Abstract
Most of what is written and believed about pain and nociceptors originates from studies of the “somatic” (non-visceral) sensory system. As a result, the unique features of visceral pain are often overlooked. In the clinic, the management of visceral pain is typically poor, and drugs that are used with some efficacy to treat somatic pain often present unwanted effects on the viscera. For these reasons, a better understanding of visceral sensory neurons—particularly visceral nociceptors—is required. This review provides evidence of functional, morphological, and biochemical differences between visceral and non-visceral afferents, with a focus on potential nociceptive roles, and also considers some of the potential mechanisms of visceral mechanosensation.
Introduction
The majority of what is known about pain and nociceptors originates from studies of “somatic” structures (i.e., non-visceral components of the body, principally skin). Nevertheless, the most common pain produced by disease (and the most difficult to manage) is that originating from the internal organs (i.e., visceral pain), and the characteristics of visceral innervation differ significantly from other tissues. Visceral pain may result from direct inflammation of a visceral organ (e.g., inflammatory bowel disease, pancreatitis, appendicitis), occlusion of bile or urine flow (e.g., kidney stones), or from functional visceral disorders [e.g., irritable bowel syndrome (IBS)]. Add to this list angina, painful bladder syndrome (interstitial cystitis), gastroesophageal reflux disease, endometriosis, and dyspepsia, and the widespread impact of visceral disease becomes clear. Most basic and clinical pain research has focused on somatic (principally cutaneous) tissue, which has significantly influenced strategies for pain management. As a result, the unique features of visceral pain and innervation have remained underappreciated, and thus visceral pain management is typically poor. Moreover, visceral nociceptors are intrinsically different from cutaneous and most other non-visceral nociceptors. We provide here a review of the visceral sensory system and highlight some of the features that distinguish it from non-visceral systems. The visceral system encompasses a large number of organs, from the eyes (technically, the brain is also a visceral organ) down to the genitourinary organs, and so this review will focus on our laboratory’s current primary area of experimental expertise: the lower gastrointestinal tract (principally colon) and bladder.
The Visceral Sensory System
The principal extrinsic afferent nerves innervating visceral organs are anatomically associated with sympathetic and parasympathetic nerves and are accordingly named (e.g., pelvic nerve afferent), although they are not part of these efferent, autonomic, pathways. Most extrinsic visceral afferent neurons have cell bodies in dorsal root ganglia (DRG) and terminate in the spinal cord (spinal afferents); visceral afferent fibers in the vagus nerve, with cell bodies in the nodose and adjacent jugular ganglia, terminate in the brainstem nucleus tractus solitarius. There are two features that are unique to the visceral sensory innervation: 1) most organs also have an intrinsic innervation (e.g., the enteric nervous system of the gastrointestinal tract) and 2) each organ is innervated by two different extrinsic nerves (e.g., the distal colon and urinary bladder are innervated by the pelvic and splanchnic nerves). Although there are likely important functional interactions between the intrinsic and extrinsic visceral innervations, their anatomical relationship and means of intercommunication are largely unknown. Figure 1 summarizes the visceral sensory innervation, using the gut as an example.
Figure 1. Functional neuroanatomy of the visceral sensory system.
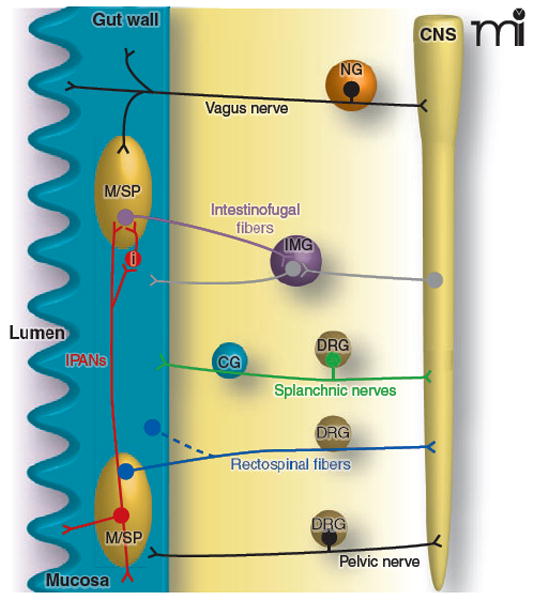
The gut is depicted here as an example of the sensory innervation of the visceral system. Vagal afferent neurons, which do not innervate the urinary bladder or the distal gut, have cell bodies in the nodose ganglia (NG) bilaterally and travel alongside parasympathetic efferent pathways to organs in the thoracic and abdominal cavities. Once in the gut wall, vagal afferent fibers innervate neurons in the myenteric or submucosal plexus (M/SP), circular and longitudinal muscle layers, and the mucosa. Pelvic afferent neurons also travel alongside parasympathetic efferent pathways, but their cell bodies are in dorsal root ganglia (DRG). Other spinal nerves (e.g., greater splanchnic) travel alongside sympathetic efferent pathways, have cell bodies in DRG, and pass through prevertebral ganglia (e.g., the celiac ganglion, CG). Intestinofugal afferents (purple) synapse onto efferent sympathetic neurons in prevertebral ganglia, such as the inferior mesenteric ganglion (IMG) and have their cell bodies in M/SP. Afferent fibers of the intrinsic (or enteric) nervous system, termed intrinsic primary afferent neurons (IPANs), synapse onto intestinofugal fibers, either directly, or via interneurons (i). Rectospinal fibers (blue) have cell bodies in the myenteric plexus or muscle layers, with axons terminating in the spinal cord (CNS). Note that not all these nerves and fibers will terminate in the same areas of the gut, and inputs to the spinal cord may traverse a number of different levels; this figure has been simplified for clarity.
Visceral Afferents are Anatomically Different from Non-Visceral Afferents
A key difference between visceral and non-visceral sensory neurons is the degree to which their peripheral terminals are specialized. For example, cutaneous afferents can have one of many different sensory endings to transduce stimuli into electrical energy (e.g., Merkel cells, Ruffini endings, Pacinian corpuscles), whereas only two types of specialized ending have been reported in visceral afferents: intraganglionic laminar endings (IGLEs) and intramuscular arrays (IMAs). Both types have limited distributions (e.g., near sphincters), are specific to muscular vagal or pelvic innervation, and are less intricate than their non-visceral counterparts [for review, see (1, 2)]. IGLEs and IMAs appear to be low-threshold mechanoreceptors and are therefore less likely to be involved in detecting noxious events. Most spinal visceral afferents are believed to have primitive, unencapsulated endings (like non-visceral nociceptors), with no specialized structure and one or a few punctate receptive fields.
Visceral Afferents Transmit Unique Sensations
Visceral and non-visceral afferents encode different types of information: the conscious experiences generated by the visceral sensory system are not initiated by non-visceral afferents. For example, the sensation of nausea does not arise from the skin, and vice versa, one cannot detect cutting of the gut [for review, see (3, 4)]. Conscious sensations arising from the viscera, in addition to pain, include organ filling, bloating and distension, dyspnea, and nausea, whereas non-visceral afferent activity gives rise to sensations such as touch, pinch, heat, cutting, crush, and vibration. Both sensory systems can detect chemical stimuli.
The Visceral Nociceptor Defined
Nociceptors were originally defined as receptors that respond to noxious stimuli, particularly those that damage or threaten to damage skin. Thus, they were defined in a functional context. As our understanding of nociceptors has increased, however, attempts to redefine the nociceptor have generated much debate and little agreement. Furthermore, a “noxious stimulus” is semantically distinct from a “painful stimulus,” a concept that has evolved from the descriptions of “nocicipient” cutaneous receptors by Sherrington at the beginning of the twentieth century (5).
“Pain” is a psychological state, defined by the International Association for the Study of Pain (IASP) as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” (6). This concept, and the distinction between nociception and pain, has been appreciated for some time, as the following quotation from 1900 shows (7):
The stimuli which evoke pain may be characterised as ‘excessive.’ It might almost be asserted that ‘excess’ is that quality of a stimulus in virtue of which it becomes ‘adequate’ for the sense of pain. ‘Excessive’ in this application connotes ‘harmful,’ or ‘to be avoided,’ e.g. by muscular action for resistance or escape. The ‘excess’ of the stimulus may lie in its intensity, or in its extensity (spatial or temporal).
Potential confusion arises from the IASP’s definition (6) of a noxious stimulus as “one which is damaging to normal tissues,” thereby excluding the potential for damage that is considered under the definition of “pain”. Thus, a nociceptor is a peripheral sensory receptor (considered colloquially to be the entire neuron, including its peripheral and central terminals and soma) that signals actual tissue damage.
One problem with these definitions is demonstrated by the existence in visceral organs of low-threshold mechanosensory afferents (sometimes also called “wide dynamic range”) that proportionally encode organ distension from low, physiological (non-noxious), distending pressures through to pressures that are noxious (Figure 2A and B). Similarly, joint afferents and some cutaneous afferents also have low mechanical response thresholds and encode well into the noxious range; however, neither slowly nor rapidly adapting mechanosensitive afferents in skin encode into the noxious range, or sensitize (see below). The current definition thus appears to omit low-threshold visceral afferents from the classification of nociceptor, as no matter how prolonged a non-noxious stimulus may be, low-threshold mechanoreceptors will not signal a noxious event until the stimulus intensity increases. Silent (or sleeping) nociceptors offer another complication. These neurons are unusual in that they are insensitive to all but the highest intensity of mechanical stimulation. However, inflammatory chemicals “awaken” these nociceptors and induce spontaneous activity and mechanosensitivity in the noxious range.
Figure 2. The viscera are innervated by low- and high-threshold mechanoreceptors.
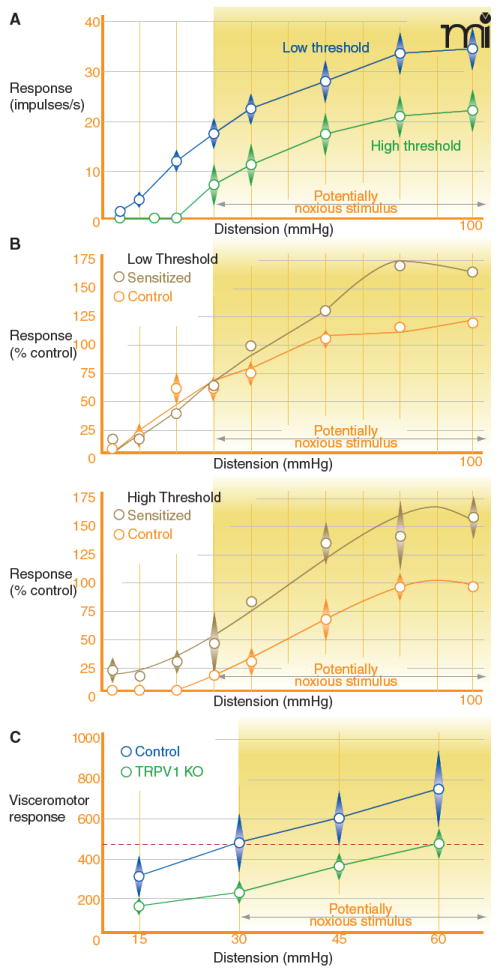
Hollow viscera are exquisitely sensitive to distension, a phenomenon that can be observed experimentally by recording the electrical activity induced in pelvic nerve afferent fibers (mechanoreceptors) during distension of the visceral organ under study. A) Low-threshold mechanoreceptors (blue) detect both non-noxious and noxious distension pressures, whereas high-threshold mechanoreceptors (green) only respond to noxious distention pressures. Data are recorded from distension of the rat bladder. B) Mechanoreceptors (of either the high- or low-threshold type) in the pelvic nerve can be sensitized by the addition of irritants (e.g., xylenes) into the bladder. C) Mice lacking the TRPV1 receptor show impaired visceral nociception. The visceromotor response (electrical activity recorded in the abdominal musculature) produced during distension of the colon to a noxious pressure (e.g., 60 mmHg) is lower in mice that do not have functional TRPV1 receptors compared to wild-type controls. Even at the highest tested colonic distension pressure, TRPV1 knockout mice only show a level of response equivalent to that of the wild-type mice at 30 mmHg, the pressure at which this stimulus is likely to be noxious (dashed red line). Panels A and B are adapted with permission from (64), and panel C is adapted with permission from (48).
Accordingly, we propose that a visceral nociceptor (or indeed, a nociceptor in any tissue) is a sensory receptor that, when activated, can produce a reflex or response that is protective or adaptive (e.g., withdrawal, guarding, vocalization); can encode stimulus intensity in the noxious range; and can sensitize (i.e., give increased responses to noxious intensities of stimulation after insult or exposure to chemical mediators such as those produced during inflammation). Requirement for the latter two capabilities reveals that most of the visceral sensory innervation is nociceptive in character, particularly during organ insult. Most (70-80%) mechanosensitive visceral afferents have low thresholds for activation in the physiological range; the remainder have high thresholds and are commonly considered to represent the population of visceral nociceptors. Nevertheless, most low-threshold mechanosensitive visceral afferents encode into the noxious range and generally give greater responses than their high-threshold counterparts (Figure 2A). They also sensitize after organ insult, giving increased responses to both innocuous and noxious intensities of stimulation. These findings argue for potential roles of both low- and high-threshold mechanoreceptive visceral afferents in visceral pain conditions.
With an ever-increasing number of in vitro methods available to the pain researcher, the identification of nociceptors often relies on cellular characterization, such as size or biochemical markers, rather than functional definitions. For example, all small-diameter, capsaicin-sensitive DRG neurons (that is, those that either express the capsaicin receptor TRPV1 or respond to application of capsaicin) are sometimes considered as nociceptors. The reliability of biochemical targets, such as TRPV1, to act as nociceptor markers is discussed below.
Visceral Pain is Different from Non-Visceral Pain
The ability to identify the source (spatial location) of cutaneous pain is excellent, and the ability to identify that of joint and muscle pain is generally good; in contrast, visceral pain is diffuse in character and poorly localized. Two factors contribute to this difference. First, relative to non-visceral structures, the viscera are sparsely innervated. It is estimated that fewer than seven percent of spinal afferents in the DRG project to the viscera [see (1,8,9,10)], and only a fraction of these convey input to the central nervous system that will be perceived. This sparse innervation is compensated for in the spinal cord, where visceral terminations arborize widely over several spinal segments and even to the contralateral spinal cord (11). Second, spinal neurons that receive visceral input also receive convergent input from skin or deeper structures (including other viscera), producing referred pain. For example, cardiac pain (angina) is typically referred to the left arm and shoulder (but skin, joint, or muscle pain is not referred from shoulder to heart). In addition, whereas pain can be evoked from virtually all non-visceral structures, parenchymous viscera (e.g., liver and pancreas) do not give rise to pain unless the organ is inflamed or the organ capsule is distorted, for example by a tumor. Finally, visceral pain is commonly associated with greater emotional valence and exaggerated autonomic reflexes, although the former is a central phenomenon not to be confused with nociception.
Distinguishing Visceral Nociceptors from Their Non-Visceral Counterparts
We discuss here some of the differences between visceral and non-visceral nociceptors—with the caveat that the majority of studies are done in the absence of a physiologically (functionally) defined nociceptor population.
Morphological Considerations
The most obvious parameter for distinguishing cell types is size. It is generally accepted that DRG neurons are bimodally distributed in terms of soma size, resulting in the designation of “(large) light” or “small dark” neurons. The former have myelinated A-fibers and somata with dense neurofilament (typically detected using antibodies raised against neurofilament protein, such as RT97 (12)); the latter have unmyelinated C-fibers and are significantly less dense. Generally, the somata of visceral afferents in DRG are larger than those considered to be non-visceral nociceptors (13, 14).
Generally, smaller-diameter neurons have myelinated Aδ- or unmyelinated C-fiber processes, whereas myelinated Aα/β-fibers can be found on cells of most sizes. Up to eighty percent of visceral DRG somata can have C-fibers, whereas fewer than forty percent generally have Aδ-fibers (15,16). An exception, however, can be found in the perianal mucosa, where the distribution is reversed: 23% C-fibers, 77% Aδ-fibers (16). Visceral Aβ-fibers are rarely encountered. In contrast, L4 DRG neurons with projections in the sciatic nerve (a non-visceral nerve that innervates skin and muscle) show a bias towards Aα/β-fibers (≥ 69%), with few Aδ- (approximately 15%) or C- (7-17%) fibers (17, 18). A similar pattern has been shown in guinea pig neurons that innervate the left hind limb and flank; the bias shifts to C-fibers in an identified nociceptive population of these afferents (19). In addition to nociceptive Aδ- and C-fibers, non-visceral nociceptors can also have Aβ-fibers [for review, see (20)]. It is important to appreciate that studies such as these do not necessarily reflect the exact proportions of each type of fiber present; the data are subject to varying selection dynamics and other experimental parameters chosen by the investigator.
Visceral and non-visceral afferents also differ in their spinal cord terminations. Spinal visceral afferent fibers terminate in the superficial dorsal horn, lamina V, and around the central canal, an area also referred to as lamina X (21, 22). In contrast, the terminal fields of cutaneous nociceptive afferent neurons are much smaller than those of visceral afferents and terminate throughout the spinal dorsal horn (11, 23). Non-visceral nociceptors also terminate in the superficial dorsal horn and lamina V, and thus converge on some of the same second order spinal neurons as visceral afferents; this convergence may account for the perception of pain from referred visceral sensations. Furthermore, visceral C-fibers have significantly more extensive spinal terminations (more terminal regions in different laminae and at multiple levels of the spinal cord) than the “nest-like” terminations of non-visceral C-fibers that are found principally in laminae I and II (11).
Biochemical Considerations
Calcitonin Gene–Related Peptide
The vast majority (typically, 70–90%) of visceral afferent cell bodies in the DRG stain positive with antibody for the calcitonin gene-related peptide (CGRP) (Table 1) (24-35). In contrast, DRG cell bodies of the non-visceral sensory system are far less likely to manifest positive immunostaining for CGRP. For example, approximately four-fifths of mouse colonic DRG somata are positive for CGRP, compared to about one-fourth of cells in the whole DRG population (24), one-third to one-half of cutaneous afferents (25, 36, 37), and one-fifth to one-third of muscle afferents (37). It has also been noted that visceral neurons that are positive for CGRP show a more intense signal than do positive non-visceral neurons (38).
Table 1.
a Biochemical Differences between Visceral and Non-Visceral Spinal Afferents
| Biochemical marker | Visceral afferents | Percent positive | Non-visceral afferents | Percent positive | Reference(s) |
|---|---|---|---|---|---|
| CGRP | mouse colon | 81% | rat ankle skin | 51% | (24, 25) |
| mouse colon | 79% | rat plantar skin | 41% | (34, 36 | |
| mouse colon | 63% | rat dorsal skin | 35% | (35, 37) | |
| rat colon | 88% | rat skin b | 51% | (35, 38) | |
| rat bladder | 60% | mouse skin b | 30% c | (26, 39) | |
| rat bladder | 52–63% | rat skin b | 20% | (27, 40) | |
| rat bladder | 69% | rat skin d | 19% | (25, 41) | |
| rat ureter and urethra | 90% | rat trapezius muscle | 33% | (26, 37) | |
| rat kidney, ureter, or bladder | >90% | rat longissimus muscle | 22% | (26, 37) | |
| rat kidney | 93% | mouse muscle e | 39% | (30, 39) | |
| rat stomach | 47–88% | rat muscle f | 70% | (28, 38) | |
| mouse stomach | 69–95% | rat muscle f | 30% | (28, 40) | |
| guinea pig stomach | 69–87% | (28) | |||
| rat stomach | 36–84% | (33) | |||
| rat stomach | 74% | (29) | |||
| rat esophagus | 54–90% | (33) | |||
| dog testes | ~80% | (31) | |||
| lamb ileum | 57% | (32) | |||
| rat splanchnic n. | 99% | (38) | |||
| rat splanchnic n. | 95% | (40) | |||
| rat splanchnic n. | 88% | (41) | |||
| trkA | rat bladder | 75% | rat ankle skin | 43% | (25) |
| rat pelvic n. | 90% | rat skin b | 48% | (43) | |
| rat muscle f | 20% | (43) | |||
| rat skin/muscle g | 44% | (43) | |||
| IB4 binding | mouse colon | 20% | rat ankle skin | 43% | (24, 25) |
| mouse colon | 14% | rat plantar skin | 20% | (34, 36) | |
| mouse colon | 6% | rat skin b | 37–42% h | (35, 44) | |
| rat colon | 12% | mouse skin b | 14% | (35, 39) | |
| rat bladder | 29% | rat muscle | 3% i | (25, 44) | |
| rat splanchnic n. | 1–3%h | (44) | |||
| rat splanchnic n. | 4 5% i | (44) | |||
| TRPV1 | mouse colon | 82% | rat plantar skin | 35% | (24, 36) |
| mouse colon | 67% | rat skin j | 32% | (35, 45) | |
| mouse colon | 62% | mouse skin b | 28% c | (65, 39) | |
| rat colon | 83% | mouse muscle e | 39% | (35, 39) | |
| rat bladder | 69% | (45) | |||
| rat stomach | 71% | (46) |
This table shows examples from previous studies that have reported the immunoreactivity or presence of the biochemical markers discussed here. These studies were performed by retrogradely labeling either from specific organs or muscles, or by labeling whole nerves (indicated by “n.” or the appropriate footnote; see also box 1)
saphenous nerve;
values reduced to 14% (CGRP) and 6% (TRPV1) when the saphenous nerve was retrogradely labeled with IB4 instead of wheat germ agglutinin;
retrograde labeling from the celiac ganglion or left cutaneous branches of the spinal nerves;
femoral nerve;
gastrocnemius nerve;
sciatic nerve;
72 hours after injecting the retrograde tracer;
48 hours after injecting the retrograde tracer;
labeled from the L6 dermatome.
Using techniques that label whole nerve bundles (Table 1 and Box 1), rather than just cell bodies in the DRG, ninety percent or more of visceral afferents are reactive to anti-CGRP immunostaining, compared to only about 20–50 percent of skin and 30–70 percent of muscle afferents (38-41). Approximately one-third of functionally identified non-visceral nociceptors in the guinea pig are CGRP-positive (42), although proportions vary among tissues (e.g., hairy versus glabrous skin). If only C- and Aδ-fibers are considered, this value is just under half of nociceptive units. We are unaware of any visceral correlate to these experiments for comparison.
Box 1. Spinal Nerves Innervating Visceral and Non-visceral Tissues.
One method to identify visceral or non-visceral spinal afferents is to use retrograde labeling of whole nerve bundles and study their cell bodies in the DRG. Some of these nerves are described here, including their afferent innervation and the DRG levels at which labeled neurons have been found in the rat. Data are collated from a number of studies [72-76].
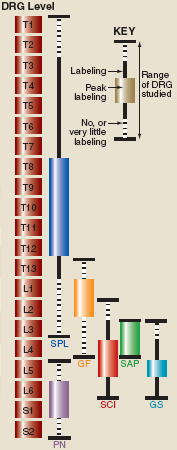
| Population | Nerve | Aferent innervation | Principal DRG levels |
| Visceral | splanchnic (SPL) | visceral organs | T8–T12 |
| pelvic (PN) | visceral organs | L6 and S1 | |
| Non-visceral | genitofemoral (GF) | muscle | L1 and L2 |
| gastrocnemius (GS) | muscle | L5 | |
| saphenous (SAP) | skin | L3–L4 | |
| sciatic (SCI) | muscle and skin | L4–L5 | |
Subpopulations of afferent neurons that bear the CGRP marker are known as “peptidergic. ” Classically, peptidergic neurons are reported to express the nerve growth factor receptor trkA; in contrast, non-peptidergic neurons express Ret, the receptor for glial cell line–derived neurotrophic factor. It has been suggested that nonpeptidergic neurons also bind the Griffonia simplicifolia-dervied isolectin IB4 and express the purinergic P2X3 receptor. Immunoreactivity patterns for trkA correlate well with those for CGRP; for example, 75% of bladder and 43% of skin DRG somata express trkA (25), and in situ hybridization studies show trkA mRNA in ninety percent of visceral (pelvic nerve), 20% of muscle (gastrocnemius nerve), and 48% of cutaneous (saphenous nerve) afferents (43). Thus, a greater proportion of visceral DRG somata, relative to non-visceral DRG somata, contain CGRP and are, therefore, potential nociceptors. Any line of argument that maintains that visceral afferents are predominantly peptidergic, however, should be tempered by the caveat that a strict distinction between “peptidergic” and “nonpeptidergic” may not be completely suitable for this group of neurons.
Isolectin B4
Among DRG somata, cutaneous afferents are over ten times more likely to bind IB4 than are visceral afferents [40 percent vs 3 percent; 72 hours after retrogradely labeling whole nerve trunks (44)]. Few muscle DRG somata (approximately 3%) bind this lectin (44). There are currently few studies detailing the binding of IB4 in afferents retrogradely labeled from specific organs, but in the case of colon and bladder, the proportion of afferents that bind IB4 has been estimated at six to thirty percent (24, 25, 34, 35), whereas retrograde labeling from skin indicates that twenty to forty percent of cutaneous afferents bind IB4 (25,36). These data suggest that visceral afferents are biased towards the peptidergic classification; however, it is worth noting that over ninety percent of colonic DRG somata that bind IB4 (i.e., presumed to be “non-peptidergic”) stain positive for CGRP (whereas only about twenty percent of all IB4-binding DRG somata test positive for CGRP; David R. Robinson, PhD thesis, University of Cambridge, 2004). Also, although the functional significance is unknown, these IB4- and CGRP-positive neurons display lower-intensity IB4 staining than afferents that bind IB4 but are not CGRP-positive (24). In any event, these observations suggest that the biochemical phenotype visceral afferents can be distinguished from that of non-visceral sensory neurons. This difference is reinforced by the finding that colonic afferents are far less likely to test positive for both the P2X3 receptor and IB4 binding, relative to the rate that both markers appear together in the general DRG population (24).
Transient Receptor Potential Vanilloid 1
The capsaicin receptor, TRPV1, is often regarded as a marker for nociceptors. The majority of visceral DRG somata test positive for TRPV1 (Table 1) (24, 39, 45, 46). In contrast, cutaneous (36, 39,45) and muscle afferents (39) are far less likely to contain the receptor. The few studies that have functionally identified nociceptors report that only a very small number of cutaneous nociceptors contain TRPV1. For example, one study showed only thirteen percent of tested nociceptors (both C- and A-fiber afferents) innervating mouse skin were TRPV1-positive (47). In the colon, animals lacking the TRPV1 receptor are significantly less sensitive to colorectal distension (Figure 2C) than wild-type littermates (48). Although not specifically identified as nociceptors, approximately half of colonic serosal mechanoreceptors respond to capsaicin (49). In innervation of cat viscera, all C-fibers and 38% of A-fibers are capsaicin-sensitive (50).
An illustration of both the complexity of the definition of the nociceptor and the role of TRPV1 can be found in a less commonly studied animal species. The African naked mole-rat, Heterocephalus glaber, expresses functional TRPV1 receptors, but does not exhibit pain behavior in response to capsaicin applied to the hind paw, unless substance P, which is absent in cutaneous C-fibers (51), is first administered as an intrathecal injection (52). This observation raises an interesting question: Are the afferent neurons that are obviously capable of encoding a noxious stimulus to be considered nociceptors, even if such “nociception” depends on a pharmacological intervention?
Differences among Visceral Afferent Populations
Although visceral afferents can be distinguished from their non-visceral counterparts, they do not appear to form a homogeneous population (Table 1). Indeed, the colon and bladder are innervated by afferents associated with two different nerves: afferents that follow the splanchnic nerves and have cell bodies in thoracolumbar DRG, and afferents that follow the pelvic nerve and have cell bodies in lumbrosacral DRG. Studying the mechanosensitivity of single afferent fibers that innervate the colon reveals five different classes of mechanosensory primary afferent (53) (Figure 3). Two of these are expressed in specific afferent populations, with the remaining three (i.e., serosal, muscular, and mucosal) found in both the splanchnic and pelvic pathways. Mesenteric afferents, an afferent class that is not observed in the pelvic nerve, constitute half the splanchnic innervation of the colon. Similarly, muscular/mucosal afferents have been reported in the pelvic, but not splanchnic, innervation and have been likened to a population of vagal afferents. This prompts the question of whether the pelvic nerve is fulfilling a “vagal-like” role in those more distal portions of the gastrointestinal tract that the vagus nerve does not innervate. (Pelvic afferent terminals in the colon are predominantly found distal to those from the lumbar splanchnic nerve). Generally, pelvic afferents have lower mechanical thresholds for activation but respond more intensely to a given stimulus than do splanchnic afferents. Chemical differences are also apparent between these two visceral afferent subpopulations. For example, significantly more splanchnic than pelvic afferents (66% versus 11%) respond to the direct application of bradykinin (BK), a chemical mediator released following tissue injury, to the receptive ending (54). BK-responsive pelvic afferents (two of nineteen fibers tested) were mechanosensitive, whereas the BK-responsive splanchnic population included mechanically insensitive as well as mechanically sensitive afferents. If a purely serosal afferent population is studied (chosen because they are equally represented in both the pelvic and splanchnic nerves), more splanchnic than pelvic afferents respond to activation of either P2X or TRPV1 receptors (49), findings that are confirmed immunohistochemically (35, 49).
Figure 3. The colon is innervated by five different types of mechanosensory afferent.
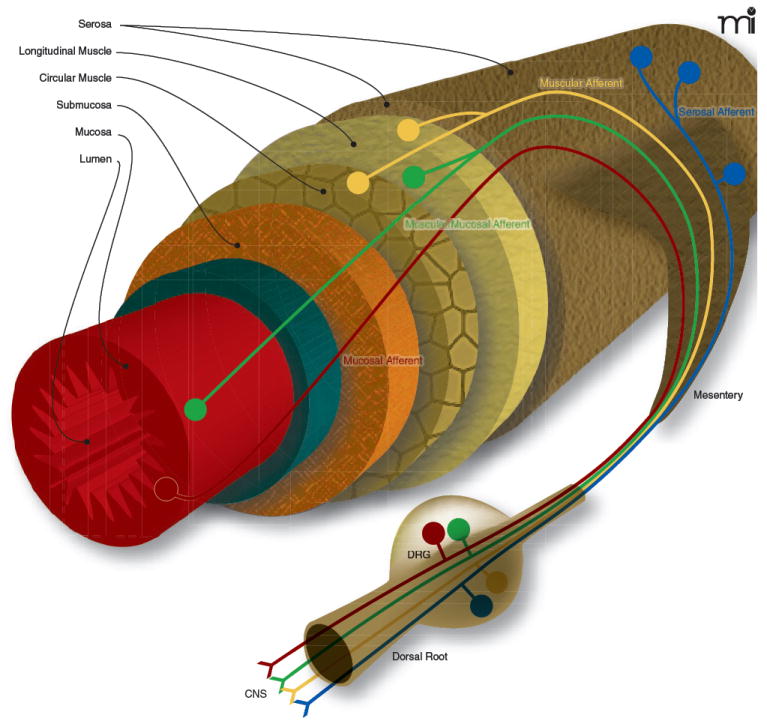
Five different types of afferent fiber have been reported in the colon: serosal, mesenteric, muscular, mucosal, and muscular/mucosal. Each has characteristic response criteria based on a protocol of probing, stretching, and stroking the colonic wall, and each has a different putative functional role. Most serosal afferents are mechanosensitive and, given that their thresholds for activation are higher than would be expected physiologically, are thought to signal short, sharp events (e.g., muscle contraction). Found only in the splanchnic afferent innervation, mesenteric afferents are predominantly found closely associated with blood vessels and likely signal twisting of the colon wall and some changes in mesenteric blood pressure, with a potential role in inflammation. Muscular afferents, named for their termination in the circular and longitudinal muscle layers of the gut, respond directly to circumferential stretch with a low threshold of activation (though can code into the noxious range). Muscular afferents exhibit slightly different properties depending upon the nerve (splanchnic or pelvic) in which they are found, but generally are considered to contribute to sustained filling, bloating, or distending sensations. Mucosal afferents are stretch-insensitive (at least circular stretch-insensitive) and respond to fine probing and stoking of the mucosal membrane. This suggests a role in providing feedback from physiological stimuli such as the normal passing of fecal material through the gastrointestinal tract. Finally, muscular/mucosal afferents, so named for their ability to detect both circular stretch and fine mucosal stroking, are a class of mechanoreceptor that, in mouse colon at least, are only found in the pelvic innervation. Presumably, these fibers provide a combination of the information that is transmitted from the muscular and mucosal fibers described above. For examples of the responses seen for each of these mechanosensitive fiber types, please see reference (53).
Similar findings have been reported in the rat bladder, an organ that also receives dual innervation through the pelvic and splanchnic nerves. Whole-cell patch clamp electrophysiology of cultured retrograde labeled DRG neurons has revealed that almost all pelvic afferent cell bodies respond to the P2X agonist α,β-methyleneATP, whereas only half of the splanchnic afferents responded (55). Although practically all the neurons studied responded to capsaicin, those from pelvic DRG evoked a significantly greater current.
The peptide content of splanchnic and pelvic afferent cell bodies in the DRG, based on immunostaining for CGRP, has been reported to be similar. On the other hand, there appears to be a difference in the non-peptidergic population, as more pelvic than splanchnic afferent cell bodies bind IB4, although variations in the use of retrograde tracers and fluorophphres have produced some inconsistencies in results (35, 56). Of the two IB4-binding populations, splanchnic afferent cell bodies tend to show lower intensity in staining than does the pelvic population (DR Robinson and GA Hicks, unpublished). A higher proportion of IB4-binding afferents has also been reported in splanchnic, as compared to pelvic, afferents that innervate the rat bladder (55).
Visceral Mechanosensation
As anyone who has experienced “gas” or bloating can attest, the distension of the gut can be an unpleasant and sometimes intensely painful experience. As genetically modified mice have become more widely available, there has been increased interest in the study of molecules that mediate visceral mechanosensation and hypersensitivity (Table 2). These studies have implicated a number of different molecules, including two members of the TRP family of receptors, TRPV1 and TRPV4, and the acid-sensing ion channels (ASICs).
Table 2.
Molecules that Mediate Mechanosensation and Hypersensitivity
| Molecule | Description | Effects in the colon | Reference(s) |
|---|---|---|---|
| 5-HT3 | Serotonin receptor | Antagonists attenuate glycerol-induced visceral nociception and prevent restraint stress–induced colonic hypersensitivity. | (66, 67) |
| ASIC3 | Acid-sensing ion channel | Knockout mice show reduced mechanosensitivity. | (48, 62) |
| Nav1.8 | Voltage-gated Na+ channel | Knockout mice show reduced response to intracolonic capsaicin or intracolonic mustard oil. | (68) |
| P2X | Purinergic P2X receptors | ATP is released from the colonic mucosa by colorectal distension, and pelvic nerve afferents are activated by α,β-methyleneATP; visceral hypersensitivity is reversed by specific P2X1, P2X3, and P2X2/3 antagonists. | (69, 70) |
| PAR | Protease-activated receptors | Luminal application of PAR2-activating peptide causes visceral hypersensitivity. | (71) |
| TRPV1 | TRPb-vanilloid 1 receptor | Knockout mice show reduced mechanosensitivity. | (48) |
| TRPV4 | TRPb-vanilloid 4 receptor | Knockout mice show attenuated mechanosensitivity and reduced response to colorectal distension; a selective agonist increases response to colorectal distension. | (56, 60) |
References provided here are representative, not exhaustive.
TRP, transient receptor potential.
As indicated above, five different types of afferent fiber have been reported in the colon, each of which is responsive to different forms of mechanical stimulation and has its own putative functional role. An overview of these, along with the differences seen between the splanchnic and pelvic innervation of the colon, can be found in Figure 3.
TRPV1 knockout mice show a significant reduction in their behavioral (visceromotor) response to colorectal distension as well as in afferent fiber responses to stretch (48); similarly, urinary bladder and jejunal afferent subpopulations exhibit reduced mechanosensitivity (57, 58). It also appears that TRPV1 plays a role in hypersensitivity to mechanical stimulation in models of colon (48) and bladder hypersensitivity (interstitial cystitis) (59). This evidence of a role for TRPV1 in visceral hypersensitivity—and thus, presumably, in visceral pain—extends to non-visceral tissues, because mice that lack the TRPV1 receptor do not exhibit the enhanced referred mechanical hypersensitivity to the hind paw following cystitis that is seen in wild type controls (59).
A relative of TRPV1, the TRPV4 receptor, has also been implicated in visceral mechanosensation and may be most important in the colon; TRPV4 receptor mRNA content is significantly greater in colonic DRG cell bodies (with more in splanchnic than pelvic nerve DRG) compared to the cell bodies of gastric or non-visceral afferents (56). Furthermore, mechanical responses of colon afferents are reduced, and response thresholds are greater, in TRPV4 receptor knockout mice, consistent with reduced behavioral responses to colorectal distension (56). Conversely, the intracolonic administration of a TRPV4 receptor–selective agonist results in a dose-dependent increase in the responses of mice to colorectal distension (60).
The proton-sensing ion channels of the ASIC family have been investigated as potential visceral mechanotransducers using knockout mice that lack ASIC1a, ASIC2, or ASIC3 channels. The loss of ASIC1a appears to result in an increase in mechanosensitivity throughout the gastrointestinal tract, including the colon, whereas deficiency in ASIC2 results in different mechanical responses, depending on the target (in the colon it also results in increased mechanosensitivity) (61, 62). CGRP release from the colon is unchanged by knockout of ASIC2 (63). In contrast, knocking out ASIC3 leads to reduced colonic mechanosensitivity, both with respect to stretch in single-fiber experiments and behavioral assessment of the visceromotor response to colorectal distension (48, 62).
Conclusion
We have reviewed evidence here that visceral nociceptors—or, more accurately, visceral afferents with the potential to transmit nociceptive information—differ from non-visceral (somatic) afferents in a number of ways, including their morphology and the channels and receptors they contain. They also differ in the consequences of their activation. In humans, visceral pain has a number of characteristics that distinguish it from pain originating from non-visceral structures, and these differences are most likely responsible for the symptoms experienced by patients with a visceral disease such as IBS. Somatic pain relief strategies typically work poorly for the management of visceral pain, and a better understanding of the visceral nociceptor (along with central mechanisms not discussed here) is vital to the development of new therapies for visceral pain management. The way forward would be easier if we were able to identify visceral nociceptors by characteristics other than response to noxious stimuli. Although CGRP- and TRPV1-containing DRG somata are more common in visceral sensory neurons, these and other potential surrogates do not reliably distinguish visceral from non-visceral nociceptors. Until such a marker (or constellation of markers) is found, identification of nociceptors requires functional assessment.
Biographies

David R. Robinson, PhD, received his doctoral degree, with a PhD studentship from GlaxoSmithKline, from the University of Cambridge, where he studied the characterization of the distal colon with particular emphasis on colonic hypersensitivity. He is now a Postdoctoral Associate at the Pittsburgh Center for Pain Research, University of Pittsburgh, where his research interests remain focused around colonic hypersensitivity.

Gerald F. Gebhart, PhD, is Professor and Director of the Center for Pain Research at the University of Pittsburgh School of Medicine. He has published more than 350 articles in journals and books. His interests in the pain field include descending modulation of pain (mechanisms of descending facilitation, secondary hyperalgesia, and chronic pain conditions) and visceral pain.
References
- 1.Grundy D. Signalling the state of the digestive tract. Auton Neurosci. 2006;125:76–80. doi: 10.1016/j.autneu.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19(Suppl 1):1–19. doi: 10.1111/j.1365-2982.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- 3.Cervero F. Sensory innervation of the viscera: Peripheral basis of visceral pain. Physiol Rev. 1994;74:95–138. doi: 10.1152/physrev.1994.74.1.95. [DOI] [PubMed] [Google Scholar]
- 4.Sengupta JN, Gebhart GF. Gastrointestinal afferent fibers and sensation. In: Johnson LR, Alpers DH, Christensen J, Jacobson ED, Walsh JH, editors. Physiology of the Gastrointestinal Tract. Raven Press; New York: 1994. pp. 483–519. [Google Scholar]
- 5.Sherrington CS. Qualitative difference of spinal reflex corresponding with qualitative difference of cutaneous stimulus. J Physiol (Lond) 1903;30:39–46. doi: 10.1113/jphysiol.1903.sp000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merskey H, Lindblom U, Mumford JM, Nathan PW, Sunderland S. Pain terms: A current list with definitions and notes on usage. In: Merskey H, Bogduk N, editors. Classification of Chronic Pain. IASP Press; Seattle, USA: 1994. pp. 207–213. [Google Scholar]
- 7.Sherrington CS. Cutaneous Sensations. In: Schäffer EA, editor. Text-Book of Physiology. Young J. Pentland; Edinburgh: 1900. pp. 920–1001.. Nobel Laureate Sir Charles Sherrington’s essays and lectures were fundamental to today’s understanding of pain, and his research on cutaneous nociception led the way for many generations of pain scientists.
- 8.Cervero F, Connell LA, Lawson SN. Somatic and visceral primary afferents in the lower thoracic dorsal root ganglia of the cat. J Comp Neurol. 1984;228:422–431. doi: 10.1002/cne.902280309. [DOI] [PubMed] [Google Scholar]
- 9.Jänig W, Morrison JF. Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on visceral nociception. Prog Brain Res. 1986;67:87–114. doi: 10.1016/s0079-6123(08)62758-2. [DOI] [PubMed] [Google Scholar]
- 10.Jänig W. Neurobiology of visceral afferent neurons: Neuroanatomy, functions, organ regulations and sensations. Biol Psychol. 1996;42:29–51. doi: 10.1016/0301-0511(95)05145-7. [DOI] [PubMed] [Google Scholar]
- 11.Sugiura Y, Terui N, Hosoya Y. Difference in distribution of central terminals between visceral and somatic unmyelinated (C) primary afferent fibers. J Neurophysiol. 1989;62:834–840. doi: 10.1152/jn.1989.62.4.834.. This article examined the central termination patterns and morphology of both visceral and non-visceral afferent neurons. The authors filled individual axons and also performed intracellular recordings to measure conduction velocity.
- 12.Lawson SN, Harper AA, Harper EI, Garson JA, Anderton BH. A monoclonal antibody against neurofilament protein specifically labels a subpopulation of rat sensory neurones. J Comp Neurol. 1984;228:263–272. doi: 10.1002/cne.902280211. [DOI] [PubMed] [Google Scholar]
- 13.Sugiura T, Dang K, Lamb K, Bielefeldt K, Gebhart GF. Acid-sensing properties in rat gastric sensory neurons from normal and ulcerated stomach. J Neurosci. 2005;25:2617–2627. doi: 10.1523/JNEUROSCI.2894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gold MS, Traub RJ. Cutaneous and colonic rat DRG neurons differ with respect to both baseline and PGE2-induced changes in passive and active electrophysiological properties. J Neurophysiol. 2004;91:2524–2531. doi: 10.1152/jn.00866.2003. [DOI] [PubMed] [Google Scholar]
- 15.Sengupta JN, Saha JK, Goyal RK. Stimulus-response function studies of esophageal mechanosensitive nociceptors in sympathetic afferents of opossum. J Neurophysiol. 1990;64:796–812. doi: 10.1152/jn.1990.64.3.796. [DOI] [PubMed] [Google Scholar]
- 16.Sengupta JN, Gebhart GF. Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J Neurophysiol. 1994;71:2046–2060. doi: 10.1152/jn.1994.71.6.2046.. This study incorporated a functional approach to the study of colon and bladder afferent physiology. Electrophysiological recordings were taken from single fibers in decentralized dorsal rootlets (L6 and S1) and were examined for responses to mechanical (distension) and chemical stimulation. The paper also investigates the possibility of silent nociceptors in these populations.
- 17.Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol (Lond) 1985;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harper AA, Lawson SN. Electrical properties of rat dorsal root ganglion neurones with different peripheral nerve conduction velocities. J Physiol (Lond) 1985;359:47–63. doi: 10.1113/jphysiol.1985.sp015574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Djouhri L, Bleazard L, Lawson SN. Association of somatic action potential shape with sensory receptive properties in guinea-pig dorsal root ganglion neurones. J Physiol (Lond) 1998;513:857–872. doi: 10.1111/j.1469-7793.1998.857ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Djouhri L, Lawson SN. Abeta-fiber nociceptive primary afferent neurons: A review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Rev. 2004;46:131–145. doi: 10.1016/j.brainresrev.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Jänig W. Neurobiology of visceral afferent neurons: Neuroanatomy, functions, organ regulations and sensations. Biol Psychol. 1996;42:29–51. doi: 10.1016/0301-0511(95)05145-7. [DOI] [PubMed] [Google Scholar]
- 22.Cervero F, Connell LA. Fine afferent fibers from viscera do not terminate in the substantia gelatinosa of the thoracic spinal cord. Brain Res. 1984;294:370–374. doi: 10.1016/0006-8993(84)91053-9. [DOI] [PubMed] [Google Scholar]
- 23.Willis WD, Jr, Coggeshall RE. Sensory mechanisms of the spinal cord: Ascending sensory tracts and their descending control. Kluwer Academic / Plenum Publishers; New York: 2004. [Google Scholar]
- 24.Robinson DR, McNaughton PA, Evans ML, Hicks GA. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol Motil. 2004;16:113–124. doi: 10.1046/j.1365-2982.2003.00456.x.. This article documents the first morphological characterization of the spinal afferent neurons that innervate the mouse colon and has provided the basis for both functional and morphological studies of visceral nociception and hypersensitivity.
- 25.Bennett DLH, Dmietrieva N, Priestley JV, Clary D, McMahon SB. trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neurosci Lett. 1996;206:33–36. doi: 10.1016/0304-3940(96)12418-6. [DOI] [PubMed] [Google Scholar]
- 26.Su HC, Wharton J, Polak JM, et al. Calcitonin gene-related peptide immunoreactivity in afferent neurons supplying the urinary tract: Combined retrograde tracing and immunohistochemistry. Neuroscience. 1986;18:727–747. doi: 10.1016/0306-4522(86)90066-7. [DOI] [PubMed] [Google Scholar]
- 27.Callsen-Cencic P, Mense S. Expression of neuropeptides and nitric oxide synthase in neurones innervating the inflamed rat urinary bladder. J Auton Nerv Syst. 1997;65:33–44. doi: 10.1016/s0165-1838(97)00032-5. [DOI] [PubMed] [Google Scholar]
- 28.Green T, Dockray GJ. Characterization of the peptidergic afferent innervation of the stomach in the rat, mouse and guinea-pig. Neuroscience. 1988;25:181–193. doi: 10.1016/0306-4522(88)90017-6. [DOI] [PubMed] [Google Scholar]
- 29.Schicho R, Donnerer J, Liebmann I, Lippe IT. Nociceptive transmitter release in the dorsal spinal cord by capsaicin-sensitive fibers after noxious gastric stimulation. Brain Res. 2005;1039:108–115. doi: 10.1016/j.brainres.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 30.Zheng F, Lawson SN. Immunocytochemical properties of rat renal afferent neurons in dorsal root ganglia: A quantitative study. Neuroscience. 1994;63:295–306. doi: 10.1016/0306-4522(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 31.Tamura R, Mizumura K, Kumazawa T. Coexistence of calcitonin gene-related peptide- and substance P-like immunoreactivity in retrogradely labeled superior spermatic neurons in the dog. Neurosci Res. 1996;25:293–299. doi: 10.1016/0168-0102(96)01055-3. [DOI] [PubMed] [Google Scholar]
- 32.Chiocchetti R, Grandis A, Bombardi C, Lucchi ML, Dal Lago DT, Bortolami R, Furness JB. Extrinsic and intrinsic sources of calcitonin gene-related peptide immunoreactivity in the lamb ileum: A morphometric and neurochemical investigation. Cell Tissue Res. 2006;323:183–196. doi: 10.1007/s00441-005-0075-2. [DOI] [PubMed] [Google Scholar]
- 33.Green T, Dockray GJ. Calcitonin gene-related peptide and substance P in afferents to the upper gastrointestinal tract in the rat. Neurosci Lett. 1987;76:151–156. doi: 10.1016/0304-3940(87)90707-5. [DOI] [PubMed] [Google Scholar]
- 34.Beyak MJ, Ramji N, Krol KM, Kawaja MD, Vanner SJ. Two TTX-resistant sodium currents in mouse colonic dorsal root ganglia neurons and their role in colitis induced hyperexcitability. Am J Physiol Gastrointest Liver Physiol. 2004;287:G845–G855. doi: 10.1152/ajpgi.00154.2004. [DOI] [PubMed] [Google Scholar]
- 35.Christianson JA, Traub RJ, Davis BM. Differences in spinal distribution and neurochemical phenotype of colonic afferents in mouse and rat. J Comp Neurol. 2006;494:246–259. doi: 10.1002/cne.20816. [DOI] [PubMed] [Google Scholar]
- 36.Aoki Y, Ohtori S, Takahashi K, Ino H, Douya H, Ozawa T, Saito T, Moriya H. Expression and co-expression of VR1, CGRP, and IB4-binding glycoprotein in dorsal root ganglion neurons in rats: Differences between the disc afferents and the cutaneous afferents. Spine. 2005;30:1496–1500. doi: 10.1097/01.brs.0000167532.96540.31. [DOI] [PubMed] [Google Scholar]
- 37.Tsukagoshi M, Goris RC, Funakoshi K. Differential distribution of vanilloid receptors in the primary sensory neurons projecting to the dorsal skin and muscles. Histochem Cell Biol. 2006;126:343–352. doi: 10.1007/s00418-006-0167-4. [DOI] [PubMed] [Google Scholar]
- 38.Perry MJ, Lawson SN. Differences in expression of oligosaccharides, neuropeptides, carbonic anhydrase and neurofilament in rat primary afferent neurons retrogradely labelled via skin, muscle or visceral nerves. Neuroscience. 1998;85:293–310. doi: 10.1016/s0306-4522(97)00629-5.. This study was the first to note the difference in cell-body size of visceral afferent neurons. Visceral sensory neuron cell bodies are now known generally to be larger in diameter than their cutaneous nociceptor counterparts.
- 39.Christianson JA, McIlwrath SL, Koerber HR, Davis BM. Transient receptor potential vanilloid 1-immunopositive neurons in the mouse are more prevalent within colon afferents compared to skin and muscle afferents. Neuroscience. 2006;140:247–257. doi: 10.1016/j.neuroscience.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Molander C, Ygge J, Dalsgaard CJ. Substance P-, somatostatin- and calcitonin gene-related peptide-like immunoreactivity and fluoride resistant acid phosphatase-activity in relation to retrogradely labeled cutaneous, muscular and visceral primary sensory neurons in the rat. Neurosci Lett. 1987;74:37–42. doi: 10.1016/0304-3940(87)90047-4. [DOI] [PubMed] [Google Scholar]
- 41.Kashiba H, Senba E, Ueda Y, Tohyama M. Cell size and cell type analysis of calcitonin gene-related peptide-containing cutaneous and splanchnic sensory neurons in the rat. Peptides. 1991;12:101–106. doi: 10.1016/0196-9781(91)90174-n. [DOI] [PubMed] [Google Scholar]
- 42.Lawson SN, Crepps B, Perl ER. Calcitonin gene-related peptide immunoreactivity and afferent receptive properties of dorsal root ganglion neurones in guinea-pigs. J Physiol (Lond) 2002;540:989–1002. doi: 10.1113/jphysiol.2001.013086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 44.Plenderleith MB, Snow PJ. The plant lectin Bandeiraea simplicifolia I-B4 identifies a subpopulation of small diameter primary sensory neurones which innervate the skin in the rat. Neurosci Lett. 1993;159:17–20. doi: 10.1016/0304-3940(93)90787-l. [DOI] [PubMed] [Google Scholar]
- 45.Hwang SJ, Oh JM, Valtschanoff JG. Expression of the vanilloid receptor TRPV1 in rat dorsal root ganglion neurons supports different roles of the receptor in visceral and cutaneous afferents. Brain Res. 2005;1047:261–266. doi: 10.1016/j.brainres.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 46.Schicho R, Florian W, Liebmann I, Holzer P, Lippe IT. Increased expression of TRPV1 receptor in dorsal root ganglia by acid insult of the rat gastric mucosa. Eur J Neurosci. 2004;19:1811–1818. doi: 10.1111/j.1460-9568.2004.03290.x. [DOI] [PubMed] [Google Scholar]
- 47.Lawson JJ, McIlwrath SL, Woodbury CJ, Davis BM, Koerber HR. TRPV1 unlike TRPV2 is restricted to a subset of mechanically insensitive cutaneous nociceptors responding to heat. J Pain. 2008;9:298–308. doi: 10.1016/j.jpain.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones RCW, III, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brierley SM, Carter R, Jones W, III, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol (Lond) 2005;567:267–281. doi: 10.1113/jphysiol.2005.089714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Longhurst JC, Kaufman MP, Ordway GA, Musch TI. Effects of bradykinin and capsaicin on endings of afferent fibers from abdominal visceral organs. Am J Physiol Regul Integr Comp Physiol. 1984;247:R552–R559. doi: 10.1152/ajpregu.1984.247.3.R552. [DOI] [PubMed] [Google Scholar]
- 51.Park TJ, Comer C, Carol A, Lu Y, Hong HS, Rice FL. Somatosensory organization and behavior in naked mole-rats: II. Peripheral structures, innervation, and selective lack of neuropeptides associated with thermoregulation and pain. J Comp Neurol. 2003;465:104–120. doi: 10.1002/cne.10824. [DOI] [PubMed] [Google Scholar]
- 52.Park TJ, Lu Y, Juttner R, Smith ES et al. Selective inflammatory pain insensitivity in the African naked mole-rat (Heterocephalus glaber) PLoS Biol. 2008;6:e13. doi: 10.1371/journal.pbio.0060013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brierley SM, Jones RC, III, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology. 2004;127:166–178. doi: 10.1053/j.gastro.2004.04.008.. This study was the first to investigate directly, and document differences in, mechanosensitivities and topographical location of receptive endings of the different nerves innervating the same organ. In this case, the pelvic and splanchnic innervations of the lower gastrointestinal tract of the mouse.
- 54.Brierley SM, Jones RC, III, Xu L, Gebhart GF, Blackshaw LA. Activation of splanchnic and pelvic colonic afferents by bradykinin in mice. Neurogastroenterol Motil. 2005;17:854–862. doi: 10.1111/j.1365-2982.2005.00710.x. [DOI] [PubMed] [Google Scholar]
- 55.Dang K, Bielefeldt K, Gebhart GF. Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J Neurosci. 2005;25:3973–3984. doi: 10.1523/JNEUROSCI.5239-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brierley SM, Page AJ, Hughes PA, Adam B, Liebregts T, Cooper NJ, Holtmann G, Liedtke W, Blackshaw LA. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology. 2008;134:2059–2069. doi: 10.1053/j.gastro.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daly D, Rong W, Chess-Williams R, Chapple C, Grundy D. Bladder afferent sensitivity in wild-type and TRPV1 knockout mice. J Physiol (Lond) 2007;583:663–674. doi: 10.1113/jphysiol.2007.139147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rong W, Hillsley K, Davis JB, Hicks G, Winchester WJ, Grundy D. Jejunal afferent nerve sensitivity in wild-type and TRPV1 knockout mice. J Physiol (Lond) 2004;560:867–881. doi: 10.1113/jphysiol.2004.071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang ZY, Wang P, Merriam FV, Bjorling DE. Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain. 2008;139:158–167. doi: 10.1016/j.pain.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 60.Cenac N, Altier C, Chapman K, Liedtke W, Zamponi G, Vergnolle N. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology. 2008;135:937–946. 946. doi: 10.1053/j.gastro.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 61.Page AJ, Brierley SM, Martin CM, et al. The ion channel ASIC1 contributes to visceral but not cutaneous mechanoreceptor function. Gastroenterology. 2004;127:1739–1747. doi: 10.1053/j.gastro.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 62.Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, Wemmie JA, Blackshaw LA. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54:1408–1415. doi: 10.1136/gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roza C, Puel JL, Kress M, Baron A, Diochot S, Lazdunski M, Waldmann R. Knockout of the ASIC2 channel in mice does not impair cutaneous mechanosensation, visceral mechanonociception and hearing. J Physiol (Lond) 2004;558:659–669. doi: 10.1113/jphysiol.2004.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su X, Sengupta JN, Gebhart GF. Effects of opioids on mechanosensitive pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol. 1997;77:1566–1580. doi: 10.1152/jn.1997.77.3.1566. [DOI] [PubMed] [Google Scholar]
- 65.Tan LL, Bornstein JC, Anderson CR. Distinct chemical classes of medium-sized transient receptor potential channel vanilloid 1-immunoreactive dorsal root ganglion neurons innervate the adult mouse jejunum and colon. Neuroscience. 2008;156:334–343. doi: 10.1016/j.neuroscience.2008.06.071. [DOI] [PubMed] [Google Scholar]
- 66.Mori T, Kawano K, Shishikura T. 5-HT3-receptor antagonist inhibits visceral pain differently in chemical and mechanical stimuli in rats. J Pharmacol Sci. 2004;94:73–76. doi: 10.1254/jphs.94.73. [DOI] [PubMed] [Google Scholar]
- 67.Hirata T, Keto Y, Nakata M, Takeuchi A, Funatsu T, Akuzawa S, Sasamata M, Miyata K. Effects of serotonin 5-HT3 receptor antagonists on stress-induced colonic hyperalgesia and diarrhoea in rats: A comparative study with opioid receptor agonists, a muscarinic receptor antagonist and a synthetic polymer. Neurogastroenterol Motil. 2008;20:557–565. doi: 10.1111/j.1365-2982.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 68.Laird JMA, Souslova V, Wood JN, Cervero F. Deficits in visceral pain and referred hyperalgesia in Nav1.8 (SNS/PN3)-null mice. J Neurosci. 2002;22:8352–8356. doi: 10.1523/JNEUROSCI.22-19-08352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wynn G, Rong W, Xiang Z, Burnstock G. Purinergic mechanisms contribute to mechanosensory transduction in the rat colorectum. Gastroenterology. 2003;125:1398–1409. doi: 10.1016/j.gastro.2003.07.008.. This paper uncovered a role for ATP in mechanosensation in the colorectum and showed that ATP was released from the colon wall in a pressure-dependent manner during distension that also excited pelvic nerve afferents innervating the rat colorectum.
- 70.Xu GY, Shenoy M, Winston JH, Mittal S, Pasricha PJ. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut. 2008;57:1230–1237. doi: 10.1136/gut.2007.134221. [DOI] [PubMed] [Google Scholar]
- 71.Coelho AM, Vergnolle N, Guiard B, Fioramonti J, Bueno L. Proteinases and proteinase-activated receptor 2: A possible role to promote visceral hyperalgesia in rats. Gastroenterology. 2002;122:1035–1047. doi: 10.1053/gast.2002.32387. [DOI] [PubMed] [Google Scholar]
- 72.Neuhuber WL, Sandoz PA, Fryscak T. The central projections of primary afferent neurons of greater splanchnic and intercostal nerves in the rat. A horseradish peroxidase study. Anat Embryol (Berl) 1986;174:123–144. doi: 10.1007/BF00318344. [DOI] [PubMed] [Google Scholar]
- 73.Nadelhaft I, Booth AM. The location and morphology of preganglionic neurons and the distribution of visceral afferents from the rat pelvic nerve: A horseradish peroxidase study. J Comp Neurol. 1984;226:238–245. doi: 10.1002/cne.902260207. [DOI] [PubMed] [Google Scholar]
- 74.Nagy JI, Senba E. Neural relations of cremaster motoneurons, spinal cord systems and the genitofemoral nerve in the rat. Brain Res Bull. 1985;15:609–627. doi: 10.1016/0361-9230(85)90211-4. [DOI] [PubMed] [Google Scholar]
- 75.Baron R, Jänig W, Kollmann W. Sympathetic and afferent somata projecting in hindlimb nerves and the anatomical organization of the lumbar sympathetic nervous system of the rat. J Comp Neurol. 1988;275:460–468. doi: 10.1002/cne.902750310. [DOI] [PubMed] [Google Scholar]
- 76.Swett JE, Torigoe Y, Elie VR, Bourassa CM, Miller PG. Sensory neurons of the rat sciatic nerve. Exp Neurol. 1991;114:82–103. doi: 10.1016/0014-4886(91)90087-s. [DOI] [PubMed] [Google Scholar]


