through two transmembrane domains (Santel and Fuller, 2001) orienting their N-terminal GTPase domains and C-terminal coiled-coil regions towards the cytosol (Rojo et al., 2002) and facilitate the docking of adjacent mitochondria through a tethering mechanism (Koshiba et al., 2004). Trans-mitochondrial tethering occurs when the C-terminal coiled-coil regions of Mfn1 and Mfn2 interact (Chen et al., 2003, Ishihara et al., 2004, Koshiba et al., 2004, Meeusen et al., 2004). The GTPase activity of the mitofusins is necessary for fusion activity in addition to the maintenance of mitochondrial membrane potential (Hales and Fuller, 1997, Hermann et al., 1998, Santel and Fuller, 2001). Some experiments suggest that Mfn1 and Mfn2 proteins are functionally redundant (Chen et al., 2003); however, Mfn1 and Mfn2 seem to have specialized functions despite their similarities in protein structure (Cipolat et al., 2006, Eura et al., 2003). It has been shown that increased GTPase activity of Mfn1 mediates more efficient GTP-dependent mitochondrial tethering than Mfn2 (Ishihara et al., 2004). Using co-immunoprecipitation, anti-apoptotic Bcl-2 and Bcl-xL have been shown to specifically interact with Mfn2 to enhance mitochondrial fusion (Delivani et al., 2006). Mitofusin interactors also include the pro-apoptotic Bcl-2 family members Bax and Bak, which have been shown to interact with both Mfn1 and Mfn2 (Brooks et al., 2007). It has been proposed that mitofusins and Bcl-2 family proteins interact to regulate mitochondrial fusion in healthy cells (Karbowski et al., 2006).
Opa1 is located in the inner mitochondrial membrane, facing the intermembrane space (Olichon et al., 2002) and is required for fusion but not mitochondrial docking (Chen et al., 2005, Cipolat et al., 2004, Olichon et al., 2003). A single gene encodes mammalian Opa1 with 8 transcript variants resulting from alternative splicing (Delettre et al., 2001). Opa1 splice variants are differentially proteolyzed and yield various long and short isoforms that influence membrane association (Satoh et al., 2003). Longer isoforms, which retain the N-terminal transmembrane segment and the MTS, are more tightly connected to the inner mitochondrial membrane when compared to the shorter isoforms lacking residues required for membrane insertion (Ishihara et al., 2004). Numerous proteases have been identified in Opa1 processing that include paraplegin (Ishihara et al., 2004), PARL (presenilin-associated rhomboid-like) (Cipolat et al., 2006, McQuibban et al., 2003) and Yme1 (Griparic et al., 2007, Song et al., 2007). The identification of multiple splice variants of Opa1 in mammalian cells suggests that many versions of Opa1 may be necessary to differentially regulate mitochondrial morphology with respect to damaging cellular triggers. Cleavage of Opa1 is enhanced under conditions of decreased inner mitochondrial membrane potential suggesting its activity is coordinated with the energetic state of the mitochondria, supporting the notion that high membrane potential is required for fusion (Duvezin-Caubet et al., 2006, Griparic et al., 2004, Ishihara et al., 2004, Meeusen et al., 2006).
Recently it has been proposed that the parkinsonism-associated genes PINK1 and parkin regulate mitochondrial dynamics through interaction with the fission/fusion machinery. Specifically, it is thought that PINK1 and parkin promote mitochondrial fission in Drosophila. Introducing an extra copy of Drp1 rescues wing posture abnormalities caused by degeneration of indirect flight muscle in PINK1 mutants with both PINK1 and parkin deficient mutants (Deng et al., 2008, Karbowski et al., 2006, Poole et al., 2008, Yang et al., 2008). Fewer swollen and more intact cristae structures were observed in tissue with Drp1 overexpression when compared to the flight muscle in wild type flies (Poole et al., 2008, Deng et al., 2008). However, Drp1 overexpression had no effect on mitochondrial morphology in the wild type (ie PINK1 +/+) background (Yang et al., 2008).
As an alternative to the dPINK1 knockdown model, dPINK1 overexpression was examined in the compound eye of Drosophila, where ectopic dPINK1 expression causes disruption of ommatidia. Genetic perturbations of dDrp1 that reduce its activity suppress the dPINK1 eye overexpression phenotype (Poole et al., 2008). However, although the compound eye is a useful structure to assess genetic relationships, phenotypes associated with dPINK1 overexpression compared to knockout are functionally different, as parkin does not rescue PINK1 overexpression effects (Yang et al., 2008).
Inactivation of PINK1 in Drosophila also leads to loss of dopaminergic neurons (Park et al., 2006, Yang et al., 2006) and overexpression of Drp1 restores dopamine levels to normal in dPINK1 null mutant (Yang et al., 2008). GFP labelled mitochondria in dopaminergic neurons in flies are aggregated and tubular in dPINK1 mutant or dPINK1 RNAi lines (Yang et al., 2008). Increasing Drp1 dosage in this mutant dPINK1 background promotes uniform mitochondrial distribution and eliminates aggregate formation (Yang et al., 2008). Drp1 overexpression in a wild type background did not influence mitochondrial morphology in this study. Studies in cultured S2R Drosophila cells indicate that downregulation of Drp1 results in a singular perinuclear mitochondrial aggregate that resembles the phenotype demonstrated by dPINK1 silencing (Yang et al., 2008). However, double knockdown of dPINK1 and dDrp1 demonstrates extreme fusion of the mitochondrial networking (Yang et al., 2008). Additionally, expression of the Fis1 Drosophila homolog in PINK1 silenced S2R cells suppresses the mitochondrial phenotype. Together these data suggest that Fis1 and Drp1 are epistatic to PINK1.
Some studies characterizing the genetic interactions between PINK1 and parkin in Drosophila testes suggest that these parkinsonism associated genes may inhibit mitochondrial fusion (Deng et al., 2008) rather than promote mitochondrial fission. In Drosophila, mitochondrial morphology can be examined during spermatogenesis, where germ cells undergo multiple rounds of mitosis and meiosis with incomplete cytokinesis to yield interconnected early spermatids (Fuller, 1993). During spermatid maturation at the ‘onion stage’, the mitochondria aggregate adjacent to the nucleus (Fuller, 1993) and fuse into two large mitochondrial masses that are arranged by layers of wrapped mitochondrial membrane into a structure called the nebenkern (Lindley, 1980). Dramatic morphological changes occur when the two interlocked subunits of the nebenkern elongate with the growing microtubule-based axenome to promote membrane remodeling and individualization (Fuller, 1998). The Drosophila genome encodes two Mfn homologs that regulate mitochondrial fusion, Fuzzy onion (Fzo) and Marf (Mitochondrial assembly regulatory factor). Fzo expression is limited to the testes and mutations cause mitochondrial fusion deficits and male sterility due to defective nebenkern formation (Hales and Fuller, 1997). Marf is expressed in germline and somatic cells (Hwa et al., 2002). During spermatogenesis, dPINK1 or dparkin loss of function results in defects in mitochondria morphology as observed by the vacuolated ‘onion stage’ nebenkern (Clark et al., 2006, Deng et al., 2008, Riparbelli and Callaini, 2007). Fzo mutants exhibit onion-staged nebenkern composed of many small mitochondria and, as a consequence, nebenkern borders appear irregular but this is morphologically different from the PINK1 phenotype (vacuolated with regular borders). Double mutants with dPINK1 and Fzo loss of function exhibit a single elongated mitochondrial derivative and irregular nebenkern borders associated with Fzo mutants were rescued by dPINK1 mutation (Deng et al., 2008). These data support the notion that PINK1 plays a role in mitochondrial morphology but suggest that PINK1 is not a core component of the fusion/fission machinery but rather plays a modulatory role.
The Drosophila genome also encodes a single homolog of Opa1 (Yarosh et al., 2008) that functions to regulate mitochondrial fusion of the inner mitochondrial membrane. Loss of function mutations in Drosophila Opa1 or Mfn2 homologs suppresses the dPINK1 and dparkin flight and climbing phenotypes (Poole et al., 2008). Moreover using the dPINK1 overexpression eye phenotype, alterations that decrease dOpa1 and Marf activity enhance the eye phenotype associated with PINK1/parkin pathway activation (Poole et al., 2008). PINK1-dependent inhibition of fusion was further studied in the indirect flight muscle using mitochondrial directed GFP and phalloidin to simultaneously label mitochondria and actin, respectively. In addition to muscle specific downregulation of Marf in flies, dOpa1 RNAi in muscle resulted in mitochondrial fragmentation that is relatively similar to dDrp1 overexpression in transgenic flies (Deng et al., 2008).
These studies of mitochondrial dynamics in Drosophila models have therefore provided a great deal of insight into the relationships of PINK1 and parkin to mitochondrial morphology, especially in flight muscles and in testes. However, PINK1 silencing studies performed in mammalian cells (Exner et al., 2007, Weihofen et al., 2009) or C. elegans (Ichishita et al., 2008) have shown very different effects of PINK1/parkin on morphology. The genetic interaction between PINK1 and parkin initially identified in Drosophila is conserved in mammalian cells in that parkin can rescue loss of PINK1. However, PINK1 deficient mammalian cells have a fragmented and truncated mitochondrial morphology, which would suggest an imbalance towards fission (Exner et al., 2007, Weihofen et al., 2009). Data from our own studies in mammalian cells also supports a fragmented mitochondrial phenotype after PINK1 silencing (KJ Thomas, unpublished observations) and parkin deficient fibroblasts have decreased mitochondrial connectivity (Mortiboys et al., 2008). Additionally, in a high throughput system for examining modulators of mitochondrial morphology in C. elegans, knockdown of the PINK1 homolog in that species also resulted in fission, not fusion. What possible explanations could there be for apparently disparate results in different models?
The dynamic life cycle of a mitochondrion is heavily dependent upon mitochondrial membrane potential (Duvezin-Caubet et al., 2006, Griparic et al., 2004, Ishihara et al., 2004, Meeusen et al., 2006, Twig et al., 2008). The downregulation of PINK1 causes loss of mitochondrial membrane potential in several mammalian systems (Abou-Sleiman et al., 2006, Exner et al., 2007, Valente et al., 2004b). Therefore, loss of Δψm associated with the loss of PINK1 may be a primary event that leads to secondary effects mitochondrial morphology that may result in apparent fission or fusion depending on the tissue/cell type or status. As recent data suggests that fusion and fission are linked (Twig et al., 2008), the apparent occurrence of either fusion or fission may not be instructive as to which is the primary event. Coupled with evidence comparing PINK1 mutants with mutations in the fission/fusion machinery (Deng et al., 2008), the most parsimonious interpretation is that the effects of PINK1 on morphology are indirect.
An additional question regarding the relationship between PINK1 and mitochondrial morphology occurs because of evidence of multiple roles of mitochondria in apoptosis. Mitochondrial membrane permeabilization is often considered the “point of no return” in events leading to cell death as during apoptosis, the permeability of the outer mitochondrial membrane increases allowing soluble, intermembrane space proteins to be released into the cytoplasm (Adrain et al., 2001, Patterson et al., 2000, Susin et al., 1999). PINK1 has been shown to block apoptosis induced by staurosporine, a general kinase inhibitor (Gelmetti et al., 2008, Petit et al., 2005, Wood-Kaczmar et al., 2008) and degeneration of indirect fly muscles in Drosophila from knockout animals includes apoptosis. Overexpression of PINK1 and parkin in cultured cells can prevent the release of cytochrome c and caspase activation (Darios et al., 2003, Gelmetti et al., 2008, Petit et al., 2005, Pridgeon et al., 2007). However, apoptosis is associated with mitochondrial fission and there is therefore difficult to understand how a molecule could promote fission but limit apoptosis. It is known that Drp1-mediated mitochondrial fission and fragmentation of the mitochondrial network occurs during programmed cell death in Drosophila (Abdelwahid et al., 2007, Goyal et al., 2007). In recent studies on overexpression of parkin, increased expression did not induce fission (as would be predicted from the idea that PINK1/parkin promotes fission or limits fusion) but instead resulted in the formation of extended organelles that were then degraded by autophagy (Narendra et al., 2008). One possibility is, again, that the effects on morphology are secondary to the direct effects of PINK1, which may include protection of the organelle. Clearly, the resolution of these questions will require a more detailed understanding of the mechanism(s) by which PINK1 and its partner, parkin, influences both cellular viability and mitochondrial morphology in different tissues and cells.
Mechanisms involved in the PINK1/parkin pathway and effects on mitochondria
How do PINK1 and parkin influence mitochondria? One current controversy surrounds the localization of PINK1 and parkin. The majority of parkin is cytoplasmic in most cells, including neurons (Cookson et al., 2003, Hase et al., 2002), although a fraction is found at the outer mitochondrial membrane (Darios et al., 2003, Narendra et al., 2008). Kuroda et al. suggested that parkin preferentially localizes to the mitochondrial matrix, and mitochondrial biogenesis is enhanced through an association with mitochondria transcription factor A in dividing cells (Kuroda et al., 2006). As previously described, PINK1 is found in both the mitochondria (Beilina et al., 2005, Gandhi et al., 2006, Plun-Favreau et al., 2007, Lin and Kang, 2008, Muqit et al., 2006, Pridgeon et al., 2007, Silvestri et al., 2005, Weihofen et al., 2009) and the cytoplasm (Beilina et al., 2005, Haque et al., 2008, Lin and Kang, 2008, Takatori et al., 2008, Weihofen et al., 2008, Weihofen et al., 2009, Zhou et al., 2008). Whether PINK1 and parkin proteins physically interact is unclear. It is plausible that they associate in the cytoplasm before PINK1 is transported to the mitochondria or perhaps they interact after mitochondrial import and processing of PINK1.
Alternatively, PINK1 and parkin might modify one another indirectly. For example, they may share substrates, such as the mitochondrial proteins released into the cytoplasm under stress conditions or other proteins mediating cell death and/or survival. Parkin may only ubiquitylate substrates that have been phosphorylated by PINK1. It is also possible that parkin can only function as a ubiquitin ligase after phosphorylation by PINK1 whose kinase activity is upregulated in response to oxidative stress.
Might post-translational regulation of key players in the mitochondrial dynamics process be targets for phosphorylation or ubiquitylation by PINK1/parkin, respectively? Drp1 activity is enhanced by cyclin-dependent kinase 1/cyclin B phosphorylation of Ser618 (human Drp1; Ser585 in rat Drp1) during mitosis (Taguchi et al., 2007) and cAMP-dependent protein kinase A (PKA) inactivates Drp1 GTPase activity by phosphorylation of Ser637 (human Drp1; Ser656 in rat Drp1) which results in mitochondrial fusion (Chang and Blackstone, 2007, Cribbs and Strack, 2007). The Ser637 residue is conserved in Drosophila, but according to these reports if Drp1 phosphorylation in flies mimics that which occurs in mammalian cells, PINK1 would limit mitochondrial fission. Whether PINK1 influences mitochondrial morphology through direct phosphorylation of Drp1 remains to be determined.
SUMOylation also regulates Drp1 activity and mitochondrial morphology (Harder et al., 2004) (Zunino et al., 2007). SUMOylation of Drp1 enhances its activity, positively regulating mitochondrial fission. During apoptosis, more Drp1 protein associates with the outer mitochondrial membrane and Drp1 SUMOylation increases (Wasiak et al., 2007), resulting in increased mitochondrial fission. Parkin has been shown to physically interact in vitro and in vivo with SUMO1 to increase its nuclear transport and self-ubiquitination (Um and Chung, 2006). Downregulation of SUMO1 may inhibit mitochondrial fission caused by excess parkin turnover in the dPINK1/dparkin pathway and the epistatic Drp1.
The mitofusins, which regulate fusion events in yeast (Fzo1) and mammals, may be regulated by ubiquitylation. During the mating of yeast, mitochondria fragment and the levels of Fzo1 decrease. Chemical inhibition (Neutzner and Youle, 2005) or genetic inactivation (Escobar-Henriques et al., 2006) of the proteasome attenuates the loss of Fzo1. Fzo1 is removed from the outer mitochondrial membrane and degraded following lysine 48-dependent ubiquitination to regulate its function in a process similar to ERAD (ER-associated degradation) removal and degradation of membrane spanning proteins. Mammalian mitofusin protein levels increase after exposure to proteasome inhibitors in culture (Karbowski et al., 2007), suggesting the ubiquitin proteasome pathway regulates these fusion proteins.
As an E3 ligase, parkin can promote both degradative and nondegradative forms of ubiquitination (Doss-Pepe et al., 2005, Joch et al., 2007, Lim et al., 2006). For instance, ubiquitin lysine 48-linked polyubiquitin chains target substrates to the proteasome (Imai et al., 2000), whereas ubiquitin lysine 63-linked chains control non-degradative processes including kinase activation, DNA repair, translational regulation and endocytosis of membrane proteins (Doss-Pepe et al., 2005, Joch et al., 2007, Moore et al., 2008, Pickart and Fushman, 2004). It is notable that ubiquitin lysine 63-linked chains promote the degradation of membrane proteins by the lysosome, a putative link supporting the notion that parkin may be a quality control mechanism in Drosophila whereby the PINK1/parkin pathway promotes fission to degrade impaired mitochondria by autophagy. In fact, it has been shown that overexpression of parkin in mammalian cells selectively recruits dysfunctional mitochondria with low membrane potential and promotes their autophagy (Narendra et al., 2008). The loss of parkin associated with recessive parkinsonism suggests that parkin-mediated ubiquitination of specific cellular substrates is required for the survival of dopaminergic neurons. At the present it is unclear how parkin deficiencies might precipitate defects in mitochondrial function and whether this relates to the accumulation of toxic parkin substrates or loss of a non-degradative regulatory role of parkin-mediated ubiquitination.
Morphological changes in mitochondria can be influenced by directed movement or anchoring of these organelles by various components of the cytoskeleton (Rube and van der Bliek, 2004). In cultured cells, disruption of the microtubule network affects the distribution of mitochondria (Heggeness et al., 1978), suggesting that mitochondria require microtubules for normal cellular distribution. Secondary messengers, such as calcium, can influence movement of mitochondria along microtubule tracks. For instance, mitochondrial motility is blocked by increased mitochondrial calcium uptake (Rintoul et al., 2003), suggesting that local calcium buffering limits mitochondrial movement. Moreover, calcium signaling can influence the function of molecular motors (Marston, 1995) that facilitate mitochondrial movement along cytoskeletal elements. Recently, it was shown that PINK1 forms a multi-protein complex with an atypical calcium-dependent GTPase Miro and the adaptor protein Milton (Weihofen et al., 2009), a complex which links the heavy chain of kinesin to mitochondria for anterograde transport of mitochondria along microtubules (Glater et al., 2006, Stowers et al., 2002). Overexpression of Miro and Milton rescued mitochondrial fragmentation associated with the loss of PINK1 and increased mitochondrial localized PINK1 protein, further suggesting a role for PINK1 at the outer mitochondrial membrane and linking PINK1 function to mitochondrial trafficking (Weihofen et al., 2009). These aspects of mitochondrial transport may be especially important in neurons where mitochondria are transported out of synapses. Future studies should address the effects of PINK1/parkin pathway perturbations not only on mitochondrial calcium regulation and buffering but also investigate the effect of the differences between metabolically homogeneous and heterogeneous pools of mitochondria on mitochondrial transport and trafficking.
Other putative links between defects in mitochondrial transport and recessive parkinsonism include the association of parkin with the cytoskeleton or the influence of oxidative stress on cytoskeletal function. Parkin is reported to associate with cytoskeletal elements, such as actin filaments (Huynh et al., 2000) and microtubules (Ren et al., 2003). Studies suggest that the binding of parkin stabilizes microtubules (Yang et al., 2005). Stabilization of the cytoskeletal network may be an important link between parkin and mitochondrial dynamics because disruption of microtubules can cause aberrant mitochondrial distribution (Yaffe et al., 1996). On the other hand, this interaction may simply provide transport of misfolded proteins for degradation (Yang et al., 2005). Additionally, oxidative stress is associated with microtubule disorganization (Annunen-Rasila et al., 2007) and oxidative insult can impair mitochondrial trafficking (Liu et al., 2008). The idea of oxidation being detrimental to mitochondrial plasticity is further supported by the notion that rotenone selectively kills neurons via a microtubule-dependent mechanism (Ren and Feng, 2007). While the shape and connectivity of the mitochondria are likely to influence the ability of the cytoskeleton to move the organelle, it is unknown if altered transport is the result of defects in morphology or vice versa.
Concluding remarks
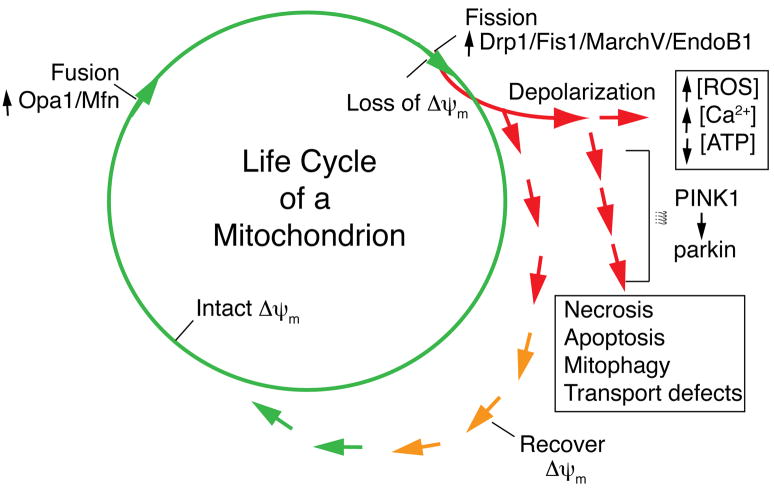
In addition to summarizing the role of PINK1 in mitochondrial function, this review has attempted to critically analyze the newly proposed relationship between parkinsonism and mitochondrial dynamics. Evolutionarily conserved from yeast to humans, mitochondrial morphogens regulate the genome, membrane integrity, bioenergetic connectivity and turnover of mitochondria. We suggest here that the PINK1/parkin pathway is also conserved from Drosophila to mammals but those differences in the output, in terms of mitochondrial morphology, imply that it is likely that the primary effect is on mitochondrial function and any changes in morphology are probably secondary. Figure 2 illustrates the possible direct effects of PINK1/parkin on the life cycle of a mitochondrion, which may influence other aspects of organellar function, such as transport or degradation (mitophagy), in addition to mitochondrial membrane potential. Future work needs to address in detail the biochemical roles of PINK1/parkin in terms of substrates for both proteins, as well as their relationship to each other.
Figure 2. Model for the life cycle of a mitochondrion.
This proposed model for the life cycle of a mitochondrion reflects mitochondrial dynamics and turnover (adapted from (Twig et al., 2008)). In a healthy cell, the antagonizing events of mitochondrial fusion and fission are dependent upon mitochondrial membrane potential. Following a mitochondrial fission event, fragmented mitochondria may either maintain an intact membrane potential (green line) or depolarize (red line). Should mitochondrial membrane depolarization take place prior to fission, it is unlikely to proceed to fusion without repolarization. Consequences of depolarization include the production of reactive oxygen species (ROS), increased cytosolic [Ca2+] or loss of ATP with subsequent effects on various types of cell death and/or impaired mitochondrial transport and trafficking. PINK1 lies upstream of parkin in a conserved genetic pathway as indicated. Exactly how this relates to mitochondrial morphology is unclear, but we suggest here that the major effect of PINK1/parkin might be on the turnover of damaged mitochondria and thus may be tangential to the fusion/fission machinery.
Acknowledgments
This work was supported by the National Institutes on Aging, Intramural Research Program, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelwahid E, Yokokura T, Krieser RJ, Balasundaram S, Fowle WH, White K. Mitochondrial disruption in Drosophila apoptosis. Dev Cell. 2007;12:793–806. doi: 10.1016/j.devcel.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Abou-Sleiman PM, Muqit MM, McDonald NQ, Yang YX, Gandhi S, Healy DG, Harvey K, Harvey RJ, Deas E, Bhatia K, Quinn N, Lees A, Latchman DS, Wood NW. A heterozygous effect for PINK1 mutations in Parkinson’s disease? Ann Neurol. 2006;60:414–419. doi: 10.1002/ana.20960. [DOI] [PubMed] [Google Scholar]
- Adrain C, Creagh EM, Martin SJ. Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2. Embo J. 2001;20:6627–6636. doi: 10.1093/emboj/20.23.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunen-Rasila J, Ohlmeier S, Tuokko H, Veijola J, Majamaa K. Proteome and cytoskeleton responses in osteosarcoma cells with reduced OXPHOS activity. Proteomics. 2007;7:2189–2200. doi: 10.1002/pmic.200601031. [DOI] [PubMed] [Google Scholar]
- Beilina A, Van Der Brug M, Ahmad R, Kesavapany S, Miller DW, Petsko GA, Cookson MR. Mutations in PTEN-induced putative kinase 1 associated with recessive parkinsonism have differential effects on protein stability. Proc Natl Acad Sci U S A. 2005;102:5703–5708. doi: 10.1073/pnas.0500617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard G, Rossignol R. Ultrastructure of the mitochondrion and its bearing on function and bioenergetics. Antioxid Redox Signal. 2008;10:1313–1342. doi: 10.1089/ars.2007.2000. [DOI] [PubMed] [Google Scholar]
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Blackinton JG, Anvret A, Beilina A, Olson L, Cookson MR, Galter D. Expression of PINK1 mRNA in human and rodent brain and in Parkinson’s disease. Brain Res. 2007;1184:10–16. doi: 10.1016/j.brainres.2007.09.056. [DOI] [PubMed] [Google Scholar]
- Blake CI, Spitz E, Leehey M, Hoffer BJ, Boyson SJ. Platelet mitochondrial respiratory chain function in Parkinson’s disease. Mov Disord. 1997;12:3–8. doi: 10.1002/mds.870120103. [DOI] [PubMed] [Google Scholar]
- Brooks C, Wei Q, Feng L, Dong G, Tao Y, Mei L, Xie ZJ, Dong Z. Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc Natl Acad Sci U S A. 2007;104:11649–11654. doi: 10.1073/pnas.0703976104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetto JD, Soltys BJ, Gupta RS. Localization of mitochondrial 60-kD heat shock chaperonin protein (Hsp60) in pituitary growth hormone secretory granules and pancreatic zymogen granules. J Histochem Cytochem. 2000;48:45–56. doi: 10.1177/002215540004800105. [DOI] [PubMed] [Google Scholar]
- Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem. 2007;282:21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A. 2004;101:15927–15932. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D’Adamio L, Derks C, Dejaegere T, Pellegrini L, D’Hooge R, Scorrano L, De Strooper B. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126:163–175. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- Cookson MR, Lockhart PJ, McLendon C, O’Farrell C, Schlossmacher M, Farrer MJ. RING finger 1 mutations in Parkin produce altered localization of the protein. Hum Mol Genet. 2003;12:2957–2965. doi: 10.1093/hmg/ddg328. [DOI] [PubMed] [Google Scholar]
- Coonrod EM, Karren MA, Shaw JM. Ugo1p is a multipass transmembrane protein with a single carrier domain required for mitochondrial fusion. Traffic. 2007;8:500–511. doi: 10.1111/j.1600-0854.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danino D, Moon KH, Hinshaw JE. Rapid constriction of lipid bilayers by the mechanochemical enzyme dynamin. J Struct Biol. 2004;147:259–267. doi: 10.1016/j.jsb.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Darios F, Corti O, Lucking CB, Hampe C, Muriel MP, Abbas N, Gu WJ, Hirsch EC, Rooney T, Ruberg M, Brice A. Parkin prevents mitochondrial swelling and cytochrome c release in mitochondria-dependent cell death. Hum Mol Genet. 2003;12:517–526. doi: 10.1093/hmg/ddg044. [DOI] [PubMed] [Google Scholar]
- De Vos KJ, Allan VJ, Grierson AJ, Sheetz MP. Mitochondrial function and actin regulate dynamin-related protein 1-dependent mitochondrial fission. Curr Biol. 2005;15:678–683. doi: 10.1016/j.cub.2005.02.064. [DOI] [PubMed] [Google Scholar]
- Delettre C, Griffoin JM, Kaplan J, Dollfus H, Lorenz B, Faivre L, Lenaers G, Belenguer P, Hamel CP. Mutation spectrum and splicing variants in the OPA1 gene. Hum Genet. 2001;109:584–591. doi: 10.1007/s00439-001-0633-y. [DOI] [PubMed] [Google Scholar]
- Delivani P, Adrain C, Taylor RC, Duriez PJ, Martin SJ. Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics. Mol Cell. 2006;21:761–773. doi: 10.1016/j.molcel.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Deng H, Dodson MW, Huang H, Guo M. The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A. 2008;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Jankovic J, Guo Y, Xie W, Le W. Small interfering RNA targeting the PINK1 induces apoptosis in dopaminergic cells SH-SY5Y. Biochem Biophys Res Commun. 2005;337:1133–1138. doi: 10.1016/j.bbrc.2005.09.178. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, Jenner P, Marsden CD. Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J Neurochem. 1989;52:381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- Dohm JA, Lee SJ, Hardwick JM, Hill RB, Gittis AG. Cytosolic domain of the human mitochondrial fission protein fis1 adopts a TPR fold. Proteins. 2004;54:153–156. doi: 10.1002/prot.10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doss-Pepe EW, Chen L, Madura K. Alpha-synuclein and parkin contribute to the assembly of ubiquitin lysine 63-linked multiubiquitin chains. J Biol Chem. 2005;280:16619–16624. doi: 10.1074/jbc.M413591200. [DOI] [PubMed] [Google Scholar]
- Duvezin-Caubet S, Jagasia R, Wagener J, Hofmann S, Trifunovic A, Hansson A, Chomyn A, Bauer MF, Attardi G, Larsson NG, Neupert W, Reichert AS. Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J Biol Chem. 2006;281:37972–37979. doi: 10.1074/jbc.M606059200. [DOI] [PubMed] [Google Scholar]
- Escobar-Henriques M, Westermann B, Langer T. Regulation of mitochondrial fusion by the F-box protein Mdm30 involves proteasome-independent turnover of Fzo1. J Cell Biol. 2006;173:645–650. doi: 10.1083/jcb.200512079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eura Y, Ishihara N, Yokota S, Mihara K. Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J Biochem. 2003;134:333–344. doi: 10.1093/jb/mvg150. [DOI] [PubMed] [Google Scholar]
- Exner N, Treske B, Paquet D, Holmstrom K, Schiesling C, Gispert S, Carballo-Carbajal I, Berg D, Hoepken HH, Gasser T, Kruger R, Winklhofer KF, Vogel F, Reichert AS, Auburger G, Kahle PJ, Schmid B, Haass C. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci. 2007;27:12413–12418. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller M. Spermatogenesis. New York: Cold Spring Harbor Laboratory Press; 1993. [Google Scholar]
- Fuller MT. Genetic control of cell proliferation and differentiation in Drosophila spermatogenesis. Semin Cell Dev Biol. 1998;9:433–444. doi: 10.1006/scdb.1998.0227. [DOI] [PubMed] [Google Scholar]
- Gandhi S, Muqit MM, Stanyer L, Healy DG, Abou-Sleiman PM, Hargreaves I, Heales S, Ganguly M, Parsons L, Lees AJ, Latchman DS, Holton JL, Wood NW, Revesz T. PINK1 protein in normal human brain and Parkinson’s disease. Brain. 2006;129:1720–1731. doi: 10.1093/brain/awl114. [DOI] [PubMed] [Google Scholar]
- Gardner PR, Fridovich I. Superoxide sensitivity of the Escherichia coli aconitase. J Biol Chem. 1991;266:19328–19333. [PubMed] [Google Scholar]
- Gautier CA, Kitada T, Shen J. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci U S A. 2008;105:11364–11369. doi: 10.1073/pnas.0802076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelmetti V, Ferraris A, Brusa L, Romano F, Lombardi F, Barzaghi C, Stanzione P, Garavaglia B, Dallapiccola B, Valente EM. Late onset sporadic Parkinson’s disease caused by PINK1 mutations: clinical and functional study. Mov Disord. 2008;23:881–885. doi: 10.1002/mds.21960. [DOI] [PubMed] [Google Scholar]
- Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal G, Fell B, Sarin A, Youle RJ, Sriram V. Role of mitochondrial remodeling in programmed cell death in Drosophila melanogaster. Dev Cell. 2007;12:807–816. doi: 10.1016/j.devcel.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci U S A. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griparic L, Kanazawa T, van der Bliek AM. Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J Cell Biol. 2007;178:757–764. doi: 10.1083/jcb.200704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griparic L, van der Wel NN, Orozco IJ, Peters PJ, van der Bliek AM. Loss of the intermembrane space protein Mgm1/OPA1 induces swelling and localized constrictions along the lengths of mitochondria. J Biol Chem. 2004;279:18792–18798. doi: 10.1074/jbc.M400920200. [DOI] [PubMed] [Google Scholar]
- Hales KG, Fuller MT. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell. 1997;90:121–129. doi: 10.1016/s0092-8674(00)80319-0. [DOI] [PubMed] [Google Scholar]
- Haque ME, Thomas KJ, D’Souza C, Callaghan S, Kitada T, Slack RS, Fraser P, Cookson MR, Tandon A, Park DS. Cytoplasmic Pink1 activity protects neurons from dopaminergic neurotoxin MPTP. Proc Natl Acad Sci U S A. 2008;105:1716–1721. doi: 10.1073/pnas.0705363105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder Z, Zunino R, McBride H. Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr Biol. 2004;14:340–345. doi: 10.1016/j.cub.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Hase A, Yamada H, Arai K, Sunada Y, Shimizu T, Matsumura K. Characterization of parkin in bovine peripheral nerve. Brain Res. 2002;930:143–149. doi: 10.1016/s0006-8993(02)02241-2. [DOI] [PubMed] [Google Scholar]
- Healy DG, Abou-Sleiman PM, Gibson JM, Ross OA, Jain S, Gandhi S, Gosal D, Muqit MM, Wood NW, Lynch T. PINK1 (PARK6) associated Parkinson disease in Ireland. Neurology. 2004;63:1486–1488. doi: 10.1212/01.wnl.0000142089.38301.8e. [DOI] [PubMed] [Google Scholar]
- Heggeness MH, Simon M, Singer SJ. Association of mitochondria with microtubules in cultured cells. Proc Natl Acad Sci U S A. 1978;75:3863–3866. doi: 10.1073/pnas.75.8.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann GJ, Thatcher JW, Mills JP, Hales KG, Fuller MT, Nunnari J, Shaw JM. Mitochondrial fusion in yeast requires the transmembrane GTPase Fzo1p. J Cell Biol. 1998;143:359–373. doi: 10.1083/jcb.143.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepken HH, Gispert S, Azizov M, Klinkenberg M, Ricciardi F, Kurz A, Morales-Gordo B, Bonin M, Riess O, Gasser T, Kogel D, Steinmetz H, Auburger G. Parkinson patient fibroblasts show increased alpha-synuclein expression. Exp Neurol. 2008;212:307–313. doi: 10.1016/j.expneurol.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Hoepken HH, Gispert S, Morales B, Wingerter O, Del Turco D, Mulsch A, Nussbaum RL, Muller K, Drose S, Brandt U, Deller T, Wirth B, Kudin AP, Kunz WS, Auburger G. Mitochondrial dysfunction, peroxidation damage and changes in glutathione metabolism in PARK6. Neurobiol Dis. 2007;25:401–411. doi: 10.1016/j.nbd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem. 2007;76:751–780. doi: 10.1146/annurev.biochem.76.071905.090048. [DOI] [PubMed] [Google Scholar]
- Huynh DP, Scoles DR, Ho TH, Del Bigio MR, Pulst SM. Parkin is associated with actin filaments in neuronal and nonneural cells. Ann Neurol. 2000;48:737–744. [PubMed] [Google Scholar]
- Hwa JJ, Hiller MA, Fuller MT, Santel A. Differential expression of the Drosophila mitofusin genes fuzzy onions (fzo) and dmfn. Mech Dev. 2002;116:213–216. doi: 10.1016/s0925-4773(02)00141-7. [DOI] [PubMed] [Google Scholar]
- Ichishita R, Tanaka K, Sugiura Y, Sayano T, Mihara K, Oka T. An RNAi screen for mitochondrial proteins required to maintain the morphology of the organelle in Caenorhabditis elegans. J Biochem. 2008;143:449–454. doi: 10.1093/jb/mvm245. [DOI] [PubMed] [Google Scholar]
- Imai Y, Soda M, Takahashi R. Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J Biol Chem. 2000;275:35661–35664. doi: 10.1074/jbc.C000447200. [DOI] [PubMed] [Google Scholar]
- Ingerman E, Perkins EM, Marino M, Mears JA, McCaffery JM, Hinshaw JE, Nunnari J. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol. 2005;170:1021–1027. doi: 10.1083/jcb.200506078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004;117:6535–6546. doi: 10.1242/jcs.01565. [DOI] [PubMed] [Google Scholar]
- Jeffrey PD, Russo AA, Polyak K, Gibbs E, Hurwitz J, Massague J, Pavletich NP. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature. 1995;376:313–320. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- Jezek P, Hlavata L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int J Biochem Cell Biol. 2005;37:2478–2503. doi: 10.1016/j.biocel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Joch M, Ase AR, Chen CX, MacDonald PA, Kontogiannea M, Corera AT, Brice A, Seguela P, Fon EA. Parkin-mediated monoubiquitination of the PDZ protein PICK1 regulates the activity of acid-sensing ion channels. Mol Biol Cell. 2007;18:3105–3118. doi: 10.1091/mbc.E05-11-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Jeong SY, Youle RJ. Endophilin B1 is required for the maintenance of mitochondrial morphology. J Cell Biol. 2004;166:1027–1039. doi: 10.1083/jcb.200407046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Neutzner A, Youle RJ. The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J Cell Biol. 2007;178:71–84. doi: 10.1083/jcb.200611064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443:658–662. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci U S A. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Grunewald A, Hedrich K. Early-onset parkinsonism associated with PINK1 mutations: frequency, genotypes, and phenotypes. Neurology. 2006;66:1129–1130. doi: 10.1212/01.wnl.0000220157.81513.85. author reply 1129–1130. [DOI] [PubMed] [Google Scholar]
- Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004;305:858–862. doi: 10.1126/science.1099793. [DOI] [PubMed] [Google Scholar]
- Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet. 2006;38:518–520. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- Krige D, Carroll MT, Cooper JM, Marsden CD, Schapira AH. Platelet mitochondrial function in Parkinson’s disease. The Royal Kings and Queens Parkinson Disease Research Group. Ann Neurol. 1992;32:782–788. doi: 10.1002/ana.410320612. [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Mitsui T, Kunishige M, Shono M, Akaike M, Azuma H, Matsumoto T. Parkin enhances mitochondrial biogenesis in proliferating cells. Hum Mol Genet. 2006;15:883–895. doi: 10.1093/hmg/ddl006. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Langston JW, Forno LS, Rebert CS, Irwin I. Selective nigral toxicity after systemic administration of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyrine (MPTP) in the squirrel monkey. Brain Res. 1984;292:390–394. doi: 10.1016/0006-8993(84)90777-7. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15:5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tomiyama H, Sato K, Hatano Y, Yoshino H, Atsumi M, Kitaguchi M, Sasaki S, Kawaguchi S, Miyajima H, Toda T, Mizuno Y, Hattori N. Clinicogenetic study of PINK1 mutations in autosomal recessive early-onset parkinsonism. Neurology. 2005;64:1955–1957. doi: 10.1212/01.WNL.0000164009.36740.4E. [DOI] [PubMed] [Google Scholar]
- Lim KL, Dawson VL, Dawson TM. Parkin-mediated lysine 63-linked polyubiquitination: a link to protein inclusions formation in Parkinson’s and other conformational diseases? Neurobiol Aging. 2006;27:524–529. doi: 10.1016/j.neurobiolaging.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Lin W, Kang UJ. Characterization of PINK1 processing, stability, and subcellular localization. J Neurochem. 2008;106:464–474. doi: 10.1111/j.1471-4159.2008.05398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindley DaTKT. Spermatogenesis. New York: Academic Press; 1980. [Google Scholar]
- Liou HH, Chen RC, Tsai YF, Chen WP, Chang YC, Tsai MC. Effects of paraquat on the substantia nigra of the wistar rats: neurochemical, histological, and behavioral studies. Toxicol Appl Pharmacol. 1996;137:34–41. doi: 10.1006/taap.1996.0054. [DOI] [PubMed] [Google Scholar]
- Liu BN, Ismailov SB, Liu MB. [The state of cytoskeleton and its links “oxygen-peroxide” effects in some pathologies and apoptosis] Biomed Khim. 2008;54:58–77. [PubMed] [Google Scholar]
- Marongiu R, Brancati F, Antonini A, Ialongo T, Ceccarini C, Scarciolla O, Capalbo A, Benti R, Pezzoli G, Dallapiccola B, Goldwurm S, Valente EM. Whole gene deletion and splicing mutations expand the PINK1 genotypic spectrum. Hum Mutat. 2007;28:98. doi: 10.1002/humu.9472. [DOI] [PubMed] [Google Scholar]
- Marston S. Ca(2+)-dependent protein switches in actomyosin based contractile systems. Int J Biochem Cell Biol. 1995;27:97–108. doi: 10.1016/1357-2725(94)00080-u. [DOI] [PubMed] [Google Scholar]
- McQuibban GA, Saurya S, Freeman M. Mitochondrial membrane remodelling regulated by a conserved rhomboid protease. Nature. 2003;423:537–541. doi: 10.1038/nature01633. [DOI] [PubMed] [Google Scholar]
- Meeusen S, DeVay R, Block J, Cassidy-Stone A, Wayson S, McCaffery JM, Nunnari J. Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell. 2006;127:383–395. doi: 10.1016/j.cell.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Meeusen S, McCaffery JM, Nunnari J. Mitochondrial fusion intermediates revealed in vitro. Science. 2004;305:1747–1752. doi: 10.1126/science.1100612. [DOI] [PubMed] [Google Scholar]
- Moore DJ, West AB, Dikeman DA, Dawson VL, Dawson TM. Parkin mediates the degradation-independent ubiquitination of Hsp70. J Neurochem. 2008;105:1806–1819. doi: 10.1111/j.1471-4159.2008.05261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki Y, Kim YJ, Ido Y, Misawa H, Kawashima K, Endo S, Takahashi R. L347P PINK1 mutant that fails to bind to Hsp90/Cdc37 chaperones is rapidly degraded in a proteasome-dependent manner. Neurosci Res. 2008;61:43–48. doi: 10.1016/j.neures.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Mortiboys H, Thomas KJ, Koopman WJ, Klaffke S, Abou-Sleiman P, Olpin S, Wood NW, Willems PH, Smeitink JA, Cookson MR, Bandmann O. Mitochondrial function and morphology are impaired in parkin-mutant fibroblasts. Ann Neurol. 2008;64:555–565. doi: 10.1002/ana.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muqit MM, Abou-Sleiman PM, Saurin AT, Harvey K, Gandhi S, Deas E, Eaton S, Payne Smith MD, Venner K, Matilla A, Healy DG, Gilks WP, Lees AJ, Holton J, Revesz T, Parker PJ, Harvey RJ, Wood NW, Latchman DS. Altered cleavage and localization of PINK1 to aggresomes in the presence of proteasomal stress. J Neurochem. 2006;98:156–169. doi: 10.1111/j.1471-4159.2006.03845.x. [DOI] [PubMed] [Google Scholar]
- Nakajima A, Kataoka K, Hong M, Sakaguchi M, Huh NH. BRPK, a novel protein kinase showing increased expression in mouse cancer cell lines with higher metastatic potential. Cancer Lett. 2003;201:195–201. doi: 10.1016/s0304-3835(03)00443-9. [DOI] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neutzner A, Youle RJ. Instability of the mitofusin Fzo1 regulates mitochondrial morphology during the mating response of the yeast Saccharomyces cerevisiae. J Biol Chem. 2005;280:18598–18603. doi: 10.1074/jbc.M500807200. [DOI] [PubMed] [Google Scholar]
- Niefind K, Guerra B, Pinna LA, Issinger OG, Schomburg D. Crystal structure of the catalytic subunit of protein kinase CK2 from Zea mays at 2.1 A resolution. Embo J. 1998;17:2451–2462. doi: 10.1093/emboj/17.9.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen AL, Byrne AM, Herman B, Lemasters JJ. Mitochondrial permeability transition in hepatocytes induced by t-BuOOH: NAD(P)H and reactive oxygen species. Am J Physiol. 1997;272:C1286–1294. doi: 10.1152/ajpcell.1997.272.4.C1286. [DOI] [PubMed] [Google Scholar]
- Nolen B, Yun CY, Wong CF, McCammon JA, Fu XD, Ghosh G. The structure of Sky1p reveals a novel mechanism for constitutive activity. Nat Struct Biol. 2001;8:176–183. doi: 10.1038/84178. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Shaw JM. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet. 2005;39:503–536. doi: 10.1146/annurev.genet.38.072902.093019. [DOI] [PubMed] [Google Scholar]
- Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, Lenaers G. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem. 2003;278:7743–7746. doi: 10.1074/jbc.C200677200. [DOI] [PubMed] [Google Scholar]
- Olichon A, Emorine LJ, Descoins E, Pelloquin L, Brichese L, Gas N, Guillou E, Delettre C, Valette A, Hamel CP, Ducommun B, Lenaers G, Belenguer P. The human dynamin-related protein OPA1 is anchored to the mitochondrial inner membrane facing the inter-membrane space. FEBS Lett. 2002;523:171–176. doi: 10.1016/s0014-5793(02)02985-x. [DOI] [PubMed] [Google Scholar]
- Otsuga D, Keegan BR, Brisch E, Thatcher JW, Hermann GJ, Bleazard W, Shaw JM. The dynamin-related GTPase, Dnm1p, controls mitochondrial morphology in yeast. J Cell Biol. 1998;143:333–349. doi: 10.1083/jcb.143.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann Neurol. 1989;26:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- Patterson SD, Spahr CS, Daugas E, Susin SA, Irinopoulou T, Koehler C, Kroemer G. Mass spectrometric identification of proteins released from mitochondria undergoing permeability transition. Cell Death Differ. 2000;7:137–144. doi: 10.1038/sj.cdd.4400640. [DOI] [PubMed] [Google Scholar]
- Pesah Y, Pham T, Burgess H, Middlebrooks B, Verstreken P, Zhou Y, Harding M, Bellen H, Mardon G. Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development. 2004;131:2183–2194. doi: 10.1242/dev.01095. [DOI] [PubMed] [Google Scholar]
- Petit A, Kawarai T, Paitel E, Sanjo N, Maj M, Scheid M, Chen F, Gu Y, Hasegawa H, Salehi-Rad S, Wang L, Rogaeva E, Fraser P, Robinson B, St George-Hyslop P, Tandon A. Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson disease-related mutations. J Biol Chem. 2005;280:34025–34032. doi: 10.1074/jbc.M505143200. [DOI] [PubMed] [Google Scholar]
- Petronilli V, Miotto G, Canton M, Brini M, Colonna R, Bernardi P, Di Lisa F. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J. 1999;76:725–734. doi: 10.1016/S0006-3495(99)77239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli C, Sardanelli A, Scrima R, Ripoli M, Quarato G, D’Aprile A, Bellomo F, Scacco S, De Michele G, Filla A, Iuso A, Boffoli D, Capitanio N, Papa S. Mitochondrial Respiratory Dysfunction in Familiar Parkinsonism Associated with PINK1 Mutation. Neurochem Res. 2008 doi: 10.1007/s11064-008-9729-2. [DOI] [PubMed] [Google Scholar]
- Pickart CM, Fushman D. Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol. 2004;8:610–616. doi: 10.1016/j.cbpa.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Pletjushkina OY, Lyamzaev KG, Popova EN, Nepryakhina OK, Ivanova OY, Domnina LV, Chernyak BV, Skulachev VP. Effect of oxidative stress on dynamics of mitochondrial reticulum. Biochim Biophys Acta. 2006;1757:518–524. doi: 10.1016/j.bbabio.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Plun-Favreau H, Klupsch K, Moisoi N, Gandhi S, Kjaer S, Frith D, Harvey K, Deas E, Harvey RJ, McDonald N, Wood NW, Martins LM, Downward J. The mitochondrial protease HtrA2 is regulated by Parkinson’s disease-associated kinase PINK1. Nat Cell Biol. 2007;9:1243–1252. doi: 10.1038/ncb1644. [DOI] [PubMed] [Google Scholar]
- Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praefcke GJ, McMahon HT. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat Rev Mol Cell Biol. 2004;5:133–147. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 Protects against Oxidative Stress by Phosphorylating Mitochondrial Chaperone TRAP1. PLoS Biol. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana M, de Coo I, Diaz F, Smeets H, Moraes CT. An out-of-frame cytochrome b gene deletion from a patient with parkinsonism is associated with impaired complex III assembly and an increase in free radical production. Ann Neurol. 2000;48:774–781. [PubMed] [Google Scholar]
- Ren Y, Feng J. Rotenone selectively kills serotonergic neurons through a microtubule-dependent mechanism. J Neurochem. 2007;103:303–311. doi: 10.1111/j.1471-4159.2007.04741.x. [DOI] [PubMed] [Google Scholar]
- Ren Y, Zhao J, Feng J. Parkin binds to alpha/beta tubulin and increases their ubiquitination and degradation. J Neurosci. 2003;23:3316–3324. doi: 10.1523/JNEUROSCI.23-08-03316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricaurte GA, Langston JW, Delanney LE, Irwin I, Peroutka SJ, Forno LS. Fate of nigrostriatal neurons in young mature mice given 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: a neurochemical and morphological reassessment. Brain Res. 1986;376:117–124. doi: 10.1016/0006-8993(86)90905-4. [DOI] [PubMed] [Google Scholar]
- Rintoul GL, Filiano AJ, Brocard JB, Kress GJ, Reynolds IJ. Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J Neurosci. 2003;23:7881–7888. doi: 10.1523/JNEUROSCI.23-21-07881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riparbelli MG, Callaini G. The Drosophila parkin homologue is required for normal mitochondrial dynamics during spermiogenesis. Dev Biol. 2007;303:108–120. doi: 10.1016/j.ydbio.2006.10.038. [DOI] [PubMed] [Google Scholar]
- Rohe CF, Montagna P, Breedveld G, Cortelli P, Oostra BA, Bonifati V. Homozygous PINK1 C-terminus mutation causing early-onset parkinsonism. Ann Neurol. 2004;56:427–431. doi: 10.1002/ana.20247. [DOI] [PubMed] [Google Scholar]
- Rojo M, Legros F, Chateau D, Lombes A. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci. 2002;115:1663–1674. doi: 10.1242/jcs.115.8.1663. [DOI] [PubMed] [Google Scholar]
- Rossignol R, Gilkerson R, Aggeler R, Yamagata K, Remington SJ, Capaldi RA. Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res. 2004;64:985–993. doi: 10.1158/0008-5472.can-03-1101. [DOI] [PubMed] [Google Scholar]
- Roux A, Uyhazi K, Frost A, De Camilli P. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature. 2006;441:528–531. doi: 10.1038/nature04718. [DOI] [PubMed] [Google Scholar]
- Rube DA, van der Bliek AM. Mitochondrial morphology is dynamic and varied. Mol Cell Biochem. 2004;256–257:331–339. doi: 10.1023/b:mcbi.0000009879.01256.f6. [DOI] [PubMed] [Google Scholar]
- Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J Cell Sci. 2001;114:867–874. doi: 10.1242/jcs.114.5.867. [DOI] [PubMed] [Google Scholar]
- Satoh M, Hamamoto T, Seo N, Kagawa Y, Endo H. Differential sublocalization of the dynamin-related protein OPA1 isoforms in mitochondria. Biochem Biophys Res Commun. 2003;300:482–493. doi: 10.1016/s0006-291x(02)02874-7. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- Shimura-Miura H, Hattori N, Kang D, Miyako K, Nakabeppu Y, Mizuno Y. Increased 8-oxo-dGTPase in the mitochondria of substantia nigral neurons in Parkinson’s disease. Ann Neurol. 1999;46:920–924. [PubMed] [Google Scholar]
- Silvestri L, Caputo V, Bellacchio E, Atorino L, Dallapiccola B, Valente EM, Casari G. Mitochondrial import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Hum Mol Genet. 2005;14:3477–3492. doi: 10.1093/hmg/ddi377. [DOI] [PubMed] [Google Scholar]
- Sim CH, Lio DS, Mok SS, Masters CL, Hill AF, Culvenor JG, Cheng HC. C-terminal truncation and Parkinson’s disease-associated mutations down-regulate the protein serine/threonine kinase activity of PTEN-induced kinase-1. Hum Mol Genet. 2006;15:3251–3262. doi: 10.1093/hmg/ddl398. [DOI] [PubMed] [Google Scholar]
- Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofic E, Lange KW, Jellinger K, Riederer P. Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson’s disease. Neurosci Lett. 1992;142:128–130. doi: 10.1016/0304-3940(92)90355-b. [DOI] [PubMed] [Google Scholar]
- Song Z, Chen H, Fiket M, Alexander C, Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol. 2007;178:749–755. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovski D, Koutsopoulos OS, Okamoto K, Ryan MT. Levels of human Fis1 at the mitochondrial outer membrane regulate mitochondrial morphology. J Cell Sci. 2004;117:1201–1210. doi: 10.1242/jcs.01058. [DOI] [PubMed] [Google Scholar]
- Stowers RS, Megeath LJ, Gorska-Andrzejak J, Meinertzhagen IA, Schwarz TL. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36:1063–1077. doi: 10.1016/s0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- Strauss KM, Martins LM, Plun-Favreau H, Marx FP, Kautzmann S, Berg D, Gasser T, Wszolek Z, Muller T, Bornemann A, Wolburg H, Downward J, Riess O, Schulz JB, Kruger R. Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson’s disease. Hum Mol Genet. 2005;14:2099–2111. doi: 10.1093/hmg/ddi215. [DOI] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Brenner C, Larochette N, Prevost MC, Alzari PM, Kroemer G. Mitochondrial release of caspase-2 and -9 during the apoptotic process. J Exp Med. 1999;189:381–394. doi: 10.1084/jem.189.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Jeong SY, Karbowski M, Youle RJ, Tjandra N. The solution structure of human mitochondria fission protein Fis1 reveals a novel TPR-like helix bundle. J Mol Biol. 2003;334:445–458. doi: 10.1016/j.jmb.2003.09.064. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Davis JN, 2, Cassarino DS, Trimmer PA, Currie LJ, Dougherty J, Bridges WS, Bennett JP, Jr, Wooten GF, Parker WD. Matrilineal inheritance of complex I dysfunction in a multigenerational Parkinson’s disease family. Ann Neurol. 1998;44:873–881. doi: 10.1002/ana.410440605. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Miller SW, Tuttle JB, Trimmer PA, Sheehan JP, Bennett JP, Jr, Davis RE, Parker WD., Jr Origin and functional consequences of the complex I defect in Parkinson’s disease. Ann Neurol. 1996;40:663–671. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- Tai HC, Schuman EM. Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nat Rev Neurosci. 2008;9:826–838. doi: 10.1038/nrn2499. [DOI] [PubMed] [Google Scholar]
- Takatori S, Ito G, Iwatsubo T. Cytoplasmic localization and proteasomal degradation of N-terminally cleaved form of PINK1. Neurosci Lett. 2008;430:13–17. doi: 10.1016/j.neulet.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Tan EK, Khajavi M, Thornby JI, Nagamitsu S, Jankovic J, Ashizawa T. Variability and validity of polymorphism association studies in Parkinson’s disease. Neurology. 2000;55:533–538. doi: 10.1212/wnl.55.4.533. [DOI] [PubMed] [Google Scholar]
- Tan EK, Yew K, Chua E, Puvan K, Shen H, Lee E, Puong KY, Zhao Y, Pavanni R, Wong MC, Jamora D, de Silva D, Moe KT, Woon FP, Yuen Y, Tan L. PINK1 mutations in sporadic early-onset Parkinson’s disease. Mov Disord. 2006;21:789–793. doi: 10.1002/mds.20810. [DOI] [PubMed] [Google Scholar]
- Tan EK, Yew K, Chua E, Shen H, Jamora RD, Lee E, Puong KY, Zhao Y, Pavanni R, Wong MC, Puvan K, Yih Y, Tan LC. Analysis of PINK1 in Asian patients with familial parkinsonism. Clin Genet. 2005;68:468–470. doi: 10.1111/j.1399-0004.2005.00500.x. [DOI] [PubMed] [Google Scholar]
- Tang B, Xiong H, Sun P, Zhang Y, Wang D, Hu Z, Zhu Z, Ma H, Pan Q, Xia JH, Xia K, Zhang Z. Association of PINK1 and DJ-1 confers digenic inheritance of early-onset Parkinson’s disease. Hum Mol Genet. 2006;15:1816–1825. doi: 10.1093/hmg/ddl104. [DOI] [PubMed] [Google Scholar]
- Taymans JM, Van den Haute C, Baekelandt V. Distribution of PINK1 and LRRK2 in rat and mouse brain. J Neurochem. 2006;98:951–961. doi: 10.1111/j.1471-4159.2006.03919.x. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis. 2007;12:835–840. doi: 10.1007/s10495-006-0525-7. [DOI] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. Embo J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JW, Chung KC. Functional modulation of parkin through physical interaction with SUMO-1. J Neurosci Res. 2006;84:1543–1554. doi: 10.1002/jnr.21041. [DOI] [PubMed] [Google Scholar]
- Unoki M, Nakamura Y. Growth-suppressive effects of BPOZ and EGR2, two genes involved in the PTEN signaling pathway. Oncogene. 2001;20:4457–4465. doi: 10.1038/sj.onc.1204608. [DOI] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004a;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Valente EM, Salvi S, Ialongo T, Marongiu R, Elia AE, Caputo V, Romito L, Albanese A, Dallapiccola B, Bentivoglio AR. PINK1 mutations are associated with sporadic early-onset parkinsonism. Ann Neurol. 2004b;56:336–341. doi: 10.1002/ana.20256. [DOI] [PubMed] [Google Scholar]
- Varadi A, Johnson-Cadwell LI, Cirulli V, Yoon Y, Allan VJ, Rutter GA. Cytoplasmic dynein regulates the subcellular distribution of mitochondria by controlling the recruitment of the fission factor dynamin-related protein-1. J Cell Sci. 2004;117:4389–4400. doi: 10.1242/jcs.01299. [DOI] [PubMed] [Google Scholar]
- Wallace DC. Mitochondrial DNA in aging and disease. Sci Am. 1997;277:40–47. doi: 10.1038/scientificamerican0897-40. [DOI] [PubMed] [Google Scholar]
- Wallace DC, Shoffner JM, Watts RL, Juncos JL, Torroni A. Mitochondrial oxidative phosphorylation defects in Parkinson’s disease. Ann Neurol. 1992;32:113–114. doi: 10.1002/ana.410320123. [DOI] [PubMed] [Google Scholar]
- Wang D, Qian L, Xiong H, Liu J, Neckameyer WS, Oldham S, Xia K, Wang J, Bodmer R, Zhang Z. Antioxidants protect PINK1-dependent dopaminergic neurons in Drosophila. Proc Natl Acad Sci U S A. 2006;103:13520–13525. doi: 10.1073/pnas.0604661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Chou AH, Yeh TH, Li AH, Chen YL, Kuo YL, Tsai SR, Yu ST. PINK1 mutants associated with recessive Parkinson’s disease are defective in inhibiting mitochondrial release of cytochrome c. Neurobiol Dis. 2007;28:216–226. doi: 10.1016/j.nbd.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Wasiak S, Zunino R, McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol. 2007;177:439–450. doi: 10.1083/jcb.200610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihofen A, Ostaszewski B, Minami Y, Selkoe DJ. Pink1 Parkinson mutations, the Cdc37/Hsp90 chaperones and Parkin all influence the maturation or subcellular distribution of Pink1. Hum Mol Genet. 2008;17:602–616. doi: 10.1093/hmg/ddm334. [DOI] [PubMed] [Google Scholar]
- Weihofen A, Thomas KJ, Ostaszewski BL, Cookson MR, Selkoe DJ. Pink1 Forms a Multiprotein Complex with Miro and Milton, Linking Pink1 Function to Mitochondrial Trafficking (dagger) Biochemistry. 2009 doi: 10.1021/bi8019178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann B. Molecular machinery of mitochondrial fusion and fission. J Biol Chem. 2008;283:13501–13505. doi: 10.1074/jbc.R800011200. [DOI] [PubMed] [Google Scholar]
- Wood-Kaczmar A, Gandhi S, Yao Z, Abramov AS, Miljan EA, Keen G, Stanyer L, Hargreaves I, Klupsch K, Deas E, Downward J, Mansfield L, Jat P, Taylor J, Heales S, Duchen MR, Latchman D, Tabrizi SJ, Wood NW. PINK1 is necessary for long term survival and mitochondrial function in human dopaminergic neurons. PLoS ONE. 2008;3:e2455. doi: 10.1371/journal.pone.0002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe MP, Harata D, Verde F, Eddison M, Toda T, Nurse P. Microtubules mediate mitochondrial distribution in fission yeast. Proc Natl Acad Sci U S A. 1996;93:11664–11668. doi: 10.1073/pnas.93.21.11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Jiang Q, Zhao J, Ren Y, Sutton MD, Feng J. Parkin stabilizes microtubules through strong binding mediated by three independent domains. J Biol Chem. 2005;280:17154–17162. doi: 10.1074/jbc.M500843200. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gehrke S, Imai Y, Huang Z, Ouyang Y, Wang JW, Yang L, Beal MF, Vogel H, Lu B. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci U S A. 2006;103:10793–10798. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, Vogel H, Lu B. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci U S A. 2008;105:7070–7075. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarosh W, Monserrate J, Tong JJ, Tse S, Le PK, Nguyen K, Brachmann CB, Wallace DC, Huang T. The molecular mechanisms of OPA1-mediated optic atrophy in Drosophila model and prospects for antioxidant treatment. PLoS Genet. 2008;4:e6. doi: 10.1371/journal.pgen.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol. 2003;23:5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci U S A. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim MB, Ben-Shachar D, Riederer P. Is Parkinson’s disease a progressive siderosis of substantia nigra resulting in iron and melanin induced neurodegeneration? Acta Neurol Scand Suppl. 1989;126:47–54. doi: 10.1111/j.1600-0404.1989.tb01782.x. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol. 2005;6:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- Yu T, Fox RJ, Burwell LS, Yoon Y. Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hFis1. J Cell Sci. 2005;118:4141–4151. doi: 10.1242/jcs.02537. [DOI] [PubMed] [Google Scholar]
- Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadikoff C, Rogaeva E, Djarmati A, Sato C, Salehi-Rad S, St George-Hyslop P, Klein C, Lang AE. Homozygous and heterozygous PINK1 mutations: considerations for diagnosis and care of Parkinson’s disease patients. Mov Disord. 2006;21:875–879. doi: 10.1002/mds.20854. [DOI] [PubMed] [Google Scholar]
- Zhou C, Huang Y, Shao Y, May J, Prou D, Perier C, Dauer W, Schon EA, Przedborski S. The kinase domain of mitochondrial PINK1 faces the cytoplasm. Proc Natl Acad Sci U S A. 2008;105:12022–12027. doi: 10.1073/pnas.0802814105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu PP, Patterson A, Stadler J, Seeburg DP, Sheng M, Blackstone C. Intra- and intermolecular domain interactions of the C-terminal GTPase effector domain of the multimeric dynamin-like GTPase Drp1. J Biol Chem. 2004;279:35967–35974. doi: 10.1074/jbc.M404105200. [DOI] [PubMed] [Google Scholar]
- Zunino R, Schauss A, Rippstein P, Andrade-Navarro M, McBride HM. The SUMO protease SENP5 is required to maintain mitochondrial morphology and function. J Cell Sci. 2007;120:1178–1188. doi: 10.1242/jcs.03418. [DOI] [PubMed] [Google Scholar]