Abstract
Interstitial cystitis (IC)/painful bladder syndrome (PBS) is a painful debilitating chronic visceral pain disorder of unknown etiology that affects an estimated 1 million people in the, United States alone. It is characterized by inflammation of the bladder that results in chronic pelvic pain associated with bladder symptoms of urinary frequency and urgency. Regardless of the etiology, IC/PBS involves either increased and/or abnormal activity in afferent nociceptive sensory neurons. Pain-related symptoms in patients with IC/PBS are often very difficult to treat. Both medical and surgical therapies have had limited clinical utility in this debilitating disease and numerous drug treatments, such as heparin, dimethylsulfoxide and amitriptyline, have proven to be palliative at best, and in some IC/PBS patients provide no relief whatsoever. Although opiate narcotics have been employed to help alleviate IC/PBS pain, this strategy is fraught with problems as systemic narcotic administration causes multiple unwanted side effects including mental status change and constipation. Moreover, chronic systemic narcotic use leads to dependency and need for dose escalation due to tolerance: therefore, new therapies are desperately needed to treat refractory IC/PBS. This has led our group to develop a gene therapy strategy that could potentially alleviate chronic pelvic pain using the herpes simplex virus-directed delivery of analgesic proteins to the bladder.
Keywords: interstitial cystitis, painful bladder syndrome, visceral pain, dorsal root ganglia, herpes simplex virus
Interstitial cystitis/painful bladder syndrome
Interstitial cystitis (1C) or painful bladder syndrome (PBS) is a non-malignant chronic inflammatory pain condition of the visceral organs of the lower urinary tract (LUT), characterized by a constellation of symptoms including bladder/pelvic pain most commonly associated with urinary urgency and frequency that alters urinary output due to pain upon voiding.1–4 In many instances, the pain and discomfort felt by patients with IC/PBS is substantial, with their quality of life being similar to patients with end-stage renal disease. Although IC/PBS affects both men and women of all ages, it predominantly affects women, with up to 12% of women showing some symptoms of the disease during their lifetime. Diagnosis is usually based on the presence of the prior listed symptoms, which unfortunately are characteristic of numerous other bladder conditions, such as cancer and infectious disease, making definitive diagnosis of IC/PBS difficult. Once these other disorders have been ruled out, IC/PBS is then diagnosed as painful bladder symptoms in the absence of infection or other identifiable conditions. Urinalysis including urine culture can be employed to rule out infection, whereas tests such as the prostate-specific antigen can be employed in men to rule out cancer as the cause. In some instances, other clinical tests, such as urodynamic studies, cytoscopy with hydrodistension and the potassium chloride sensitivity test, can be employed in the diagnosis. However, the variability of the potassium chloride sensitivity and specificity of this test for IC/PBS and the fact that hydrodistension testing generates a painful response in patients and can generate lesions similar to those found in IC/PBS patients make these tests less attractive for use in the diagnosis of IC.
Epidemiology of IC/PBS
Oravisto (1975)5 reported an incidence of 18.1 per 100 000 female individuals and an overall incidence of 10.6 per 100 000 in Finland. In 1987, Held et al.6 performing population-based studies in the United States reported a prevalence of 20 000–90 000 documented cases of IC/PBS where the duration of patient symptoms was for 30–50 months before diagnosis. Curhan et al. (1999)7 also reported that the incidence of IC/PBS is much more prevalent than was initially thought. IC/PBS affects over 700 000 people in the United States, an overall prevalence 50% higher than earlier estimates and more than three times that reported in Europe. More than 90% of IC/PBS patients are women, but IC/PBS occurs in people of both sexes and all ages. More recently, Payne et al.8 documented that the prevalence of IC-like symptoms is much higher at approximately 5000/100 000 although the prevalence of a formal physician diagnosis of IC is relatively low at approximately 200/100 000 population. Thus, it is likely that the prevalence of IC/PBS or chronic pelvic pain is greater than was first anticipated and that IC/PBS has now become more readily recognized as an important diagnosis with greater awareness of this disorder.
Pathophysiology of IC/PBS
Although the etiology of IC/PBS remains unknown it is likely to be a multifactorial and not a single-source disease that can be initiated by various triggers such as autoimmune response to host antigens, allergic reaction to substances that have leaked into the bladder, neurogenic inflammation, sensitization of the afferent pathways, dysfunction of the bladder epithelium, inherited susceptibility9 and also bladder mucosal ischemia as potential mediators of the observed disease pathology. In the past, bladder infection was proposed as a primary mechanism for the creation of the IC/PBS pathophysiologic state; it has been difficult to ascribe support for this hypothesis compared with the other possible triggering mechanisms.
It has been proposed that a defect in the protective glycosaminoglycan (GAG) layer that lines the epithelium of the bladder, resulting from a wide range of stimuli, may lead to increased permeability across the uroepithelium (Figure 1). This change in membrane permeability then can result in irritating substances present within urine breaching this layer leaking into the surrounding tissues, triggering the painful symptoms observed in IC/PBS.10 This initial injury to the bladder epithelium9 thereby induces other events, such as irritation of the afferent pathways responsible for neurogenic control of bladder function as well as the release of soluble mediators at the site of injury, which in turn results in an increase in and further activation of mast cells, all of which are signs of immune involvement in the disease process and in some instances point to an autoimmune or allergic function that may contribute to IC/PBS pathophysiology.9,11 The role of the host immune response in IC/PBS pathogenesis is further supported by the fact that numerous patients with IC/PBS also suffer from allergies, various autoimmune disorders such as inflammatory/irritable bowel syndrome (IBS), asthma, sensitive skin and lupus as well as fibromyalgia, vulvodynia and migraine headaches,11,12 which may further lead to mast cell activation and overall inflammation in the region of the initial insult and the afferents innervating this site.
Figure 1.
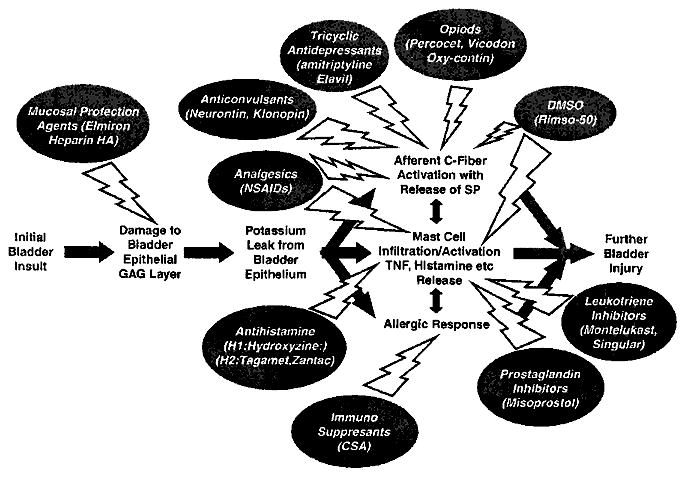
The proposed pathogonesis of IC/PBS and standard therapies for IC/PBS. An initial insult to the bladder results in the damage of the bladder epithelial layer that, for example, allows potassium to leak in and to prompt a cascade of events, each contributing to tissue inflammation and sensory fiber activation thereby inducing pain sensation in the lower urinary tract (LUT). Various standard drug treatments that have been employed for IC/PBS are depicted showing the level(s) at which these factors are involved in blocking the inflammatory or neurogenic processes that lead to IC/PBS pain. IC/PBS, interstitial cystitis/painful bladder syndrome.
The stimulation of mast cell infiltrate to the bladder urothelium site of initial injury and the subsequent release of soluble mediators by these cells, such as cytokines like tumor necrosis factor, histamines and prostaglandins, contributes to further local inflammation, resulting in additional damage to the bladder mucosal lining. This in turn results in an additional immune cell infiltration primarily consisting of mast cells, the further activation of the release of soluble mediators as well as the release of tachykinins such as substance P (SP), which ultimately leads to the sensitization of sensory nerves.9,12 Thus, the combination of SP stimulation of afferent processes with a cycle of increasing local inflammation surrounding these processes, further contributes to the role of the inflammatory process in the disease pathogenesis.
Standard IC/PBS treatment regimens
Treatment strategies for IC/PBS have been just as puzzling as the diagnosis of this syndrome. Although a variety of treatment regimens have been used to manage other chronic painful symptoms, none uniformly eradicate the symptoms of severe suprapubic pain, urinary frequency, and urgency experienced by patients with IC/PBS.3,4,13 Thus, the treatment regimen of IC/PBS is often specific to the individual patient and frequently involves a combination of therapies. However, some patients are not helped by any of the current treatment regimens.
Although surgery has proven an effective therapy for some forms of chronic lower back pain, it is rarely used to treat IC/PBS pain only as a last resort should other standard therapies fail. However, even following surgery, many patients still experience urinary frequency, urgency and pain even phantom bladder pain after cystectomy. Neuromodulation strategies are also usually employed only when other standard therapies have failed. These approaches use either the external transcutaneous electrical nerve stimulator (TENS) units or InterStim implants to send varying modulatory impulses to the nerves controlling bladder function in the hope of regulating urinary frequency and urgency. In addition, these devices have shown some relief in patients with mild-to-moderate IC/PBS pain, but controlled studies to assess the number of patients with improved pain scores have not been initiated and some adverse side effects have been observed with these devices.
As the initial injury leading to IC/PBS occurs through damage to the urothelium, agents like GAGs, such as heparin,14 Uracyst (chondroitin sulfate)15 and Cystistat, 16,17 have been administered by intravesical instillation over a 20-min period weekly for 1–2 months, then monthly thereafter in an effort to help restore or maintain the lining of the bladder. These agents also appear to have some anti-inflammatory action in addition to being potent protective factors for repairing the bladder surface. Another drug that acts at the same level is Elmiron (pentosan polysulfate sodium), the only oral medication specifically approved by the Food and Drug Administration to treat IC/PBS. In addition to the use of Elmiron as an oral agent, it can also be given by direct instillation into the bladder. Clinical trials using Elmiron have shown promise with ~50% of patients displaying some form of improvement in their bladder symptoms; however, it may take more than 6 months for these results to occur.18–21 Bladder instillation with RIMSO-50, a 50% solution of dimethylsulfoxide, the only other Food, and Drug Administration-approved treatment for IC/PBS or other agents,3,4,22 also has helped relieve the symptoms of IC/PBS because these agents may not only possess anti-inflammatory and analgesic properties but also may act by relaxing bladder detrusor muscle activity.23 Some unwanted side effects have been observed in patients treated with the 50% dimethylsulfoxide solution that seem to lessen over time; however, the use of a 25% solution fails to induce these harmful effects yet maintains some activity toward managing the IC/PBS symptoms.24 Dimethylsulfoxide has been used in many combination therapies in which it increases the uptake of other drugs in the cocktail across the bladder lining following intravesicular instillation.25,26
Histamines, leukotrienes and prostaglandins are generated upon mast cell infiltration of the site of bladder epithelium injury; thus, drugs that block their inflammatory activities have been employed to treat IC/PBS. The histamine H1R blocker hydroxyzine (Atarax) has been employed alone or in combination with other drugs, such as Elmiron,27 with limited success while possessing mild sedation as an unwanted side effect. Even H2R-specific histamine blockers, such as Tagamet, have resulted in a reduction in pain in ~50% of IC/PBS patients tested.28 The leukotriene inhibitor Montelukast (Singulair), which is taken for allergy relief, has shown some reduction in IC/PBS patient bladder-related symptoms29 as well as the prostaglandin analog Misoprostol.30 Other agents that may offer relief include tricyclic antidepressants that have analgesic properties with both anti-cholinergic and anti-histamine effects such as amitriptyline (elavil).31,32
Non-opioid pain medications employed in the treatment of other chronic pain syndromes, such as the drugs gabapentin (Neurontin), aspirin (Bufferin), acetominophen (Tylenol) and non-steroidal anti-inflammatory drugs (Advil, Motrin, Aleve), have also been prescribed for IC/PBS pain, with gabapentin showing the greatest efficacy (~50% pain reduction) due to its action against neuropathic pain.33 Opioid analgesics, such as tramadol (Ultram), codeine (+/− aspirin, acetaminophen) and hydrocodone (Vicodin), have been used to treat moderate bladder pain with a modicum of success. Although all possess narcotic and dependency issues, tramadol seems to possess a superior safety profile compared with the other opioids and has just recently been proven to be effective in the treatment of IC/PBS-related bladder pain.34,35 Patients with frequent or unremitting pain may require more aggressive pain management, such as long-acting opioids, for example morphine (MS Contin and Oramorph) and oxycodone (OxyContin).3,36 However, the use of systemic opioid therapy has been limited because of its untoward side effects and dependency.37,38
On account of the multiple factors that have been shown to play a role in the initiation of IC/PBS, it has been suggested that multimodal therapeutic intervention may hold the best promise in treating patients with IC/PBS. Today, several such approaches have shown varying degrees of efficacy, including the combination of Elmiron with other meds such as heparin,31 hydroxyzine27 or steroids such as cyclosporin A (CSA).39 Other treatments have combined a membrane-protective agent like heparin with lidocaine14 or heparin and hydrocortisone,40 which has higher efficacies than using either agent alone.
C-fiber activation in IC/PBS pathology and pain management
The activation of C-fiber afferents that innervate the bladder by a variety of potential mechanisms represents crucial initial steps to the development of pain associated with IC/PBS. This process may involve components of an inflammatory nature or may also involve the activation of chemo- and mechanoreceptors.
Afferent pathways innervating bladder and urethral function
Sensory information including the feeling of bladder fullness or bladder pain is conveyed to the spinal cord through afferent axons in the pelvic and hypogastric nerves.41,42 Neuronal somata of these afferent nerves are located in the dorsal root ganglia (DRG) at S2–S4 and T11–L2 spinal segmental levels in humans. These afferent fibers carry impulses from tension receptors and nociceptors in the bladder wall to neurons in the dorsal horn of the spinal cord (Figure 2). Afferent axons in the pelvic, hypogastric and pudendal nerves transmit information from the LUT to the spinal cord. The primary afferent neurons of the pelvic and pudendal nerves are present within the sacral DRG, whereas afferent innervation in the hypogastric nerves arises in the rostral-lumbar DRG.42–44 The central axons of the DRG neurons carry the sensory information from the LUT to second-order neurons in the spinal cord43,45–47 (Figure 2). Visceral afferent fibers of the pelvic46 and pudendal47 nerves enter the cord and travel rostrocaudally within Lissauer’s tract.
Figure 2.
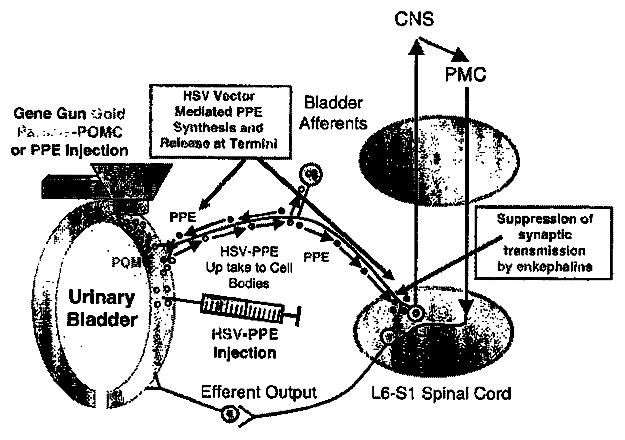
Schematic diagram of gene therapy strategies for IC/PBS. Gold particles coated with POMC or PPE DNA were used to bombard the bladder wall. These plasmid DNAs encode the peptides (POMC and PPE) that can only locally block pain by suppressing further neuropeptide release. HSV vectors expressing either the PPE gene (HSV-PPE) or the lacZ control (HSV-lacZ) are injected into bladder wall where viral genomes can encode enkephalins locally and virus can be transported to bladder afferent pathways. In contrast to using gold particles with the gene gun technology, HSV-PPE vector genomes present within L6-S1 DRG bladder afferents synthesize and release enkephalins within the dorsal horn of spinal cord, and binding of met- and leu-enk to opioid receptors present on postsynaptic second-order spinal tract neurons and presynaptic bladder afferents may allow better suppression of synaptic transmission of the bladder pain responses. DRG, dorsal root ganglia; HSV, herpes simplex virus; IC/PBS, interstitial cystitis/painful bladder syndrome; POMC, pro-opiomelanocortin; PPE, preproenkephalin.
Afferent fibers passing in the pelvic nerve to the sacral cord are responsible for initiating the micturition reflex. These bladder afferents are composed of myelinated Aδ-fiber or unmyelinated C-fiber axons.45,48 In rats, there is now evidence that many C-fiber bladder afferents are volume receptors that do not respond to bladder contractions, a property that distinguishes them from ‘in series tension receptors’.49 In cats, Aδ-bladder afferents appear to be low-threshold mechanoreceptors,50 whereas C-fiber bladder afferents41 are generally mechanoinsensitive (‘silent C-fibers’). Some of the latter may be nociceptive, and are sensitized by intravesical administration of chemicals such as high potassium, low pH, high osmolality and irritants such as capsaicin and turpentine.41,51–53 Following exposure to these substances, the sensitivity of bladder mechanoreceptors to distension, increases and some ‘silent’ afferents become mechanoceptive. Immunohistochemical studies indicate that bladder afferent neurons contain various neuropeptides, such as SP, calcitonin gene-related peptide and vasoactive intestinal peptide.51,54 The distribution of these peptidergic afferent terminals in the spinal cord is similar to that of central projections of bladder afferent neurons.43,55 The release of these neuropeptides in the bladder wall is known to trigger inflammatory responses similar to those characteristic of IC/PBS, including plasma extravasation or vasodilation.56 However, the release of these neuropeptides in central afferent nerve terminals activates second-order neurons in the spinal cord to transmit pain sensation to the brain. Thus, during inflammation and possibly other pathological conditions including IC/PBS or chronic pelvic pain, there is recruitment of mechanosensitive C-fibers that form a new functional afferent pathway.57
Hyperexcitability of C-fiber afferent pathways as a mechanism for LUT pain
Pain is a defining characteristic of IC/PBS or chronic pelvic pain syndrome. One mechanism by which pain is induced is postulated to involve chronic tissue inflammation that can lead to functional changes in C-fiber afferents.12,58–60 These normally silent fibers appear to have a specific function in signaling noxious events in the bladder. Hyperactivity of C-fiber afferents may, therefore, lead to increased pain sensation. Chronic conditions that involve tissue inflammation or irritation can induce changes in sensory pathways that lead to hyperalgesia (heightened response to painful stimuli) and allodynia (pain in response to normally non-painful stimuli). For example, tissue inflammation in visceral organs such as urinary bladder can increase afferent nerve excitability in response to both noxious and non-noxious stimuli.41,61 Therefore, changes in afferent nerves might contribute to painful symptoms in patients with IC/PBS or chronic pelvic pain syndrome, a chronic pain syndrome of unknown etiology that appears to have an inflammatory component. It has also been reported that C-fiber desensitization induced by intravesical application of high-dose capsaicin and resiniferatoxin is effective for treating painful symptoms in IC/PBS patients,62 although a more recent prospective, randomized clinical trial using intravesical resiniferatoxin application was not effective in patients with IC.63
Indirect evidence for this postulate comes from histologic analysis of bladders from patients with IC/PBS, which is marked by edema, vasodilation, proliferation of nerve fibers and infiltration of mast cells,64,65 and from chemically induced cystitis in animals, in which increased urinary frequency is initiated by sensitizing mechanosensitive afferents and/or recruitment of afferents normally unresponsive to mechanical stimulation.41,61,66 In addition, proinflammatory agents, such as prostaglandin E2, serotonin, histamine and adenosine, as well as neurotrophic factors such as nerve growth factor (NGF), can induce functional changes in C-fiber afferents that can lead to these relatively unexcitable afferents becoming hyperactive or hyperexcitable.66–70
A more direct evidence linking chronic inflammation with functional changes in C-fiber afferents has been derived from a rat model of chronic cystitis induced by systemic application of cyclophosphamide, which undergoes hepatic metabolism to acrolein, an irritant excreted in the urine71 or intravesical application of hydrochloric acid (HCI).72 In the cyclophosphamide model, it has been documented that the majority of bladder afferent neurons from both control and chronic cystitis rats are capsaicin sensitive and exhibit tetrodotoxin-resistant action potentials, whereas those from treated rats exhibit significantly lower thresholds for spike activation and show high-frequency firing characteristics.73 Other significant changes in bladder afferents from cyclophosphamide-treated rats include increased somal diameter, increased input capacitance and decreased density of slowly inactivating A-type K+ (KA) currents. Together, these data suggested that chronic inflammation induces both cell hypertrophy and hyperexcitability of C-fiber visceral afferent neurons.73 More recently, hyperexcitability of C-fiber afferent neurons due to decreased K+ currents was also reported in cats with IC/PBS.74 C-fiber-mediated nociceptive responses, such as urinary frequency and C-fiber afferent hyperexcitability, were also identified in rats with urethral inflammation or pudendal nerve injury.60 Thus, targeting hyperexcitable C-fiber activity represents a mechanism to treat LUT pain.
Opioid mechanisms in the peripheral nervous system and central nervous system
Multiple opioid peptides and receptors
Opioid peptides are derived from three different precursor proteins: pro-opiomelanocortin, prodynorphin and proenkephalin.75–77 The main groups of opioid peptides, enkephalins, dynorphins and β-endorphin, derive from proenkephalin, prodynorphin and pro-opiomelanocortin, respectively. Proenkephalin is the source of [Met5]- and [Leu5]-enkephalins and several longer peptides. Three members of the receptor family were cloned. The cloned μ-opioid receptor is a morphine-like receptor78–82 and endomorphins may be its endogenous ligands. The enkephalins bind to the δ-opioid receptor80,83–86 with great affinity and, therefore, are considered to be endogenous δ-opioid receptor agonists. The affinity of β-endorphin binding to μ- and δ-opioid receptors was found to be similar. Dynorphins bind to κ-opioid receptors83,87–91 and therefore appear to function as its endogenous ligands. However, opioid peptides do not bind exclusively to one specific receptor type but have some affinity for other opioid receptors as well, similar to that of the neurotrophins for their cognate receptors.
High expression levels of opioid receptors (μ-, κ- and δ-binding) were found in the substantia gelatinosa where sensory afferents terminate as well as on the terminals of primary afferents,92 explaining the significant binding of opioid peptides to the dorsal sensory roots in the DRG.93,94 Opioid receptors have also been shown on peripheral terminals of nociceptive sensory nerves in animals and humans.95,96 Earlier studies have shown that all three opioid receptor types (μ, κ and δ) can be functionally active in peripheral tissues97,98 although some other studies suggested that κ-opioid receptor mechanisms, rather than μ- or δ-receptors, are more likely to be involved at the peripheral site in visceral pain induced in the bladder or colon.99,100
Central afferent terminals, opioids and presynaptic inhibition
It has been well documented that opioids have a direct inhibitory effect upon the excitability of the afferent terminals, leading to a net decrease in the release of neurotransmitter into the synapse. Changes in central terminal excitability could reflect three general mechanisms: (1) a hyperpolarization of the terminal by the activation of terminal receptors that alter ion permeability, leading to a shunting of membrane currents and thus making the terminal more difficult to excite; (2) depolarization of the terminal such that voltage-sensitive Ca2+ channels may become inactivated, negating the ability of any subsequent terminal depolarization to evoke neurotransmitter release; or (3) direct inhibitory coupling to voltage-sensitive Ca2+ channels, blocking Ca2+-dependent exocytosis,92,101–103 Consequence of these events could then reduce the amount of neurotransmitter that gets released secondary to depolarization.
Populations of unmyelinated C-fiber afferents are known to contain a variety of neuropeptides such as SP and calcitonin gene-related peptide, which are released in a Ca2+-dependent fashion following the physiological or pharmacological activation of nociceptive afferents.104,105 The presence of opioid receptors on the terminals and the coupling of opiate receptors with voltage-sensitive Ca2+ channels suggest that opioids might act to block directly the release of the neurotransmitters contained in the respective terminals. Thus, it appears that opioids acting through μ- and δ-receptors act presynaptically to inhibit the release of peptides from nociceptive C-fibers that release neuropeptides such as SP.
Role of opioids in dorsal horn neuron postsynaptic inhibition
Various studies have shown that opioids at or near the terminals of the C-fibers in the dorsal horn of the spinal cord reduced both spontaneous activity and the firing of spinothalamic projection dorsal horn neurons evoked by noxious and innocuous stimuli.92 Both μ- and δ-agonists exerted a suppressive effect upon nociceptive-specific neurons in lamina I of the spinal cord. Opioid peptides also produce a hyperpolarization and a decrease in cell firing of the dorsal horn neurons in the substantia gelatinosa.106
Opioid peptides in the control of LUT function
Various neurotransmitters at the spinal and supraspinal level are involved in the regulation of LUT activities.107,108 Among them, opioid peptides and GABA have been shown to be important inhibitory transmitters suppressing LUT activity. Enkephalinergic pathways in the central nervous system exert an inhibitory control on the micturition reflex.107,108 Enkephalinergic varicosities have been shown by immunocytochemistry at various sites including the primary motor cortex (PMC), the sacral parasympathetic nucleus and urethral sphincter motor nucleus in the spinal cord. Administration of opioid drugs or enkephalins to the brain or spinal cord suppresses micturition and sphincter reflexes.l09–112 In the brain, both μ- and δ-opioid receptors mediate inhibitory effects, which are blocked by naloxone.110,113,114 In the cat spinal cord, δ-opioid receptors mediate the inhibition of bladder activity and κ-receptors mediate the inhibition of sphincter activity.112 In the rat spinal cord, δ- and μ-receptors but not κ-receptors are involved in the suppression of bladder reflexes.108,110,111 In conscious dogs, intrathecal administration of morphine increases the volume threshold for inducing micturition without altering voiding pressure that can be blocked by naloxone. These observations indicate that spinal opioid mechanisms can control the afferent limb of the micturition reflex110 but not C-fiber bladder afferents, which are reportedly not involved in normal voiding in rats.115
It has been shown that visceral pain induced by colon or bladder noxious distention was suppressed by the activation of spinal μ- and δ-opioid receptors and peripheral κ-receptors in rats.99,100 Craft et al.116 also reported that behavioral pain responses induced by the intravesical application of resiniferatoxin, a potent analog of capsaicin, were suppressed by the activation of systemic μ-, δ- and κ-opioid receptors and peripheral δ-receptors in rats. Meen et al117 have also shown that bladder nociceptive responses induced by cyclophosphamide in rats were suppressed by the intrathecal application of opioids.
Gene therapy: an alternative therapy for LUT pain
Gene therapy offers considerable promise for treating otherwise intractable diseases of the human nervous system, including the treatment of chronic pain, compared with standard drug-related therapies.118–121 In many instances in which drug therapies have been employed, the therapeutic factor delivered systematically cannot pass the blood–brain barrier in sufficient quantities to be therapeutic or systemic delivery of the agent results in side effects associated with expression of the agent in cells or tissues in which its expression is unwanted or deleterious.
Both non-viral and viral delivery vehicles have been employed to deliver genes to bladder and other tissues. Each delivery system has its own advantages and disadvantages for delivery to different target cells and tissues. Overall, a major advantage of the non-viral delivery systems has been the low immunogenicity of this approach compared with viral vectors that all show some level of host response to the vector itself, which may only amplify any host response to the therapeutic gene. In addition, the non-viral systems have not been plagued by the short-term nature of vector-delivered transgene expression due to the limited maintenance of the delivery vehicle in the transduced cells. On the other hand, viral vectors are exceedingly more efficient at delivering their genetic payload to the target cell compared with non-viral systems because over millennia viruses have acquired efficient methods to deliver their own genetic material to cells to replicate their genomes and further propagate themselves. Another advantage is that most, viral vectors can be readily produced and purified for in vivo gene transfer, whereas the non-viral methods are limited by the production of sufficient quantities of DNA for transduction.
The majority of gene transfer studies for the bladder have revolved around gene delivery to bladder tumors, in which the goal is to achieve high-level transduction of the tumor in the absence of gene delivery to normal bladder epithelium; both non-viral and viral vectors have been used for gene transfer to urothelium. Non-viral approaches to transfer to the bladder have used direct injection of naked, liposome-mediated transfer of DNA as well as electroporation of DNA into the bladder wall and gene gun-mediated delivery of DNA linked to gold particles, although most of these approaches have met with limited success in achieving high-level transduction of either the urothelium or the smooth muscle layer. The GAG layer present on the uroplakin-covered umbrella cells has limited access of agents to the urothelium in undamaged bladder, and thus simple administration of genes by cationic liposomes122,123 led to poor transduction of the bladder suggesting that other means of abrogating this physical barrier are needed for effective gene transfer. To bypass this barrier, various treatments have been employed; however, their damage to the urothelium would be detrimental to the already damaged urothelium present in IC/PBS patients, and thus would not be of clinical use to improve transduction. Other groups have exposed the bladder by a lower midline incision followed by the direct injection of plasmid DNA expressing a luciferase, eGFP or lacZ reporter gene combined with electroporation to achieve gene delivery, where the voltage, number, duration and frequency of the electric pulses were optimized to provide efficient delivery primarily to cells of the smooth muscle layer.124–126 Moreover, the groups did not detect any difference in KCl-induced detrusor muscle activity following electroporation, suggesting that this approach did not damage the urothelium. Otani et al.125 showed that the transduction of bladder by this method with a plasmid encoding the muscarinic M3 receptor did result in detrusor muscle changes that may potentially be therapeutic, whereas Iwashita et al.124 showed that nNOS gene transfer led to an increase in NO production within the bladder smooth muscle layer. Finally, gene gun delivery of gold particle-linked DNA (Figure 2) to the bladders of animals in which the bladder has been exposed by a midline incision enabled delivery of molecules that alter the painful responses to bladder nociceptive stimuli in animal models of cystitis127,128 discussed in detail below.
Viral vectors (adenoviruses, pox viruses and herpes simplex virus (HSV)) have been employed in gene transfer studies to the bladder and have met with greater success than the various non-viral methods, although the main goal of many of these studies was to efficiently transduce urogenic tumors over that of the normal bladder urothelium. The same GAG barrier that blocked transduction using non-viral methods also proved troublesome for initial studies using intravesical delivery of adenoviral vectors,129,130 so continued studies to improve transduction employed agents to disrupt this barrier such as ethanol,130 polyamines such as CHAP and Big-CHAP,131 HCI32 and dodecyl-β-D-maltoside or SDS.133 Engler et al.130 found that while 40% ethanol provided enhanced gene transfer, it also resulted in hemorrhagic cystitis, but its reduction to 22% yielded a high-level expression (84% of surface expression of lacZ), either as a pretreatment or when co-administered with the vector. It is interesting to note that the alcohol had no effect on vector-mediated gene transfer in vitro, supporting the role of the agent in altering the GAG barrier in vivo. Various cationic, anionic, zwitterionic and non-ionic agents were employed to break down the GAG layer hydrophilic polyanionic barrier restricting adenovirus transduction; however, most either gave little improvement or led to cystitis.131 One agent, Big CHAP, yielded high transduction in the absence of toxicity, but more-purified Big CHAP preparations of the agent failed to enhance vector-mediated transduction, whereas the impurities Syn3-Lac and Syn3-Mel were found to possess the ability to enhance the transduction of adenovirus. Another study employed 60 mM HCl to alter the GAG barrier,132 which did improve adenovirus-mediated gene transfer, which could be blocked using Elmiron, yet the authors did not examine the effects of this short (10 min) acid treatment on bladder integrity/toxicity as many groups actually employ acid treatment to create an animal model of cystitis. Ramesh et al.133 used a variety of agents including alcohols, chlorpactin, tween, oxychlorosene, SDS and even dodecyl-β-D-maltoside. Most of these did not significantly improve transduction but rather acted as bladder irritants. Even treatment with 0.1–0.2% oxychlorosene, which gave a modest enhancement to vector-mediated transgene expression, resulted in edema, neutrophil infiltration, ulceration and mucosal erosion. However, pretreatment of with 0.1% dodecyl-β-D-maltoside, an alkyl disaccharide, yielded increased adenoviral transduction without measurable signs of bladder irritation. Initial studies using Pox virus vectors delivered intravesically failed to transduce the urothelium,134 which is likely to be the result of a block in vaccinia infection by the GAG barrier. The use of similar agents employed to increase adenovirus transduction to make that barrier more penetrable to the Pox vector, such as HCl, NH4Cl, chlorpactin and oxychlorosene,135 yielded similar results, with oxychlorosene providing the greatest enhancement with the least toxicity, whereas agents such as acids tended to produce severe changes to the urothelium that in turn result in cystitis.
Herpes simplex virus vectors have been employed for delivery of genes to the bladder136 and bladder afferents.137–139 Unlike studies using Adeno and Pox viral vectors, the main goal of the HSV studies were the delivery of therapeutic genes to the bladder and the DRG afferents that innervate the bladder either for treatment of diabetic neuropathy138,139 or for cystitis/PBS.137 Delivery of vector in these studies was through the injection of the vector directly into the bladder wall following a lower midline incision to expose the bladder (Figure 2). Brooks et al.136 showed high-level expression of either the lacZ reporter gene or the tumor necrosis factor therapeutic gene within the bladder and not any other tissue, demonstrating the specificity achieved using HSV, yet they did not examine whether vector injection led to transgene expression within bladder afferents. The studies examining the effects of NGF delivery to the bladder and its afferents by replication-defective HSV vectors showed both short-term and long-term NGF expression in both bladder smooth muscle layer and L6 and S1 DRG small-medium neurons, with the length of expression determined by the promoter used to drive transgene expression.138,139 In functional studies using the streptozotocin (STZ)-treated diabetic animal rat model, NGF vector-treated animals displayed reduced bladder capacity and postvoid volumes in metabolic cage studies and also cystometry compared with STZ-treated diabetic animals that received the control vector expressing the lacZ reporter gene,138 suggesting a partial restoration of function that correlated with the expression of the NGF transgene within the bladder and afferents. Using similar delivery approaches, replication-defective HSV vectors expressing opioid gene products have also been recently employed in a cystitis model of bladder pain137 described in detail below.
Non-viral delivery methods for treating LUT pain
On account of the clinical observation that opiate drugs are effective in suppressing painful symptoms in patients with IC/PBS,3 and that opioids were able to block pain signaling in animal models,95,98,109 it seems reasonable to assume that opiate gene therapy could reduce LUT pain induced by chronic inflammation if the therapy is able to provide enough of the transgene products in the target organ-specific afferent pathways following the local inoculation of the vectors into target organs in the LUT such as the bladder, urethra or pelvic floor. Toward this goal, non-viral vectors using the gene gun technology to transfer either the pro-opiomelanocortin127 or preproenkephalin (PPE)128 expression cassette plasmid DNAs linked to gold particles into the bladder of rats where the bladder is exposed by a low midline incision were employed in cystitis models. Cystometric measurements in rats receiving pro-opiomelanocortin gold particles127 showed an increased ICI compared with control animals following the infusion of 0.3% acetic acid irritant into the bladder at 3 days after the introduction of three gold particle-DNA bullets into three sites within the bladder wall (Figure 3a), which correlated with β-endorphin expression within the bladder. A lesser effect was seen at 7 days, which disappeared by 2 weeks, attesting to the rather short-term nature of using non-viral methodologies. Moreover, the specificity of the response could be reversed by treatment of these animals with opioid receptor antagonist naloxone hydrochloride. In an attempt to block δ-rather than μ-opioid receptor pain signaling, PPE plasmid DNA linked to gold particles were employed in a similar cystitis model except that 15 μM capsaicin was used as a bladder irritant instead of acetic acid.128 As seen earlier, opioid gene expression at 4–7 days postinjection resulted in a lessening of the effects of capsaicin of δ-opioid receptor activation/signaling that again was specifically blocked by naloxone HCI (Figure 3b). The use of non-viral vectors for opioid gene delivery in these two approaches was limited by the short-term nature of transgene expression and moreover by the expression of the opioid gene only within the bladder itself with none of the product being released within the synaptic cleft of the spinal cord.
Figure 3.
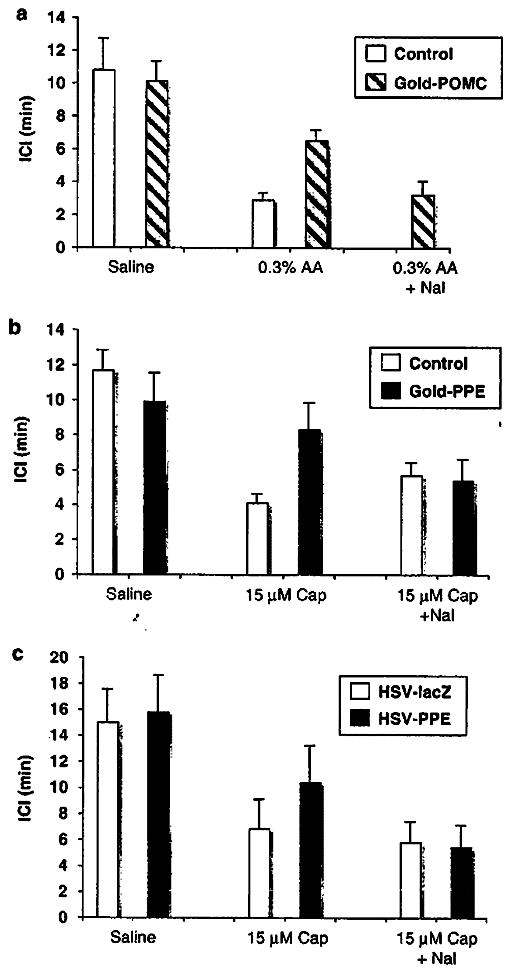
Effects of non-viral and viral vector-mediated opioid gene expression on bladder hyperactivity induced by nociceptive stimuli in rats. Gene gun-mediated delivery of gold particles linked either to the (a) POMC or (b) PPE gene expression cassettes was used to deliver three particles to three different sites within the bladder wall of rats with a lower midline incision to expose the bladder. In similar studies, (c) HSV-PPE or HSV-lacZ control vector were injected into the rat bladder wall Intercontraction intervals (ICIs) during cystometry were decreased by intravesical application of acetic acid (AA) or capsaicin (Cap). However, gene gun (GOLD-PPE or GOLD-POMC) or HSV-PPE-treated animals exhibited a smaller reduction in ICI compared with controls, which was reversed by naloxon (Nal). HSV, herpes simplex virus; POMC, pro-opiomelanocortin; PPE, preproenkephalin.
HSV vector-mediated delivery of enkephalin to inhibit LUT pain
HSV represents an alternative viral vector system that has many biological features that make it attractive for gene delivery to the peripheral nervous system as the virus naturally establishes latency in DRG sensory neurons, in which viral genomes persist for the life of the host in a non-integrated state without altering host cell metabolism. Completely replication-defective genomic viruses can be constructed that retain the ability to establish latency without the threat of viral growth in vivo or the potential of reactivation from the latent state. As part of the virus’s natural life cycle, it possesses the ability to spread from cells of the periphery such as epithelial or mucosal cells to the sensory nerves that innervate the site of primary infection by retrograde axonal transport, thus these vectors can be applied peripherally. Moreover, by selecting the correct dermatome for direct vector inoculation, one can specifically target the sensory neurons in which one wants to express the therapeutic product. For example, inoculation of the footpad with an HSV vector expressing PPE leads to transgene expression in the lumbar DRG and subsequent transport and release of enkephalin from both central (spinal cord) and peripheral (foot) processes of sensory bipolar neurons. Such an approach has been used to treat forms of peripheral nerve pain including formalin-induced pain,140 pain associated with bone cancer141 and neuropathic pain due to spinal nerve ligation.142 In this last example, the effective dose (ED50) of morphine was reduced 10-fold in animals treated with the HSV vector.142
On account of the previous experience using HSV vectors to enable efficient gene transfer in the bladder injection model and the ability of the HSV vectors expressing PPE to block sensory nerve pain in other peripheral models, we employed the identical vectors in a model of capsaicin-induced LUT visceral pain. In this study,137 animals received injection of enkephalin-expressing vector (HSV-PPE) or a control vector expressing the β-galactosidase reporter gene (HSV-lacZ) and cystometry was performed 7–14 days post-transduction before and following infusion of 15 μM capsaicin. Similar to previous non-viral vector studies, we observed a lessening of capsaicin-induced pain response in the HSV-PPE-injected rats compared with the control vector (HSV-lacZ)-injected animals (Figure 3c) that was once again antagonized by naloxone HCI. It is interesting to note that behavioral studies showed a decrease in motionless freezing events but no effect on lower abdominal licking events as we previously showed that freezing and licking events are predominantly correlated with bladder and urethral pain, respectively.143 Both viral genomes and transgene expression were found in both bladder smooth muscle tissue and in L6 and S1 small-medium DRG neurons, mostly corresponding to C-fiber afferent neurons, which correlated with the observed changes in function and behavior.
Summary
Considerable progress has been made in better defining IC/PBS and the components involved in establishing the complex series of symptoms/behaviors associated with this disorder. With the development of new and improved drug therapies for inflammatory and neuropathic pain, the number of patients who receive some relief of their IC/PBS symptoms is increasing. However, a significant number of patients either briefly respond, display numerous unwanted side effects or fail to respond even to the new drug treatments. Gene therapy, using either non-viral or viral vectors represents a new and potentially promising way to deliver anti-nociceptive products directly to the bladder or more importantly the bladder afferents involved in pain signaling, with the viral vector system holding more promise than non-viral methodologies. In particular, HSV vectors represent the ideal delivery vehicle based on their effective transduction of the DRG afferents as a part of that viruses’ natural biology. Replication-defective vectors expressing the PPE opioid gene are currently in use in a phase-I human clinical trial for chronic cancer-induced pain, and their success in such patient trials will have a direct impact on their future use to treat cystitis pain.
References
- 1.Doggweiler-Wiygul R, Blankenship J, MacDiarmid SA. Review on chronic pelvic pain from a urological point of view. World J Urol. 2001;19:160–165. doi: 10.1007/s003450100198. [DOI] [PubMed] [Google Scholar]
- 2.Batra AK, Hanno PM, de Groat WC. Interstitial cystitis. In: Sant GR, editor. AUA Update Series. 2. Vol. 19. American Urological Association; 1999. [Google Scholar]
- 3.Ratner V. Interstitial cystitis: a chronic inflammatory bladder condition. World J Urol. 2001;19:157–159. doi: 10.1007/pl00007096. [DOI] [PubMed] [Google Scholar]
- 4.Rivas DA, Chancellor MB, Blaivas JG. Interstitial cystitis. Adv Urol. 1994:229–265. [Google Scholar]
- 5.Oravisto KJ. Epidemiology of interstitial cystitis. Ann Chir Gynaecol Fenn. 1975;64:75–77. [PubMed] [Google Scholar]
- 6.Held PJ, Hanno PM, Wein AJ, Pauly MV, Cahn MA. Epidemiology of interstitial cystitis. In: Hanno PM, Staskin DR, Krane RJ, editors. Interstitial Cystitis. Springer-Verlag; London, New York: 2002. pp. 29–48. [Google Scholar]
- 7.Curhan GC, Speizer FE, Hunter DJ, Curhan SG, Stampfer MJ. Epidemiology of interstitial cystitis: a population based study. J Urol. 1999;161:549–552. [PubMed] [Google Scholar]
- 8.Payne CK, Joyce GF, Wise M, Clemens JQ. Interstitial cystitis and painful bladder syndrome. J Urol. 2007;177:2042–2049. doi: 10.1016/j.juro.2007.01.124. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura N, Birder LA. Interstitial cystitis and related painful bladder syndromes: pathophysiology. In: Pasricha PJ, Willis WD, Gebhart GF, editors. Chronic Abdominal and Visceral Pain: Theory and Practice. Informa Healthcare; New York: 2007. pp. 495–520. [Google Scholar]
- 10.Rajasekaran M, Stein P, Parsons CL. Toxic factors in human urine that injure urothelium. Int J Urol. 2006;13:409–414. doi: 10.1111/j.1442-2042.2006.01301.x. [DOI] [PubMed] [Google Scholar]
- 11.Erickson DR. Urine markers of interstitial cystitis. Urology. 2001;57:15–21. doi: 10.1016/s0090-4295(01)01128-1. [DOI] [PubMed] [Google Scholar]
- 12.Sant GR, Theoharides TC. Interstitial cystitis. Curr Opin Urol. 1999;9:297–302. doi: 10.1097/00042307-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Sant GR, Hanno PM. Interstitial cystitis: current issues and controversies in diagnosis. Urology. 2001;57:82–88. doi: 10.1016/s0090-4295(01)01131-1. [DOI] [PubMed] [Google Scholar]
- 14.Parsons CL. Successful downregulation of bladder sensory nerves with combination of heparin and alkalinized lidocaine in patients with interstitial cystitis. Urology. 2005;65:45–48. doi: 10.1016/j.urology.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 15.Nickel JC, Egerdie B, Downey J, Singh R, Skehan A, Carr L, et al. A real-life multicentre clinical practice study to evaluate the efficacy and safety of intravesical chondroitin sulphate for the treatment of interstitial cystitis. BJU Int. 2009;103:56–60. doi: 10.1111/j.1464-410X.2008.08028.x. [DOI] [PubMed] [Google Scholar]
- 16.Nordling J, Jorgensen S, Kallestrup E. Cystistat for the treatment of interstitial cystitis: a 3-year follow-up study. Urology. 2001;57:123. doi: 10.1016/s0090-4295(01)01079-2. [DOI] [PubMed] [Google Scholar]
- 17.Morales A, Emerson L, Nickel JC, Lundie M. Intravesical hyaluronic acid in the treatment of refractory interstitial cystitis. J Urol. 1996;156:45–48. [PubMed] [Google Scholar]
- 18.Nickel JC, Forrest J, Barkin J, Payne C, Mosbaugh P. Safety and efficacy of up to 900 mg/day polysulfate sodium (elmiron) in patients with interstitial cystitis. Urology. 2001;57:122–123. doi: 10.1016/s0090-4295(01)01078-0. [DOI] [PubMed] [Google Scholar]
- 19.Nickel JC, Barkin J, Forrest J, Mosbaugh PG, Hernandez-Graulau J, Kaufman D, et al. Randomized, double-blind, dose-ranging study of pentosan polysulfate sodium for interstitial cystitis. Urology. 2005;65:654–658. doi: 10.1016/j.urology.2004.10.071. [DOI] [PubMed] [Google Scholar]
- 20.Parsons CL, Forrest J, Nickel JC, Evans R, Lloyd LK, Barkin J, et al. Effect of pentosan polysulfate therapy on intravesical potassium sensitivity. Urology. 2002;59:329–333. doi: 10.1016/s0090-4295(01)01586-2. [DOI] [PubMed] [Google Scholar]
- 21.Davis EL, El Khoudary SR, Talbott EO, Davis J, Regan LJ. Safety and efficacy of the use of intravesical and oral pentosan polysulfate sodium for interstitial cystitis: a randomized double-blind clinical trial. J Urol. 2008;179:177–185. doi: 10.1016/j.juro.2007.08.170. [DOI] [PubMed] [Google Scholar]
- 22.Rossberger J, Fall M, Peeker R. Critical appraisal of dimethyl sulfoxide treatment for interstitial cystitis: discomfort, side-effects and treatment outcome. Scand J Urol Nephrol. 2005;39:73–77. doi: 10.1080/00365590410018738. [DOI] [PubMed] [Google Scholar]
- 23.Shiga KI, Hirano K, Nishimura J, Niiro N, Naito S, Kanaide H. Dimethyl sulphoxide relaxes rabbit detrusor muscle by decreasing the Ca2+ sensitivity of the contractile apparatus. Br J Pharmacol. 2007;151:1014–1024. doi: 10.1038/sj.bjp.0707317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melchior D, Packer CS, Johnson TC, Kaefer M. Dimethyl sulfoxide: does it change the functional properties of the bladder wall? J Urol. 2003;170:253–258. doi: 10.1097/01.ju.0000071520.73686.3d. [DOI] [PubMed] [Google Scholar]
- 25.Ghoniem GM, McBride D, Sood OP, Lewis V. Clinical experience with multiagent intravesical therapy in interstitial cystitis patients unresponsive to single-agent therapy. World J Urol. 1993;11:178–182. doi: 10.1007/BF00211416. [DOI] [PubMed] [Google Scholar]
- 26.Dimitrakov J, Kroenke K, Steers WD, Berde C, Zurakowski D, Freeman MR, et al. Pharmacologic management of painful bladder syndrome/interstitial cystitis: a systematic review. Arch Intern Med. 2007;167:1922–1929. doi: 10.1001/archinte.167.18.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sant GR, Propert KJ, Hanno PM, Burks D, Culkin D, Diokno AC, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170:810–815. doi: 10.1097/01.ju.0000083020.06212.3d. [DOI] [PubMed] [Google Scholar]
- 28.Dasgupta P, Sharma SD, Womack C, Blackford HN, Dennis P. Cimetidine in painful bladder syndrome: a histopathological study. BJU Int. 2001;88:183–186. doi: 10.1046/j.1464-410x.2001.02258.x. [DOI] [PubMed] [Google Scholar]
- 29.Bouchelouche K, Horn T, Nordling J, Larsen S, Hald T. Cysteinyl-leukotriene D(4) receptors in smooth muscle cells. Urology. 2001;57:109. doi: 10.1016/s0090-4295(01)01037-8. [DOI] [PubMed] [Google Scholar]
- 30.Kelly JD, Young MR, Johnston SR, Keane PF. Clinical response to an oral prostaglandin analogue in patients with interstitial cystitis. Eur Urol. 1998;34:53–56. doi: 10.1159/000019679. [DOI] [PubMed] [Google Scholar]
- 31.van Ophoven A, Hertle L. Long-term results of amitriptyline treatment for interstitial cystitis. J Urol. 2005;174:1837–1840. doi: 10.1097/01.ju.0000176741.10094.e0. [DOI] [PubMed] [Google Scholar]
- 32.van Ophoven A, Pokupic S, Heinecke A, Hertle L. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol. 2004;172:533–536. doi: 10.1097/01.ju.0000132388.54703.4d. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki K, Smith CP, Chuang YC, Lee JY, Kim JC, Chancellor MB. Oral gabapentin (neurontin) treatment of refractory genitourinary tract pain. Tech Urol. 2001;7:47–49. [PubMed] [Google Scholar]
- 34.Agarwal A, Yadav G, Gupta D, Singh PK, Singh U. Evaluation of intra-operative tramadol for prevention of catheter-related bladder discomfort: a prospective, randomized, double-blind study. Br J Anaesth. 2008;101:506–510. doi: 10.1093/bja/aen217. [DOI] [PubMed] [Google Scholar]
- 35.Singh SK, Agarwal MM, Batra YK, Kishore AV, Mandal AK. Effect of lumbar-epidural administration of tramadol on lower urinary tract function. Neurourol Urodyn. 2008;27:65–70. doi: 10.1002/nau.20465. [DOI] [PubMed] [Google Scholar]
- 36.Erickson DR. Interstitial cystitis: update on etiologies and therapeutic options. J Womens Health Gend Based Med. 1999;8:745–758. doi: 10.1089/152460999319075. [DOI] [PubMed] [Google Scholar]
- 37.Foley KM. Opiod analgesics in clinical pain management. In: Akil H, Herz A, Simon EJ, editors. Opioids II. Springer-Verlag; Berlin Heidelberg: New York: 1993. pp. 697–744. [Google Scholar]
- 38.Way EL. Opoid tolerance and physical dependence and their relationship. In: Akil H, Herz A, Simon EJ, editors. Opioids II. Springer-Verlag; Berlin, Heidelberg, New York: 1993. pp. 573–596. [Google Scholar]
- 39.Sairanen J, Tammela TL, Leppilahti M, Multanen M, Paananen I, Lehtoranta K, et al. Cyclosporine A and pentosan polysulfate sodium for the treatment of interstitial cystitis: a randomized comparative study. J Urol. 2005;174:2235–2238. doi: 10.1097/01.ju.0000181808.45786.84. [DOI] [PubMed] [Google Scholar]
- 40.Taneja R, Jawade KK. A rational combination of intravesical and systemic agents for the treatment of interstitial cystitis. Scand J Urol Nephrol. 2007;41:511–515. doi: 10.1080/00365590701435918. [DOI] [PubMed] [Google Scholar]
- 41.Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janig W, Morrison JF. Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on visceral nociception. Prog Brain Res. 1986;67:87–114. doi: 10.1016/s0079-6123(08)62758-2. [DOI] [PubMed] [Google Scholar]
- 43.de Groat WC. Spinal cord projections and neuropeptides in visceral afferent neurons. Prog Brain Res. 1986;67:165–187. doi: 10.1016/s0079-6123(08)62762-4. [DOI] [PubMed] [Google Scholar]
- 44.Yoshimura N, de Groat WC. Neural control of the lower urinary tract. Int J Urol. 1997;4:111–125. doi: 10.1111/j.1442-2042.1997.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 45.de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst. 1981;3:135–160. doi: 10.1016/0165-1838(81)90059-x. [DOI] [PubMed] [Google Scholar]
- 46.Morgan C, Nadelhaft I, de Groat WC. The distribution of visceral primary afferents from the pelvic nerve to Lissauer’s tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J Comp Neurol. 1981;201:415–440. doi: 10.1002/cne.902010308. [DOI] [PubMed] [Google Scholar]
- 47.Thor KB, Hisamitsu T, Roppolo JR, Tuttle P, Nagel J, deGroat WC. Selective inhibitory effects of ethylketocyclazocine on reflex pathways to the external urethral sphincter of the cat. J Pharmacol Exp Ther. 1989;248:1018–1025. [PubMed] [Google Scholar]
- 48.Mallory B, Steers WD, de Groat WC. Electrophysiological study of micturition reflexes in rats. Am J Physiol. 1989;257:R410–R421. doi: 10.1152/ajpregu.1989.257.2.R410. [DOI] [PubMed] [Google Scholar]
- 49.Morrison JF. The physiological mechanisms involved in bladder emptying. Scand J Urol Nephrol Suppl. 1997;184:15–18. [PubMed] [Google Scholar]
- 50.Habler HJ, Janig W, Koltzenburg M. Myelinated primary afferents of the sacral spinal cord responding to slow filling and distension of the cat urinary bladder. J Physiol. 1993;463:449–460. doi: 10.1113/jphysiol.1993.sp019604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maggi CA. The dual, sensory and efferent function of the capsaicin-sensitive primary sensory nerves in the bladder and the urethra. In: Maggi CA, editor. Nervous Control of the Urogenital System. Harwood Academic Publishers; Chur, Switzerland, Langhorne, PA, USA: 1993. pp. 383–422. [Google Scholar]
- 52.McMahon SB, Abel C. A model for the study of visceral pain states: chronic inflammation of the chronic decerebrate rat urinary bladder by irritant chemicals. Pain. 1987;28:109–127. doi: 10.1016/0304-3959(87)91065-7. [DOI] [PubMed] [Google Scholar]
- 53.Wen J, Morrison JFB. Sensitization of pelvic afferent neurons from the rat bladder. J Auton Nerv Syst. 1996;58:187–198. [Google Scholar]
- 54.Keast JR, de Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol. 1992;319:615–623. doi: 10.1002/cne.903190411. [DOI] [PubMed] [Google Scholar]
- 55.Steers WD, Mackway-Gerardi AM, Ciambotti J, de Groat WC. Alterations in neural pathways to the urinary bladder of the rat in response to streptozotocin-induced diabetes. J Auton Nerv Syst. 1994;47:83–94. doi: 10.1016/0165-1838(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 56.Lundberg JM. Pharmacology of cotransmission in the autonomic nervous system: integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol Rev. 1996;48:113–178. [PubMed] [Google Scholar]
- 57.Chancellor MB, de Groat WC. Intravesical capsaicin and resiniferatoxin therapy: spicing up the ways to treat the overactive bladder. J Urol. 1999;162:3–11. doi: 10.1097/00005392-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 58.Elbadawi A. Interstitial cystitis: a critique of current concepts with a new proposal for pathologic diagnosis and pathogenesis. Urology. 1997;49:14–40. doi: 10.1016/s0090-4295(99)80329-x. [DOI] [PubMed] [Google Scholar]
- 59.Steers WD, Tuttle JB. Neurogenic inflammation and nerve growth factor: possible roles in interstitial cystitis. In: Sant GR, editor. Interstitial Cystitis. Lippincott-Raven; Philadelphia: 1997. pp. 67–75. [Google Scholar]
- 60.Yoshimura N, Seki S, de Groat WC. Society for Neuroscience 2002. Abstract Viewer/Itinerary Planner; Washington, DC: 2002. (ed. 451.5, P. N.) [Google Scholar]
- 61.Sengupta JN, Gebhart GF. Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol. 1994;72:2420–2430. doi: 10.1152/jn.1994.72.5.2420. [DOI] [PubMed] [Google Scholar]
- 62.Lazzeri M, Beneforti P, Spinelli M, Zanollo A, Barbagli G, Turini D. Intravesical resiniferatoxin for the treatment of hypersensitive disorder: a randomized placebo controlled study. J Urol. 2000;164:676–679. doi: 10.1097/00005392-200009010-00014. [DOI] [PubMed] [Google Scholar]
- 63.Payne CK, Mosbaugh PG, Forrest JB, Evans RJ, Whitmore KE, Antoci JP, et al. Intravesical resiniferatoxin for the treatment of interstitial cystitis: a randomized, double-blind, placebo controlled trial. J Urol. 2005;173:1590–1594. doi: 10.1097/01.ju.0000154631.92150.ef. [DOI] [PubMed] [Google Scholar]
- 64.Johansson SL, Ogawa K, Fall M. The pathology of interstitial cystitis. In: Sant GR, editor. Interstitial Cystitis. Lippincott-Raven; Philadelphia: 1997. pp. 143–151. [Google Scholar]
- 65.Theoharides TC, Kempuraj D, Sant GR. Mast cell involvement in interstitial cystitis: a review of human and experimental evidence. Urology. 2001;57:47–55. doi: 10.1016/s0090-4295(01)01129-3. [DOI] [PubMed] [Google Scholar]
- 66.Dmitrieva N, McMahon SB. Sensitisation of visceral afferents by nerve growth factor in the adult rat. Pain. 1996;66:87–97. doi: 10.1016/0304-3959(96)02993-4. [DOI] [PubMed] [Google Scholar]
- 67.England S, Bevan S, Docherty RJ. PGE2 modulates the tetrodotoxin-resistant sodium current in neonatal rat dorsal root ganglion neurones via the cyclic AMP-protein kinase A cascade. J Physiol. 1996;495 (Part 2):429–440. doi: 10.1113/jphysiol.1996.sp021604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gold MS, Reichling DB, Shuster MJ, Levine JD. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci USA. 1996;93:1108–1112. doi: 10.1073/pnas.93.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nicol GD, Vasko MR, Evans AR. Prostaglandins suppress an outward potassium current in embryonic rat sensory neurons. J Neurophysiol. 1997;77:167–176. doi: 10.1152/jn.1997.77.1.167. [DOI] [PubMed] [Google Scholar]
- 70.Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, et al. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci. 2006;26:10847–10855. doi: 10.1523/JNEUROSCI.3023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lanteri-Minet M, Bon K, de Pommery J, Michiels JF, Menetrey D. Cyclophosphamide cystitis as a model of visceral pain in rats: model elaboration and spinal structures involved as revealed by the expression of c-Fos and Krox-24 proteins. Exp Brain Res. 1995;105:220–232. doi: 10.1007/BF00240958. [DOI] [PubMed] [Google Scholar]
- 72.Kirimoto T, Nakano K, Irimura K, Hayashi Y, Matsuura N, Kiniwa M, et al. Beneficial effects of suplatast tosilate (1PD-1151T) in a rat cystitis model induced by intravesical hydrochloric acid. BJU Int. 2007;100:935–939. doi: 10.1111/j.1464-410X.2007.07044.x. [DOI] [PubMed] [Google Scholar]
- 73.Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sculptoreanu A, de Groat WC, Buffington CA, Birder LA. Abnormal excitability in capsaicin-responsive DRG neurons from cats with feline interstitial cystitis. Exp Neurol. 2005;193:437–443. doi: 10.1016/j.expneurol.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 75.Kakidani H, Furutani Y, Takahashi H, Noda M, Morimoto Y, Hirose T, et al. Cloning and sequence analysis of cDNA for porcine beta-neo-endorphin/dynorphin precursor. Nature. 1982;298:245–249. doi: 10.1038/298245a0. [DOI] [PubMed] [Google Scholar]
- 76.Nakanishi S, Inoue A, Kita T, Nakamura M, Chang AC, Cohen SN, et al. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979;278:423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- 77.Noda M, Furutani Y, Takahashi H, Toyosato M, Hirose T, Inayama S, et al. Cloning and sequence analysis of cDNA for bovine adrenal preproenkephalin. Nature. 1982;295:202–206. doi: 10.1038/295202a0. [DOI] [PubMed] [Google Scholar]
- 78.Wang JB, Imai Y, Eppler CM, Gregor P, Spivak CE, Uhl GR. Mu opiate receptor: cDNA cloning and expression. Proc Natl Acad Sci USA. 1993;90:10230–10234. doi: 10.1073/pnas.90.21.10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen Y, Mestek A, Liu J, Hurley JA, Yu L. Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Mol Pharmacol. 1993;44:8–12. [PubMed] [Google Scholar]
- 80.Fukuda K, Kato S, Mori K, Nishi M, Takeshima H. Primary structures and expression from cDNAs of rat opioid receptor delta- and mu-subtypes. FEBS Lett. 1993;327:311–314. doi: 10.1016/0014-5793(93)81011-n. [DOI] [PubMed] [Google Scholar]
- 81.Thompson RC, Mansour A, Akil H, Watson SJ. Cloning and pharmacological characterization of a rat mu opioid receptor. Neuron. 1993;11:903–913. doi: 10.1016/0896-6273(93)90120-g. [DOI] [PubMed] [Google Scholar]
- 82.Minami M, Onogi T, Toya T, Katao Y, Hosoi Y, Maekawa K, et al. Molecular cloning and in situ hybridization histochemistry for rat mu-opioid receptor. Neurosci Res. 1994;18:315–322. doi: 10.1016/0168-0102(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 83.Yasuda K, Raynor K, Kong H, Breder CD, Takeda J, Reisine T, et al. Cloning and functional comparison of kappa and delta opioid receptors from mouse brain. Proc Natl Acad Sci USA. 1993;90:6736–6740. doi: 10.1073/pnas.90.14.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mansour A, Thompson RC, Akil H, Watson SJ. Delta opioid receptor mRNA distribution in the brain: comparison to delta receptor binding and proenkephalin mRNA. J Chem Neuroanat. 1993;6:351–362. doi: 10.1016/0891-0618(93)90010-2. [DOI] [PubMed] [Google Scholar]
- 85.Kieffer BL, Befort K, Gaveriaux-Ruff C, Hirth CG. The delta-opioid receptor: isolation of a cDNA by expression cloning and pharmacological characterization. Proc Natl Acad Sci USA. 1992;89:12048–12052. doi: 10.1073/pnas.89.24.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Evans CJ, Keith DE, Jr, Morrison H, Magendzo K, Edwards RH. Cloning of a delta opioid receptor by functional expression. Science. 1992;258:1952–1955. doi: 10.1126/science.1335167. [DOI] [PubMed] [Google Scholar]
- 87.Nishi M, Takeshima H, Fukuda K, Kato S, Mori K. cDNA cloning and pharmacological characterization of an opioid receptor with high affinities for kappa-subtype-selective ligands. FEBS Lett. 1993;330:77–80. doi: 10.1016/0014-5793(93)80923-i. [DOI] [PubMed] [Google Scholar]
- 88.Minami M, Toya T, Katao Y, Maekawa K, Nakamura S, Onogi T, et al. Cloning and expression of a cDNA for the rat kappa-opioid receptor. FEBS Lett. 1993;329:291–295. doi: 10.1016/0014-5793(93)80240-u. [DOI] [PubMed] [Google Scholar]
- 89.Meng F, Xie GX, Thompson RC, Mansour A, Goldstein A, Watson SJ, et al. Cloning and pharmacological characterization of a rat kappa opioid receptor. Proc Natl Acad Sci USA. 1993;90:9954–9958. doi: 10.1073/pnas.90.21.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li S, Zhu J, Chen C, Chen YW, Deriel JK, Ashby B, et al. Molecular cloning and expression of a rat kappa opioid receptor. Biochem J. 1993;295 (Part 3):629–633. doi: 10.1042/bj2950629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen Y, Mestek A, Liu J, Yu L. Molecular cloning of a rat kappa opioid receptor reveals sequence similarities to the mu and delta opioid receptors. Biochem J. 1993;295 (Part 3):625–628. doi: 10.1042/bj2950625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yaksh TL. The spinal actions of opiods. In: Akil H, Herz A, Simon EJ, editors. Opioids II. Springer-Verlag; Berlin, Heidelberg, New York: 1993. pp. 53–90. [Google Scholar]
- 93.Hiller JM, Simon EJ, Crain SM, Peterson ER. Opiate receptors in cultures of fetal mouse dorsal root ganglia (DRG) and spinal cord: predominance in DRG neurites. Brain Res. 1978;145:396–400. doi: 10.1016/0006-8993(78)90875-2. [DOI] [PubMed] [Google Scholar]
- 94.Fields HL, Emson PC, Leigh BK, Gilbert RF, Iversen LL. Multiple opiate receptor sites on primary afferent fibres. Nature. 1980;284:351–353. doi: 10.1038/284351a0. [DOI] [PubMed] [Google Scholar]
- 95.Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med. 1995;332:1685–1690. doi: 10.1056/NEJM199506223322506. [DOI] [PubMed] [Google Scholar]
- 96.Stein C, Yassouridis A. Peripheral morphine analgesia. Pain. 1997;71:119–121. [PubMed] [Google Scholar]
- 97.Barber A, Gottschlich R. Opioid agonists and antagonists: an evaluation of their peripheral actions in inflammation. Med Res Rev. 1992;12:525–562. doi: 10.1002/med.2610120505. [DOI] [PubMed] [Google Scholar]
- 98.Stein C. Peripheral mechanisms of opioid analgesia. Anesth Analg. 1993;76:182–191. doi: 10.1213/00000539-199301000-00031. [DOI] [PubMed] [Google Scholar]
- 99.Su X, Sengupta JN, Gebhart GF. Effects of opioids on mechanosensitive pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol. 1997;77:1566–1580. doi: 10.1152/jn.1997.77.3.1566. [DOI] [PubMed] [Google Scholar]
- 100.Su X, Sengupta JN, Gebhart GF. Effects of kappa opioid receptor-selective agonists on responses of pelvic nerve afferents to noxious colorectal distension. J Neurophysiol. 1997;78:1003–1012. doi: 10.1152/jn.1997.78.2.1003. [DOI] [PubMed] [Google Scholar]
- 101.Attali B, Saya D, Nah SY, Vogel Z. Kappa opiate agonists inhibit Ca2+ influx in rat spinal cord-dorsal root ganglion cocultures. Involvement of a GTP-binding protein. J Biol Chem. 1989;264:347–353. [PubMed] [Google Scholar]
- 102.Dunlap K, Fischbach GD. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol. 1981;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Werz MA, Macdonald RL. Heterogeneous sensitivity of cultured dorsal root ganglion neurones to opioid peptides selective for mu- and delta-opiate receptors. Nature. 1982;299:730–733. doi: 10.1038/299730a0. [DOI] [PubMed] [Google Scholar]
- 104.Jessell TM, Dodd J. Neurotransmitters and differentiation antigens in subsets of sensory neurons projecting to the spinal dorsal horn. Res Publ Assoc Res Nerv Ment Dis. 1986;64:111–133. [PubMed] [Google Scholar]
- 105.Yaksh TL, Jessell TM, Gamse R, Mudge AW, Leeman SE. Intrathecal morphine inhibits substance P release from mammalian spinal cord in vivo. Nature. 1980;286:155–157. doi: 10.1038/286155a0. [DOI] [PubMed] [Google Scholar]
- 106.Zieglgansberger W, Sutor B. Responses of substantia gelatinosa neurons to putative neurotransmitters in an in vitro preparation of the adult rat spinal cord. Brain Res. 1983;279:316–320. doi: 10.1016/0006-8993(83)90201-9. [DOI] [PubMed] [Google Scholar]
- 107.de Groat WC, Yoshimura N. Pharmacology of the lower urinary tract. Annu Rev Pharmacol Toxicol. 2001;41:691–721. doi: 10.1146/annurev.pharmtox.41.1.691. [DOI] [PubMed] [Google Scholar]
- 108.de Groat WC, Booth AM, Yoshimura N. In: Nervous Control of the Urogenital System. Maggi CA, editor. Harwood Academic Publishers; Chur, Switzerland; Langhorne, PA, USA: 1993. pp. 227–290. [Google Scholar]
- 109.Booth AM, Hisamitsu T, Kawatani M, de Groat WC. Regulation of urinary bladder capacity by endogenous opioid peptides. J Urol. 1985;133:339–342. doi: 10.1016/s0022-5347(17)48935-x. [DOI] [PubMed] [Google Scholar]
- 110.Downie JW. Pharmacological manipulation of central micturition circuitry. Curr Opin Central Periph Nerv Sys. 1999;1:231–239. [Google Scholar]
- 111.Dray A, Metsch R. Inhibition of urinary bladder contractions by a spinal action of morphine and other opioids. J Pharmacol Exp Ther. 1984;231:254–260. [PubMed] [Google Scholar]
- 112.Thor KB, Morgan C, Nadelhaft I, Houston M, de Groat WC. Organization of afferent and efferent pathways in the pudendal nerve of the female cat. J Comp Neurol. 1989;288:263–279. doi: 10.1002/cne.902880206. [DOI] [PubMed] [Google Scholar]
- 113.Hisamitsu T, de Groat WC. The inhibitory effect of opioid peptides and morphine applied intrathecally and intracerebro-ventricularly on the micturition reflex in the cat. Brain Res. 1984;298:51–65. doi: 10.1016/0006-8993(84)91146-6. [DOI] [PubMed] [Google Scholar]
- 114.Mallory BS, Roppolo JR, de Groat WC. Pharmacological modulation of the pontine micturition center. Brain Res. 1991;546:310–320. doi: 10.1016/0006-8993(91)91495-m. [DOI] [PubMed] [Google Scholar]
- 115.Chuang YC, Fraser MO, Yu Y, Beckel JM, Seki S, Nakanishi Y, et al. Analysis of the afferent limb of the vesicovascular reflex using neurotoxins, resiniferatoxin and capsaicin. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1302–R1310. doi: 10.1152/ajpregu.2001.281.4.R1302. [DOI] [PubMed] [Google Scholar]
- 116.Craft RM, Henley SR, Haaseth RC, Hruby VJ, Porreca F. Opioid antinociception in a rat model of visceral pain: systemic versus local drug administration. J Pharmacol Exp Ther. 1995;275:1535–1542. [PubMed] [Google Scholar]
- 117.Meen M, Coudore-Civiale MA, Eschalier A, Boucher M. Involvement of hypogastric and pelvic nerves for conveying cystitis induced nociception in conscious rats. J Urol. 2001;166:318–322. [PubMed] [Google Scholar]
- 118.Chancellor MB, Yoshimura N, Pruchnic R, Huard J. Gene therapy strategies for urological dysfunction. Trends Mol Med. 2001;7:301–306. doi: 10.1016/s1471-4914(01)02088-3. [DOI] [PubMed] [Google Scholar]
- 119.Burton EA, Wechuck JB, Wendell SK, Goins WF, Fink DJ, Glorioso JC. Multiple applications for replication-defective herpes simplex virus vectors. Stem Cells. 2001;19:358–377. doi: 10.1634/stemcells.19-5-358. [DOI] [PubMed] [Google Scholar]
- 120.Goss JR, Goins WF, Glorioso JC. Gene therapy applications for the treatment of neuropathic pain. Expert Rev Neurother. 2007;7:487–506. doi: 10.1586/14737175.7.5.487. [DOI] [PubMed] [Google Scholar]
- 121.Fraser MO, Lavelle JP, Sacks MS, Chancellor MB. The future of bladder control-intravesical drug delivery, a pinch of pepper, and gene therapy. Rev Urol. 2002;4:1–11. [PMC free article] [PubMed] [Google Scholar]
- 122.Nogawa M, Yuasa T, Kimura S, Tanaka M, Kuroda J, Sato K, et al. Intravesical administration of small interfering RNA targeting PLK-1 successfully prevents the growth of bladder cancer. J Clin Invest. 2005;115:978–985. doi: 10.1172/JCI23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tyagi P, Wu PC, Chancellor M, Yoshimura N, Huang L. Recent advances in intravesical drug/gene delivery. Mol Pharm. 2006;3:369–379. doi: 10.1021/mp060001j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Iwashita H, Yoshida M, Nishi T, Otani M, Ueda S. In vivo transfer of a neuronal nitric oxide synthase expression vector into the rat bladder by electroporation. BJU Int. 2004;93:1098–1103. doi: 10.1111/j.1464-410X.2003.04788.x. [DOI] [PubMed] [Google Scholar]
- 125.Otani M, Yoshida M, Iwashita H, Kawano Y, Miyamae K, Inadome A, et al. Electroporation-mediated muscarinic M3 receptor gene transfer into rat urinary bladder. Int J Urol. 2004;11:1001–1008. doi: 10.1111/j.1442-2042.2004.00924.x. [DOI] [PubMed] [Google Scholar]
- 126.Yoo JJ, Soker S, Lin LF, Mehegan K, Guthrie PD, Atala A. Direct in vivo gene transfer to urological organs. J Urol. 1999;162:1115–1118. doi: 10.1016/S0022-5347(01)68088-1. [DOI] [PubMed] [Google Scholar]
- 127.Chuang YC, Chou AK, Wu PC, Chiang PH, Yu TJ, Yang LC, et al. Gene therapy for bladder pain with gene gun particle encoding pro-opiomelanocortin cDNA. J Urol. 2003;170:2044–2048. doi: 10.1097/01.ju.0000092945.76827.47. [DOI] [PubMed] [Google Scholar]
- 128.Chuang YC, Chuang YC, Yang LC, Chiang PH, Kang HY, Ma WL, et al. Gene gun particle encoding preproenkephalin cDNA produces analgesia against capsaicin-induced bladder pain in rats. Urology. 2005;65:804–810. doi: 10.1016/j.urology.2004.10.070. [DOI] [PubMed] [Google Scholar]
- 129.Werthman PE, Drazan KE, Rosenthal JT, Khalili R, Shaked A. Adenoviral-p53 gene transfer to orthotopic and peritoneal murine bladder cancer. J Urol. 1996;155:753–756. [PubMed] [Google Scholar]
- 130.Engler H, Anderson SC, Machemer TR, Philopena JM, Connor RJ, Wen SF, et al. Ethanol improves adenovirus-mediated gene transfer and expression to the bladder epithelium of rodents. Urology. 1999;53:1049–1053. doi: 10.1016/s0090-4295(98)00641-4. [DOI] [PubMed] [Google Scholar]
- 131.Connor RJ, Connor RJ, Engler H, Machemer T, Philopena JM, Horn MT, et al. Identification of polyamides that enhance adenovirus-mediated gene expression in the urothelium. Gene Therapy. 2001;8:41–48. doi: 10.1038/sj.gt.3301348. [DOI] [PubMed] [Google Scholar]
- 132.Lin LF, Zhu G, Yoo JJ, Soker S, Sukhatme VP, Atala A. A system for the enhancement of adenovirus mediated gene transfer to uro-epithelium. J Urol. 2002;168:813–818. [PubMed] [Google Scholar]
- 133.Ramesh N, Memarzadeh B, Ge Y, Frey D, VanRoey M, Rojas V, et al. Identification of pretreatment agents to enhance adenovirus infection of bladder epithelium. Mol Ther. 2004;10:697–705. doi: 10.1016/j.ymthe.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 134.Lee SS, Eisenlohr LC, McCue PA, Mastrangelo MJ, Lattime EC. Intravesical gene therapy: in vivo gene transfer using recombinant vaccinia virus vectors. Cancer Res. 1994;54:3325–3328. [PubMed] [Google Scholar]
- 135.Siemens DR, Austin JC, See WA, Tartaglia J, Ratliff TL. Evaluation of gene transfer efficiency by viral vectors to murine bladder epithelium. J Urol. 2001;165:667–671. doi: 10.1097/00005392-200102000-00091. [DOI] [PubMed] [Google Scholar]
- 136.Brooks AD, Ng B, Liu D, Brownlee M, Burt M, Federoff HJ, et al. Specific organ gene transfer in vivo by regional organ perfusion with herpes viral amplicon vectors: implications for local gene therapy. Surgery. 2001;129:324–334. doi: 10.1067/msy.2001.111697. [DOI] [PubMed] [Google Scholar]
- 137.Yokoyama H, Sasaki K, Franks ME, Goins WF, Goss JR, Degroat WC, et al. Gene therapy for bladder overactivity and nociception with herpes simplex virus vectors expressing preproenkephalin. Hum Gene Ther. 2008;20:63–71. doi: 10.1089/hum.2008.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sasaki K, Chancellor MB, Goins WF, Phelan MW, Glorioso JC, de Groat WC, et al. Gene therapy using replication-defective herpes simplex virus vectors expressing nerve growth factor in a rat model of diabetic cystopathy. Diabetes. 2004;53:2723–2730. doi: 10.2337/diabetes.53.10.2723. [DOI] [PubMed] [Google Scholar]
- 139.Goins WF, Yoshimura N, Phelan MW, Yokoyama T, Fraser MO, Ozawa H, et al. Herpes simplex virus mediated nerve growth factor expression in bladder and afferent neurons: potential treatment for diabetic bladder dysfunction. J Urol. 2001;165:1748–1754. [PubMed] [Google Scholar]
- 140.Goss JR, Mata M, Goins WF, Wu HH, Glorioso JC, Fink DJ. Antinociceptive effect of a genomic herpes simplex virus-based vector expressing human proenkephalin in rat dorsal root ganglion. Gene Therapy. 2001;8:551–556. doi: 10.1038/sj.gt.3301430. [DOI] [PubMed] [Google Scholar]
- 141.Goss JR, Harley CF, Mata M, O’Malley ME, Goins WF, Hu X, et al. Herpes vector-mediated expression of proenkephalin reduces bone cancer pain. Ann Neurol. 2002;52:662–665. doi: 10.1002/ana.10343. [DOI] [PubMed] [Google Scholar]
- 142.Hao S, Mata M, Goins W, Glorioso JC, Fink DJ. Transgene-mediated enkephalin release enhances the effect of morphine and evades tolerance to produce a sustained antiallodynic effect in neuropathic pain. Pain. 2003;102:135–142. doi: 10.1016/s0304-3959(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 143.Saitoh C, Chancellor MB, de Groat WC, Yoshimura N. Effects of intravesical instillation of resiniferatoxin on bladder function and nociceptive behavior in freely moving, conscious rats. J Urol. 2008;179:359–364. doi: 10.1016/j.juro.2007.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]


