SUMMARY
The recognition of odors is accomplished in the sensory epithelium where individual olfactory neurons express only one of 1,300 odorant receptor genes. Neurons expressing a given receptor project to two spatially invariant glomeruli in the olfactory bulb such that each odor elicits a distinct and sparse pattern of glomerular activity. We have altered the neural representation of odors in the brain by generating a mouse with a “monoclonal nose” in which greater than 95% of the sensory neurons express a single odorant receptor, M71. As a consequence, the frequency of sensory neurons expressing endogenous receptor genes is reduced twenty-fold. We observe that these mice can smell but odor discrimination and performance in associative olfactory learning tasks are impaired. However, these mice cannot detect the M71 ligand acetophenone despite the observation that virtually all sensory neurons and glomeruli are activated by this odor. The M71 transgenic mice readily detect other odors in the presence of acetophenone. These observations have implications for how receptor activation in the periphery is represented in the brain and how these representations encode odors.
INTRODUCTION
Olfactory perception requires the recognition of odors in the periphery and more central mechanisms in the brain that allow the discrimination of odors and appropriate behavioral output. Odor recognition is accomplished in the sensory epithelium where individual olfactory neurons choose only one of 1,300 odorant receptor (OR) genes from only one allele (Buck and Axel, 1991; Chess et al., 1994; Malnic et al., 1999; Young and Trask, 2002; Zhang and Firestein, 2002). The expression of a functional receptor is thought to elicit a feedback signal that stabilizes odorant receptor gene choice and suppresses the transcription of additional receptors, assuring that all neurons will ultimately express a single functional receptor and the choice of this receptor will remain stable for the life of the cell (Lewcock and Reed, 2004; Nguyen et al., 2007; Serizawa et al., 2003; Shykind, 2005; Shykind et al., 2004).
Neurons expressing a given receptor project to two topographically fixed loci (glomeruli) in the olfactory bulb, the first relay station for olfactory information in the brain (Ressler et al., 1993, 1994; Vassar et al., 1994; Vassar et al., 1993). The pattern of projections is conserved between different individuals and provides a two-dimensional representation of receptor activation in the periphery in the olfactory bulb. This anatomic map is functional: imaging studies reveal that each odor elicits a distinct pattern of glomerular activity. Most odorants at native concentrations activate fewer than 5% of the glomeruli in the olfactory bulb such that odor representations in the bulb are relatively sparse (Lin da et al., 2006). Sparse sensory distributions minimize overlap between different stimuli and have been postulated to maximize discrimination and enhance sensory-evoked associative learning (Barlow, 1972). These sparse, spatially invariant patterns of neural activity in the bulb may reflect a feature critical for the processing of olfactory information in the brain. We have used a genetic approach in mice to alter the sparse representation of odors in the brain and examined the consequence of these perturbations in the patterns of neural activity on odor discrimination and olfactory driven behaviors.
We have generated a mouse with a “monoclonal nose” in which greater than 95% of all olfactory sensory neurons express a single odorant receptor, M71, which is responsive to the ligand acetophenone. As a consequence, the frequency of sensory neurons expressing endogenous receptor genes is reduced twenty-fold. Exposure of these M71 transgenic animals to acetophenone results in a striking increase in activity in the sensory epithelium and dense rather than sparse patterns of neural activity in the bulb. Virtually all sensory neurons and glomeruli are activated by acetophenone. In contrast, the twenty-fold decrease in the number of neurons expressing endogenous receptor strongly diminishes the glomerular response to odors that do not activate the M71 receptor.
This transgenic model allows us to examine the effect of the striking reduction in neurons expressing the endogenous repertoire of odorant receptors on odor-evoked behavior. The pervasive neural activity evoked by acetophenone in these mice provides a novel scenario in which we can analyze odor detection in the context of dense rather sparse sensory representation. Finally, the concomitant increase in neural activity in response to acetophenone, and decrease in activity in response to all other odors provides a genetic formulation of an extreme version of the “cocktail party problem” (Haykin and Chen, 2005) for olfactory perception. Can the M71 transgenic mice distinguish odors in the presence of acetophenone, which elicits pervasive background activity?
We have therefore examined the consequence of these alterations in the patterns of neural activity, in M71 transgenic mice, on olfactory discrimination and odor-driven behaviors. We observe that these mice can indeed smell in the face of twenty-fold reduction in neurons expressing the endogenous receptor repertoire, but odor discrimination and performance in associative olfactory learning tasks are impaired. Innate olfactory-driven behaviors, including mating and aggression, are severely diminished. These mice cannot detect acetophenone despite a 1,000-fold increase in neurons expressing the M71 receptor. However, the M71 transgenic mice readily detect other odors in the presence of acetophenone. These observations have implications for how receptor activation in the periphery is represented in the brain and how these representations may encode odors.
RESULTS
Expression of the Acetophenone Receptor, M71, in the Vast Majority of Sensory Neurons
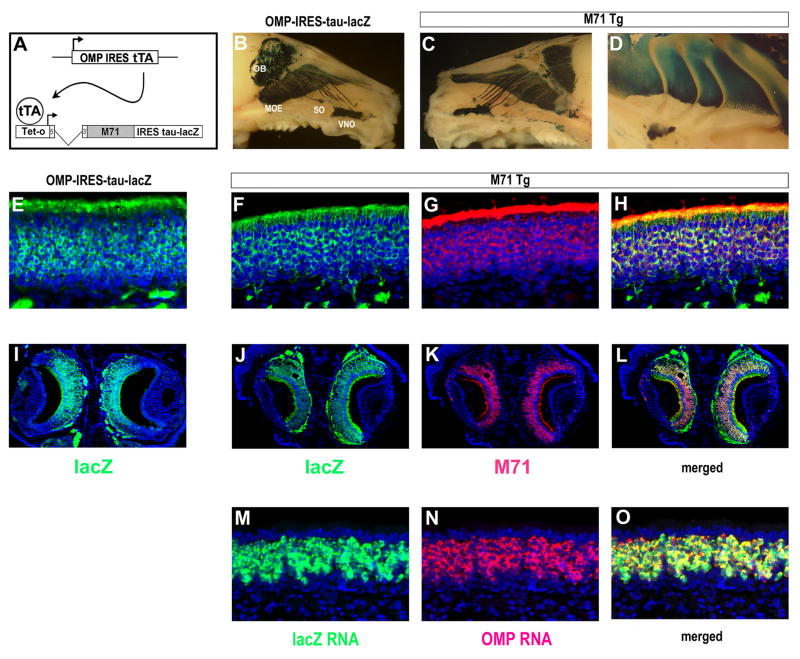
We have generated transgenic mouse lines bearing the construct tet0-M71-IRES-tau-lacZ in which the odorant receptor M71 and the marker protein tau-lacZ are both under the control of a tet-transactivator (tTA) responsive promoter, tet0 (Gossen et al., 1995), (Figure 1A). These transgenic lines were crossed with a mouse strain previously generated in our lab (OMP-IRES-tTA) that harbors the tTA gene under the control of the OMP promoter (Yu et al., 2004). OMP is specifically expressed in all mature olfactory neurons such that cells that activate OMP gene transcription will express a bicistronic RNA encoding both OMP and tTA. The expression of tTA should direct the coordinate synthesis of both M71 and tau-lacZ protein in all olfactory sensory neurons. A similar strategy has been used to homogenize the olfactory epithelium in recent experiments to examine the mechanisms of feedback repression of OR gene expression (Nguyen et al., 2007).
Figure 1.
Expression of the teto-M71-IRES-tau-lacZ transgene (M71 Tg) in olfactory sensory epithelia. (A) Schematic of the genetic strategy to express the M71 odorant receptor in all olfactory sensory neurons. The transgene teto-M71-IRES-tau-lacZ can be activated in all sensory neurons by the expression of tTA from the OMP-IRES-tTA locus. (B) Expression of OMP-IRES-tau-lacZ (detected by x-gal staining, blue) marks olfactory sensory neurons in a whole mount preparation in: the main olfactory epithelium (MOE), septal organ (SO), and vomeronasal organ (VNO), as well as in axons of sensory neurons from these areas as they project to the main (OB) and accessory olfactory bulb. (C) The expression of the M71 transgene in all the sensory epithelia detected by x-gal staining (blue) of a whole mount preparation. (D) Expression of the M71 transgene across all zones of the MOE as detected by x-gal staining in a whole mount preparation. (E) Immunohistochemical detection of the expression of lacZ (green) in coronal sections through the olfactory epithelium of control, OMP-IRES-tau-lacZ mice, counterstained with Toto-3 (blue). (F-H) Immunohistochemical detection of the expression of lacZ and M71 in coronal sections through the main olfactory epithelium of M71 transgenic mice. (F) Staining with antibody to lacZ (green). (G) Staining with antibody directed against the M71 receptor (red). (H) Merged fields of F and G. Nuclei are counterstained with Toto-3 (blue). (I) Immunohistochemical detection of the expression of lacZ (green) in coronal sections through the VNO of control OMP-IRES-tau-lacZ mice, counterstained with Toto-3 (blue) (J-L) Immunohistochemical detection of the expression of lacZ and M71 in coronal sections through the VNO of M71 transgenic mice. (J) Staining with antibody to lacZ (green). (K) Staining with antibody directed against the M71 receptor (red). (L) Merged fields of J and K. Nuclei are counterstained with Toto-3 (blue). (M-O) Two-color RNA in situ hybridization detects the ubiquitous expression the M71-IRES-tau-lacZ transgene and the OMP gene in sections through the olfactory epithelium of M71 transgenic mice. (M) RNA in situ hybridization with antisense probe to lacZ RNA (green). (N) RNA in situ hybridization with antisense probe for OMP RNA (red). (O) Merged fields of G and H with nuclei counterstained by Toto-3 (blue).
Ten independent transgenic lines bearing the tet0-M71-IRES-tau-lacZ construct were generated and crossed with mice harboring OMP-IRES-tTA. Analysis of the resulting compound heterozygotes identified seven lines that express the M71 transgene in the olfactory epithelium. The frequency of neurons expressing the transgene displayed considerable variation in the different lines (1-60%). One line however showed coordinate expression of M71 and tau-lacZ in the vast majority of olfactory sensory neurons. The expression of tau-lacZ from this line is apparent upon X-gal staining of a whole-mount preparation in the three sensory epithelia in the nose: the main olfactory epithelium (MOE), vomeronasal organ (VNO), and the septal organ, as well as in the axon fibers that project to the main and accessory olfactory bulb. The pattern of lacZ expression is comparable to the pattern observed in mice expressing OMP-IRES-tau-lacZ (Figure 1B and C, and (Mombaerts et al., 1996). The M71 transgene is expressed ubiquitously across the entire neuroepithelium with no evidence of the zonal segregation characteristic of the endogenous M71 gene (Vassalli et al., 2002), (Figure 1D). Immunohistochemical staining with antibodies directed against either lacZ or the M71 receptor reveals expression of the two genes in greater than 95% of the cells in the main olfactory epithelium (Figure 1E-H, and data not shown). The level of expression of M71 receptor from the transgene is comparable to that of the endogenous allele, as demonstrated by immunohistochemistry and RNA in situ hybridization (data not shown). We also observe the expression of the M71 transgene in 90% of the sensory neurons in the VNO (Figure 1I-L, and data not shown). RNA in situ hybridization with differentially labeled probes to OMP and lacZ mRNA directly shows that greater than 95% of the mature sensory neurons in the MOE express the bicistronic message from the transgene (Figure 1M-O).
Neurons that Express the M71 Transgene do not Express Endogenous Receptor Genes
In wild type mice, individual olfactory sensory neurons express only one of the 1,300 endogenous receptor genes. The singularity of receptor gene expression is thought to result, at least in part, from a mechanism in which the expression of a functional receptor elicits a feedback signal that stabilizes receptor gene choice and suppresses the expression of additional receptor genes (Lewcock and Reed, 2004; Nguyen et al., 2007; Serizawa et al., 2003; Shykind et al., 2004). In the M71 transgenic strain 95% of sensory neurons express the exogenous M71 gene. If this exogenous receptor were expressed before an endogenous gene is chosen, the feedback mechanism would suppress endogenous receptor expression in the vast majority of sensory neurons (Nguyen et al., 2007).
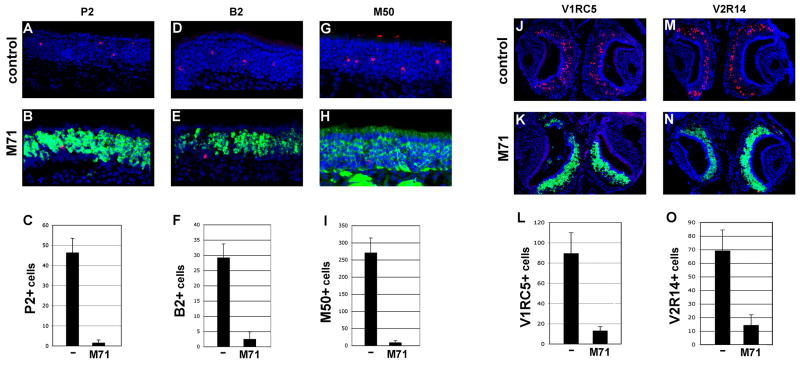
We therefore examined the frequency of cells expressing endogenous odorant receptor genes in the M71 transgenic mice using two-color RNA in situ hybridization and immunohistochemistry. In wild type animals, each OR gene is expressed in about 0.1% of the neurons. In the epithelium of the M71 transgenic mice, the frequency of cells expressing the endogenous receptors B2, P2 and M50 is reduced 12, 30 and 31-fold respectively when compared to the wild type sensory epithelium (Figure 2A-I). These data demonstrate that the expression of the M71 transgene suppresses endogenous OR gene choice and results in a near monoclonal the sensory epithelium.
Figure 2.
Expression of the endogenous odorant receptor genes in M71 transgenic and control mice detected in coronal sections through the main olfactory and vomeronasal epithelia. Average number of cells expressing the endogenous OR gene per section (n=10) is shown for each receptor in the graphs below. (A, B) Two-color RNA in situ hybridization with differentially labeled riboprobes, lacZ (green) and OR P2 (red), in sections from control (A) and M71 transgenic (B) mice. Nuclei are counterstained with Toto-3 (blue). (C) P2+ cells in controls = 46.5 +/- 7.1 (s.d.), in M71 transgenics = 1.6 +/-1.4 (s.d.).
(D, E) Two-color RNA in situ hybridization with differentially labeled riboprobes, lacZ (green) and OR B2 (red), in sections from control (D) and M71 transgenic mice (E). (F) B2+ cells in controls = 29.2 +/- 4.2 (s.d.), in M71 transgenics = 2.5 +/- 1.7 (s.d.).
(G, H) Immunohistochemical detection of lacZ and OR M50. Antibody directed against lacZ (green) and M50 receptor (red) in sections from control (G) and M71 transgenic mice (H). (I) M50+ cells in controls = 271 +/- 43 (s.d.), in M71 transgenics = 8.8 +/- 5.2 (s.d.). Nuclei are counterstained with Toto-3 (blue).
(J, K) Two-color RNA in situ hybridization with differentially labeled RNA probes for lacZ (green) and the vomeronasal receptor V1RC5 RNA (red) in control (J) and M71 transgenic mice (K). (L) V1RC5+ cells in controls = 89.6+/- 20.4 (s.d.), in M71 transgenics = 13.2 +/- 4.0 (s.d.). (M, N) Two-color RNA in situ hybridization with riboprobes for lacZ RNA (green), and the vomeronasal receptor V2R14 RNA (red), in control (M) and M71 transgenic mice (N). (O) V2R14+ cells in controls = 69.2 +/- 15.3 (s.d.), and in M71 transgenics = 14.4 +/- 7.6 (s.d.).
Recent data suggests that the pheromone receptors expressed in the vomeronasal organ also elicit a feedback signal that assures that only a single receptor is expressed in the neurons of this sensory epithelium (Roppolo et al., 2007). If M71, a receptor from the main olfactory epithelium, can similarly elicit feedback in the VNO we would predict a decrease in the frequency of cells expressing endogenous pheromone receptors. Two-color RNA in situ hybridization reveals an eight-fold reduction in the frequency of cells expressing V1RC5, a receptor expressed in the apical zone of the VNO (Dulac and Axel, 1995), (Figure 2J-L). We observe a five-fold reduction in the frequency of cells expressing V2R14, a receptor restricted to the more basal vomeronasal epithelium (Herrada and Dulac, 1997), (Figure 2M-O). These data suggest that a mechanism of feedback suppression elicited by the M71 transgene is also operative in the vomeronasal organ.
The Glomerular Map in M71 Transgenic Mice
In wild type animals, individual sensory neurons expressing a given receptor project with precision to two spatially invariant glomeruli creating a topographic map in the olfactory bulb (Ressler et al., 1994; Vassar et al., 1994). We have examined the pattern of projections of sensory neurons in the olfactory bulb of M71 transgenic mice. X-Gal staining of whole mount preparations of the M71 transgenic bulb reveals that lacZ+ fibers from the neurons expressing the transgene course over the entire surface of the main olfactory bulb and innervate large numbers of glomeruli. This pattern of projections is comparable to the pattern observed in the bulb of the OMP-IRES-tau-lacZ line (Figure 3A, B). Similarly, tau-lacZ+ fibers emanating from the VNO of M71 transgenic animals innervate the entire AOB (Figure 3D, and control C). The size of glomeruli are comparable between the M71 transgenic and control bulbs (M71 bulbs 53μm +/-1.5, SEM, and control bulbs 51μm +/- 1.6, SEM). Immunohistochemical staining for lacZ in coronal sections of main and accessory olfactory bulbs demonstrates that the lacZ+ fibers innervate the vast majority of glomeruli (Figure 3E-H).
Figure 3.
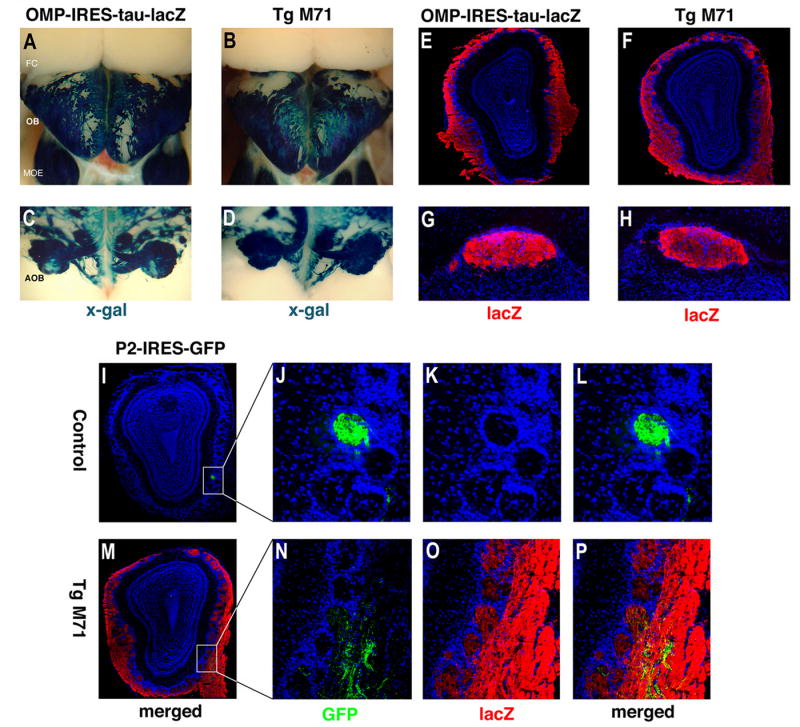
Pervasive innervation of the olfactory bulb in M71 transgenic mice. (A) Dorsal view of a x-gal stained (blue) whole mount preparation revealing the olfactory bulb (OB), main olfactory epithelium (MOE) and frontal cortex (FC) of a control OMP-IRES-tau-lacZ animal reveals the extent of sensory input to the bulb. (B) Dorsal view of an x-gal stained whole mount preparation of an M71 transgenic animal. (C) Dorsocaudal view of an x-gal stained whole mount preparation of a control OMP-IRES-tau-lacZ animal reveals the extent of sensory input to the accessory olfactory bulb (AOB). (D) Dorsocaudal view of an x-gal stained whole mount preparation of an M71 transgenic animal. (E) Immunohistochemical staining with antibody directed against lacZ (red) of a coronal section through the main olfactory bulb of a control OMP-IRES-tau-lacZ animal, counter stained for nuclei with Toto-3 (blue). (F) Immunohistochemical detection of lacZ+ fibers (red) in a coronal section through the main olfactory bulb of an M71 transgenic animal. (G) Immunohistochemical staining with antibody directed against lacZ (red) of a coronal section through the accessory olfactory bulb of a control OMP-IRES-tau-lacZ animal, counter stained for nuclei with Toto-3 (blue). (H) Immunohistochemical detection of lacZ+ fibers (red) in a coronal section through the accessory olfactory bulb of an M71 transgenic animal.
(I-P) Diminished sensory input from fibers expressing endogenous OR and co-innervation of glomeruli in M71 transgenic animals. (I) Coronal sections of olfactory bulb of P2-IRES-GFP control mice reveal P2 axons converging to form a single glomerulus as visualized by antibody to GFP (green) and Toto-3 nuclear counterstain, in low-power and (J-L) high-power images of region (white box) of same section. (M) In a low power image of a coronal section through the olfactory bulb of an M71 transgenic animal, also bearing the P2-IRES-GFP allele, diminished numbers of P2+ axons (green) converge on the P2 glomerulus in the presence of lacZ+ axons (red). (N-P) High-power images of boxed region in M reveal co-innervation of P2 glomerulus, as detected by anti-GFP antibody (green, N), by lacZ+ fibers detected by antiserum to lacZ (red, O) and merged in P. Nuclei are counterstained by Toto-3 (blue).
We have also examined the pattern of projections of neurons that express the endogenous P2 receptor in M71 transgenic mice. Mice bearing a genetically modified allele of the P2 receptor, P2-IRES-GFP (Gogos et al., 2000), were crossed into the M71 transgenic background. In these mice, the frequency of cells expressing P2-IRES-GFP was about 5% of that observed in controls bearing the P2-IRES-GFP allele alone (data not shown). Nonetheless, most P2+ neurons faithfully project axons to a single glomerulus on the medial (shown) and lateral surface of the bulb at a position roughly equivalent to that of the P2 glomerulus in wild type mice (Figure 3I-P). The glomeruli targeted by the P2+ axons were co-innervated by neurons expressing the M71 transgene (Figure 3N-P). Similar results were obtained for sensory neurons expressing the MOR23 and MOR28 receptors (data not shown). Thus, the olfactory bulb of the M71 transgenic mice receives diminished sensory input from cells expressing endogenous receptors, but the fibers from these neurons project appropriately to individual glomeruli, creating a topographic map. Superimposed upon this map are the pervasive projections from neurons expressing the M71 transgene. The observation that the M71 fibers are not constrained to a single enormous glomerulus but project to virtually all glomeruli suggests that the formation of a glomerulus is not solely determined by the convergence of like axons but may also be limited in size by properties intrinsic to the olfactory bulb.
Odor-evoked Activity in the Olfactory Epithelium and Olfactory Bulb of M71 Transgenic Mice
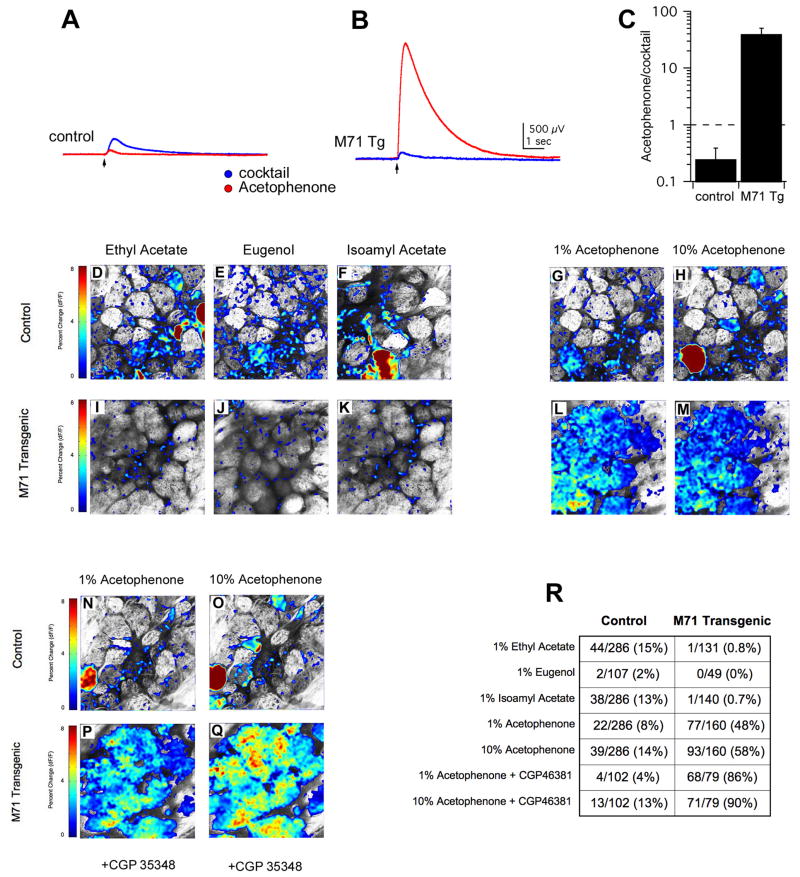
We next asked whether the exogenous expression of the M71 transgene results in the insertion of functional M71 receptors in the dendrites of olfactory sensory neurons by performing electro-olfactogram (EOG) recordings on olfactory epithelia of M71 transgenic and control mice. We compared responses elicited by a brief puff of the M71 ligand, acetophenone (Bozza et al., 2002; 10 μM; Figure 4A) with those obtained with a cocktail of five odorants (carvone, lyral, limonene, iso-eugenol and heptanal, 10 μM each; Figure 4A). The ratio of the integrated field potentials evoked by acetophenone to those elicited by the odorant cocktail was determined. In control animals, acetophenone evoked a far smaller response than that of the cocktail (ratio of 0.25 ± 0.14, n = 7 pairings from 4 animals, Figure. 4A and C). In marked contrast, acetophenone produced a dramatically larger response than the odorant cocktail in M71 transgenic animals (ratio of 40 ± 10, n = 9 pairings from 4 animals, Figure 4B and C). This 160-fold change in the ratio of the field potential is likely to result from the large increase in the frequency of cells expressing the acetophenone receptor, M71. These data indicate that exogenously expressed M71 receptors can respond to odor and render the olfactory epithelium exquisitely sensitive to acetophenone.
Figure 4.
Odor-evoked activity in the epithelium and olfactory bulb of M71 transgenic mice. (A-C) Representative electro-olfactogram (EOG) recordings from control (A) and M71 transgenic (B) mice in response to either a cocktail of odorants (blue) or to acetophenone (red). (C) Acetophenone sensitivity in M71 transgenic and control mice, expressed as the ratio of integrated EOG responses to acetophenone and to an odorant cocktail. (D-R) Two-photon imaging of odor-evoked activity in the olfactory bulb in response to 1% ethyl acetate, 1% eugenol and 1% isoamyl acetate in control (D-F) and M71 transgenic animals (I-K). Pseudo-colored heat maps show mean percent change in fluorescence (ΔF/F) for each odor. Activity evoked by 1% acetophenone and 10% acetophenone in control (G, H) and M71 transgenic bulbs (L, M). Activity evoked by 1% acetophenone and 10% acetophenone in the presence of the GABAB-receptor antagonist CGP46381 in control (N,O) and M71 transgenic bulbs (P, Q); same image fields as before antagonist application as shown in G-M. Summary table of the fraction of glomeruli responding to each odor in control and M71 transgenic mice.
We then asked whether the strong response to acetophenone observed in the sensory epithelium of M71 transgenic animals is reflected in the patterns of glomerular activity of sensory axons in the olfactory bulb. The M71 transgenic line was crossed with mice that express synapto-pHluorin, a pH-sensitive fluorescent indicator of synaptic release, in olfactory sensory neurons (Bozza et al., 2004). Two-photon imaging in these mice allows us to monitor presynaptic glomerular activity in response to odor. Multiple fields, each encompassing about 30 glomeruli on the dorsal surface of the bulb were imaged in response to odor. Glomerular responses were considered positive if their mean percent change in fluorescence (ΔF/F) was greater than two standard deviations of the mean above baseline. In control animals, individual odors (ethyl acetate, isoamyl acetate, eugenol, and acetophenone) at a concentration of 1% typically activated 2-3 glomeruli per field with a ΔF/F of 5% (Figure 4D-G, R). In contrast, exposure of the M71 transgenic animals to ethyl acetate, isoamyl acetate, or eugenol failed to elicit a discernable glomerular response (Figure 4I-K, R). This result is likely a consequence of the marked reduction in sensory input from neurons expressing the endogenous receptor repertoire. However, exposure of the M71 transgenic mice to 1% acetophenone resulted on average in the activation of 51% of the glomeruli imaged, with three animals exhibiting activation of 75% of the glomeruli and one animal revealing activation of only 10% of glomeruli (Figure 4L and R). The level of acetophenone-evoked activity (ΔF/F = 1.9%) in transgenic mice was substantially lower then acetophenone-induced glomerular activity in controls (ΔF/F = 6.5%). Exposure to 10% acetophenone evoked activity in the 75% of the glomeruli (Figure 4M and R) in transgenic animals but the level of activity remained low (ΔF/F = 1.8%) when compared to the more sparse but robust response (ΔF/F = 5.9%) in the control bulbs (Figure 4H and R).
These observations suggest that despite the presynaptic activation of the majority of glomeruli, inhibition of synaptic release may be occurring at sensory axon termini in the bulb of M71 transgenic mice upon exposure to acetophenone. The major presynaptic inhibitory input onto sensory neurons is thought to be mediated by GABAergic periglomerular cells (Aroniadou-Anderjaska et al., 2000; McGann et al., 2005; Murphy et al., 2005; Vucinic et al., 2006). We therefore examined odor-evoked activity in the presence or absence of the GABAB receptor antagonist, CGP46381. In control mice, odor-evoked responses to all odors tested, including acetophenone, exhibited only modest (13.3%) elevations in glomerular activity in the presence of CGP46381 (Figure 4N and data not shown). In contrast, in the M71 transgenic mouse, we observe dramatic enhancement in the level of glomerular activity and recruitment of additional active glomeruli. We observe a 210% increase in the glomerular responses to 1% acetophenone and a 170% increase to 10% acetophenone in virtually all responsive glomeruli upon application of the GABAB receptor inhibitor (Figure 4P and Q). Moreover, the frequency of acetophenone activated glomeruli, in response to 1% acetophenone, increases from 51% to 90% (Figure 4R and data not shown). We do not observe significant changes in fluorescence in response to any other odor tested in the transgenic mice, either in the presence or absence of inhibitor (data not shown), a finding consistent with the 20-fold reduction in input from neurons bearing endogenous receptors. Taken together, the data demonstrate that exogenously expressed M71 receptors in the transgenic mice function to activate sensory neurons in the olfactory epithelium, which then transmit this information to the olfactory bulb. M71 transgenic mice reveal a significant decrease in presynaptic glomerular activity in response to all odors tested, other than acetophenone. Acetophenone, however, elicits activity in multiple glomeruli, but this response is likely to reflect strong GABAergic presynaptic inhibition.
Associative Olfactory Discrimination in M71 Transgenic Mice
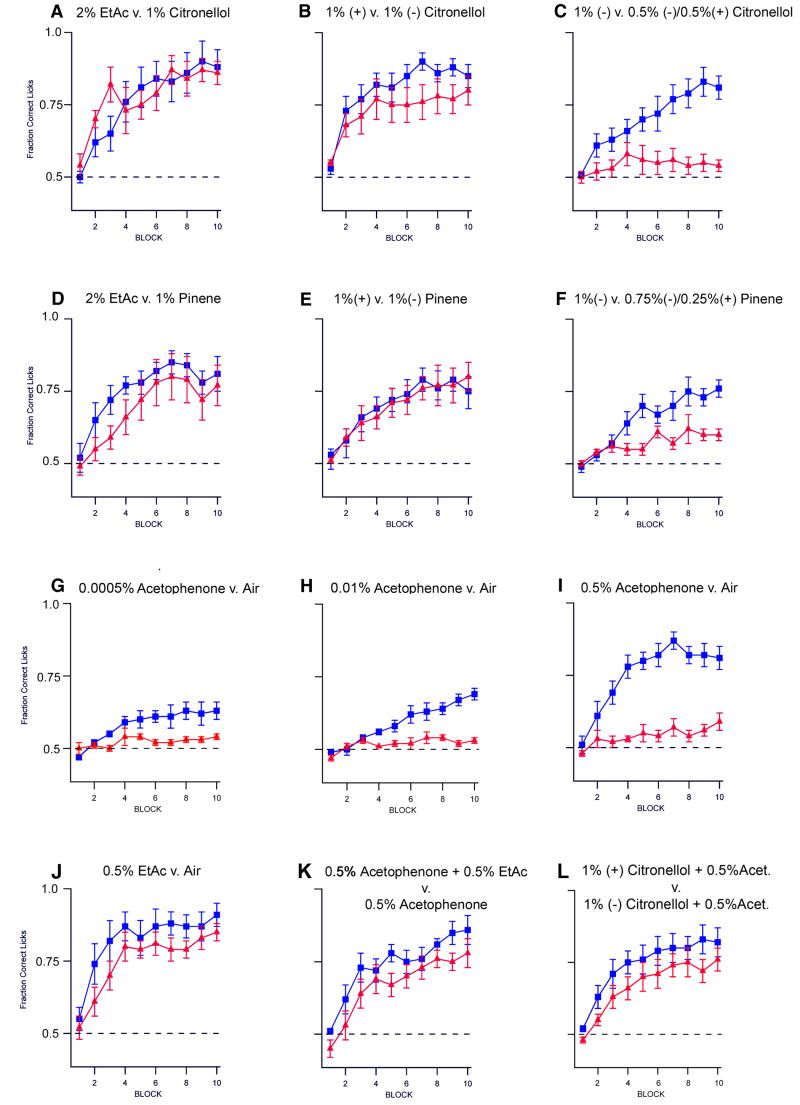
The pervasive expression of M71 receptor in the transgenic line results in dramatic alterations in the patterns of glomerular activity in response to odor. As a consequence, we might expect defects in both odor discrimination and innate olfactory-driven behavior. We have therefore examined the performance of M71 transgenic mice in an olfactory associative learning task. A go/no-go discrimination assay was employed in which water-restricted mice were exposed to two odors, one of which was followed by a water reward (Bodyak and Slotnick, 1999). Mice were trained to sample both odor stimuli and lick only in response to the rewarded odor. Initially, mice were tested for their ability to distinguish two structurally unrelated odors: ethyl acetate and citronellol, or ethyl acetate and pinene. M71 transgenic and control animals performed this task with near maximal accuracy (Figure 5A and D). Next, the olfactory discrimination task was made more difficult, with mice required to discriminate between pairs of enantiomers. We observed that M71 transgenic and control mice were able to distinguish between 1% (-) citronellol and 1% (+) citronellol (Figure 5B), and between 1% (-) pinene and 1% (+) pinene (Figure 5E). Olfactory deficits in M71 transgenic mice became apparent when the mice were required to discriminate between mixtures of enantiomer pairs. Control mice accurately discriminated between 1% (-) citronellol and a mixture of 0.5% (-) citronellol and 0.5% (+) citronellol (Figure 5C), and between 1% (-) pinene and a mixture of 0.75% (-) pinene and 0.25% (+) pinene (Figure 5F), whereas the performance of M71 transgenic mice in these tasks is greatly reduced. These data demonstrate that despite a drastic reduction in the expression of the endogenous OR repertoire the M71 transgenic mice are not anosmic; they are capable of learned olfactory discrimination but their performance is compromised.
Figure 5.
M71 transgenic mice display deficits in olfactory discrimination. (A-C) Control mice (blue) can discriminate between 2% ethyl acetate and 1% citronellol (A), between 1%(-) citronellol and 1%(+) citronellol (B), as well as between 1% (-) citronellol and a mix of 0.5% (+)/ 0.5% (-) citronellol (C). M71 transgenic mice (red) can discriminate between 2% ethyl acetate and 1% citronellol (A), between 1%(-) citronellol and 1%(+) citronellol (B), but fail to discriminate between 1% (-) citronellol and a mix of 0.5% (+)/ 0.5% (-) citronellol (C). (D-F) Control mice can discriminate between 2% ethyl acetate and 1% pinene (D), between 1%(-) pinene and 1%(+) pinene (E), as well as between 1% (-) pinene and a mix of 0.25% (+)/ 0.75% (-) pinene (F). M71 transgenic mice can discriminate between 2% ethyl acetate and 1% pinene (D), between 1%(-) pinene and 1%(+) pinene (E), but fail to discriminate between 1% (-) pinene and a mix of 0.25% (+)/ 0.75% (-) pinene (F). (G-I) Control mice (blue) show increasing accuracy in the discrimination of acetophenone and air (no odor stimulus) as acetophenone concentration is increased from 0.0005% to 0.5% (G-I). M71 transgenic mice (red) fail to discriminate between acetophenone and air at all concentrations tested (G-I). Both M71 transgenic (red) as well as control animals (blue) are capable of discriminating between 0.5% ethyl acetate and air (J), between 0.5% acetophenone and a mix of 0.5% acetophenone and 0.5% ethyl acetate (K), and between a mix of 1% (+) citronellol and acetophenone and a mix of 1% (-) citronellol and acetophenone (L). The fraction of correct licks in response to rewarded versus unrewarded odor is shown. For each discrimination task, n≧6 for control and M71 transgenic animals.
The expression of the acetophenone receptor, M71, in the vast majority of sensory neurons results in a dramatic enhancement in acetophenone-evoked activity in the epithelium and widespread activation of glomeruli in the bulb. We therefore asked whether these alterations in patterns of glomerular activity are reflected in the sensitivity of the transgenic mouse to acetophenone. We exposed wild type and M71 transgenic mice to increasing concentrations of acetophenone (0.0005%-0.5%) in the go/no-go discrimination task. Wild type mice discriminated between acetophenone and air with increasing accuracy in the assay as the concentration of acetophenone was increased. Surprisingly however, M71 transgenic mice failed to detect acetophenone at all concentrations tested (Figure 5G-I). This deficit is specific for acetophenone since M71 transgenic mice readily discriminate ethyl acetate and other odors from air (Figure 5J and data not shown). Thus despite a 1,000-fold increase in sensory neurons expressing the M71 receptor and the global activation of glomeruli, the M71 transgenic mice fail to detect acetophenone.
We next asked whether M71 transgenic mice could detect and discriminate other odors in the presence of acetophenone. The addition of a second odor along with acetophenone might elicit contrast: a small number of glomeruli may be activated above a background of widespread glomerular activity elicited by acetophenone. We therefore asked whether mice could discriminate a mix of ethyl acetate and acetophenone from acetophenone alone. In the go/no-go discrimination task, both M71 transgenic and control animals were able to discriminate these odors (Figure 5K). We also asked whether M71 transgenic mice could discriminate (+) citronellol from (-) citronellol, both in the presence of acetophenone. We observed that M71 transgenic and control animals were equally able to discriminate these enantiomers in the presence of acetophenone (Figure 5L). Thus the M71 transgenic mice fail to detect acetophenone despite the observation that most sensory neurons and glomeruli are activated by this odor but readily detect and discriminate other odors in its presence.
Innate Olfactory-Driven Behaviors
M71 transgenic mice are capable of associative olfactory discrimination of odors despite widespread alterations in their olfactory circuit. The effect of these alterations on the hard-wired neural circuits that dictate innate odor-driven behaviors may be more pronounced. We therefore examined the performance of the M71 transgenic mice in mating and aggression, two olfactory-driven innate behaviors. Mating behavior in male mice consists of an initial phase of olfactory exploration followed by mounting and intromission (Leypold et al., 2002; Stone 1922; Stowers et al., 2002). Group-housed male mice (three months old) were presented with estrous females and observed for 30 minutes. M71 transgenic males explored females for half the duration exhibited by control mice (Figure 6A). Moreover, transgenic males mounted females at one-tenth the frequency of control mice and with one-twentieth the duration (Figure 6B and C). Similar results were obtained with males that were singly housed for three weeks prior to the assay (data not shown).
Figure 6.
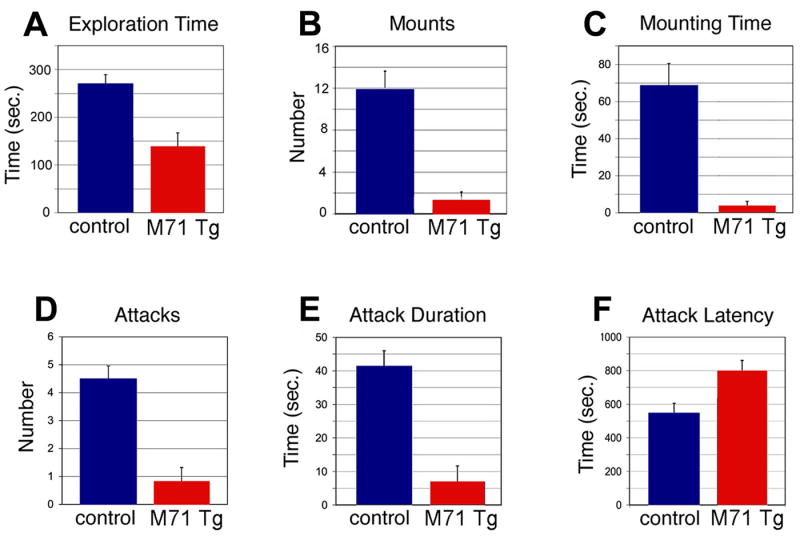
Innate male behaviors are drastically reduced in M71 transgenic mice. (A-C) Male sexual behavior is reduced in M71 transgenic mice (red bars) compared to controls (blue bars). Exploration time (A) in controls: 268.8 sec. ± 20.6 sec. (SEM) and in M71 transgenic males: 138 sec. ± 29.1 sec. (SEM), p=0.0009. Number of mounts (B) in controls: 11.8 ± 1.78 (SEM), and in M71 transgenic males: 1.27 ± 0.82 (SEM), p=0.00004. Total mounting time (C) in controls: 68 sec. ± 12.6 sec. (SEM), and in M71 transgenic males: 3.6 sec. ± 2.51 sec. (SEM), p=0.00015. (D-F) Aggressive behavior in a resident-intruder assay is reduced in M71 transgenic mice (red bars) compared to controls (blue bars). Number of attacks (D) in controls: 4.5 ± 0.7 (SEM), and in M71 transgenic males: 0.8 ± 0.5 (SEM), p=0.0002. Attack duration (E) in controls: 41.2 sec. ± 8.9 sec. (SEM), and in M71 transgenic males: 6.9 sec. ± 4.8 sec. (SEM), p=0.001. Attack latency (F) in controls: 542 sec. ± 63.2 sec. (SEM), and in M71 transgenic males: 797 sec. ± 63.1 sec. (SEM), p=0.0048.
Aggressive behavior was examined in wild type and M71 transgenic mice in a resident-intruder assay (Leypold et al., 2002). The behavior of resident males was observed for a period of 15 minutes after the introduction of an intruder male into their home cage. After initial olfactory exploration, wild type resident males initiated vigorous attacks against intruder mice (Figure 6D-F). In contrast, M71 transgenic males showed large deficits in aggressive behavior (Figure 6D-F), including a reduction in the number of attacks and in attack duration, and an increase in attack latency. These data suggest that the decrease in the frequency of expression of endogenous receptors in M71 transgenic mice results in significant deficits in innate olfactory-driven behavior.
DISCUSSION
We have exploited the mechanism of feedback suppression of odorant receptor gene expression to generate a mouse with a “monoclonal nose.” The vast majority of sensory neurons express the acetophenone-responsive receptor M71, and exposure to this odor results in a dense map of glomerular activity rather than the sparse patterns of activity in control mice. In these animals, the frequency of sensory neurons expressing endogenous receptor genes is reduced twenty-fold and glomerular activity in response to most odors is dramatically diminished. These perturbations allow us to address questions concerning receptor choice in olfactory sensory neurons, the representation of odors in the brain, and how these representations result in olfactory discrimination and olfactory-driven behaviors.
Feedback Repression of Receptor Expression
The odorant receptor not only defines the chemical receptive field of the neuron, but also assures the singularity of receptor choice and contributes to the specificity of axon targeting in the olfactory bulb. What mechanisms have evolved to assure the expression of only one of the 1,300 OR genes in a sensory neuron? Recent data suggest a model in which a singular transcriptional machine consisting of DNA enhancer elements is capable of associating in trans with the promoters of odorant receptor genes (Lomvardas et al., 2006). A unique transcriptional compartment which harbors a single copy of a DNA regulatory element could allow the stochastic activation of only one OR allele in an olfactory sensory neuron. Once a functional receptor is expressed, feedback stabilizes odorant receptor gene choice and suppresses the transcription of additional receptors (Lewcock and Reed, 2004; Nguyen et al., 2007; Serizawa et al., 2003; Shykind, 2005; Shykind et al., 2004). This mechanism assures that all neurons will ultimately express a single functional receptor and the choice of this receptor will remain stable for the life of the cell.
In the M71 transgenic mice, greater than 95% of the sensory neurons express only the M71 gene. These data suggest that the M71 transgene is expressed prior to endogenous receptor gene choice. The expression of a functional receptor from the M71 transgene will elicit a feedback signal that suppresses the transcription of endogenous receptor genes. This mechanism results in a twenty-fold diminution in the frequency of sensory cells expressing endogenous receptor genes. A similar process of feedback control appears to be operative in the VNO (Roppolo et al., 2007). Our data imply that the expression of an MOE receptor, M71, elicits feedback in the VNO despite the fact that the VNO and MOE receptors use different G protein signaling cascades for odor transduction. The feedback cascade may therefore use yet another signaling pathway that is shared by the two sensory epithelia.
An alternative model of OR gene regulation proposes a mechanism for the stability of OR expression without the requirement for feedback suppression. In this model, receptor choice is a kinetically slow process that occurs over a narrow window of developmental time and the rare cells that express two receptors die (Feinstein et al., 2004). Our experiments with the M71 transgene render this alternative unlikely. In models that invoke negative selection rather than feedback suppression, the vast majority of cells in the M71 transgenic mice would express two functional receptors leading to massive cell death in the MOE. In TUNEL assays for cell death, we observed the same frequency of apoptotic cells in the sensory epithelia of M71 transgenic mice and controls (data not shown). Our data therefore support a model in which the expression of either exogenous or endogenous receptor elicits a feedback signal suppressing subsequent OR gene choice.
Olfactory Discrimination and Olfactory Behaviors by a “Monoclonal Nose”
In M71 transgenic mice, the singular expression of the acetophenone receptor in most neurons perturbs the organization and function of the sensory epithelium and olfactory bulb. Can we relate these alterations in sensory neurons and sensory representations to the changes we observe in odor discrimination and olfactory-driven behaviors? The striking decrease in the number of neurons expressing the endogenous receptor repertoire is likely to diminish the sensitivity to odors at the level of the sensory epithelium. This may afford an explanation for the observation that the M71 transgenic mice can smell but their capacity to discriminate odors is debilitated.
Chemical ablation of the sensory epithelium, as well as surgical removal of large portions of the olfactory bulb have provided an alternative strategy to diminish olfactory sensory input (McBride et al., 2003; Lu and Slotnick, 1998). In these studies, despite significant physical damage, animals retain the ability to smell with only minimally impaired discriminatory consequences. These physical interventions differ from our genetic approach in that surgery will completely deplete one set of inputs leaving others intact, whereas the genetic depletion removes 95% of all sensory cells creating a defined, reproducible decrease in all neurons expressing the endogenous receptor repertoire. Moreover, physical ablations result in significant damage to the epithelium or bulb and cannot reproducibly deplete defined sensory input.
Innate olfactory-driven behaviors, including mating and aggression, are severely impaired in M71transgenic mice. The decrease in mating behavior in the M71 transgenics is also observed in mutants that silence the main olfactory epithelium (Mandiyan et al., 2005). Mutants in vomeronasal function do not exhibit a reduction in mating behavior, but rather reveal difficulty in discriminating between the sexes of the mating partner (Kimchi et al., 2007; Leypold et al., 2002; Stowers et al., 2002). Taken together, these observations suggest that the abolition of mating behavior in the M71 transgenic mice results from functional defects in the main olfactory system. Aggression appears to be controlled both by the MOE and the VNO (Leypold et al., 2002; Mandiyan et al., 2005; Stowers et al., 2002) and we therefore cannot dissect the relative contributions of the two olfactory epithelia to this behavioral deficit.
Learned olfactory behaviors are impaired whereas innate behaviors are more severely diminished or abolished. One relatively simple explanation for the differential sensitivity of the two classes of olfactory driven behavior argues that the concentration of natural odors that elicit mating and aggression is far lower than the levels of odor we employ in the more artificial associative learning tasks. A more interesting explanation invokes the greater plasticity of the circuits engaged in associative behaviors to explain the differential sensitivity of the two sorts of olfactory driven behaviors. Innate behavior is exhibited by naïve animals, without prior learning or experience, suggesting that the neural circuits responsible for these behaviors are developmentally programmed and invariant in different individuals. Hardwired neural circuits that exhibit limited plasticity are likely to be most severely affected by the perturbations in the organization of the olfactory system we observe in M71 transgenic mice. Associative olfactory learning however is plastic. Therefore, it may suffice that different odors elicit different patterns of neural activity but these odor-specific patterns need not be identical in different individuals. The alteration in the patterns of neural activity elicited by odors in the M71 transgenic mice may not abolish odor discrimination despite the fact that these patterns are perturbed and may differ from those observed in wild type mice. These arguments suggest that mutations that alter the organization of the olfactory system may affect innate behaviors more profoundly than the more plastic associative behaviors, a finding consistent with the behavior observed in the M71 transgenic mice.
M71 Transgenic Mice Fail to Detect Acetophenone
The M71 transgenic mice fail to detect acetophenone despite a 1,000-fold increase in neurons expressing the acetophenone receptor. One mechanism responsible for the inability to detect acetophenone may involve suppression at any of the various olfactory stations from peripheral sensory neuron to cortex. Suppression, a property often observed in other sensory systems, may be artificially exaggerated in the M71 mouse where 95% of the sensory inputs to every glomerulus derive from cells expressing the acetophenone receptor. The diminished response to acetophenone may be a property intrinsic to the neurons that define the olfactory circuit or may reflect a more dynamic network property. In the olfactory bulb several classes of inhibitory neurons, including periglomerular cells and granule cells, result in odor-evoked inhibition of either sensory neurons or mitral cells (Aroniadou-Anderjaska et al., 2000; Aungst et al., 2003; Murphy et al., 2005). This inhibition may either act locally (intra-glomerular) or over long distances (inter-glomerular).
We observe significant suppression of the response to acetophenone at the presynaptic axonal arbors in the glomerulus. Synapto-pHluorin imaging in the bulb reveals only weak responses in most glomeruli of M71 transgenic mice upon exposure to 1% acetophenone. The administration of GABAB receptor antagonists to the bulb of these mice results in a two-fold elevation in the glomerular response to acetophenone in M71 mice and only modest elevations (13%) in control animals. Thus, significant presynaptic inhibition, presumably mediated by GABAergic periglomerular and short axon cells, arises upon exposure to acetophenone as a consequence of the ubiquitous activation of glomeruli in the M71 mouse. Strong suppression is already apparent in the termini of the most peripheral neurons in the olfactory circuit and inhibition of the multiple downstream neurons within this pathway could further diminish the organism’s response to acetophenone.
Despite the evidence for presynaptic inhibition at the termini of sensory neurons, exposure of M71 transgenic mice to acetophenone results in significant activation of at least half the glomeruli. Presynaptic inhibition, although strong, is incomplete suggesting that additional mechanisms may therefore be operative and may explain the inability of M71 mice to detect acetophenone. One model argues that the recognition of patterns of neural activity, or contrast, in higher olfactory centers is required for odor detection (Haberly, 2001). In the olfactory bulb, a given odor activates a sparse, spatially invariant subpopulation of glomeruli. In piriform cortex, physiological concentrations of odors activate a unique ensemble comprised of 5-10% of the layer 2 cortical neurons (D. Stettler and R. Axel, unpublished data). Higher processing centers may recognize odors by interpreting patterns of neural activity defined by the distribution of active and inactive neurons. In M71 transgenic mice, acetophenone may activate most of the neurons in the olfactory pathway. As a consequence, a contrasting pattern of neuronal activation, which may be required for odor discrimination, is replaced by pervasive neural activity.
Although the M71 transgenic mice cannot detect acetophenone, they are able to distinguish other odors in the presence of acetophenone. In these mice, 95% of the sensory input in most glomeruli derives from neurons expressing the M71 receptor and only 5% of the axons project from neurons bearing one of the 1,300 endogenous receptors. In the absence of suppression, a given odor in the context of acetophenone would therefore activate a subset of glomeruli to levels only 5% higher than glomeruli activated by acetophenone alone. It is possible that ubiquitous activation of glomeruli by acetophenone results in a global suppression above which small signals in a subpopulation of glomeruli (local excitation) elicited by a second odor may be detected.
This scenario may afford the M71 transgenic mouse the ability to discriminate a mix of ethyl acetate in acetophenone from acetophenone alone. However, acetophenone alone will not only elicit pervasive activation of the bulb but will also specifically activate the subset of glomeruli that receive input from other endogenous acetophenone receptors. Thus acetophenone alone, as well as in mixtures, will elicit patterns of glomerular activity in which subsets of glomeruli activated by endogenous receptors stand out on a background of pervasive M71 activity. Why do the M71 transgenic mice fail to detect acetophenone? Acetophenone is normally found in mouse urine such that all mice are continually exposed to this odor. In M71 transgenic mice this results in global activation of the bulb with slightly greater activity in the subset of glomeruli receiving input from the neurons expressing endogenous acetophenone receptors. This specific pattern of glomerular activation can never occur without pervasive activity and may be subject to suppression. This could result in the failure of M71 transgenic mice to detect acetophenone but still allow the detection of other odors in the presence of acetophenone.
One mechanism to accomplish the exquisite sensitivity reflected in the detection of odors in the presence of acetophenone is gain control (McGann et al., 2005; Olsen and Wilson, 2008; Root et al., 2008). Sensory systems respond to natural stimuli that convey information over several orders of magnitude yet employ neurons that exhibit a far narrower dynamic range. Gain control can be achieved through adaptation, inhibition, or specific enhancement. Adaptation has been invoked to explain the ability to detect vastly different intensities in vision and amplitudes in audition (Frisina, 2001; Shapley and Enroth-Cugell, 1984). We observe significant presynaptic inhibition of sensory neurons upon exposure to acetophenone in M71 mice. The small changes in signal elicited by a second odor, in only a subpopulation of neurons, may generate contrast and provide an informative pattern salient to the organism. The olfactory circuit may then employ mechanisms of contrast enhancement to detect relevant signals in the face of olfactory “noise” (acetophenone). The mechanisms of inhibition and enhancement we invoke in the exaggerated genetic setting of the M71 transgenic mouse might, under normal conditions, underlie the ability to detect individual odors in “noisy” odiferous environments.
EXPERIMENTAL PROCEDURES
Generation of transgenic tet0-M71-IRES-tau-lacZ mice
The M71 odorant receptor cDNA (with an N-terminal Flag-rho tag) followed by an internal ribosome entry site (IRES) - tau-lacZ cassette (Mombaerts et al., 1996) was inserted into a plasmid containing the teto promoter (Yu et al., 2004) and an exogenous intron with splice donor and acceptor sites. An SV40 polyadenylation sequence was placed directly after the stop codon of the tau-lacZ gene. The construct was separated from vector sequence by agarose gel electrophoresis and microinjected into the pronuclei of fertilized eggs. Genomic DNA isolated from the tails of resultant mice was analyzed by PCR and Southern blotting to identify transgenic founders by standard protocols.
X-gal and Immunohistochemical Staining
M71-IRES-tau-lacZ expressing neurons were visualized in whole mount preparation using X-gal (Gibco-BRL), a chromogenic substrate for lacZ, as previously described (Gogos et al., 2000). Immunohistochemistry was performed on 16μm cryosections of tissue that was prefixed in 1% freshly prepared paraformaldeyde (EMS) for 60 min. followed by decalcification in 0.5M EDTA, 1X PBS for 18 hours at 4°C and embedding in OCT (Sakura) on dry ice. Anti-lacZ antiserum (Biogenesis) was used at 1:1,000 dilution, anti-M50 antiserum (Lomvardas et al., 2006) was used at 1:2,000 dilution, and anti-GFP antiserum at 1:1,000 dilution (Molecular Probes). Primary antibodies were visualized using Alexa 488 and Cy3-conjugated secondary antibodies (Molecular Probes and Jackson Labs) and counterstained with Toto-3, 1:1,000 (Molecular Probes). Stained sections were visualized using a Bio-Rad MRC 1024ES confocal microscope.
RNA In Situ Hybridization
Tissue was prepared as described for immunohistochemistry. Two-color RNA in situ hybridization was carried out on 16μm cryosections using riboprobes labeled with either digoxigenin or FITC (Roche) by either T7 or SP6 RNA polymerase (Promega). Hybridizations were carried out as described (Vassar et al., 1993) and dig-labeled probes were detected using sheep anti-digoxigenin-HRP (horse radish peroxidase, Roche) and visualized with the fluorogenic HRP substrate, Cy3-tyramide, following manufacturer’s instructions (Perkin-Elmer TSA system). For the second color, slides were treated with sodium azide (0.05%) in TNB buffer (Perkin-Elmer TSA kit) for 60 min. at room temperature, to inactivate the first HRP-labeled antibody. FITC-labeled riboprobes were then detected by sheep anti-FITC-HRP (Roche) and visualized with the fluorogenic HRP substrate FITC-tyramide. Nuclei were counterstained with Toto-3 at 1:1,000 (Molecular Probes).
EOG Recordings
A preparation of the medial surface of the olfactory turbinates was superfused (150 mL/hr) with artificial cerebrospinal fluid (ACSF) at room temperature. Local field potentials were recorded with an ACSF-filled glass pipette (2-4 MΩ) placed on the surface of the olfactory epithelium, and were amplified by a Multiclamp 700A amplifier (Molecular Devices, Sunnyvale, CA), filtered at 1 kHz and digitized at 5 kHz. (ITC-18; Instrutech, Mineola, NY). Data were collected and analyzed using Axograph X and IGOR Pro (Wavemetrics, Lake Oswego, OR). A second pipette containing the odorant cocktail diluted in ACSF was placed within 100 μm of the recording electrode using a micromanipulator. Odorants were delivered as brief puffs (30 psi, 100 ms) using a Picospritzer III (Parker). Reponses to three odorant puffs, delivered one minute apart, were obtained at each location and averaged. Puffs of ACSF alone did not elicit changes in local field potential.
In Vivo Imaging
Imaging experiments were performed on adult mice (8-10 weeks old). Homozygous OMP-synapto-pHluorin mice (Bozza et al., 2004) were acquired from The Jackson Laboratory (Bar Harbor, ME) and crossed with mice harboring the M71 transgene and OMP-IRES-tTA. Mice were anaesthetized using ketamine/xylazine (100mg/kg and 10mg/kg, Henry Schein Veterinary, Inc.), the dorsal-lateral surface of the olfactory bulb was exposed and a custom-cut glass coverslip was placed over the area and sealed in place with a layer of 2% agarose. For CGP46381 use, the coverslip was removed and 1mM CGP46381 in ACSF was bath-applied for 30 minutes. Animals were maintained at 37°C using a feedback-controlled heating pad (Fine Science Tools, Inc.) and depth of anesthesia was monitored by foot pinch and whisking responses throughout the imaging.
Imaging was performed using a custom-modified Prairie Technologies Ultima two-photon microscope with two tunable pulsed IR lasers (910nm; Coherent, Chameleon Ultra II). Images concurrent with odor administration were acquired as a T-series via the PrairieView software; for each trial, 90 images were taken at a rate of ~2.5 Hz and a resolution of 256 × 256 pixels using a 16x water immersion objective (Olympus, 0.8 N.A.). Odors (ethyl acetate, isoamyl acetate, acetophenone, eugenol) were diluted in dipropylene glycol to achieve a final concentration of 1% or 10% (weight/volume) (Sigma-Aldrich, Inc.) and were delivered to the animals from a distance of approximately 1cm using a custom-made manifold. Each odor, as well as a blank consisting of dipropylene glycol only, was pseudorandomly presented 8 times to the animal at each imaging site. Odor delivery lasted for 3 seconds in each trial. A Z-stack was taken at each imaging site to aid in offline analysis and alignment.
Image and Data Analysis
All image processing and data analysis was performed in ImageJ and Matlab using custom-written software. Pseudocolor maps were generated by calculating the average percent change (ΔF/F) elicited by an odor at each site on a pixel-by-pixel by calculating an average image for the pre-odor baseline (images 5-11 of the 90-image series) and an average image for the odor sampling period (images 31-37), and dividing the average odor-sampling image by the average pre-odor baseline image for each trial. The resulting pictures, consisting of matrices of ΔF/F values in response to odor, were then averaged across all 8 trials, pseudocolored, and superimposed upon an image of the glomeruli during resting fluorescence at the site.
An ROI-based analysis was performed to determine the mean ΔF/F in response to each odor, as well as the percent change after drug application. Circular ROI’s encompassing identifiable glomeruli were manually chosen for each site. For each ROI, the mean value of all the pixels falling within the boundaries of the ROI was calculated for each image in the T-series, and then used to calculate a ΔF/F value as described previously (pixel value at time T/mean baseline pixel value). The ΔF/F values were then averaged across trials and plotted across time, and the maximum ΔF/F value for each glomerulus was calculated.
Glomeruli were classified as responsive or non-responsive based on the mean and standard deviation of the distribution of the maximum ΔF/F values in response to dipropylene glycol. The response threshold was set at 2 standard deviations above the mean ΔF/F, corresponding to a value of 0.84% in M71 transgenics and 0.63% in controls. Glomeruli from all experiments (M71 transgenic N=4, control N=5) were then classified as responsive or non-responsive using an automatic thresholding program in Matlab. Mean ΔF/F in response to odor was calculated by averaging the maximum ΔF/F values of responsive glomeruli to an odor in control animals, and averaging the maximum ΔF/F values of all glomeruli to acetophenone in M71 transgenics.
Mating and aggression assay
For all behavioral assays, heterozygous OMP-IRES-tTA and hemizygous M71 transgenic mice of a mixed 129SvEv X C57BL6/J background were bred to generate OMP-IRES-tTA/M71-IRES-taul-lacZ compound heterozygotes and control (wild type, OMP-tTA heterozygous, tetO-M71 hemizygous) animals. Male mice were weaned at 3 weeks and littermates were group-housed until 3 months of age. One day prior to the experiment, mice were separated and housed singly. Males were presented with estrous females for 30 min., and parameters of sexual behavior (exploration, mounting, intromission) were scored from video recordings (Leypold et al., 2002). Data from control genotypes were pooled since no difference was observed among the three groups (one-way ANOVA).
For the resident-intruder assay, male mice were isolated at 9 weeks of age and housed singly for 3 weeks. An age-matched, wild type male intruder of 129SvEv genetic background was added to the home cage of the resident test mouse and the mice were observed for 15 min. Aggressive behavior was defined as biting, chasing, or wrestling.
Olfactometry
Mice were adapted to a reverse 12-hour light/dark cycle and water restricted (approximately 1–1.5 ml per day to maintain 85–90% of baseline weight) for one week prior to training and testing. Training and testing were performed using the Slotnick operant conditioning paradigm (Bodyak and Slotnick, 1999) and a liquid-dilution, eight-channel olfactometer (Knosys, Lutz, FL). In this paradigm one of two odors was paired with a water reward following a two second delay (S+ odor). The other odor was not paired with a reward (S− odor). The S+ and S− odors were presented in a fixed random order, and the readout of the assay was the number of licks during the two-second interval following the odor pulse. Each experiment consisted of 200 odor presentations, and the data are presented in blocks of 20 trials. All odorants were purchased from Sigma-Aldrich (highest grade available) and were dissolved in light mineral oil. The fraction of correct licks was calculated as the number of correct licks/total number of licks and averaged for mice with the same genotype. Successful odor discrimination was defined when fraction of correct licks surpassed 75% correct.
Acknowledgments
The authors acknowledge P.J. Kisloff for assistance in preparation of the manuscript. We thank T. Jessell, S. Siegelbaum, Dan Stettler, and members of the Axel laboratory for critical reading of the manuscript and discussions. We also thank T. Cutforth and G. Barnea for reagents and A. Schaefer, J. Schwarz and B. Nwachukwu for help with behavioral assays. This work was supported by the Howard Hughes Medical Institute and a grant from the NIH (R.A., B.S.). A. F. was supported by long-term Postdoctoral fellowships from EMBO and the Human Frontiers Science Program. K. F. was supported by the Robert Leet and Clara Guthrie Patterson Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT. Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABA(B) heteroreceptors. Journal of neurophysiology. 2000;84:1194–1203. doi: 10.1152/jn.2000.84.3.1194. [DOI] [PubMed] [Google Scholar]
- Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT. Centre-surround inhibition among olfactory bulb glomeruli. Nature. 2003;426:623–629. doi: 10.1038/nature02185. [DOI] [PubMed] [Google Scholar]
- Barlow HB. Single units and sensation: a neuron doctrine for perceptual psychology? Perception. 1972;1:371–394. doi: 10.1068/p010371. [DOI] [PubMed] [Google Scholar]
- Bodyak N, Slotnick B. Performance of mice in an automated olfactometer: odor detection, discrimination and odor memory. Chemical senses. 1999;24:637–645. doi: 10.1093/chemse/24.6.637. [DOI] [PubMed] [Google Scholar]
- Bozza T, McGann JP, Mombaerts P, Wachowiak M. In vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron. 2004;42:9–21. doi: 10.1016/s0896-6273(04)00144-8. [DOI] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Chess A, Simon I, Cedar H, Axel R. Allelic inactivation regulates olfactory receptor gene expression. Cell. 1994;78:823–834. doi: 10.1016/s0092-8674(94)90562-2. [DOI] [PubMed] [Google Scholar]
- Dulac C, Axel R. A novel family of genes encoding putative pheromone receptors in mammals. Cell. 1995;83:195–206. doi: 10.1016/0092-8674(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Feinstein P, Bozza T, Rodriguez I, Vassalli A, Mombaerts P. Axon guidance of mouse olfactory sensory neurons by odorant receptors and the beta2 adrenergic receptor. Cell. 2004;117:833–846. doi: 10.1016/j.cell.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Frisina RD. Subcortical neural coding mechanisms for auditory temporal processing. Hearing research. 2001;158:1–27. doi: 10.1016/s0378-5955(01)00296-9. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Osborne J, Nemes A, Mendelsohn M, Axel R. Genetic ablation and restoration of the olfactory topographic map. Cell. 2000;103:609–620. doi: 10.1016/s0092-8674(00)00164-1. [DOI] [PubMed] [Google Scholar]
- Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chemical senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Haykin S, Chen Z. The cocktail party problem. Neural computation. 2005;17:1875–1902. doi: 10.1162/0899766054322964. [DOI] [PubMed] [Google Scholar]
- Herrada G, Dulac C. A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell. 1997;90:763–773. doi: 10.1016/s0092-8674(00)80536-x. [DOI] [PubMed] [Google Scholar]
- Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- Lewcock JW, Reed RR. A feedback mechanism regulates monoallelic odorant receptor expression. Proc Natl Acad Sci U S A. 2004;101:1069–1074. doi: 10.1073/pnas.0307986100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci U S A. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin da Y, Shea SD, Katz LC. Representation of natural stimuli in the rodent main olfactory bulb. Neuron. 2006;50:937–949. doi: 10.1016/j.neuron.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Lomvardas S, Barnea G, Pisapia DJ, Mendelsohn M, Kirkland J, Axel R. Interchromosomal interactions and olfactory receptor choice. Cell. 2006;126:403–413. doi: 10.1016/j.cell.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- Lu XC, Slotnick BM. Olfaction in rats with extensive lesions of the olfactory bulbs: implications for odor coding. Neuroscience. 1998;84:849–866. doi: 10.1016/s0306-4522(97)00520-4. [DOI] [PubMed] [Google Scholar]
- Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nature neuroscience. 2005;8:1660–1662. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- McBride K, Slotnick B, Margolis FL. Does intranasal application of zinc sulfate produce anosmia in the mouse? An olfactometric and anatomical study. Chemical senses. 2003;28:659–670. doi: 10.1093/chemse/bjg053. [DOI] [PubMed] [Google Scholar]
- McGann JP, Pirez N, Gainey MA, Muratore C, Elias AS, Wachowiak M. Odorant representations are modulated by intra- but not interglomerular presynaptic inhibition of olfactory sensory neurons. Neuron. 2005;48:1039–1053. doi: 10.1016/j.neuron.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Darcy DP, Isaacson JS. Intraglomerular inhibition: signaling mechanisms of an olfactory microcircuit. Nature neuroscience. 2005;8:354–364. doi: 10.1038/nn1403. [DOI] [PubMed] [Google Scholar]
- Nguyen MQ, Zhou Z, Marks CA, Ryba NJ, Belluscio L. Prominent roles for odorant receptor coding sequences in allelic exclusion. Cell. 2007;131:1009–1017. doi: 10.1016/j.cell.2007.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452:956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 1993;73:597–609. doi: 10.1016/0092-8674(93)90145-g. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell. 1994;79:1245–1255. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Root CM, Masuyama K, Green DS, Enell LE, Nassel DR, Lee CH, Wang JW. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59:311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roppolo D, Vollery S, Kan CD, Luscher C, Broillet MC, Rodriguez I. Gene cluster lock after pheromone receptor gene choice. Embo J. 2007;26:3423–3430. doi: 10.1038/sj.emboj.7601782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Nakatani H, Suzuki M, Saito M, Yoshihara Y, Sakano H. Negative feedback regulation ensures the one receptor-one olfactory neuron rule in mouse. Science. 2003;302:2088–2094. doi: 10.1126/science.1089122. [DOI] [PubMed] [Google Scholar]
- Shapley R, Enroth-Cugell C. Visual adaptation and retinal gain controls. Progress in Retinal Research. 1984;3:263–346. [Google Scholar]
- Shykind BM. Regulation of odorant receptors: one allele at a time. Hum Mol Genet. 2005;14(Spec No 1):R33–39. doi: 10.1093/hmg/ddi105. [DOI] [PubMed] [Google Scholar]
- Shykind BM, Rohani SC, O’Donnell S, Nemes A, Mendelsohn M, Sun Y, Axel R, Barnea G. Gene switching and the stability of odorant receptor gene choice. Cell. 2004;117:801–815. doi: 10.1016/j.cell.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Stone CP. J Comp Psychol. 1922;2:95–153. [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Vassalli A, Rothman A, Feinstein P, Zapotocky M, Mombaerts P. Minigenes impart odorant receptor-specific axon guidance in the olfactory bulb. Neuron. 2002;35:681–696. doi: 10.1016/s0896-6273(02)00793-6. [DOI] [PubMed] [Google Scholar]
- Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Vassar R, Ngai J, Axel R. Spatial segregation of odorant receptor expression in the mammalian olfactory epithelium. Cell. 1993:309–318. doi: 10.1016/0092-8674(93)90422-m. [DOI] [PubMed] [Google Scholar]
- Vucinic D, Cohen LB, Kosmidis EK. Interglomerular center-surround inhibition shapes odorant-evoked input to the mouse olfactory bulb in vivo. Journal of neurophysiology. 2006;95:1881–1887. doi: 10.1152/jn.00918.2005. [DOI] [PubMed] [Google Scholar]
- Young JM, Trask BJ. The sense of smell: genomics of vertebrate odorant receptors. Hum Mol Genet. 2002;11:1153–1160. doi: 10.1093/hmg/11.10.1153. [DOI] [PubMed] [Google Scholar]
- Yu CR, Power J, Barnea G, O’Donnell S, Brown HE, Osborne J, Axel R, Gogos JA. Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron. 2004;42:553–566. doi: 10.1016/s0896-6273(04)00224-7. [DOI] [PubMed] [Google Scholar]
- Zhang X, Firestein S. The olfactory receptor gene superfamily of the mouse. Nature neuroscience. 2002;5:124–133. doi: 10.1038/nn800. [DOI] [PubMed] [Google Scholar]