Abstract
The innexin family of gap junction proteins has 25 members in Caenorhabditis elegans. Here, we describe the first high-resolution expression map of all members through analysis of live worms transformed with green fluorescent protein under the control of entire promoter regions. Our analyses show that innexins have dynamic expression patterns throughout development and are found in virtually all cell types and tissues. Complex tissues, such as the pharynx, intestine, gonad, as well as scaffolding tissues and guidepost cells express a variety of innexins in overlapping or complementary patterns, suggesting they may form heteromeric and heterotypic channels. Innexin expression occurs in several types of cells that are not known to form gap junctions as well as in a pair of migrating cells, suggesting they may have hemichannel function. Therefore, innexins likely play roles in almost all body functions, including embryonic development, cell fate determination, oogenesis, egg laying, pharyngeal pumping, excretion, and locomotion.
Keywords: C. elegans, innexin, gap junction, intercellular signaling, electrical coupling
INTRODUCTION
Gap junctions are intercellular channels, which are widely expressed in vertebrate and invertebrate tissues and allow passage of ions and small molecules, including amino acids, metabolites, and certain fluorescent and signaling molecules between two cells. Each gap junction is formed by oligomerization of six subunits on each cell membrane in homotypic (made of a single protein species on both cell membranes), heterotypic (made of two different homomeric hemichannels on each membrane), and heteromeric (made of a mixture of subunit composition on both membranes) manner (Fig. 1; Phelan and Starich, 2001). Gap junction subunits belong to two superfamilies of proteins; connexins (found only in vertebrates) and pannexins (found both in vertebrates and invertebrates; Starich et al., 1996; Phelan et al., 1998; Willecke et al., 2002; Bruzzone et al., 2003; Barbe et al., 2006). The invertebrate pannexins are called “innexins,” and in C. elegans, the genes that encode them adopt the prefix “inx.” Although connexins, vertebrate pannexins, and innexins are very distinct in their primary sequences, they share major structural and functional properties. For example, all of them have four putative membrane spanning domains with two or three cysteine residues in the extracellular loops (Panchin et al., 2000). They are suggested to be modulated by similar physiological factors (e.g., junctional voltage, intracellular pH and Ca2+) and blocked by similar pharmacological factors (e.g., glycyrrhetinic acid, octanol, and carbenoxolone; Bohrmann and Haas-Assenbaum, 1993; Gho, 1994; Landesman et al., 1999; Harris, 2001; Bruzzone et al., 2005; Liu et al., 2006). In addition to gap junctions, these molecules are suggested to form hemichannels that connect a cell’s interior to the extracellular space, providing a pathway for release and uptake of molecules and ions in a controlled manner (Saez et al., 2005; Spray et al., 2006).
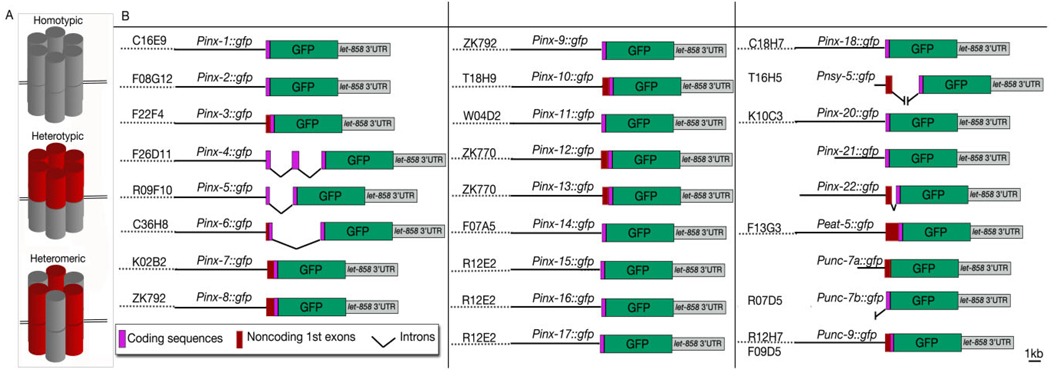
Fig. 1.
A: Graphic rendition of subunit composition of homotypic, heterotypic, and heteromeric gap junctions. B: Transcriptional fusion constructs used in this study. For all innexin genes except inx-21 and inx-22, a genomic DNA fragment containing a short upstream regulatory sequence of each innexin gene (black straight bars) was subcloned into a green fluorescent protein (GFP) expression vector which was then coinjected with the corresponding cosmid (dotted lines) into hermaphrodites allowing for in vivo homologous recombination. Because there were no suitable cosmids for inx-21 and inx-22, two long fragments upstream of the initiation sites of these two genes were polymerase chain reaction (PCR)-cloned and used for subcloning. T16H5 only contains the 706-bp sequence upstream of the nsy-5 initiation site, which might have revealed activating sequences in two neurons (AVK and AVB), which were not observed to express nsy-5 previously (Chuang et al., 2007). The transcription products of Punc-7a∷GFP and Punc-7b∷GFP are referred to as unc-7a and unc-7b, respectively, throughout this study.
Invertebrate systems, in particular Drosophila and C. elegans, have played important roles in understanding gap junction properties. The Drosophila and C. elegans genomes contain 8 and 25 innexin genes, respectively. Functions have been assigned to several of the innexins through analyses of phenotypes caused by mutations or RNA interference-induced silencing of the innexin genes. In Drosophila, innexin 1 (ogre) is involved in the development of the visual system (Curtin et al., 2002), innexin 2 and innexin 3 in epithelial tissue organization (Bauer et al., 2002, 2004; Lehmann et al., 2006), innexin 4 in germ cell differentiation (Tazuke et al., 2002; Gilboa et al., 2003), and innexin 8 (Shaking B) in electrical transmission in the giant fiber system (Blagburn et al., 1999) and the visual system (Curtin et al., 2002). In C. elegans, INX-3 is essential to embryonic morphogenesis as well as pharyngeal development and proper pumping (Starich et al., 2003), EAT-5 and INX-6 are required for synchronized pharyngeal muscle contractions (Avery, 1993; Starich et al., 1996; Li et al., 2003), INX-16 is important to intestinal muscle contractions by propagating Ca2+ waves between intestinal cells (Peters et al., 2007), INX-14 and INX-22 are essential for oocyte maturation and fertilization (Whitten and Miller, 2007), INX-19 (also known as NSY-5) is involved in a neuronal (AWC) cell fate determination (Chuang et al., 2007), and UNC-7 and UNC-9 appear to be involved in several neural functions, including locomotion, and sensitivities to volatile anesthetics and the anthelminthic ivermectin (Starich et al., 2001, 2003; Li et al., 2003; Phelan, 2005; Norman and Maricq, 2007; Whitten and Miller, 2007). Recent electrophysiological analyses show that UNC-9 but not UNC-7 is required for electrical coupling of body-wall muscle cells (Liu et al., 2006), suggesting that the two innexins do not have identical functions, although they are sometimes coexpressed. The functions of many C. elegans innexins remain completely unknown.
Here, we describe a comprehensive, in vivo analysis of expression of all innexins in C. elegans at high resolution by expressing transcriptional innexin–green fluorescent protein (GFP) fusion markers. Although the expression patterns of some C. elegans innexins have been analyzed previously by antibody staining of fixed animals or by fluorescent microscopy of live animals expressing transcriptional or translational GFP fusion markers, most of these studies tended to focus on analyzing innexins associated with a particular cell group and function, and did not include complete descriptions of cellular identities. In the present study, by using an in vivo homologous recombination approach to generate the promoter∷gfp transcriptional fusions, we were able to include the entire promoter regions in most cases (Fig. 1; Supp. Table S1, which is available online). Our analyses revealed unexpected dynamism, range, and complexity of the innexin expression patterns, suggesting that, even for the innexins with assigned functions, there might be additional biological roles. Because subtle phenotypes associated with specific innexin deficiencies can be more easily detected if the types of cells/tissues expressing them are known, our current description of the expression patterns for C. elegans innexins at various developmental stages can also guide future studies analyzing innexin functions through reverse genetics.
RESULTS
C. elegans Innexins Have Homologs in C. briggsae and C. remanei
The completion of genomic sequencing of C. elegans revealed 22 innexin genes in addition to eat-5, unc-7, and unc-9, which had been discovered previously through mutant analyses (C. elegans Sequencing Consortium, 1998; Bargmann, 1998). These additional innexins were numbered arbitrarily from inx-1 to inx-22. Further sequencing and genomic analysis of two additional Caenorhabditis species (C. briggsae and C. remanei) revealed that each of these species has retained at least one member of each type of these innexins, except inx-8 and inx-9, which share a single ortholog in C. briggsae, but have distinct orthologs in C. remanei (www.Wormbase.org). This strongly suggests that each innexin gene is a true gene rather than a pseudogene. Among C. elegans innexins, there are 3 sets of polycistronic ones: inx-12 and inx-13, inx-16 and inx-17, and inx-21, and inx-22. inx-21 and inx-22 have open reading frames separated by only 65 bp; however, they are unlikely to be recent duplications because their predicted amino acid sequences are only 35% identical (www.Wormbase.org). They also seem to have distinct expression patterns (see below). inx-12 and inx-13 and inx-16 and inx-17 have partially overlapping expression patterns (see below). Although inx-8 and inx-9 are only 819 bp away in the genome and their translational products are 84% identical, they are not polycistronic and their expression patterns only partially overlap.
Innexins in the C. elegans Alimentary System
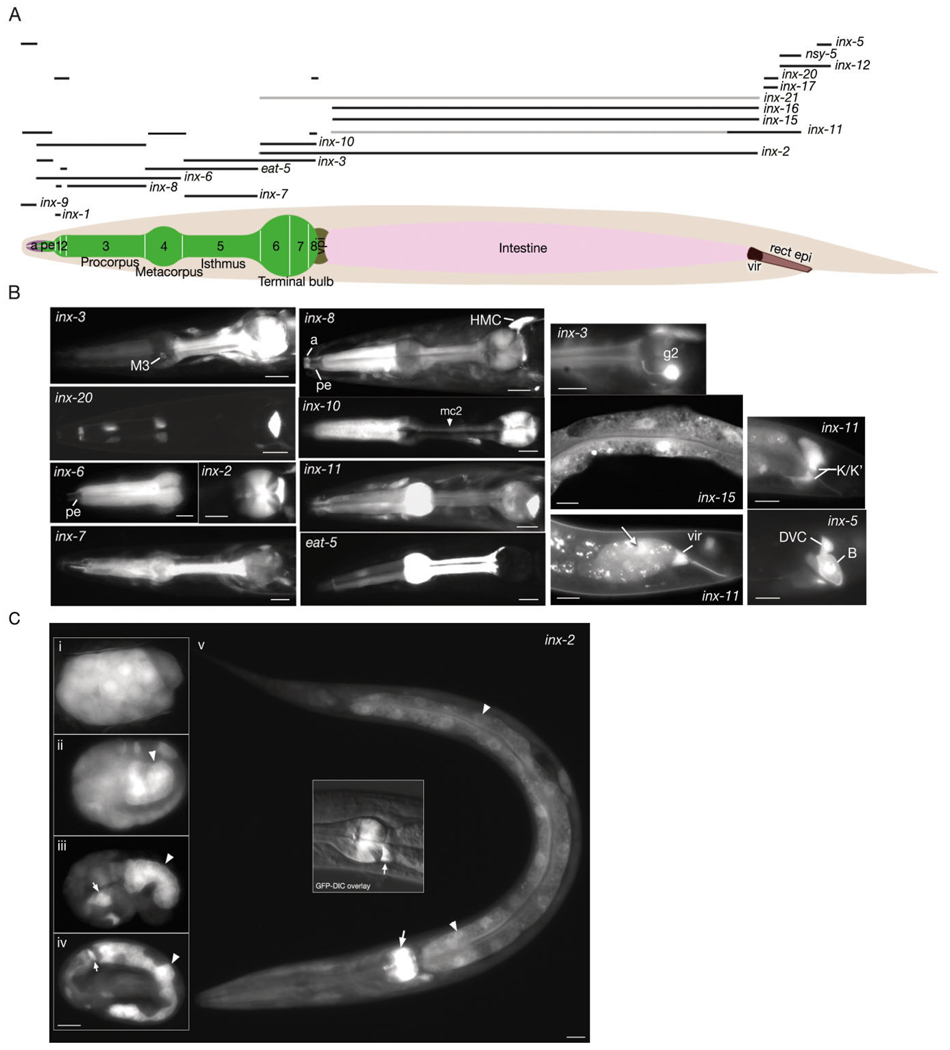
The C. elegans alimentary system is made of three major tissues: transitional epithelia at the anterior and posterior ends, and the pharynx and intestine between them. To date, several innexins, some with important physiological functions, have been identified in the alimentary system through antibody staining, expression of GFP transcriptional and translational fusions, and forward genetics. These include inx-3, inx-6, inx-10, inx-11, inx-16, and eat-5 (Starich et al., 1996, 2001, 2003; Li et al., 2003; Phelan, 2005). In our study, we were able to detect all these innexins in previously described locations, as well as many other innexins in various parts of the alimentary system (Fig. 2; Table 1). The two juxtaposed anterior transitional epithelia of the alimentary system express different sets of innexins; anterior and posterior arcades of the buccal cavity express inx-5, inx-6, and inx-11, while the pharyngeal epithelium expresses inx-3, inx-6, and inx-10. In pharynx proper, pharyngeal muscles express inx-1, inx-2, inx-3, inx-6, inx-7, inx-8, inx-10, inx-11, and eat-5 in overlapping patterns. All marginal cells (mc) express inx-10, mc1 and mc2 express eat-5, while mc2 also expresses inx-6 (Fig. 2). In our study, we detected unc-9 in M5, I1, I6, and NSM neurons, inx-5 in M1, inx-9 in I3, inx-7 in MC, inx-12 in M2, and inx-13 in both M1 and M2 neurons. Both pharyngeal gland cells were found to express inx-11. Additionally, g1 gland cells express inx-18 while g2 gland cells express inx-3. The pharyngeal–intestinal valve, which constitutes the passageway from the pharynx into the intestine, expresses inx-2 (Fig. 2). Intestinal cells primarily express four types of innexins; inx-2, inx-11, inx-15 and inx-16. Of these, inx-2 is expressed at high levels during embryonic stages (see below). Its intestinal expression levels start to decrease after hatching, becoming barely detectable in the adult, although its pharyngeal expression continues in the adult (Fig. 2). The expression domain of inx-15 is restricted to the intestine in the whole body and similar to inx-2 it is more strongly expressed during the embryonic development (data not shown). inx-11 is mainly expressed in the posterior intestinal cells, which are suggested to function as a pacemaker for rhythmic defecation (Dal Santo et al., 1999). inx-16 is expressed at high levels in the intestine throughout the lifespan of the animal starting from two-fold stage of embryogenesis. Some other innexins were detected at low levels in the intestine (Table 1). Among these, inx-21 is unique in that its expression was found to be limited to the body wall muscle in three-fold stage embryo and the intestine in the adult. No other expression pattern was seen for this innexin. The intestinal-rectal valve, which forms the passageway from the intestine to the rectum, expresses three different innexins: inx-11, inx-17, and inx-20. Similar to pharyngeal g2, the rectal gland expresses inx-3 and during early larval stages expresses inx-9. Within the posterior transitional (rectal) epithelium, inx-12 is expressed broadly, while inx-5 seemed to be expressed at higher levels in B epithelial cell and inx-11 and nsy-5 were seen at higher levels in K.a and K′ cells.
Fig. 2. Innexins in the alimentary canal.
A: Graphic representation of expression domains of innexins in epithelial and muscle tissue is shown as black bars above the hermaphrodite image. Darker bars represent stronger adult expression. Thus, inx-11 is expressed strongly in the posterior intestine and faintly in the rest of the intestine. inx-21 is expressed solely in the intestine (light pink) in the adult, however the expression level is low. The numbers shown on the bi-lobed pharynx (green) indicate the pharyngeal muscles. A, arcades (light purple); pe, pharyngeal epithelium; vpi, pharyngeal valve (brown); vir, intestinal rectal valve (dark brown); rect epi, rectal epithelium (light brown). B: Fluorescent micrographs of transgenic animals expressing p inx∷GFP reporters. Left two columns show pharyngeal images. M3, M3 motor neuron; mc2, marginal cell 2; HMC, head mesodermal cell. Third column top panel shows g2 gland that expresses inx-3. Middle panel shows inx-15 expression in intestine and bottom panel shows inx-11 expression in intestinal rectal valve and also the posterior intestine (arrow). Rectal epithelial (K/K′ and B) innexin expressions are shown on the rightmost column. DVC, DVC neuron. C: Fluorescent micrographs of pinx-2∷GFP expression through developmental stages. i: Approximately 30-cell stage. inx-2 is expressed throughout the embryo. ii: Approximately embryonic day (E) 16 stage. Expression of inx-2 is stronger in the intestinal precursors (arrowhead) than the rest of the embryo although it continues to be expressed outside the alimentary canal. iii: Comma stage embryo. inx-2 expression is restricted to intestinal cells (arrowhead) and some pharyngeal precursors (arrow). iv: Three-fold stage. inx-2 expression continues at high levels in the intestinal cells (arrowhead) and terminal bulb pharyngeal muscle (arrow). v: L1 larva. inx-2 expression is decreased in the intestinal cells (arrowheads) although it continues to be expressed at relatively high levels in the pharyngeal muscle (arrow) and the pharyngeal–intestinal valve (middle inset, arrow). Scale bars = 10 µm.
TABLE 1.
Innexin Expression in the Alimentary Canala
| Strong and consistent | Weak or rare | |
|---|---|---|
| Anterior arcades | inx-5, inx-9, inx-11 | inx-3, inx-12 |
| Posterior arcades | inx-5, inx-9, inx-11 | inx-3, inx-12, unc-7a, unc-9 |
| Pharyngeal epithelium | inx-3, inx-6, inx-10, inx-11 | inx-7, inx-8, inx-20 |
| pm1 | inx-1, inx-6, inx-8, inx-10, inx-20 | inx-11, inx-7 |
| pm2 | inx-6, inx-10, inx-20, eat-5 | inx-3, inx-7, inx- 8, inx-9, inx-11 |
| pm3 | inx-6, inx-8, inx-10 | inx-2, inx-3, inx-7, inx-9, inx-11, eat-5 |
| pm4 | inx-6, inx-11, eat-5 | inx-3, inx-7, inx-8, inx-9, inx-10 |
| pm5 | inx-3, inx-7, eat-5 | inx-8, inx-9, inx-10, inx-11, inx-14 |
| pm6 | inx-2, inx-3, inx-10 | inx-6, inx-7, inx-8, inx-9, inx-11, inx-14, eat-5 |
| pm7 | inx-2, inx-3, inx-10 | inx-7, inx-8, inx-9, inx-11, inx-14, eat-5 |
| pm8 | inx-2, inx-3, inx-10, inx-11, inx-20 | inx-7, inx-8, inx-9, eat-5 |
| mc1 | inx-10, eat-5 | inx-9 |
| mc2 | inx-6, inx-10, eat-5 | |
| mc3 | inx-10 | |
| M1 | inx-5, inx-13 | |
| M2 | inx-12, inx-13 | |
| M3 | inx-3 | |
| M4 | ||
| M5 | unc-9 | |
| I1 | unc-9 | |
| I2 | ||
| I3 | inx-9 | |
| I4 | ||
| I5 | ||
| I6 | unc-9 | |
| MC | inx-7 | inx-3 |
| MI | ||
| NSM | unc-9 | |
| RIPb | inx-4, unc-9 | |
| g1 | inx-11, inx-18 | inx-7, inx-9, unc-7a |
| g2 | inx-3, inx-11 | inx-7, inx-9, unc-7a |
| vpi | inx-2 | |
| Intestine | inx-2, inx-11,c inx-15, inx-16 | inx-3, inx-5, inx-8, inx-13, inx-17, inx-21, nsy-5, unc-7b |
| vir | inx-11, inx-17, inx-20 | inx-6 |
| Rectal gl. | inx-3 | inx-7, inx-9 |
| Rectal epi. |
inx-5 (B cell), inx-11 (K.a/K’ cells), inx-12, nsy-5 (K.a/K’ cells) |
inx-3, inx-8, inx-9, inx-13, eat-5 (K.a/K’ cells) |
Epi, epithelium; gl, gland; vir, intestinal–rectal valve.
Although it is a somatic neuron, RIP is included in this list because it is the only connection between the somatic and pharyngeal nervous systems.
inx-11 is more strongly expressed in the most posterior (int 9) intestinal cell.
The enteric muscles (stomato-intestinal muscles, anal sphincter muscle, and anal depressor muscle) are coupled to each other by gap junctions (Hall and Altun, 2008) and express several classes of innexins: the anal depressor muscle expresses inx-8, inx-10 (less strongly), inx-13 (less strongly), unc-9, and inx-14; the anal sphincter muscle expresses inx-8 and unc-9; and the stomato-intestinal muscles express inx-8, unc-9, and inx-14. The enteric neuron DVB synapses onto stomato-intestinal muscles and the anal depressor, and makes gap junctions to the AVL neuron, completing a circuitry in the enteric motor system for enteric muscle contractions. DVB expresses several innexins, including inx-3, inx-7, inx-10 (less strongly), inx-13, unc-7, and unc-9. The expression of inx-8 in all enteric muscles and unc-9 in all enteric muscles as well as DVB suggests that these two innexins may be the major gap junction proteins within this circuit.
Innexins in the Reproductive System
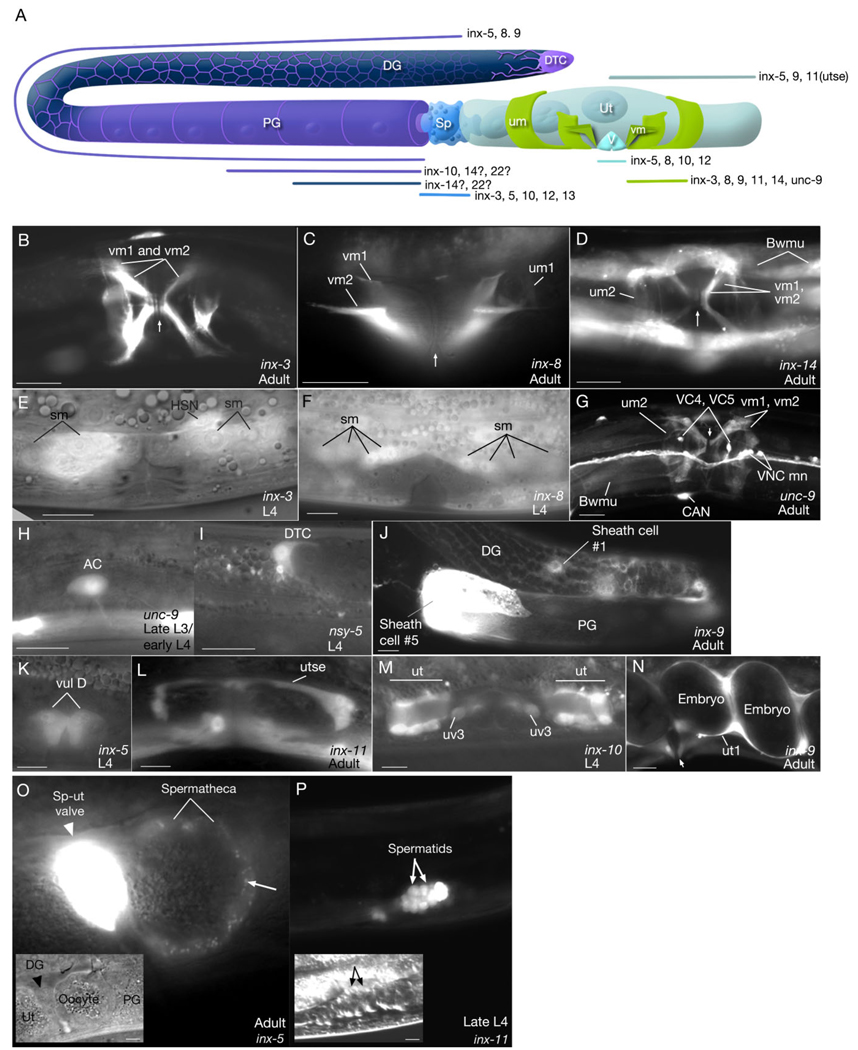
Transmission electron microscopic (TEM) studies have revealed the existence of gap junctions only between sheath/sheath, sheath/oocyte, sex muscles/egg-laying neurons, and spermathecal epithelial cells (White et al., 1986; Hall and Altun, 2008). However, we have now found much more widespread expression of innexins in the hermaphrodite reproductive system (Fig. 3; Table 2). The gonadal sheath of the hermaphrodite expresses four different innexins; inx-5, inx-8, inx-9, inx-10. Among these, inx-5 is also expressed in spermatheca, sp-ut valve, uterus, and vulva. Spermathecal cells additionally express inx-3, inx-10, inx-12, and inx-13, while uterine cells additionally express inx-9. The uterine seam cell, utse, expresses inx-11. inx-8, inx-10, and inx-12 are expressed in the vulval epithelium. Interestingly, we have found that the spermatocytes and spermatids of the developing larva express inx-11, while the sperm in the adult express inx-12 (Fig. 3; Table 2). Also, during larval development a pair of migratory distal tip cells (DTC), express nsy-5 during their migratory phase, and a transient cell that functions to pattern the cells of the vulva, the anchor cell (AC), expresses unc-9 (Fig. 3).
Fig. 3. Expression of innexins in reproductive tissues.
A: Graphic representation of expression domains of innexins in the reproductive system (after Hall and Altun, 2008). DTC, distal tip cell (violet); DG, distal gonad; PG, proximal gonad; Sp, spermatheca (bright blue); um, uterine muscle (green); vm, vulva muscle (green); Ut, uterus (pale blue); V, vulva (sky blue). Gonad sheath is shown in purple color and germline in navy blue. inx-5, inx-8, inx-9 are expressed throughout gonad sheath (including thin fingers covering distal gonad) while inx-10 expression is higher in proximal gonad. inx-14 and inx-22 may be localized to oocytes and/or proximal gonad sheath (Whitten and Miller, 2007). B–D,G,J,N: Fluorescent micrographs of transgenic animals expressing the pinx∷GFP reporter genes. E,F,H,I,K–M: Fluorescent micrographs transposed over differential interference contrast microscopy (DIC) images of the same anatomical region. Scale bars = 10 µm. (B,D,G) ventral views; (C,E,F,H,I–N) lateral views. AC, anchor cell; Bwmu, body wall muscles; DG, distal gonad; DTC, distal tip cell; PG, proximal gonad; sm, sex muscle progenitors; um, uterine muscles; ut, uv, utse, portions of uterus; vm, vulval muscles; vul, vulval epithelium; VNCmn, ventral nerve cord motor neurons. Vulva position is marked with an arrow in some panels (B,C,D,F,N). The developmental stages of the animals are indicated on each panel. O,P: Innexin expression in the spermatheca and during spermatogenesis. O: Fluorescent micrograph of pinx-5∷GFP reporter gene expression in spermatheca superimposed to the DIC image (inset) of the same region in an adult hermaphrodite. Strong expression is observed in spermathecal-uterine (sp-ut) valve. An oocyte is seen inside the spermatheca. inx-5 is seen as localized to small punctate regions on the spermathecal wall (arrow), suggesting membrane localization signal is within the 30 amino acid sequence from the N-terminus included in our expression construct. Scale bar = 1 µm. Similar punctate/aggregated localization patterns were previously observed for inx-3 and inx-6 (Starich et al., 1996; Li et al., 2003). P: Fluorescent micrograph of pinx-11∷GFP reporter gene expression in the spermatids in a late L4 stage larva. (Inset) DIC image of the same anatomical region. Scale bar = 10 µm in inset.
TABLE 2.
Innexins in the Developing and Adult C. elegans Hermaphrodite Reproductive System
| Adult | Developing larva | |
|---|---|---|
| Germline | inx-14a, inx-22a | |
| Gonad sheath | inx-5, inx-8, inx-9, inx-10b, inx-14c, inx-22c | |
| DTC | nsy-5d | |
| Uterus | inx-5, inx-9, inx-11e | inx-5, inx-10, inx-11 |
| Vulva | inx-5 (low), inx-8 (low), inx-10f, inx-12 | inx-5, inx-9, inx-12, inx-13f |
| Spermatheca | inx-3, inx-5, inx-10, inx-12, inx-13 | inx-5, inx-10 |
| Sperm (spermatocytes, spermatids) | inx-12 | inx-11 |
| Sp-ut valve | inx-5, inx-13 | inx-5 |
| vm | inx-3, inx-8, inx-9, inx-11f, inx-14, unc-9 | inx-3, inx-8 |
| um | inx-8, inx-11f, inx-14, unc-9 | inx-3, inx-8 |
| HSN | inx-3, inx-4f, inx-7, unc-9 | inx-3, unc-9 |
| VCn | unc-7a, unc-9 | unc-7a, unc-9 |
| AC | unc-9 |
Detected in another study by antibody staining (Govindan et al., 2009, in press), but not in this one which may be due to a higher sensitivity of the antibody-staining method.
Only in proximal gonad sheath.
Extrapolated from a functional study; inx-14 and inx-22 are expressed in the proximal gonad and/or the oocytes (Whitten and Miller, 2007)
Only during distal tip cell (DTC) migration.
Only in utse.
Detected in a previous study (Starich et al., 2001), but not in this one.
We have found five innexins to be expressed in the adult hermaphrodite sex muscles: inx-3, inx-8, inx-9, inx-14, and unc-9. Additionally, inx-11 was reported to be expressed in sex muscles in a previous study (Starich et al., 2001), although we were unable to detect its expression in these muscles. Of these, expression of inx-3 and inx-8 start during early larval stages and continue during migrations of the great grand-daughters of the SM blast cell to their final locations and after the sex muscles achieve their final structures (Fig. 3; Starich et al., 2003). unc-9 is expressed in both classes of neurons that innervate the egg-laying apparatus, HSN and VC. However, unc-7 is expressed only in VCs and inx-3, inx-4, and inx-7 are specifically expressed in HSNs (Fig. 3; Table 2, Table 3), which are similar to observations by others (Starich et al., 2001).
TABLE 3.
Innexin Expression in the Neurons and Glia of the Hermaphroditea
| inx-1 | AIB, AIY (until L3) |
| inx-2 | AVK |
| inx-3 | CEPsh, ALN, ASn, CAN, DAn, DBn, DDn, DVA, DVB, HSN, PDE, PLM, PVQ, PVR, PVT, URB, VAn, VBn, VDn, M3, MC |
| inx-4 | ADA, ADE, ADF (in L1), AIN, ALN (early larva), ASG (early larva), ASH (early larva), AUA, AVJ, DVC, FLP, PHA, PHB, PVR, PVT, RIC, RIG, RIM, RIP |
| inx-5 | Amsh, CEPsh, CEPso, ILso, OLso, Phso, DVC, MI |
| inx-6 | AVD, AVK, RIS, URB |
| inx-7 | ADE, AIY, ALM, ALN, AVA, AVK, AVM, BDU, CAN, DAn, DVA, DVB, DVC, FLP, HSN, LUA, PLM, PLN, PVC, PVM, PVP, PVQ, PVT, PVW, RID, RIS, SDQ, URB, MC |
| inx-8 | ILso, AIN, AVF, AVJ, AVK, PVR, SAB |
| inx-9 | Phsh, AVK, DVC (early larva), PVR, SIB (early larva), URB, I3 |
| inx-10 | DDn, DVA, DVB, DVC, PVP |
| inx-11 | CEPsh, DVC, LUA |
| inx-12 | Amsh, CEPso, ILso, OLso, PDEso, AVH (early larva), AVJ (early larva), AVK, CAN, IL1 (early larva), PDE, PVD, SIB (early larva), URB, VAn, VBn, M2 |
| inx-13 | Amsh, CEPsh, CEPso, ILsh, ILso, OLsh, OLso, Phsh, CAN, DVA, DVB, DVC, LUA, PHA, PHB, PLN, PVC, PVQ, PVR, M1, M2 |
| inx-14 | DDn, VDn |
| inx-15 | Ned |
| inx-16 | Ned |
| inx-17 | AIN, DVA (early larva), DVC (early larva), PVT (early larva) |
| inx-18 | ADL, ASI, ASK, AVJ, AVK, DVA., DVC, IL1 (L and R only), IL2 (L and R only), PVT |
| nsy-5 | AVB, AVK |
| inx-20 | Ned |
| inx-21 | Ned |
| inx-22 | Ned |
| eat-5 | Ned |
| unc-7a | AIN, AVA, AVB, AVD, AVG, AVK, DDn (3-fold to early larva), DVA, DVB, DVC, IL1, OLL, PVD, PVQ, PVT, RIV, RME, RMG, SDQ, SIA, SIB, SMB, VCn |
| unc-7b | OLso, AVD, AVG, AVJ, AVK, DVA, DVC, IL1, PVP, RIC, RIF, RMD, SIB, SMD, URA |
| unc-9 | Phsh, ADA, ADE, ADL, AIN, AIY, ALM, AUA, AVA, AVD, AVH, AVJ, AVK, AVM, AWB, BDU, CAN, CEP, DAn, DBn, DDn, DVB, DVC, FLP, HSN, IL1, IL2, LUA, OLL, PDA, PDB, PDE, PHA, PHB, PHC, PLM, PLN, PVC, PVD, PVM, PVN, PVP, PVQ, PVR, PVT, PVW, RIB, RIC, RIF, RIP, RIS, RME, SDQ, SIA (early larva), SIB (early larva), SMB (early larva), SMD (early larva), URA, URB, VAn, VBn, VCn, VDn, M5, I1, I6, NSM |
Ned, no expression detected in the nervous system; sh, sheath cell; so, socket cell.
Innexins in the Excretory System
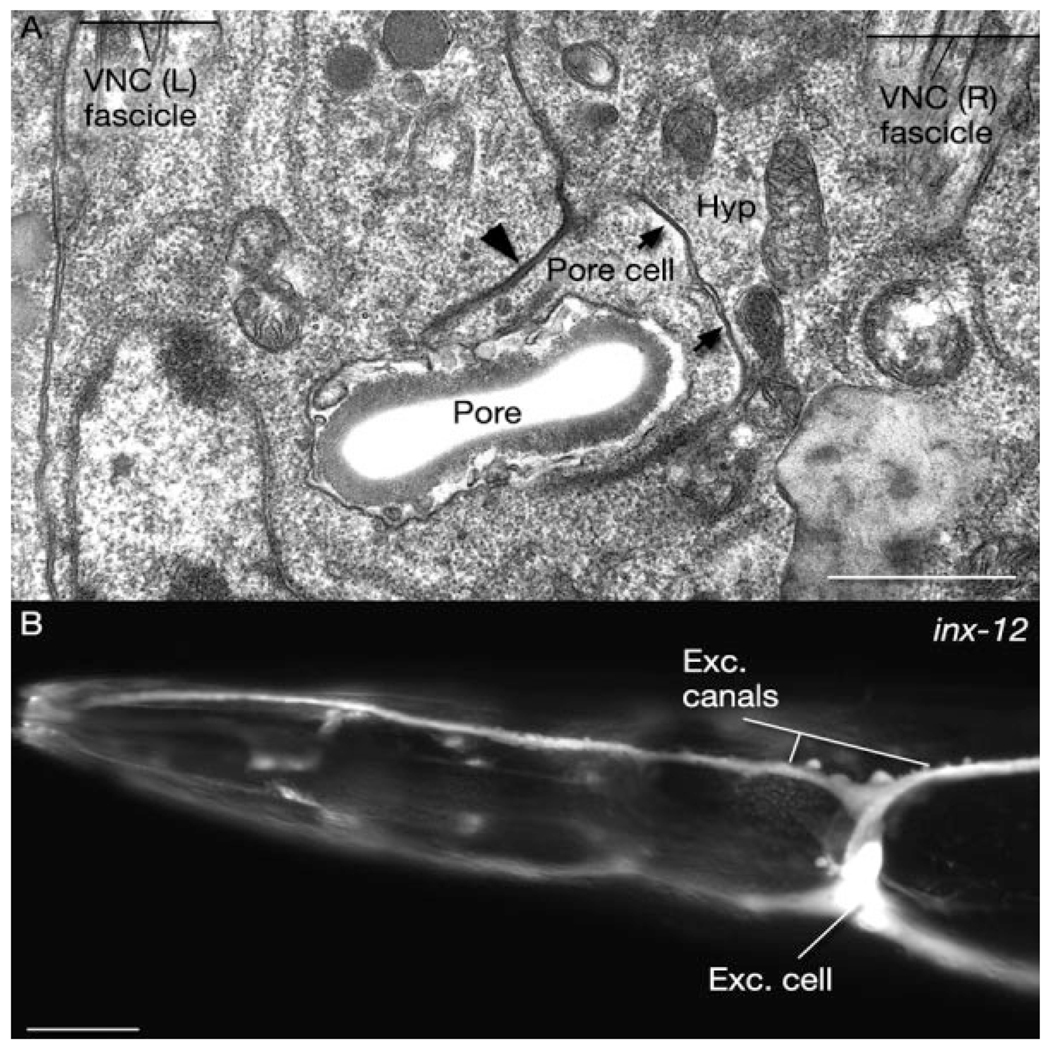
We have detected inx-3 (less strongly), inx-5, inx-12, and inx-13 in the excretory cell (Fig. 4). All except one, inx-5, are also expressed in the canal-associated neurons (CAN), while the excretory gland expresses inx-5 at low levels. We were unable to detect any innexin expression in the pore or duct cell, although these cells make gap junctions to the excretory cell (Nelson et al., 1983), and we have found by TEM that the pore cell makes gap junctions to the adjacent hypodermis (Fig. 4).
Fig. 4. Gap junctions and innexin expression in the excretory system.
A: Horizontal section. Transmission electron micrograph of gap junctions (arrows) between the pore cell and the hypodermis. Pore cell also makes adherens junctions (arrowhead) to the hypodermis. Exc, excretory; Hyp, hypodermis; VNC, ventral nerve cord. Animal name: Hall/N533 (print number 4216). B: Fluorescent micrograph of expression of pinx-12∷GFP reporter gene in the excretory cell and its canals. Scale bars = 1 µm in A, 10 µm in B.
Innexins in the Epithelium
Hypodermis
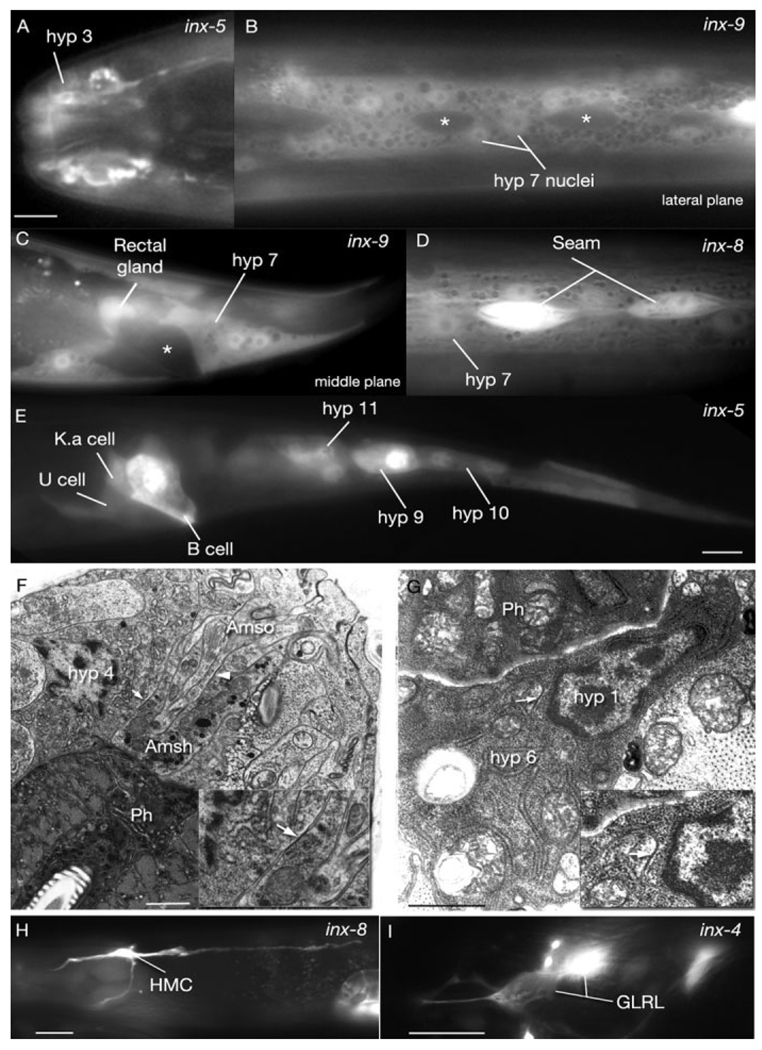
We found that inx-5, inx-8, inx-9, inx-11, inx-12, inx-13, and unc-9 are expressed in the hypodermal cells of the animal in postembryonic stages (Fig. 5). Of interest, the expression levels of inx-5 and inx-12 are stronger in the head hypodermis (hyp 1–5) and tail hypodermis (hyp 8–11) compared with the body hypodermis (hyp 7), while inx-9 is more strongly expressed in anterior hypodermis (especially hyp 1) and hyp 7 but not in tail hypodermis. These findings suggest a nonuniform function for these innexins within the hypodermis.
Fig. 5. Innexins in the hypodermal (hyp) epithelium and mesoderm (all adult images).
Fluorescent micrographs of transgenic animals expressing the pinx∷GFP reporter genes; all lateral views, anterior to the left. A: pinx-5∷GFP is expressed in the anterior hypodermis. B,C: pinx-9∷GFP is expressed in the main body hypodermis (hyp 7) and rectal gland, but excluded from the seam (asterisks in B) and the rectal epithelium (asterisk in C). D: inx-8 is coexpressed in the seam and hypodermis. E: pinx-5∷GFP is expressed in the rectal epithelium (B, K.a, and U cells) and the posterior hypodermis (hyp 9–11). F,G: Gap junctions between glia and hypodermis and between hypodermal cells in the adult head. Transmission electron micrographs. Ph, pharynx. F: Gap junctions are seen between amphid socket (Amso) and amphid sheath (Amsh) glia (arrowhead) as well as the amphid sheath and hypodermis (hyp 4 cell; arrow). Lateral section; high pressure frozen material. G: A gap junction (arrow) is seen between hyp 1 and hyp 6 cells. (Note by this stage hyp 6 has fused to hyp 7; not shown.) Transverse section; immersion fixed material (MRC/N2T). Insets show some junctional regions at higher power. H,I: Innexin expression in two mesodermal tissues. Lateral views, anterior to the left. H. pinx-8∷GFP reporter gene expression in HMC (head mesodermal cell). I. pinx-4∷GFP reporter gene expression in GLRL. Scale bars = 5 µm in A, 10 µm in E (applies to B–E), 1 µm in F,G, 10 µm in H,I.
Seam
Three innexins are detected in the seam cells: inx-8, inx-11, inx-12 (Fig. 5). By antibody staining inx-13 was also detected in these cells in a previous study, although we did not detect any inx-13 expression in the seam with our method (Starich et al., 2001). We also observed inx-5 expression in the seam precursor cells during early larval stages, but its expression becomes undetectable in later stages.
Arcades
inx-3 (weak expression), inx-5, inx-9, inx-11, inx-12 (weak expression), unc-7a, and unc-9 were detected in the arcade cells, which demarcate the transition between the hypodermis and the pharyngeal tissues.
Innexins in the Nervous System
The C. elegans nervous system contains an extensive network of gap junctions as previously revealed by electron microscopy (White et al., 1986). Although gap junctions have been shown to be sufficient to functionally couple neurons and convey information perhaps even unidirectionally within functional circuits (e.g., touch circuitry; Chalfie et al., 1985; Majewska and Yuste, 2001), apart from a few individually studied innexins, there are limited data that describe the composition of these channels between C. elegans neurons. We therefore sought to identify the types of innexins expressed by individual neuron classes to help predict the molecular identity of gap junctions within each defined network. Our study showed that most neurons that were previously described to have gap junctions in the adult animal express one or more innexins (White et al., 1986). However, in a subset of these, including AFD, AIA, AIM, AIZ, ALA, AQR, ASJ, AVE, AVL, AWA, BAG, OLQ, PQR, RIH, RIR, RMF, RMH, SAA, URX, and URY, no innexin expression was detected by our method (Table 3). Conversely, we detected innexin expression in some neurons that were previously thought to have no gap junctions in adult stage animals, such as BDU, PDA, PDB, PVD, and URA (Table 3). All innexins except inx-5, inx-15, inx-16, inx-20, inx-21, inx-22, and eat-5 were found in the nervous system. Among these, the most widely expressed innexins were inx-7, unc-7, and unc-9, while the least widely expressed ones were inx-1, inx-2, and inx-11.
Our TEM analyses revealed for the first time that gap junctions exist between glia (socket and sheath cells) and hypodermis as well as between the socket and sheath cells, but not between glia and neurons (Fig. 4)(Hall and Altun, 2008). The expression of eight innexins was detected in glia cells, including inx-3, inx-5, inx-8, inx-9, inx-11, inx-12, inx-13, unc-7, and unc-9. Among these, inx-5, inx-12, and inx-13 were expressed in a variety of glia (Table 3). However, the remaining innexins have very restricted expressions in glial tissue, such as inx-9 in phasmid sheath cells, inx-11 in cephalic sheath cells, inx-18 in inner labial sockets, unc-7 in outer labial sockets, and unc-9 in phasmid sheaths. We could not detect any innexin expression in four classes of glia: ADEsh, ADEso, Amso, and PDEsh.
Innexins in the Mesoderm
Strikingly, HMC (head mesodermal cell), a cell that contains some vestigial muscular components and is known to make gap junctions only to the dorsal and ventral neck muscles and to the excretory glands, expresses the most diverse number of innexins among all tissues (Fig. 5). With the exception of inx-5, inx-10, inx-11, inx-14, inx-15, inx-16, inx-20, inx-21, and inx-22, all innexins were expressed in this cell (Fig. 5). The six-fold symmetrical GLR cells in the head, which are presumed to be scaffolding cells that guide head and neck muscle arms during development also express a large variety of innexins, including inx-3, inx-4, inx-6, inx-7, inx-8, inx-9, inx-10, inx-12, inx-18, inx-22, and unc-7 (Fig. 5). Surprisingly, five of these (inx-4, inx-7, inx-8, inx-18, and inx-22) were seen to be only expressed in the left and right side GLR cells but not the dorsal and ventral ones. For inx-22, GLRL/R were the only cells with detectable expression within the whole animal.
Innexin Expression During Embryonic Development
Dye-coupling experiments have shown that starting from the late two-cell stage of embryogenesis, through cellular proliferation and until the early morphogenetic phase, virtually all C. elegans somatic embryonic cells are coupled to each other as a single compartment by means of gap junctions (Bossinger and Schierenberg, 1992). After the early morphogenetic phase, there is a progressive restriction of dye-coupling of cells to smaller units in forming organs. As in previous studies, we also detected that some innexins start to be expressed globally early on, while others are expressed in a time-limited and cell-and tissue-specific manner through the later stages (Starich et al., 2001). Two innexins, inx-2 and inx-3, were expressed broadly during early embryogenesis, suggesting they play roles in the initial cellular proliferation stage. It was previously shown that inx-3 mutants had no detectable phenotype, including any changes in time and extent of cellular proliferation or organization or cell fate specification (Starich et al., 2003). It is possible, therefore, that inx-2 and inx-3 act redundantly during early embryogenesis. After the beginning of morphogenesis, inx-3 expression becomes more restricted to the pharynx, hypodermis, and intestine (this study and Starich et al., 2003). By three-fold stage inx-3 expression appears in ventral cord motor neurons (strongest in DA neurons) along with continued strong pharyngeal expression. By hatching, its hypodermal expression disappears, while postembryonically born ventral cord motor neurons express it at low levels. Its pharyngeal (strong) and neuronal (faint) expressions continue to adulthood. The expression of inx-2 becomes more restricted to the intestinal and some pharyngeal precursors by embryonic day (E) 16 stage and between 1.5-fold and 3-fold stages inx-2 is strongly expressed in these tissues (Fig. 2). Its expression levels start to decline after hatching as described above.
Among remaining innexins, inx-1, inx-4, inx-6, inx-17, inx-18, inx-20, inx-21 show no expression until after early morphogenesis, suggesting they play roles in later stages, while inx-22 has no embryonic expression. inx-1 starts to be expressed in a few head neurons including AIB (brightest) and AIY (fainter; expression ends in L2) by three-fold stage, long after both cells are born, suggesting a role for inx-1 in differentiation and maturation of these neurons rather than cell-fate determination. inx-4 expression appears in neurons by hatching, many of which continue expression to adulthood. inx-5 first appears in the developing hypodermis at bean stage and then in the excretory cell at three-fold stage, while faint hypodermal expression of inx-7 is seen around two-fold stage and becomes stronger by three-fold stage. Pharyngeal and neuronal expression of inx-6 start around three-fold stage, and some of the expression in head and tail neurons disappears after L1 stage. inx-8 is expressed broadly, albeit at very low levels, around two-fold stage, and its expression becomes stronger in the pharynx, nervous tissue, HMC, and GLR cells as development continues. Arcade cells are seen to express inx-9 and inx-13 at high levels starting around two-fold stage continuing their expression throughout development and adulthood. Neuronal expression of inx-9 appears around three-fold stage. inx-10 is localized to pharyngeal precursors from early stages of embryogenesis, and by three-fold stage, all pharyngeal muscles except pm4 are seen to express it at high levels. Similarly, expression of inx-11 appears in pharyngeal tissue around two-fold stage, and by three-fold stage, strong expression becomes restricted to g1, g2, pm4, and pm8. inx-12 expression starts in seam precursors around 1.5-fold stage, followed by expression in arcade cells by 2-fold and head neurons by 3-fold stage. inx-17 and inx-18 are mainly expressed in head and tail neurons starting around three-fold stage, and the expression of inx-17 in tail neurons disappears as the larval development continues. inx-20 appears in pm1, pm2, pm8, and intestinal rectal valve at three-fold stage and continues to adulthood. unc-7 and unc-9 appear in neurons in the morphogenesis phase of embryogenesis, and their expression becomes much brighter in the nervous tissue as the embryonic development progresses. eat-5 expression is restricted to the pharyngeal tissue from early stages of pharyngeal development.
DISCUSSION
Gap junctions exist virtually in all tissues of C. elegans through all developmental stages. Although all innexins with previously described functions, except NSY-5, showed strong expression levels in involved tissues in our assays, the intensity of GFP epifluorescence should not be considered the single determinant of the importance of these proteins in vivo, because many genes that showed weak expressions in our analyses may still have essential functions. We suspect that our inability to detect GFP expression under the control of nsy-5 promoter in cells previously described to be expressing nsy-5 is due to the shorter promoter length (706 bp) used in the present study than in the previous study (5.8 kb; Chuang et al., 2007). nsy-5 mutant phenotype is only rescued when 5.8 kb of promoter sequences are included in the rescuing construct (C. Bargmann, personal communication). A GFP reporter gene under the control of this promoter is expressed in many more neurons than what we could detect with our shorter promoter (Chuang et al., 2007). We were also unable to observe any innexin expression in some cells or tissues that were previously shown to make gap junctions by TEM, which might be due to several reasons, including a low level of expression in these tissues that is undetectable by fluorescence microscopy, omission of any required 3′ sequences or introns, and inclusion of too short promoters than are required for expression in these tissues. Alternatively, the expression of innexins may be transient in these tissues. Additionally, although we examined large numbers of animals for each innexin, the mosaic nature of the expression patterns might have led to some misses. Conversely, we detected innexins in some cells that were previously described to have no gap junctions. This can be explained if innexins are used to make gap junctions in earlier or later stages of the animal in these cells, as was observed previously for nsy-5 (Chuang et al., 2007) or if they are used for building hemichannels rather than gap junctions (Spray et al., 2006). Alternatively gap junctions might have been previously missed in these cells due to technical problems including inadequate fixation for TEM, small size, or location along unexamined portions of a cell (e.g., long branches of the PVD neuron; cf. Hall and Russell, 1991; Hall and Altun, 2008).
Alimentary Canal
inx-3, inx-6, and eat-5 are among the most extensively studied C. elegans innexins. They are mainly expressed in the pharyngeal tissue and are required for synchronized contraction of the pharyngeal muscle cells numbered from pm1 to pm8 from anterior to posterior. Marginal cells, numbered from mc1 to mc3, are inserted between these muscle cells. The pharynx also contains epithelial cells in the anterior and neuronal cells with cell bodies in the anterior and terminal bulbs. Pharyngeal muscles, mc cells, and all neurons except I4 and NSM were previously shown to contain gap junctions by TEM analysis (Albertson and Thomson, 1976). Gap junctions exist between muscles and mc cells, and between neighboring mc cells while neurons make gap junctions to each other. Although identification of gap junctions between neighboring muscle cells is hindered in EM studies because of similar-looking large desmosomes, mutant and physiological analyses strongly support their existence (Avery and Horvitz, 1989). It has been shown that inx-6 is required for prevention of premature relaxation of pharyngeal procorpus muscles during contraction, while eat-5 is required for synchronization of corpus and terminal bulb contractions (Starich et al., 1996; Li et al., 2003). inx-3 is required for synchronized contraction of the terminal bulb muscles (Starich et al., 2003). Reported dye- and electrical uncoupling in inx-3, inx-6, and eat-5 mutants combined with data from this study suggest that, in the absence of each of these proteins, either no heterotypic channels form between INX-6 and INX-3 or INX-7, and between EAT-5 and INX-8 or INX-10 or the size of the remaining channels are impermeable to the dye. Also vital dye-spreading through the pharynx in these mutants suggests that the gap junctions between muscle cells are the primary route of coupling, rather than those between the muscle and mc cells because dye-spreading in these mutants stops at muscle cell boundaries and the dye is unable to continue to spread through the overlapping mc cells (Starich et al., 1996; Li et al., 2003; cf. Fig. 2). Topographically, INX-3, INX-6, and EAT-5 can cover the whole pharynx by themselves, which raises the question of what functions might be performed by the many other innexins expressed in the pharynx. It is known that INX-6 is only required during early larval stages (Li et al., 2003), although our results indicate that it is still expressed in adults. Hence, the other three innexins with expression patterns overlapping with that of INX-6 (i.e., INX-8, INX-10, and INX-11) may serve similar functions to INX-6 during other life stages.
We have found that four different types of innexins show strong and consistent expression in the intestine: inx-11, which is mostly localized to the posterior; and inx-2, inx-15, and inx-16. Of these, inx-16 has been shown previously to be required for efficient calcium wave propagation within the intestine during defecation behavior (Peters et al., 2007). However, in most inx-16 mutants, calcium waves still propagate albeit slowly. Also, the intestinal cells in inx-16 mutants continue to be dye-coupled. Any or all of the remaining four innexins in the intestine might be capable of these gap junction functions. However, because inx-2 is mainly expressed in the intestine during embryonic stages, its main function is probably in development rather than in defecation-motor cycle in later stages. Also, inx-11 is mainly restricted to the most posterior intestinal cell, int 9, which is suggested to function as the pacemaker for defecation behavior. inx-11 may have a specialized function within this cell, perhaps to supplement inx-16 for initiation of the calcium spike in posterior intestine, rather than aberrantly and for the precision of the defecation clock (Peters et al., 2007).
Reproductive System
Earlier electron microscopic studies showed that the reproductive system of the hermaphrodite has a network of gap junctions for various functions (White et al., 1986; Hall et al., 1999; Hall and Altun, 2008).
The fertilized eggs in the adult uterus are laid outside by means of the vulva with the help of uterine (four each of um1 and um2) and vulval (four each of vm1 and vm2) muscle contractions. Of the 16 sex muscles, only vm2 receive direct synaptic inputs from the egg-laying neurons, 6 VCs and 2 HSNs, while the remaining muscles are electrically coupled to the vm2 or each other (White et al., 1986; Hall and Altun, 2008). The HSN neurons also make gap junctions to each other, as well as to VC5 (White et al., 1986). Similarly, VC neurons make gap junctions with each other, and VC3 also makes a gap junction directly to the vm2 muscle. Previously, unc-7 and unc-9 were found to be required for egg-laying through mutant screens supporting the importance of these molecules for egg-laying behavior (Barnes and Hekimi, 1997). Although the egg-laying defective phenotypes of unc-9 mutants and unc-7 mutants resemble each other and therefore these two innexins were hypothesized to oligomerize within the egg-laying system, we have found that unc-7 expression is restricted to the VC neurons, while unc-9 is expressed in both types of hermaphrodite specific neurons as well as all sex muscles (Barnes and Hekimi, 1997). Thus, it would be expected that unc-9 may form homotypic channels between the sex muscles and neurons and may have a more generalized function in connecting the motor system of the egg-laying apparatus. Nevertheless, the fact that unc-7 mutants display egg-laying defects suggests that unc-7 is also essential for communication between VC neurons and the muscles and/or the HSNs.
Close inspection of the DTCs by TEM has never revealed gap junctions between these cells and the underlying germline at any stage (D.H. Hall and E. Hedgecock, unpublished observations). Nevertheless, we detected nsy-5 in migrating DTCs, which act as leader cells for directed migration of the gonad arms in the larva, suggesting a function for this innexin as a hemichannel (Kimble and White, 1981; Hedgecock et al., 1987). Our initial studies, however, failed to show any significant gonad migration defects in nsy-5 mutants (ky634 and tm1896; data not shown). It remains to be seen, however, if nsy-5 functions in a redundant or synthetic manner with other gonad migration signals. The presence of innexins in other migrating cells (sperm; Fig. 3) in the reproductive system also evokes the question that these might act as hemichannels to broadcast signals at a distance, or to monitor their environment during cell movements.
Two types of gap junction exist in the proximal gonad of the hermaphrodite: sheath/sheath and oocyte/sheath (Hall et al., 1999). Through an RNAi screen, it has recently been shown that inx-14 and inx-22 may be structural components of the transitory sheath/oocyte gap junctions, which inhibit oocyte maturation and sheath cell contraction and promote sperm guidance (Whitten and Miller, 2007). inx-14 and inx-22 mutant phenotypes are not replicated in inx-8 or inx-9 mutants, although the latter are expressed in the gonad sheath (Starich et al., 2001; Whitten and Miller, 2007; and this study). Although we were unable to detect any expression of inx-14 or inx-22 in the gonad sheath or germline, we detected two additional innexins in the sheath: inx-5 and inx-10. The function of these additional innexins is currently unknown, but it is plausible that they are involved in sheath/sheath gap junction formation. These findings support that both expression studies and functional studies are complementary and essential for comprehensive understanding of functions of gap junction molecules.
Excretory System
Robust gap junctions were previously described by TEM between the excretory canals and the neighboring hypodermis (Nelson et al., 1983; Buechner et al., 1999). The excretory cell also makes extensive gap junctions to the pore and duct cells and some to the canal-associated neurons (CAN; Nelson et al., 1983; White et al., 1986). We have also detected gap junctions between the pore cell and the adjacent hypodermis by electron microscopy. Although several innexins are expressed at high levels in the excretory cell and CANs (including inx-3, inx-12, and inx-13), we could not detect any innexin expression in the pore cell or the duct cell. CAN neurons are the only somatic neurons that are required for survival and ablation of the excretory cell leads to larval death (Nelson and Riddle, 1984; Chalfie and White, 1988). Previously, it was suggested that CANs may regulate the excretory canals (Hedgecock et al., 1987). Although the function of gap junctions in the excretory system remains unclear, it is possible that this gap junction network between CAN, excretory system, and the hypodermis, created mainly by inx-12 and inx-13, is important for osmoregulation and may therefore be essential for survival.
Epithelial System
Nematode hypodermis (also known as epidermis) is made of 11 mostly syncytial cells. The majority of the hypodermal cells fuse to make the large hyp 7 syncytium during development, while the anterior head and tail hypodermal cells stay unfused to hyp 7 (Podbilewicz and White, 1994; Hall and Altun, 2008). Hypodermal cells make a multitude of gap junctions to a variety of tissues that they share neighborhoods with, including transitional epithelia, seam cells, and excretory canals. hyp 1- hyp 3 in the head make concentric rings from internal to external within the buccal cavity. Our results suggest that inx-5 and inx-9 are the strongest candidates for forming the gap junctions between hyp 1 and arcades because these innexins coexpressed in both types of cells. inx-5 is also expressed in the rectal epithelium and tail hypodermis, although it is unlikely that there are many gap junctions between these tissues because they are mostly separated by a narrow pseudocoelom.
Seam cells are linked to the hypodermis by small gap junctions on their lateral membranes. All the innexins that are expressed in the seam cells, inx-8, inx-11, inx-12, are coexpressed in the hypodermis. Thus, it is likely that seam/hyp gap junctions are formed by a combination of these same innexins. Seam cells also undergo late cell–cell fusions, and it is possible that an innexin-based signaling may underlie this event.
Nervous System
Our analysis of innexin expression in neurons revealed some interesting findings. Although it has been shown that many neurons coexpress unc-7 and unc-9 (Starich et al., 2001), inx-7 emerged as another innexin that frequently colocalizes with these innexins. In particular, inx-7 expression seems to overlap with unc-9 and suggests they may form heteromeric channels. One candidate circuit to use inx-7 is the touch circuitry, because the touch neurons, the command interneurons that they communicate with (except AVD), as well as the touch cell connector neuron (LUA), all express inx-7. The combination of inx-4 and unc-9 are notable both at the sole connection between the somatic and pharyngeal nervous systems, i.e., gap junctions between RIP and I1, and also at the gap junctions between RIP and PVR, which function in modulation of pharyngeal pumping by the touch circuitry (Albertson and Thomson, 1976; Chalfie et al., 1985). Our study revealed that expression of some innexins may be restricted to a few neuron classes, e.g., inx-1 in AIB and AIY, and inx-2 in AVK. In general, there seems to be little correlation between the number of gap junctions that a neuron makes (judged by TEM; White et al., 1986) and the number and types of innexins that it expresses. CAN, for example, only makes sparse gap junctions to the excretory canals, but expresses five different innexins. In contrast, AVF makes at least 32 gap junctions to 4 different neuron classes, but expresses only inx-8. In our study, AVK, DVC, and PVT were found to express the highest number of innexins. Among these, PVT is a guidepost neuron that organizes left and right bundles of the ventral cord both during development and in the adult (Ren et al., 1999; Aurelio et al., 2002). It is plausible that the gap junctions made by this neuron play a role in this function. The rather large number of innexins expressed in neurons such as CAN suggests that some of these proteins may play roles apart from forming traditional intercellular gap junctions, as we discuss below.
Glial expression of innexins indicate that inx-5, inx-12, and inx-13 are the major gap junction components in these cells and that gap junctions between labial and cephalic socket and sheath cells may be composed of inx-13 homotypic channels or inx-5/inx-13, and inx-12/inx-13 heterotypic channels. Similarly, we can predict that gap junctions between the glial cells and the hypodermis are formed by inx-5, inx-12, and inx-13. The absence of innexins in four classes of glia (ADEsh, ADEso, Amso, PDEsh) may be due to the technical limitations mentioned above, because we were able to detect innexin expression in either the socket or the sheath cell (PDEso and Amsh) in two of these sensilla.
Mesodermal System
In adult animals, the six GLR cells that medially surround the muscle plate of the nerve ring make extensive gap junctions with RME motor neurons and head and neck muscle arms but not with each other (White et al., 1986; Hall and Altun, 2008). The function of these gap junctions seen in the adult is currently unknown, although it is possible they play a role in the more complex movements of the head of the animal (i.e., lateral movements as well as dorsoventral movements) compared with its body. In our study, GLRL/R were found to express additional innexins that did not seem to be expressed in the dorsal and ventral cells of the same class. The only difference between the lateral GLR cells and the dorsal and ventral ones is that the latter four align perfectly with the corresponding arms from a single row of head and neck muscles, whereas GLRL/R align with overlapping areas of muscle arms from two rows of muscle in two different quadrants (White et al., 1986). Whether the additional innexins expressed in lateral GLR cells are necessary for this muscle arm organization and whether this muscle organization contributes to the complexity of the head movements remain to be seen.
Finally, the presence of such a high number of innexins in the HMC is most puzzling. This large cell has only vestigial motor elements, but displays many large gap junctions by TEM, including some involving the excretory gland (White, 1988; Hall and Altun, 2008). HMC has been postulated to synchronize the simultaneous contractions of the dorsal and ventral head and neck muscles by means of the extensive gap junctions it forms with the somatic body wall muscles. However, HMC is also located within the pseudocoelom and is largely exposed to the pseudocoelomic fluid. It is plausible that HMC could be involved in chemical or ionic signaling by means of the pseudocoelomic fluid through various innexin hemichannels on its plasma membrane. Another possibility might place certain innexin hemichannels in the membranes of intracellular organelles to permit intracellular signaling within the cytoplasm. This suggestion goes beyond the known utility for this class of membrane channels, but would help to explicate the surprisingly large number of separate innexins expressed simultaneously in one cell. Our current method of detection did not allow for the study of trafficking between intracellular compartments, but it will be of interest to determine whether all of these innexin subunits do finally reach the plasma membrane and insert into well-defined intracellular junctions, or whether some innexin class(es) dwell solely in some interior compartment. The large size of HMC may lend itself to such explorations.
EXPERIMENTAL PROCEDURES
Strains
C. elegans strains were grown at room temperature (21–23°C) on nematode growth media (NGM) plates seeded with the Escherichia coli strain OP50.
Microscopy
For cell identification, live animals were anesthetized in M9 containing 1-phenoxy-2-propanol, mounted on agar pads and observed under Nomarski and fluorescence optics, using an Olympus AX70 upright microscope. Images were captured digitally using an Olympus Optronics camera and Magnafire 2.1 software. Image processing and analysis were performed using Adobe Photoshop.
Expression Constructs
Promoter∷GFP transcriptional fusions were expressed to evaluate the in vivo expression patterns of the innexin family (Fig. 1). The animals that were examined carried the injected constructs as extrachromosomal arrays and, therefore, expressed them as mosaics with varying copy number. To include the entire promoter in our analyses, we used the in vivo homologous recombination approach to express the transcriptional fusions (Fig. 1). A genomic DNA fragment that included ~1 kb of upstream regulatory sequence of each innexin gene followed by a short coding sequence (~50 bp) was amplified by polymerase chain reaction (PCR) and subcloned into the GFP expression vector pPD118.20. The sequences of primers for PCR are listed in Supp. Table S1.
Transgenic Strains
Transgenic strains were generated by microinjection of lin-15(n765) mutants to achieve germ line transformation. In most cases, the expression constructs were coinjected with corresponding cosmids containing the promoter region of the innexin genes to allow for homologous recombination between the plasmid and the cosmid (Supp. Table S1). Because no cosmids were available for inx-21 and inx-22, the expression constructs of the two genes were coinjected with PCR fragments that included 6.6 kb and 8 kb upstream sequences of inx-21 and inx-22, respectively. Also in the case of inx-19, the available cosmid T16H5 covered approximately 706 bp upstream of transcriptional start site. Punc-7b corresponds to R07D5, which starts from the middle of the first intron and Punc-7a corresponds to 3.3-kb sequences upstream of the first intron, including the untranslated first exon. A lin-15 rescue plasmid was also coinjected to serve as a transformation marker. Transgenic lines were identified by rescue of the Muv (multiple vulva) phenotype of lin-15(n765) mutants.
Supplementary Material
ACKNOWLEDGMENTS
The TEM image in Figure 4G comes from archival material from the MRC/LMB, Cambridge, England; kindly donated to the C. elegans Center for Anatomy by Jonathan Hodgkin and John White. We thank the C. elegans Knockout Consortium for nsy-5(tm 1896), Cori Bargmann for nsy-5(ky634) and Audrey Fraser at the Sanger Institute for sending us the cosmids. We thank Hannes Buelow for critically reading the manuscript. D.H.H. and Z.W.W. were funded by the NIH and Z.W.W. was funded by the NSF.
Grant sponsor: National Institutes of Health; Grant number: RR12596; Grant number: GM083049; Grant sponsor: National Science Foundation; Grant number: 0619427.
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Albertson DG, Thomson JN. The pharynx of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1976;275:299–325. doi: 10.1098/rstb.1976.0085. [DOI] [PubMed] [Google Scholar]
- Aurelio O, Hall DH, Hobert O. Immunoglobulin-domain proteins required for maintenance of ventral nerve cord organization. Science. 2002;295:686–690. doi: 10.1126/science.1066642. [DOI] [PubMed] [Google Scholar]
- Avery L. The genetics of feeding in Caenorhabditis elegans. Genetics. 1993;133:897–917. doi: 10.1093/genetics/133.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L, Horvitz HR. Pharyngeal pumping continues after laser killing of the pharyngeal nervous system of C. elegans. Neuron. 1989;3:473–485. doi: 10.1016/0896-6273(89)90206-7. [DOI] [PubMed] [Google Scholar]
- Barbe MT, Monyer H, Bruzzone R. Cell-cell communication beyond connexins: the pannexin channels. Physiology (Bethesda) 2006;21:103–114. doi: 10.1152/physiol.00048.2005. [DOI] [PubMed] [Google Scholar]
- Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- Barnes TM, Hekimi S. The Caenorhabditis elegans avermectin resistance and anesthetic response gene unc-9 encodes a member of a protein family implicated in electrical coupling of excitable cells. J Neurochem. 1997;69:2251–2260. doi: 10.1046/j.1471-4159.1997.69062251.x. [DOI] [PubMed] [Google Scholar]
- Bauer R, Lehmann C, Fuss B, Eckardt F, Hoch M. The Drosophila gap junction channel gene innexin 2 controls foregut development in response to Wingless signalling. J Cell Sci. 2002;115:1859–1867. doi: 10.1242/jcs.115.9.1859. [DOI] [PubMed] [Google Scholar]
- Bauer R, Lehmann C, Martini J, Eckardt F, Hoch M. Gap junction channel protein innexin 2 is essential for epithelial morphogenesis in the Drosophila embryo. Mol Biol Cell. 2004;15:2992–3004. doi: 10.1091/mbc.E04-01-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagburn JM, Alexopoulos H, Davies JA, Bacon JP. Null mutation in shaking-B eliminates electrical, but not chemical, synapses in the Drosophila giant fiber system: a structural study. J Comp Neurol. 1999;404:449–458. [PubMed] [Google Scholar]
- Bohrmann J, Haas-Assenbaum A. Gap junctions in ovarian follicles of Drosophila melanogaster: inhibition and promotion of dye-coupling between oocyte and follicle cells. Cell Tissue Res. 1993;273:163–173. doi: 10.1007/BF00304623. [DOI] [PubMed] [Google Scholar]
- Bossinger O, Schierenberg E. Cell-cell communication in the embryo of Caenorhabditis elegans. Dev Biol. 1992;151:401–409. doi: 10.1016/0012-1606(92)90180-o. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100:13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechner M, Hall DH, Bhatt H, Hedgecock EM. Cystic canal mutants in Caenorhabditis elegans are defective in the apical membrane domain of the renal (excretory) cell. Dev Biol. 1999;214:227–241. doi: 10.1006/dbio.1999.9398. [DOI] [PubMed] [Google Scholar]
- Chalfie M, White JG. The nervous system. In: Wood WB, editor. The nematode Caenorhabditis elegans. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. pp. 337–391. [Google Scholar]
- Chalfie M, Sulston JE, White JG, Southgate E, Thomson JN, Brenner S. The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci. 1985;5:956–964. doi: 10.1523/JNEUROSCI.05-04-00956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang CF, Vanhoven MK, Fetter RD, Verselis VK, Bargmann CI. An innexin-dependent cell network establishes left-right neuronal asymmetry in C. elegans. Cell. 2007;129:787–799. doi: 10.1016/j.cell.2007.02.052. [DOI] [PubMed] [Google Scholar]
- Curtin KD, Zhang Z, Wyman RJ. Gap junction proteins expressed during development are required for adult neural function in the Drosophila optic lamina. J Neurosci. 2002;22:7088–7096. doi: 10.1523/JNEUROSCI.22-16-07088.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Santo P, Logan MA, Chisholm AD, Jorgensen EM. The inositol trisphosphate receptor regulates a 50-second behavioral rhythm in C. elegans. Cell. 1999;98:757–767. doi: 10.1016/s0092-8674(00)81510-x. [DOI] [PubMed] [Google Scholar]
- Gho M. Voltage-clamp analysis of gap junctions between embryonic muscles in Drosophila. J Physiol. 1994;481(pt 2):371–383. doi: 10.1113/jphysiol.1994.sp020446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa L, Forbes A, Tazuke SI, Fuller MT, Lehmann R. Germ line stem cell differentiation in Drosophila requires gap junctions and proceeds via an intermediate state. Development. 2003;130:6625–6634. doi: 10.1242/dev.00853. [DOI] [PubMed] [Google Scholar]
- Govindan JA, Nadarajan S, Kim S, Starich TA, Greenstein D. Somatic cAMP signaling regulates MSP-dependent oocyte growth and meiotic maturation in C. elegans. Development. 2009;136 doi: 10.1242/dev.034595. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DH, Altun ZF. C. elegans atlas. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2008. p. 348. [Google Scholar]
- Hall DH, Russell RL. The posterior nervous system of the nematode Caenorhabditis elegans: serial reconstruction of identified neurons and complete pattern of synaptic interactions. J Neurosci. 1991;11:1–22. doi: 10.1523/JNEUROSCI.11-01-00001.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DH, Winfrey VP, Blaeuer G, Hoffman LH, Furuta T, Rose KL, Hobert O, Greenstein D. Ultrastructural features of the adult hermaphrodite gonad of Caenorhabditis elegans: relations between the germ line and soma. Dev Biol. 1999;212:101–123. doi: 10.1006/dbio.1999.9356. [DOI] [PubMed] [Google Scholar]
- Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- Hedgecock EM, Culotti JG, Hall DH, Stern BD. Genetics of cell and axon migrations in Caenorhabditis elegans. Development. 1987;100:365–382. doi: 10.1242/dev.100.3.365. [DOI] [PubMed] [Google Scholar]
- Kimble JE, White JG. On the control of germ cell development in Caenorhabditis elegans. Dev Biol. 1981;81:208–219. doi: 10.1016/0012-1606(81)90284-0. [DOI] [PubMed] [Google Scholar]
- Landesman Y, White TW, Starich TA, Shaw JE, Goodenough DA, Paul DL. Innexin-3 forms connexin-like intercellular channels. J Cell Sci. 1999;112(pt 14):2391–2396. doi: 10.1242/jcs.112.14.2391. [DOI] [PubMed] [Google Scholar]
- Lehmann C, Lechner H, Loer B, Knieps M, Herrmann S, Famulok M, Bauer R, Hoch M. Heteromerization of innexin gap junction proteins regulates epithelial tissue organization in Drosophila. Mol Biol Cell. 2006;17:1676–1685. doi: 10.1091/mbc.E05-11-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Dent JA, Roy R. Regulation of intermuscular electrical coupling by the Caenorhabditis elegans innexin inx-6. Mol Biol Cell. 2003;14:2630–2644. doi: 10.1091/mbc.E02-11-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Chen B, Gaier E, Joshi J, Wang ZW. Low conductance gap junctions mediate specific electrical coupling in body-wall muscle cells of Caenorhabditis elegans. J Biol Chem. 2006;281:7881–7889. doi: 10.1074/jbc.M512382200. [DOI] [PubMed] [Google Scholar]
- Majewska A, Yuste R. Topology of gap junction networks in C. elegans. J Theor Biol. 2001;212:155–167. doi: 10.1006/jtbi.2001.2364. [DOI] [PubMed] [Google Scholar]
- Nelson FK, Riddle DL. Functional study of the Caenorhabditis elegans secretory-excretory system using laser microsurgery. J Exp Zool. 1984;231:45–56. doi: 10.1002/jez.1402310107. [DOI] [PubMed] [Google Scholar]
- Nelson FK, Albert PS, Riddle DL. Fine structure of the Caenorhabditis elegans secretory-excretory system. J Ultrastruct Res. 1983;82:156–171. doi: 10.1016/s0022-5320(83)90050-3. [DOI] [PubMed] [Google Scholar]
- Norman KR, Maricq AV. Innexin function: minding the gap junction. Curr Biol. 2007;17:R812–R814. doi: 10.1016/j.cub.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol. 2000;10:R473–R474. doi: 10.1016/s0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- Peters MA, Teramoto T, White JQ, Iwasaki K, Jorgensen EM. A calcium wave mediated by gap junctions coordinates a rhythmic behavior in C. elegans. Curr Biol. 2007;17:1601–1608. doi: 10.1016/j.cub.2007.08.031. [DOI] [PubMed] [Google Scholar]
- Phelan P. Innexins: members of an evolutionarily conserved family of gap-junction proteins. Biochim Biophys Acta. 2005;1711:225–245. doi: 10.1016/j.bbamem.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Phelan P, Starich TA. Innexins get into the gap. Bioessays. 2001;23:388–396. doi: 10.1002/bies.1057. [DOI] [PubMed] [Google Scholar]
- Phelan P, Bacon JP, Davies JA, Stebbings LA, Todman MG, Avery L, Baines RA, Barnes TM, Ford C, Hekimi S, Lee R, Shaw JE, Starich TA, Curtin KD, Sun YA, Wyman RJ. Innexins: a family of invertebrate gap-junction proteins. Trends Genet. 1998;14:348–349. doi: 10.1016/s0168-9525(98)01547-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podbilewicz B, White JG. Cell fusions in the developing epithelial of C. elegans. Dev Biol. 1994;161:408–424. doi: 10.1006/dbio.1994.1041. [DOI] [PubMed] [Google Scholar]
- Ren XC, Kim S, Fox E, Hedgecock EM, Wadsworth WG. Role of netrin UNC-6 in patterning the longitudinal nerves of Caenorhabditis elegans. J Neurobiol. 1999;39:107–118. [PubMed] [Google Scholar]
- Saez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MV. Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta. 2005;1711:215–224. doi: 10.1016/j.bbamem.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: a critical appraisal. Glia. 2006;54:758–773. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- Starich TA, Lee RY, Panzarella C, Avery L, Shaw JE. eat-5 and unc-7 represent a multigene family in Caenorhabditis elegans involved in cell-cell coupling. J Cell Biol. 1996;134:537–548. doi: 10.1083/jcb.134.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starich T, Sheehan M, Jadrich J, Shaw J. Innexins in C. elegans. Cell Commun Adhes. 2001;8:311–314. doi: 10.3109/15419060109080744. [DOI] [PubMed] [Google Scholar]
- Starich TA, Miller A, Nguyen RL, Hall DH, Shaw JE. The Caenorhabditis elegans innexin INX-3 is localized to gap junctions and is essential for embryonic development. Dev Biol. 2003;256:403–417. doi: 10.1016/s0012-1606(02)00116-1. [DOI] [PubMed] [Google Scholar]
- Tazuke SI, Schulz C, Gilboa L, Fogarty M, Mahowald AP, Guichet A, Ephrussi A, Wood CG, Lehmann R, Fuller MT. A germline-specific gap junction protein required for survival of differentiating early germ cells. Development. 2002;129:2529–2539. doi: 10.1242/dev.129.10.2529. [DOI] [PubMed] [Google Scholar]
- White JG. The Anatomy. In: Wood WB, editor. The nematode Caenorhabditis elegans. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. pp. 81–122. [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Whitten SJ, Miller MA. The role of gap junctions in Caenorhabditis elegans oocyte maturation and fertilization. Dev Biol. 2007;301:432–446. doi: 10.1016/j.ydbio.2006.08.038. [DOI] [PubMed] [Google Scholar]
- Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.