Abstract
The heterotrimeric guanine nucleotide-binding protein Gαi2 is involved in regulation of immune responses against microbial and nonmicrobial stimuli. Gαi2−/− mice have a selectively impaired IgM response consistent with a disorder in B cell development yet have augmented T cell effector function associated with increased production of IFN-γ and IL-4. The goal of the present study was to determine if a deficiency in the Gαi2 protein in mice would affect the protective immune response against Strongyloides stercoralis, which is IL-4-, IL-5-, and IgM-dependent. Gαi2−/− and wild-type mice were immunized and challenged with S. stercoralis larvae and analyzed for protective immune responses against infection. Gαi2−/− mice failed to kill the larvae in the challenge infection as compared with wild-type mice, despite developing an antigen-specific Th2 response, characterized by increased IL-4, IL-5, IgM, and IgG. Transfer of serum collected from immunized Gαi2−/− mice to naïve, wild-type mice conferred passive, protective immunity against S. stercoralis infection, thus confirming the development of a protective antibody response in Gαi2−/− mice. Differential cell analyses and myeloperoxidase assays for quantification of neutrophils showed a significantly reduced recruitment of neutrophils into the microenvironment of the parasites in immunized Gαi2−/− mice. However, cell transfer studies demonstrated that neutrophils from Gαi2−/− mice are competent in killing larvae. These data demonstrate that Gαi2 signaling events are not required for the development of the protective immune responses against S. stercoralis; however, Gαi2 is essential for the recruitment of neutrophils required for host-dependent killing of larvae.
Keywords: helminthes, parasite, infection, heterotrimeric, cytokine
INTRODUCTION
Heterotrimeric guanine nucleotide-binding proteins (G proteins) are transducer stimuli from various extracellular agents (e.g., chemokines, hormones, and neurotransmitters). In return, these signal transduction events regulate many intracellular functions ranging from transcription, motility, cell activation, and tissue-specific recruitment. G proteins, a complex of three subunits termed α, β, and γ, are classified into four families: Gi, Gs, Gq, and G12/13, which receive extracellular signals through seven transmembrane domain receptors. Several studies have demonstrated the crucial role of Gi proteins in the host immune system, where signaling through these proteins regulates leukocyte chemotaxis and immune responses [1–4]. The Gαi proteins of the Gi family include Gαi1, Gαi2, and Gαi3 and are sensitive to pertussis toxin (PTX). The Gαi2 and Gαi3 proteins are expressed by mouse immune cells, whereas Gαi1 is restricted primarily to neuronal tissue [5].
Although Gαi3−/− mice show no signs of any detectable disease [2], Gαi2−/− mice have been reported to contain a discrete and profound mucosal disorder, inflammatory bowel disease, attenuation of IL-10 expression, and immune function polarized to Th1 activity [6–8]. Mice deficient in Gαi2 protein show enhanced IL-12 production [1, 9] and produce greater amounts of IFN-γ [10, 11]. Further, it has been shown that Gαi2−/− mice have a selectively impaired IgM response, consistent with the relative depletion of the marginal zone (MZ) and peritoneal B-1a B cell subpopulations, and a significant increase in follicular mature and B-1b B cells [8]. In contrast to this, several studies using PTX as an adjuvant have shown that PTX treatment at the time of immunization with microbial and nonmicrobial stimuli enhances Th1 and Th2 responses [4, 12, 13] and augments immune-mediated pathology [14, 15]. Furthermore, in an in vitro study, it has been demonstrated that splenocytes isolated from Gαi2−/− mice are hyper-responsive for IFN-γ and IL-4 production following activation through TCR, thereby suggesting that Gαi2 controls Th1- and Th2-type cytokine production [2].
Protective immunity to Strongyloides stercoralis is dependent on several immune components, which include eosinophils in the primary response [16], B-1a B cells for IgM antibody production [17, 18], CD4+ Th2 cells and their cytokines IL-4 and IL-5 [16, 19], complement component C3 in the primary and adaptive immune responses [20], and neutrophils as effector cells in the primary and adaptive immune responses [21]. Neutrophil function was tested in mice deficient in CXCR2, which is a Gαi2 receptor [22]. CXCR2−/− mice have a defect in neutrophil recruitment and thus, a deficiency in the protective innate and adaptive immune response to larval S. stercoralis in mice [21]. Further study has shown that TLR4, which is linked to activation of Gαi-coupled signaling pathways [23], is required for activation of neutrophils [24]. Mice deficient in TLR4 fail to eliminate S. stercoralis infection, despite developing T and B cell immune responses and recruiting neutrophils to the parasite microenvironment [24].
The present study used S. stercoralis infection of mice as a model to investigate the role of Gαi2 protein signaling in the host defense mechanism against helminth infections. It was hypothesized that the absence of Gαi2 protein in mice, which is required for B-1a B cell development and regulates Th1 and Th2 activity, would prevent the development of the adaptive immune response against S. stercoralis and diminish the host-mediated killing of larvae. Gαi2−/− mice were used to evaluate the role of Gαi2 protein signaling in protective adaptive immune responses against S. stercoralis infection. The present study demonstrates that Gαi2 signaling events are not required for the development of T and B cell responses during S. stercoralis infection; however, these signaling events are necessary for the recruitment of effector neutrophils required for host-mediated killing of larvae.
MATERIALS AND METHODS
Experimental animals and parasites
129 SvJ mice used in experiments were obtained from the Jackson Laboratory (Bar Harbor, ME, USA), and Gαi2−/−mice (background strain 129), generated by homologous recombination in embryonic stem cells [6], were bred at the Mayo Clinic, Scottsdale Laboratory Animal Sciences facility (Scottsdale, AZ, USA). Male mice, 7–14 weeks of age, were used in all experiments. All mice were housed at the Thomas Jefferson University Laboratory Animal Sciences facility (Philadelphia, PA, USA) in microisolator boxes under temperature- and light-controlled conditions and were allowed food and water ad libitum.
Soluble larval antigens from S. stercoralis larvae (L3) were obtained from the fresh stools of a laboratory dog infected with the parasite, according to methods described previously [25]. Larvae were collected from charcoal cultures and washed by centrifugation and resuspension in a 1:1 mixture of IMDM (Sigma Chemical Co., St. Louis, MO, USA) and NCTC-135 (Sigma Chemical Co.) with a mixture of 100 U penicillin and 100 µg streptomycin per ml (Gibco, Grand Island, NY, USA) and 25 µg levofloxacin per ml (Ortho-McNeil Pharmaceutical, Raritan, NJ, USA).
Antigen preparation
L3 were prepared as described previously [26]. Briefly, L3 were washed in PBS supplemented with 100 U penicillin and 100 µg streptomycin per ml and stored at −80°C. L3 were thawed and homogenized in the presence of a protease inhibitor cocktail (Sigma Chemical Co.) and then sonicated. The homogenized and sonicated L3 were incubated in PBS at 4°C for 18 h with continuous mixing. PBS-soluble antigens were removed, filter-sterilized, and stored at −80°C. PBS-insoluble proteins were resuspended in 20 mM Tris-HCl, 0.5% deoxycholic acid (DOC; Sigma Chemical Co.), with continuous mixing for 12 h at 4°C. The DOC-soluble antigens were then dialyzed (6–8 kDa cutoff) against PBS for 12 h, concentrated, filter-sterilized, and stored at −80°C. Protein concentration was quantified by a Micro bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA). As no difference was observed in ability of these antigens to induce T cells, DOC- or PBS-soluble antigens were used in the present study.
Immunization and challenge infections
Gαi2−/− and wild-type mice were immunized with live S. stercoralis L3 as reported earlier [25]. The immunization protocol consisted of two s.c. injections with 5000 live L3 administered 2 weeks apart. One week after the second injection, mice were challenged with 50 S. stercoralis L3 contained within diffusion chambers, which were implanted s.c. on the dorsal flank of the mice. Diffusion chambers were constructed with 0.1 or 2.0 µm pore-size Isopore membranes (Millipore, Bedford, MA, USA) and were sterilized by exposure to 100% ethylene oxide and aerated for 12 h. Implanted diffusion chambers were removed from the mice after 24 h, and larval survival was determined based on motility and morphology. Cells recovered from the diffusion chambers were quantitated, centrifuged onto slides in a Cytospin 3 apparatus (Shandon, Pittsburgh, PA, USA), and stained for differential counts with a Hema 3 stain set (Fisher Diagnostics, Middletown, VA, USA).
Spleen cell stimulation and cytokine analysis
Spleens from control and immunized Gαi2−/− and wild-type mice were removed aseptically 1 week after challenge and made into single cell suspensions. Cells, cultured in a 96-well plate at 2 × 106/well, were restimulated with S. stercoralis antigens or soluble anti-CD3 mAb (BD PharMingen, San Diego, CA, USA) in the presence of anti-IL-4 receptor α (anti-IL-4Rα) mAb (BD PharMingen) by incubating at 37°C for 3 days, and supernatants were collected and frozen at −20°C. Culture supernatants were analyzed for IL-5 and IL-4 production by sandwich ELISA using mAb TRFK.5 and TRFK.4 for measuring IL-5 and BVD6-24G2 and BVD4-1D11 for measuring IL-4 (BD PharMingen). Incubation with extravidin peroxidase (Sigma Chemical Co.) was followed by the 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid; ABTS) peroxidase substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD, USA) for color reaction, which was measured at 405 nm. IFN-γ production by spleen cells was measured using an ELISA kit (AN-18 monoclonal, BD PharMingen).
Antibody ELISA
S. stercoralis-specific antibody titers were determined by ELISA, based on a methods reported previously [26]. Briefly, maxisorp 96-well plates (Nunc Inc., Naperville, IL, USA) were coated with 50 µl S. stercoralis antigens at 10 µg/ml in PBS overnight at 4°C. The plates were washed and blocked with borate-blocking buffer (BBS). Serum samples were diluted in BBS and placed in duplicate wells at serial dilutions and incubated at 37°C for 2 h. Appropriately matched, biotinylated goat antimouse IgM (Vector Laboratories, Burlingame, CA, USA), IgG, and IgG2a (BD PharMingen) antibodies were added, and plates were incubated at 37°C for 2 h. Plates were washed, and extravidin peroxidase was added for 30 min at room temperature, followed by the peroxidase substrate ABTS; color reaction was measured at 405 nm.
Passive transfer of immunity
Serum from immunized and control Gαi2−/− and wild-type mice were pooled by the group and injected s.c. into naïve, wild-type mice at the time of challenge infection in the pocket surrounding the diffusion chamber. Each serum recipient mouse was given 100 µl serum, brought up to 200 µl with PBS. Twenty-four hours after implantation, diffusion chambers were removed from the mice, and larval survival was determined.
Myeloperoxidase (MPO) assay
ELISA was performed to measure the amount of MPO released by activated neutrophils as an alternative method for quantifying neutrophil infiltration and activity in the diffusion chamber during challenge infection. A commercially available mouse MPO ELISA kit was used for these assays (HyCult Biotechnology, The Netherlands).
Neutrophil isolation and cell transfer to diffusion chambers
Mouse bone marrow neutrophils were isolated from femurs and tibias as described previously [27] with minor modifications. Marrow cells were flushed from the bones using PBS (without Ca2+/Mg2+) containing 2% FBS, RBCs were lysed, and remaining leukocytes were washed twice and then resuspended in 3 ml 45% Percoll (Sigma Chemical Co.) solution in 1× Ca2+/Mg2+-free HBSS [1×HBSS, 15 mM HEPES (pH 7.4), and 0.003 N HCl]. The leukocytes were then loaded on top of a Percoll density gradient prepared in a 15-ml tube by layering successively 2 ml each 62%, 55%, and 50% Percoll solutions on top of 3 ml 81% Percoll solution. Cells were then centrifuged at 1200 g for 30 min at room temperature in a swinging bucket rotor. The cell band formed between the 81% and 62% layer was harvested, diluted into fresh PBS containing 2% FBS, washed, and resuspended in 5 ml. Neutrophils were purified further using MACS columns (Miltenyi Biotec, Auburn, CA, USA). Specifically, B cells (B220) and T cells (Thy 1.2) were removed by positive selection with antibody-conjugated magnetic beads. The total cell number was quantified using a hemacytometer. Purity, assessed by cytospin, was 93–95% neutrophils, along with eosinophils (1–2%), macrophages (2–4%), and lymphocytes (1–2%) as contaminating cells.
Two million purified neutrophils isolated from Gαi2−/− or wild-type mice were placed into diffusion chambers with 50 L3. Diffusion chambers used for these experiments were constructed with 0.1 µm pore-size membranes to prevent resident cells from entering and experimental cells from exiting the diffusion chamber, which with neutrophils and larvae were implanted in naïve and immunized, wild-type mice. After 24 h, the diffusion chambers were removed from the mice, and larval survival was determined.
Statistical analysis
Statistical analysis of the data was performed using multivariate, general, linear hypothesis, multifactorial ANOVA with Systat Version 11 software (Systat, Evanston, IL, USA). Fisher’s least-significant difference test was performed for post hoc analyses. Probability values of P < 0.05 were considered significant.
RESULTS
Role of Gαi2 protein signaling during adaptive immune response against S. stercoralis in mice
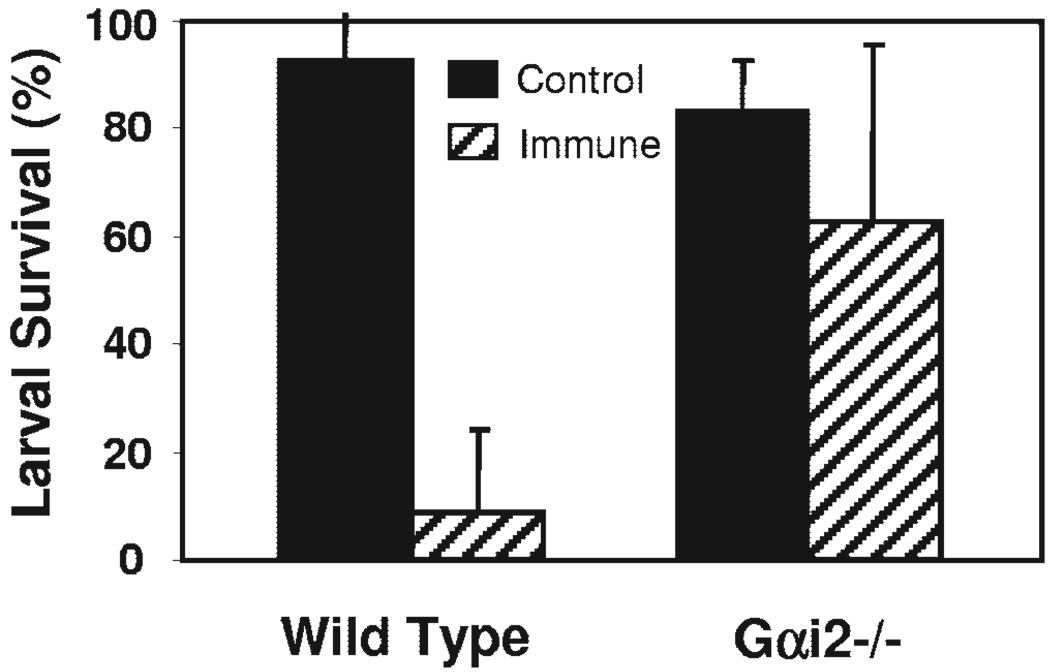
Gαi2−/− mice and wild-type mice were immunized and then challenged with larvae to determine whether signaling through Gαi2 protein plays a role in the adaptive immune response against infection with S. stercoralis. Mice challenged without any prior exposure to S. stercoralis served as controls. Immunized Gαi2−/− mice had decreased larval killing significantly (37%) as compared with immunized, wild-type mice (91%; Fig. 1). These results suggest that Gαi2 protein signaling is vital for control of S. stercoralis infection in the adaptive immune response.
Fig. 1.
Gαi2 protein signaling is required for control S. stercoralis infection. Immunized and naive Gαi2−/− and wild-type mice were challenged with 50 live S. stercoralis larvae in a diffusion chamber, and larval survival was assessed after 24 h. Error bars represent the mean ± SD of four to five mice per group and are representative of results of two separate experiments. *, Statistically significant different results between control and immunized mice at P < 0.05.
Experiments were performed to analyze the immune responses in immunized, wild-type and Gαi2−/− mice to determine the impact that the absence of the Gαi2 protein had on the development of the adaptive immune response. Spleen cells from immunized Gαi2−/− or wild-type mice were cultured in the presence of S. stercoralis antigens or anti-CD3 antibody, and supernatants were screened for IL-4, IL-5, and IFN-γ levels. Cells from immunized Gαi2−/− mice produced significantly higher levels of IL-4 (Fig. 2, A and B) and IL-5 (Fig. 2, C and D) after stimulation, as compared with cells from wild-type mice. The level of IFN-γ produced by cells without antigen stimulation or in the presence of anti-CD3 antibody was similar for control and immunized Gαi2−/− and wild-type mice, except from immunized, wild-type control mice, which had reduced IFN-γ production significantly (Fig. 2F). Addition of parasite antigens to the cell cultures ablated IFN-γ production by cells from all groups of mice (Fig. 2E).
Fig. 2.
Absence of Gαi2 protein does not impair development of the Th2 response. Spleen cells from control and immunized Gαi2−/− or wild-type mice were cultured in the presence of S. stercoralis antigens or anti-CD3 antibody (Ab), and supernatants were screened for IL-4 (A and B), IL-5 (C and D), and IFN-γ (E and F) production by ELISA. Error bars represent the mean ± SD of four to five mice per group and are representative of results of two separate experiments. *, Statistically significant different results between control and immunized mice at P < 0.05. **, Statistically significant different results between immunized, wild-type mice and Gαi2−/− mice at P < 0.05.
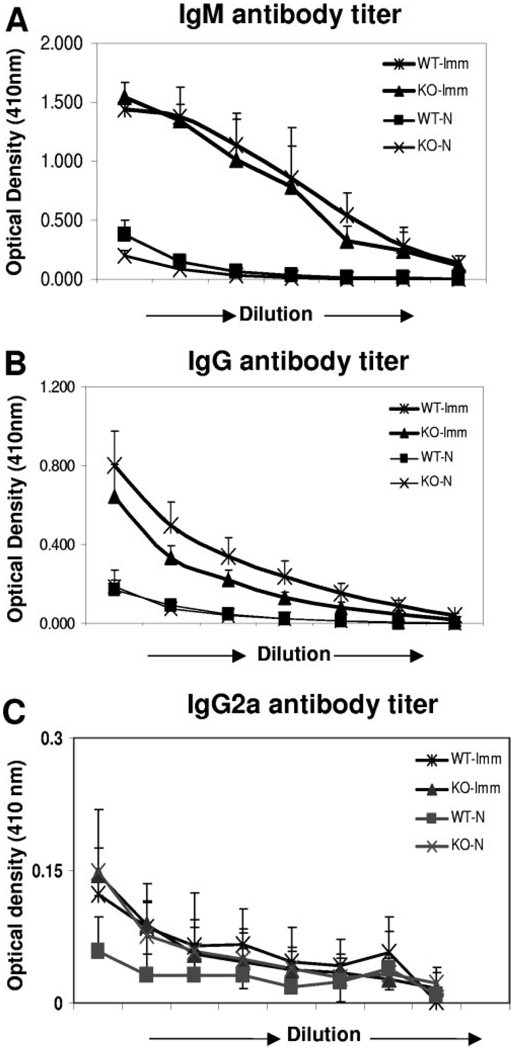
The IgM and IgG antigen-specific antibody levels in serum from immunized Gαi2−/− and wild-type mice were comparable and significantly higher as compared with control values (Fig. 3, A and B). The IgG2a antibody levels were similar in all the groups of mice (Fig. 3C).
Fig. 3.
Protective antigen-specific antibody response is unaffected in Gαi2−/− mice. Serum from control and immunized Gαi2−/− or wild-type mice (WT) were collected after challenge infection, and antigen-specific IgM (A), IgG (B), and IgG2a (C) antibody response was measured by ELISA. x-Axis shows the serial dilutions (1:2) of serum starting at 1:50. Error bars represent the mean ± SD of four to five mice per group and are representative of results of two separate experiments. KO, Knockout mice.
Serum from immunized Gαi2−/− mice conferred passive, protective immunity against S. stercoralis infection
To determine whether the antibody response generated following immunization of Gαi2−/− mice was sufficient to protect naive mice against challenge infection, serum transfer experiments were performed. Diffusion chambers containing larvae were implanted in naïve, wild-type mice and simultaneously injected s.c. with serum from immunized or control Gαi2−/− and wild-type mice. Serum from immunized Gαi2−/− and wild-type mice was able to transfer immunity to naïve, wild-type animals passively, resulting in significantly more killing of larvae as compared with mice receiving control sera (Fig. 4). These results demonstrate that Gαi2−/− mice developed normal, protective antibody responses against S. stercoralis.
Fig. 4.
Serum from immunized Gαi2−/− mice conferred passive, protective immunity against S. stercoralis infection. Naïve, wild-type mice were challenged with 50 live S. stercoralis larvae in diffusion chambers and injected simultaneously with serum from immunized or control Gαi2−/− and wild-type mice. Passive, protective immunity against S. stercoralis was determined after 24 h by assessing larval survival. Error bars represent the mean ± SD of four to five mice per group. *, Statistically significant different results between control and immunized mice at P < 0.05.
Gαi2−/− mice have reduced recruitment of neutrophils in their parasite microenvironment
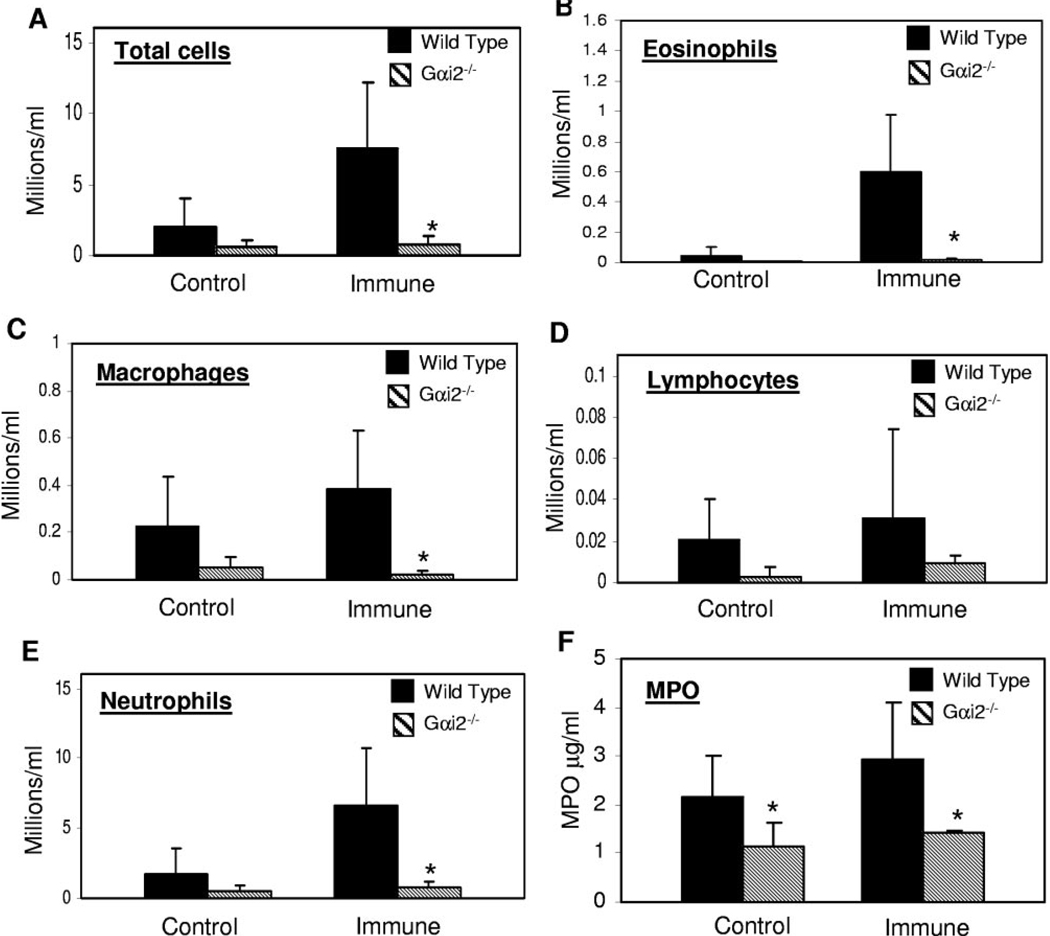
The total numbers of cells that migrated into the microenvironment of larvae were quantified, and differential cell analysis was performed to determine if there was a difference between wild-type and Gαi2−/− mice. Immunized Gαi2−/− mice had a significantly lower number of total cells recruited into the diffusion chamber (Fig. 5A). Differential cell analysis showed that reduced numbers of eosinophils, macrophages, lymphocytes, and neutrophils (Fig. 5, B–E) were recruited to the microenvironment of larvae in Gαi2−/− mice. As killing of S. stercoralis requires neutrophils during the adaptive immune response [21], fluid collected from the diffusion chambers was examined for the presence of MPO, released by activated neutrophils, for quantification of neutrophil infiltration and activity in the diffusion chambers. Fluids from diffusion chambers implanted in control or immunized Gαi2−/− mice had significantly lower levels of MPO as compared with fluid recovered from wild-type mice (Fig. 5F).
Fig. 5.
Gαi2−/− mice have reduced recruitment of cells in a parasite microenvironment. Fluid from diffusion chambers was collected and analyzed for cell recruitment and MPO production by activated neutrophils. (A) Recruitment of total cells; (B–E) recruitment of eosinophils, macrophages, lymphocytes, and neutrophils; (F) amount of MPO measured in the diffusion chamber. Error bars represent the mean ± SD of four to five mice per group and are representative of results of two separate experiments. *, Statistically significant different results between wild-type and Gαi2−/− mice at P < 0.05.
Deficiency in Gαi2 protein does not affect neutrophil function
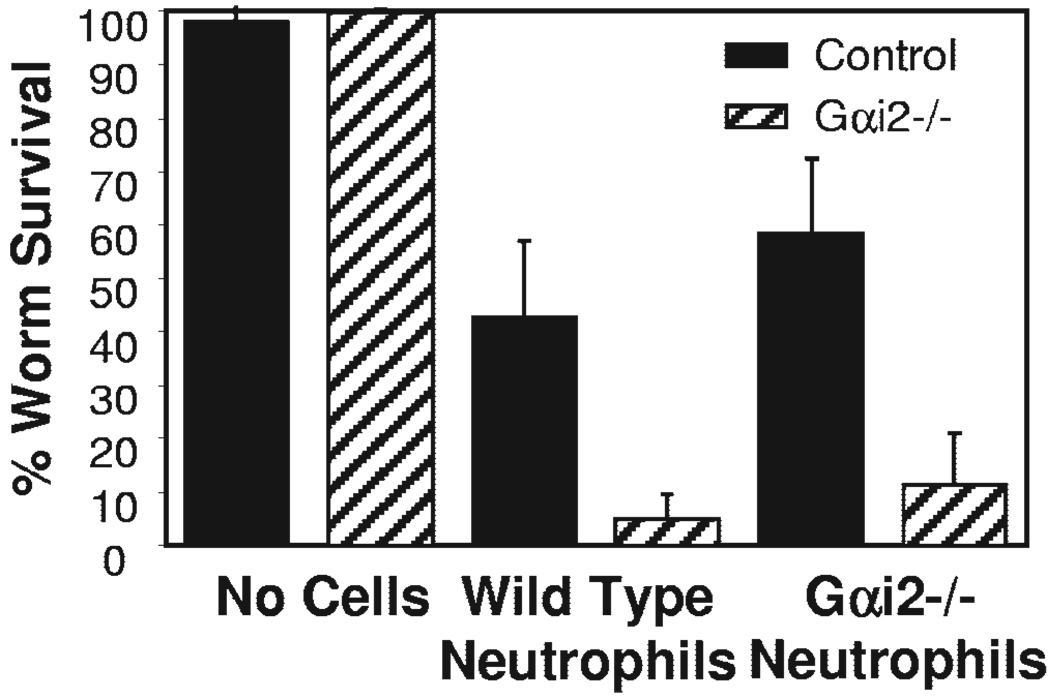
To determine if the absence of Gαi2 protein affects the ability of neutrophils to kill the worms, purified neutrophils from naïve, wild-type or Gαi2−/− mice were placed with larvae in 0.1 µm pore-size, membrane-covered diffusion chambers and implanted in immunized wild-type. The 0.1-µm pore-size membrane inhibited the influx/efflux of cells from the diffusion chamber, thereby allowing direct analysis of the ability of a specific cell population in mediating larval killing. Neutrophils from Gαi2−/− mice implanted in immunized, wild-type mice killed larvae as efficiently as neutrophils from wild-type mice, thereby demonstrating that Gαi2 protein does not regulate the ability of neutrophils to kill and eliminate the worms (Fig. 6).
Fig. 6.
Gαi2 protein does not affect neutrophil function. Purified neutrophils (2×106) isolated from Gαi2−/− mice or wild-type mice were placed into diffusion chambers (covered with 0.1 µm pore-size membranes) with 50 L3. Neutrophil function was determined after 24 h by assessing larval survival in the diffusion chamber. *, Statistically significant different results between wild-type and Gαi2−/− mice at P < 0.05. PMN, Polymorphonuclear neutrophils.
DISCUSSION
The objective of this study was to determine if the absence of Gαi2 protein signaling would affect adaptive, protective immunity to larval S. stercoralis. Signaling through the Gαi2 protein was shown to be essential for host-mediated killing of S. stercoralis larvae. Immunized Gαi2−/− mice failed to kill the larvae in the challenge infections, as compared with immunized, wild-type mice. The failure of Gαi2−/− mice to kill the larvae was not associated with impaired T cell- and B cell-mediated immune responses. Splenocytes isolated from immunized Gαi2−/− mice showed increased, antigen-specific Th2 responses, characterized by significantly elevated IL-4 and IL-5 production as compared with wild-type mice. Similar observations have been made in other studies using microbial and nonmicrobial antigens, showing that treatment with PTX promotes Th2 immune responses [4, 12, 13]. The hyper-responsive cytokine profile in Gαi2−/− mice involves an intrinsic T cell abnormality, which may cause the augmented T cell effector function. Purified, naïve T cells isolated from Gαi2−/− produce more IL-2 than naïve, wild-type T cells following TCR activation [2]. IFN-γ levels produced by unstimulated spleen cells were reduced significantly in immunized, wild-type mice as compared with immunized Gαi2−/− mice. This confirms reports that there is Th1 dysregulation in Gαi2−/− mice [1]. However, addition of S. stercoralis antigens to the cell cultures inhibited production of IFN-γ completely by cells from naïve and immune mice recovered from wild-type or Gαi2−/−mice. Stimulation through the TCR using anti-CD3 mAb did not reduce the IFN-γ response. Helminth antigens have been shown to have the capacity to suppress Th1 responses [28, 29]. It is possible that mice initially develop a mixed Th1/Th2 response against infection with S. stercoralis, as has been reported for Schistosoma mansoni and Brugia malayi [30, 31]. The mixed response is replaced by a Th2 response after repeated stimulation with the S. stercoralis antigens.
B-1a B cells and IgM have been shown to be required for adaptive, protective immunity to S. stercoralis in mice [17, 32]. Levels of antigen-specific IgM and IgG antibodies in immunized Gαi2−/− mice were similar to that observed in wild-type mice and were significantly higher compared with their control groups. The level of IgG2a antibody response, which reflects Th1 immunity, was not elevated in immunized Gαi2−/− or wild-type mice. These findings are in conflict with previous studies, which have shown that mice deficient in Gαi2 protein have increased IgM and IgG antibody levels [7, 33] or impaired IgM responses, consistent with the relative depletion of MZ and peritoneal B-1a B cells in these mice [8]. Furthermore, serum transfer experiments demonstrated that serum isolated from immunized Gαi2−/− or wild-type mice could transfer passive immunity equally to naïve, wild-type mice. Therefore, the quantity and quality of the antibody response in wild-type and Gαi2−/− mice did not differ.
Based on the observation that T and B cell responses in the Gαi2−/− mice were intact, it was next hypothesized that the defect in the ability of immunized Gαi2−/− mice to kill the larvae was in the neutrophil effector cell. Killing of S. stercoralis requires neutrophils during the adaptive immune response, whose recruitment requires the G protein-dependent receptor CXCR2 [21]. Similarly, Ascaris suum-derived chemotactic products interact with PTX-sensitive G proteins through the IL-8R pathway and induce activation and chemotaxis of human neutrophils [34]. In the present study, the total numbers of cells and specifically, neutrophils recruited into the micro-environment of the larvae were significantly lower in Gαi2−/− mice as compared with wild-type mice. This confirms previous observations that disruption of signaling through Gαi proteins inhibits cell migration and recruitment to infection sites [35–37] and contradicts studies that demonstrated that Gαi2−/− mice have equal or enhanced MPO levels, used as a measurement of neutrophil recruitment in the gut, liver, or lung following exposure to LPS [38]. It is clear from the present study that Gαi2 is required for neutrophil recruitment to the s.c. microenvironment of the nematode parasite. It remains unknown if the defect in neutrophil recruitment is related to the ability of the cells to undergo chemotaxis; however, recent studies of Gαi2−/− mice using pulmonary models of inflammation have implicated signaling events in endothelial cells as key regulators of leukocyte tissue recruitment [39].
A final hypothesis tested was that the neutrophils from Gαi2−/− mice were defective in their ability to kill the larvae. This hypothesis was tested by transferring neutrophils, recovered from wild-type and Gαi2−/− mice, into diffusion chambers implanted in immunized, wild-type mice. Neutrophils from Gαi2−/− mice were functionally competent and were able to kill larvae as efficiently as neutrophils from wild-type mice. This is in contrast to neutrophils recovered from TLR4-deficient mice, which were able to migrate to the parasite but were unable to kill the worms [24]. Although TLR4 and Gαi are linked in their activation and function [23, 38], it can be concluded from the present study that Gαi2 protein is not required for TLR4-dependent neutrophil killing of the larvae. Neutrophils from TLR4-deficient mice cannot kill larvae [24], although they migrate to the site, whereas neutrophils from Gαi2-deficient mice can kill the larvae as efficiently as wild-type cells only if they are transported experimentally to the site of infection.
In summary, the present study demonstrates that the Gαi2 signaling events are not required for the development of T and B cell responses during S. stercoralis infection. However, signaling through Gαi2 protein is necessary for recruitment of neutrophils leading to host-mediated killing of larvae. The present study extends the previous observations, which had shown that disruption of signaling through Gαi proteins inhibits cell migration and recruitment to infection sites [35–37], and demonstrates that recruitment of cells is controlled by intracellular signaling through Gαi2.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants RO1 AI47189 and RO1 A1 22662 and the Mayo Foundation. The authors thank Jessica Hess, Amy O’Connell, and Juergen Landmann for expert technical assistance.
REFERENCES
- 1.He J, Gurunathan S, Iwasaki A, Ash-Shaheed B, Kelsall BL. Primary role for Gi protein signaling in the regulation of interleukin 12 production and the induction of T helper cell type 1 responses. J. Exp. Med. 2000;191:1605–1610. doi: 10.1084/jem.191.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang TT, Zong Y, Dalwadi H, Chung C, Miceli MC, Spicher K, Birnbaumer L, Braun J, Aranda R. TCR-mediated hyper-responsiveness of autoimmune Gαi2(−/−) mice is an intrinsic naive CD4(+) T cell disorder selective for the Gαi2 subunit. Int. Immunol. 2003;15:1359–1367. doi: 10.1093/intimm/dxg135. [DOI] [PubMed] [Google Scholar]
- 3.Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu. Rev. Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 4.Ryan M, McCarthy L, Rappuoli R, Mahon BP, Mills KH. Pertussis toxin potentiates Th1 and Th2 responses to co-injected antigen: adjuvant action is associated with enhanced regulatory cytokine production and expression of the co-stimulatory molecules B7-1, B7-2 and CD28. Int. Immunol. 1998;10:651–662. doi: 10.1093/intimm/10.5.651. [DOI] [PubMed] [Google Scholar]
- 5.Birnbaumer L, Birnbaumer M. Signal transduction by G proteins: 1994 edition. J. Recept. Signal Transduct. Res. 1995;15:213–252. doi: 10.3109/10799899509045218. [DOI] [PubMed] [Google Scholar]
- 6.Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, Brabet P, Bradley A, Birnbaumer L. Gi2 α protein deficiency: a model of inflammatory bowel disease. J. Clin. Immunol. 1995;15:101S–105S. doi: 10.1007/BF01540899. [DOI] [PubMed] [Google Scholar]
- 7.Hornquist CE, Lu X, Rogers-Fani PM, Rudolph U, Shappell S, Birnbaumer L, Harriman GR. G(α)i2-deficient mice with colitis exhibit a local increase in memory CD4+ T cells and proinflammatory Th1-type cytokines. J. Immunol. 1997;158:1068–1077. [PubMed] [Google Scholar]
- 8.Dalwadi H, Wei B, Schrage M, Spicher K, Su TT, Birnbaumer L, Rawlings DJ, Braun J. B cell developmental requirement for the G α i2 gene. J. Immunol. 2003;170:1707–1715. doi: 10.4049/jimmunol.170.4.1707. [DOI] [PubMed] [Google Scholar]
- 9.Hou W, Wu Y, Sun S, Shi M, Sun Y, Yang C, Pei G, Gu Y, Zhong C, Sun B. Pertussis toxin enhances Th1 responses by stimulation of dendritic cells. J. Immunol. 2003;170:1728–1736. doi: 10.4049/jimmunol.170.4.1728. [DOI] [PubMed] [Google Scholar]
- 10.Ohman L, Franzen L, Rudolph U, Birnbaumer L, Hornquist EH. Regression of Peyer’s patches in G α i2 deficient mice prior to colitis is associated with reduced expression of Bcl-2 and increased apoptosis. Gut. 2002;51:392–397. doi: 10.1136/gut.51.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohman L, Franzen L, Rudolph U, Harriman GR, Hultgren Hornquist E. Immune activation in the intestinal mucosa before the onset of colitis in Gαi2-deficient mice. Scand. J. Immunol. 2000;52:80–90. doi: 10.1046/j.1365-3083.2000.00759.x. [DOI] [PubMed] [Google Scholar]
- 12.Mu HH, Sewell WA. Enhancement of interleukin-4 production by pertussis toxin. Infect. Immun. 1993;61:2834–2840. doi: 10.1128/iai.61.7.2834-2840.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sewell WA, de Moerloose PA, McKimm-Breschkin JL, Vadas MA. Pertussigen enhances antigen-driven interferon-γ production by sensitized lymphoid cells. Cell. Immunol. 1986;97:238–247. doi: 10.1016/0008-8749(86)90394-1. [DOI] [PubMed] [Google Scholar]
- 14.Brabb T, Goldrath AW, von Dassow P, Paez A, Liggitt HD, Goverman J. Triggers of autoimmune disease in a murine TCR-transgenic model for multiple sclerosis. J. Immunol. 1997;159:497–507. [PubMed] [Google Scholar]
- 15.McAllister CG, Vistica BP, Sekura R, Kuwabara T, Gery I. The effects of pertussis toxin on the induction and transfer of experimental autoimmune uveoretinitis. Clin. Immunol. Immunopathol. 1986;39:329–336. doi: 10.1016/0090-1229(86)90096-6. [DOI] [PubMed] [Google Scholar]
- 16.Herbert DR, Lee JJ, Lee NA, Nolan TJ, Schad GA, Abraham D. Role of IL-5 in innate and adaptive immunity to larval Strongyloides stercoralis in mice. J. Immunol. 2000;165:4544–4551. doi: 10.4049/jimmunol.165.8.4544. [DOI] [PubMed] [Google Scholar]
- 17.Herbert DR, Nolan TJ, Schad GA, Abraham D. The role of B cells in immunity against larval Strongyloides stercoralis in mice. Parasite Immunol. 2002;24:95–101. doi: 10.1046/j.0141-9838.2001.00441.x. [DOI] [PubMed] [Google Scholar]
- 18.Brigandi RA, Rotman HL, Yutanawiboonchai W, Leon O, Nolan TJ, Schad GA, Abraham D. Strongyloides stercoralis: role of antibody and complement in immunity to the third stage of larvae in BALB/cByJ mice. Exp. Parasitol. 1996;82:279–289. doi: 10.1006/expr.1996.0035. [DOI] [PubMed] [Google Scholar]
- 19.Rotman HL, Schnyder-Candrian S, Scott P, Nolan TJ, Schad GA, Abraham D. IL-12 eliminates the Th-2 dependent protective immune response of mice to larval Strongyloides stercoralis. Parasite Immunol. 1997;19:29–39. doi: 10.1046/j.1365-3024.1997.d01-142.x. [DOI] [PubMed] [Google Scholar]
- 20.Kerepesi LA, Hess JA, Nolan TJ, Schad GA, Abraham D. Complement component C3 is required for protective innate and adaptive immunity to larval Strongyloides stercoralis in mice. J. Immunol. 2006;176:4315–4322. doi: 10.4049/jimmunol.176.7.4315. [DOI] [PubMed] [Google Scholar]
- 21.Galioto AM, Hess JA, Nolan TJ, Schad GA, Lee JJ, Abraham D. Role of eosinophils and neutrophils in innate and adaptive protective immunity to larval Strongyloides stercoralis in mice. Infect. Immun. 2006;74:5730–5738. doi: 10.1128/IAI.01958-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lippert E, Jacques Y, Hermouet S. Positive regulation of human T cell activation by Gi2 proteins and interleukin-8. J. Leukoc. Biol. 2000;67:742–748. doi: 10.1002/jlb.67.5.742. [DOI] [PubMed] [Google Scholar]
- 23.Fan H, Peck OM, Tempel GE, Halushka PV, Cook JA. Toll-like receptor 4 coupled GI protein signaling pathways regulate extracellular signal-regulated kinase phosphorylation and AP-1 activation independent of NFκB activation. Shock. 2004;22:57–62. doi: 10.1097/01.shk.0000129759.58490.d6. [DOI] [PubMed] [Google Scholar]
- 24.Kerepesi LA, Hess JA, Leon O, Nolan TJ, Schad GA, Abraham D. Toll-like receptor 4 (TLR4) is required for protective immunity to larval Strongyloides stercoralis in mice. Microbes Infect. 2006 doi: 10.1016/j.micinf.2006.10.003. In press. [DOI] [PubMed] [Google Scholar]
- 25.Abraham D, Rotman HL, Haberstroh HF, Yutanawiboonchai W, Brigandi RA, Leon O, Nolan TJ, Schad GA. Strongyloides stercoralis: protective immunity to third-stage larvae inBALB/cByJ mice. Exp. Parasitol. 1995;80:297–307. doi: 10.1006/expr.1995.1036. [DOI] [PubMed] [Google Scholar]
- 26.Herbert DR, Nolan TJ, Schad GA, Lustigman S, Abraham D. Immunoaffinity-isolated antigens induce protective immunity against larval Strongyloides stercoralis in mice. Exp. Parasitol. 2002;100:112–120. doi: 10.1016/S0014-4894(02)00008-5. [DOI] [PubMed] [Google Scholar]
- 27.Lowell CA, Fumagalli L, Berton G. Deficiency of Src family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. J. Cell Biol. 1996;133:895–910. doi: 10.1083/jcb.133.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites—masters of regulation. Immunol. Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 29.Pearce EJM, Kane C, Sun JJ, Taylor J, McKee AS, Cervi L. Th2 response polarization during infection with the helminth parasite Schistosoma mansoni. Immunol. Rev. 2004;201:117–126. doi: 10.1111/j.0105-2896.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence RA, Allen JE, Osborne J, Maizels RM. Adult and microfilarial stages of the filarial parasite Brugia malayi stimulate contrasting cytokine and Ig isotype responses in BALB/c mice. J. Immunol. 1994;153:1216–1224. [PubMed] [Google Scholar]
- 31.Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J. Exp. Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ligas JA, Kerepesi LA, Galioto AM, Lustigman S, Nolan TJ, Schad GA, Abraham D. Specificity and mechanism of immunoglobulin M (IgM)- and IgG-dependent protective immunity to larval Strongyloides stercoralis in mice. Infect. Immun. 2003;71:6835–6843. doi: 10.1128/IAI.71.12.6835-6843.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, Brabet P, Boulay G, Bradley A, Birnbaumer L. Ulcerative colitis and adenocarcinoma of the colon in G α i2-deficient mice. Nat. Genet. 1995;10:143–150. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- 34.Falcone FH, Rossi AG, Sharkey R, Brown AP, Pritchard DI, Maizels RM. Ascaris suum-derived products induce human neutrophil activation via a G protein-coupled receptor that interacts with the interleukin-8 receptor pathway. Infect. Immun. 2001;69:4007–4018. doi: 10.1128/IAI.69.6.4007-4018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cyster JG, Goodnow CC. Pertussis toxin inhibits migration of B and T lymphocytes into splenic white pulp cords. J. Exp. Med. 1995;182:581–586. doi: 10.1084/jem.182.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spangrude GJ, Sacchi F, Hill HR, Van Epps DE, Daynes RA. Inhibition of lymphocyte and neutrophil chemotaxis by pertussis toxin. J. Immunol. 1985;135:4135–4143. [PubMed] [Google Scholar]
- 37.Su SB, Silver PB, Zhang M, Chan CC, Caspi RR. Pertussis toxin inhibits induction of tissue-specific autoimmune disease by disrupting G protein-coupled signals. J. Immunol. 2001;167:250–256. doi: 10.4049/jimmunol.167.1.250. [DOI] [PubMed] [Google Scholar]
- 38.Fan H, Zingarelli B, Peck OM, Teti G, Tempel GE, Halushka PV, Spicher K, Boulay G, Birnbaumer L, Cook JA. Lipopolysaccharide- and gram-positive bacteria-induced cellular inflammatory responses: role of heterotrimeric Gα(i) proteins. Am. J. Physiol. Cell Physiol. 2005;289:C293–C301. doi: 10.1152/ajpcell.00394.2004. [DOI] [PubMed] [Google Scholar]
- 39.Pero RS, Borchers MT, Spicher K, Ochkur SI, Sikora L, Rao SP, Abdala-Valencia H, O’Neill KR, Shen H, Simon MI, McGarry MP, Lee NA, Cook-Mills JM, Sriramarao P, Birnbaumer L, Lee JJ. Extravasation of leukocytes from circulation is controlled by Gαi2 signaling events in the endothelium. Proc. Natl. Acad. Sci. USA. 2006 doi: 10.1073/pnas.0700185104. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]