Abstract
Context
Individuals experiencing prodromal symptoms of schizophrenia (ultra-high-risk group) demonstrate impaired performance on tasks of executive function, attention, and working memory. The neurobiological underpinnings of such executive deficits in ultra-high-risk individuals remains unclear.
Objective
We assessed frontal and striatal functions during a visual oddball continuous performance task, in ultra-high-risk, early, and chronic schizophrenic patients with the use of functional magnetic resonance imaging.
Design
Cross-sectional case-control design.
Setting
Community; outpatient clinic.
Patients
Fifty-two individuals (control, n = 16; ultra-high risk, n = 10; early, n = 15; chronic, n = 11) from a referred clinical sample and age- and sex-matched control volunteers underwent scanning.
Main Outcome Measures
Percentage of active voxels and percentage signal change calculated for the anterior cingulate gyrus (ACG), middle frontal gyrus (MFG), inferior frontal gyrus (IFG), basal ganglia, and thalamus. Performance on the visual oddball task was measured with percentage of hits and d′ (a measure based on the hit rate and the false-alarm rate).
Results
The ultra-high-risk group showed significantly smaller differential activation between task-relevant and task-irrelevant stimuli in the frontal regions (ACG, IFG, MFG) than the control group. Frontostriatal activation associated with target stimuli in the early and chronic groups was significantly lower than the control group, while the ultra-high-risk group showed a trend toward the early group.
Conclusions
Our findings suggest that prefrontal function begins to decline before the onset of syndromally defined illness and hence may represent a vulnerability marker in assessing the risk of developing psychotic disorders among ultra-high-risk individuals.
Schizophrenia is widely considered to be a genetically mediated neurodevelopmental disorder,1,2 with symptoms typically emerging in late adolescence or early adulthood, followed by a steady clinical decline throughout life, consistent with a neuroprogressive process. 3 The prodromal phase preceding the onset of the formal symptoms of schizophrenia includes positive psychotic symptoms, negative symptoms,4,5 and cognitive deficits that include distractibility.6 Individuals experiencing prodromal symptoms have a significantly greater probability of developing the illness,4,6 suggesting that specific aspects of prodromal symptoms may reflect vulnerability markers for developing schizophrenia.7 However, prodromal symptoms, which overlap with experiences and behaviors of similar-aged individuals, many of whom do not develop schizophrenia, are mild and have low predictive value as vulnerability markers. Therefore, the current behavioral and symptomatic criteria for the prodromal stage are neither sufficiently sensitive nor specific for reliable early diagnosis of psychotic disorders. Consequently, there is a critical need for identifying markers that are more closely tied to neural function and linked to the pathophysiologic mechanisms of schizophrenia, and therein augment the validity and specificity of clinical features preceding illness onset.
It is generally accepted that interactions along the frontostriate pathways form the basis for higher executive functions and the organization, selection, and generation of task-appropriate thoughts and behaviors.8 It is well established that continuous performance–type tasks such as the forced choice visual oddball task yield robust activation that localizes brain regions associated with visual executive processing and selective attention.9–16 Studies in ultra-high-risk individuals show impaired behavioral performance on continuous performance tasks and indicate that impaired attention is (1) evident in schizophrenic patients regardless of clinical state, (2) detectable before illness onset, (3) present in first-degree relatives, and (4) predictive of later behavioral disturbances in susceptible individuals.17 Deficits on tasks of visual selective attention, executive function, working memory, and response inhibition18–21 have been associated with prefrontal functional abnormalities in schizophrenic patients,22–32 beginning as early as the first episode of illness.33 Frontal and striatal regions are thus prominently implicated in the pathophysiology of schizophrenia, and further show structurally abnormal neuroanatomic findings.34–37 Evidence of structural abnormality comes from cytoarchitectural analysis of postmortem patient brains,38,39 neurocellular alterations,40 and disorganized prefrontal white matter.41,42 Midbrain regions, which provide major afferents to prefrontal cortical regions, have reduced thalamic volumes43,44 and disrupted subcortical function in schizophrenia.45,46 Such structural43,44,47–49 and functional13,45,46,50 alterations in subcortical regions have been associated with deficits in sensory filtering, behavioral control, and flexibility.
In high-risk adolescents, performance deficits in attention and executive function tasks17 that are associated with frontostriatal cortical dysfunction and, more recently, findings of decreased gray matter in inferior frontal cortex and in the cingulate cortex51 have been reported to precede the clinical expression of illness. These attention impairments are comparable in severity with impairments observed in adult clinical cases and are the strongest predictors of evolving into schizophrenia.17 Similarly, first-degree relatives of individuals with schizophrenia and persons diagnosed as having schizotypal personality disorder display attention and executive function deficits that are early clinical markers in the diathesis for schizophrenia.52,53 Together, these findings implicate a functional deficit in brain regions responsible for attention and executive function during the prodromal stage. Examining frontostriatal dysfunction, associated with the early and chronic stages of schizophrenia, may lead to the identification of novel vulnerability markers present during the prodrome of illness. To date, there have been no published functional magnetic resonance imaging (fMRI) studies in high-risk populations experiencing prodromal symptoms. The characterization of a postulated neuroprogressive pathologic finding associated with the onset of schizophrenia motivated our efforts.
The aims of this study were (1) to use fMRI to examine the functional integrity of frontostriatal regions in an ultra-high-risk group experiencing prodromal symptoms; (2) to examine the relationship of frontostriatal function to stage of illness; and (3) to assess specific aspects of prefrontal deficits related to selective attention and executive function that facilitate goal-directed behavior through the selection of task-relevant stimuli and filtering of task-irrelevant novel stimuli. Using a cross-sectional experimental design, we studied 3 groups of patients (ultra-high-risk, early, and chronic) selected for their stage of illness, and a fourth control group. We hypothesized that patients in the early and chronic stages would show evidence of frontostriatal abnormality, reflected in reduced extent and intensity of activation, and that ultra-high-risk individuals would exhibit milder abnormality of the same form.
METHODS
SUBJECTS
We used imaging data from 52 subjects divided into 4 groups: (1) ultra-high risk (n = 10), (2) early schizophrenia (n = 15), (3) chronic schizophrenia (n = 11), and (4) control (n = 16). As expected, the chronic group was significantly older (P<.005), while the other 3 groups were not significantly different from one another. Because the chronic group was significantly older than all the other groups, we performed separate analyses to compare an older control group of 10 subjects (mean age, 34 years) with the chronic group (mean age, 38 years) that did not show a between-group age difference (P =.35). The mean duration of illness of the chronic group was significantly longer than that of the early group (P<.001). Demographic and clinical characteristics of the subject groups are summarized in the Table. Ultra-high-risk patients were recruited through local media advertisements, whereas patients for the early and chronic groups were recruited from the University of North Carolina at Chapel Hill and Dorothea Dix State Psychiatric Hospital, Raleigh, NC.
Table.
Demographic and Clinical Characteristics of Subject Sample*
| Characteristic | Ultra-High-Risk (n = 10) |
Early Schizophrenia (n = 15) |
Chronic Schizophrenia (n = 11) |
Control (n = 16) |
Older Control (n = 10) |
|---|---|---|---|---|---|
| Age, y | |||||
| Mean (SD)† | 22.6 (4.4) | 24.1 (6.5) | 38.1 (7.7) | 28.0 (11.6) | 34.0 (12.1) |
| Range | 15–31 | 19–41 | 22–52 | 19–55 | 22–55 |
| Sex, No. (%) F | 5 (50) | 5 (33) | 2 (18) | 7 (41) | 3 (33) |
| Handedness, No. (%) right-handed | 10 (100) | 11 (73) | 9 (82) | 16 (100) | 10 (100) |
| Ethnicity, No. (%) white | 8 (80) | 10 (67) | 9 (82) | 15 (88) | 9 (90) |
| Education, y‡ | 14.0 (2.6) | 12.0 (1.4) | 13.6 (2.8) | 15.4 (3.3) | 17.3 (3.3) |
| Age at illness onset, y | NA | 22.3 (5.9) | 22.9 (5.7) | NA | NA |
| Illness duration, mo§ | NA | 20.6 (19.9) | 183.5 (77.2) | NA | NA |
| No. taking antipsychotic medication (atypical) | 2 (2) | 13 (13) | 11 (10) | NA | NA |
| Dosage, mg/d chlorpromazine equivalent | 17.5 (37.4) | 448.1 (296.2) | 511.4 (345.6) | NA | NA |
| PANSS positive | 9.9 (3.0) | 11.9 (4.1) | 12.3 (5.9) | NA | NA |
| PANSS negative | 9.3 (3.8) | 12.8 (5.1) | 13.9 (5.8) | NA | NA |
| PANSS general║ | 22.8 (6.7) | 24.7 (5.0) | 25.2 (6.2) | NA | NA |
| PANSS total | 41.7 (12.1) | 48.9 (11.7) | 51.4 (13.7) | NA | NA |
Abbreviations: NA, not applicable; PANSS, Positive and Negative Syndrome Scale.
Data values represent mean (standard deviaion) except where indicated otherwise.
The chronic schizophrenia group was significantly older than the other groups (P<.005).
Education is based on incomplete information.
P<.001.
PANSS cognitive main effect for group (F2,32 = 3.250, P = .05), linear trend analysis (ultra-high-risk → early → chronic) (F1,33 = 6.605, P = .01).
Diagnoses were confirmed by the Structured Clinical Interview for DSM-IV administered by University of North Carolina staff psychiatrists and rated by means of the Positive and Negative Syndrome Scale54 and the Symptom Onset in Schizophrenia inventory.55 None of the patients was acutely psychotic at the time of testing. Subjects were excluded for past substance dependence, substance abuse within the past month, and pregnancy. Negative results of urine toxicology and serum pregnancy tests were confirmed before the fMRI session. Individuals with a history of psychiatric illness or a first-degree relative having a psychotic illness were excluded from participating as control subjects. All subjects received a complete verbal description of the study, and written informed consent was obtained for procedures approved by the institutional review boards at University of North Carolina at Chapel Hill and Duke University Medical Center, Durham, NC.
ULTRA-HIGH-RISK PATIENT EVALUATION
Ultra-high-risk subjects were screened by means of the Structured Interview for Prodromal Symptoms,56 and those who met the clinical criteria defined in the Criteria of Prodromal Symptoms57 were invited to enter the study. All patients met state criteria, while a subset of them met both state and trait criteria as summarized in this paragraph. At the time of writing, 2 of the 10 patients had already progressed to schizophrenia and therefore had actually been prodromal at the time of their fMRI sessions. Of the 10 patients in this group, 2 (not the same 2 who progressed) were taking antipsychotic medication and 4 were taking antidepressant medication. Briefly, the Criteria of Prodromal Symptoms require that individuals meet at least one of the following clinical criteria: (1) for brief intermittent psychotic state: psychotic symptoms emerging in the recent past that occur too briefly to meet official criteria for a diagnosis of psychosis; (2) for attenuated positive symptom state: nonpsychotic predelusional unusual thoughts, prehallucinatory perceptual abnormalities, or pre–thought-disordered speech disorganization; or (3) for genetic risk and deterioration state: genetic risk for psychosis (first-degree relative with schizophrenia spectrum disorder and/or schizotypal personality disorder in proband) plus a recent loss of social and/or work or school capacity equivalent to a drop in global assessment of function of 30 points sustained for at least 1 month.
STIMULI AND TASK
Participants were presented with visual stimuli from 1 of 3 categories in a semirandomized sequential manner. Stimuli consisted of frequently occurring (94%) squares of varying size and color (standards), infrequent (3%) circles of varying size and color (targets), and infrequent (3%) pictures of everyday objects (novels). All stimuli were presented centrally against a white background for 500 milliseconds, with a stimulus onset asynchrony of 1500 milliseconds, and sustained a visual angle of 6°. Each visual stimulus was accompanied by a simultaneously presented single pure auditory tone, which participants were instructed to ignore, and which was presented binaurally as part of a separate study of unattended auditory processing. In each imaging session, subjects performed 7 runs (5 minutes 12 seconds in duration) of this visual task during which they responded to target stimuli (circles) with a unique button press and responded to all other stimuli (standard and novel) with an alternate button press. All novels and targets were separated by at least 9 but not more than 11 visual standards.
ACQUISITION OF fMRI DATA
Images were acquired on a 1.5-T scanner (Signa; General Electric Co, Milwaukee, Wis) with a birdcage-type standard quadrature head coil and an advanced nuclear magnetic resonance echoplanar system. The participant’s head was positioned along the canthomeatal line and immobilized by means of a vacuum cushion and a forehead strap. T1-weighted sagittal scans were used to select 16 contiguous oblique axial slices parallel to the anterior commissure–posterior commissure plane, which provided the coplanar anatomic images. Sixteen coplanar functional images were acquired by means of a gradient echoplanar sequence (repetition time, 1500 milliseconds; echo time, 40 milliseconds; flip angle, 90°; number of signals acquired, 1; voxel dimensions, 3.75×3.75×5 mm; imaging matrix, 64×64 voxels). Each of the 7 imaging runs consisted of 200 time points. Four radiofrequency excitations were performed before image acquisition to achieve steady-state transverse relaxation. Finally, high-resolution T1-weighted anatomic images (3-dimensional spoiled gradient-recalled imaging; repetition time, 22 milliseconds; echo time, 5 milliseconds; flip angle, 20°; field of view, 24 cm; voxel dimensions, 0.9375×0.9375×1.5 mm; 256×256 voxels; 124 images) as well as 2-dimensional spinecho sequence (repetition time, 4000 milliseconds; echo time, 20 milliseconds; flip angle, 90°; field of view, 24 cm; 1.5-mm slice thickness), were acquired for future morphometric and volumetric analyses.
FUNCTIONAL IMAGE ANALYSIS
All image analyses were performed with custom analysis software written in MATLAB. Head motion was detected by center-of-mass measurements in 3 orthogonal planes. Six scans were discarded because of motion and other artifacts, constituting a 10% attrition rate of scanned subjects. Movement greater than 1mmfrom baseline was used as the rejection criterion for motion artifact and resulted in the elimination of 1 of 7 runs in 1 control subject, 1 ultra-high-risk subject, 2 early schizophrenic subjects, and 1 chronic schizophrenic subject, or about 1% of the total data. To examine the possibility that significant group differences in motion could be driving observed group differences in activation, we computed the standard deviation of the center of mass over the time course of each run and then calculated the mean of all included runs for each subject. These data were computed separately for movement in the x, y, and z directions. A repeated-measures analysis of variance (ANOVA) was performed with group as the “between” factor and the standard deviation in x, y, z as the “within” factor, which failed to show significant group differences (F3,96=1.6, P=.21).
Following the procedure of McCarthy et al,14 image time segments consisting of 5 images preceding, 9 images following, and the image coincident with each target presentation were excised from the functional runs. These segments were then averaged, and the mean of the 5 prestimulus images across all epochs was subtracted from each of the subsequent averaged 10 images to generate baseline-adjusted epochs.
Image analyses were performed with a region-of-interest approach. The following brain regions of interest were manually traced on the basis of anatomic landmarks on the coplanar high-resolution anatomic slices measured 5 mm apart traversing superiorly from the anterior commissure–posterior commissure axial plane: anterior cingulate gyrus (ACG; 5–35 mm), middle frontal gyrus (MFG; 5–35 mm), inferior frontal gyrus (IFG; 5–30 mm, including pars opercularis and pars triangularis), basal ganglia (BG; 5 mm), and thalamus (TH; 5–10 mm). Voxels activated in a task-dependent fashion in response to the 2 stimulus conditions of interest (visual target and visual novel) were identified within each region of interest by means of a correlation of the functional segments with the time course of an empirically derived hemodynamic function.9,14 Voxels with a time course that correlated with the template above a T value of 1.96 (95% confidence interval) were accepted as active voxels. For each subject, the average ratio of active voxels to total voxels in each region (ACG, IFG, MFG, BG, and TH) was computed for each of the task stimulus conditions (targets, novels) and referred to as the dependent measure of “percentage of active voxels” (PAV). In a similar manner, the average of percentage signal change for targets and novels relative to a baseline of standards was computed for all of the above regions and constituted the dependent measure of “percentage signal change” (PSC). Thus, PSC (representing the magnitude of activation) and PAV (representing a measure of the extent of activation) served as dependent measures in subsequent region-of-interest–based statistical analyses. We also used SPM99 (Statistical Parametric Mapping, 1999 Version) software (Wellcome Department of Cognitive Neurology, London, England) to normalize individual subject activation maps to a template brain and generate group-average activation maps.
STATISTICAL ANALYSIS
We hypothesized that, behaviorally, patients would demonstrate poor task-relevant target discrimination abilities relative to controls, in the absence of a strong generalized response bias. Behavioral performance analysis was conducted on percentage of hits, d′, and B′′ measures. Our use of d′ (|ZHit−ZFA|), which is based on the hit rate and the false alarm (FA) rate (incorrect response to standard or novel), was motivated by its power in measuring discriminability and evidence of its decline in ultra-high-risk and schizophrenia groups. B′′ (y[1−y]−x[1−x]/y[1−y]+x[1−x], where y equals the probability of hits and x equals the probability of false alarms), a measure of “response bias,” was calculated as an index of a subject’s tendency to overrespond or underrespond.
We hypothesized that prefrontal (MFG, IFG), medial frontal (ACG), and striatal (BG, TH) function, as measured by activation in these regions in response to target stimuli, would be diminished in all clinical groups (chronic, early, and ultra-high-risk) as compared with the control group. For prefrontal and striatal regions, our hypothesis was tested by the repeated-measure ANOVA tested separately on 2 dependent measures of activation (PAV and PSC) using 1 between factor of group (4 levels) and 1 within factor of region (2 levels for prefrontal and 2 levels for striatal). The medial frontal analysis used a simple 1-way ANOVA. All post hoc analyses of ANOVA results were performed at the 5% significance level using the Dunnett test, a specialized multiple-comparison test that performs fewer comparisons when a collection of multiple groups is compared with a single control group. Therefore, all post hoc analysis performed for this study was corrected for multiple comparisons.
The second hypothesis concerned the differential reduction between the target and novel response-related activation. Healthy individuals have significantly greater prefrontal activation to target than to novel stimuli. We hypothesized that the 3 clinical groups would have smaller differential task-dependent activation than the control group in ACG, IFG, and MFG. The differential activation was computed by subtracting PAV associated with the novels from PAV associated with the targets for each subjects in the ACG, IFG, and MFG. This hypothesis was also tested with a repeated-measures ANOVA using 1 between-factor of group (4 levels) and 1 within-factor of region (3 levels) on the PAV and PSC data.
To further test our hypotheses of decrements in frontal and striatal activation associated with illness progression, we used nonparametric statistics to perform trend analyses of the 4 groups by using the Jonckheere-Terpstra (JT) test. The goal of the JT test is to test a null hypothesis against an alternative of control>ultra-high-risk>early schizophrenia>chronic schizophrenia. In other words, it was intended to answer the question of whether there is a monotonic trend among the means of activation in each of the 4 groups. The test is a variation on the Mann-Whitney test–Wilcoxon rank sum test. The test was performed separately on each region (ACG, BG, IFG, MFG, and TH) with the use of 1 dependent variable examined across 4 groups. The quality of trend analysis was reported as z statistic and P value.
RESULTS
CLINICAL MEASURES AND BEHAVIORAL PERFORMANCE
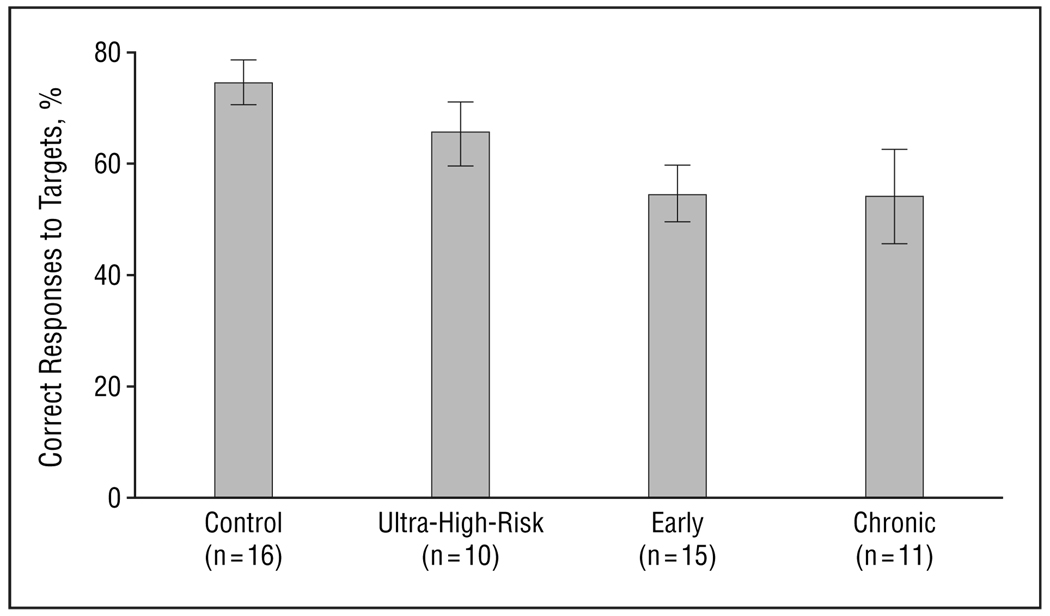
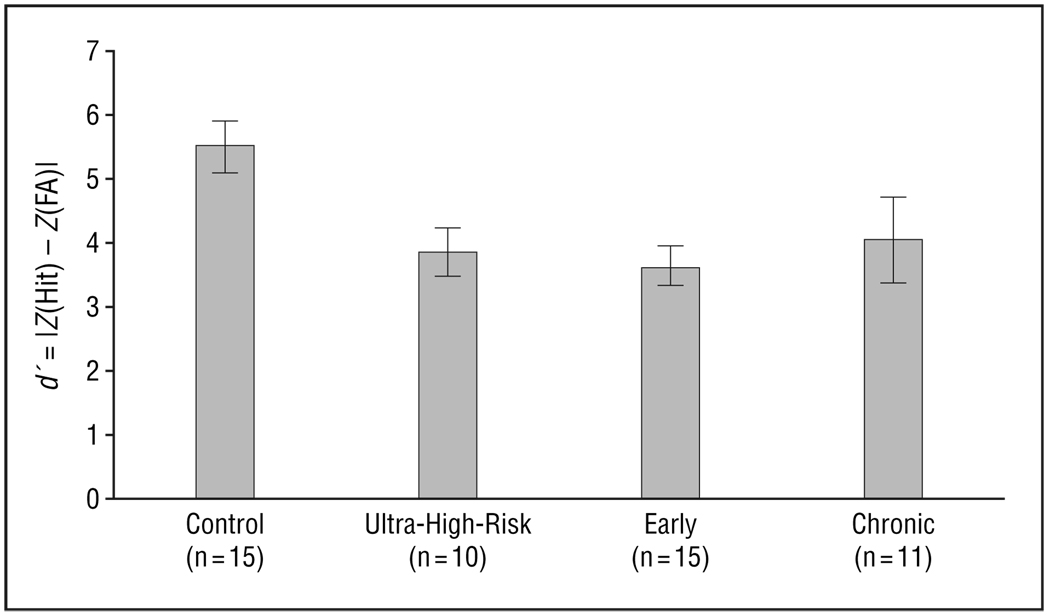
There were significant group differences in behavioral performance as measured by percentage hits (F3,48=3.4, P=.03) (Figure 1). Post hoc analysis showed that the control group had greater percentage hits than the early and chronic groups, but not the ultra-high-risk group. Trend analysis confirmed a highly significant trend of diminishing performance across the 4 groups (control>ultra-high-risk>early>chronic) of subjects (z=3.9, P<.001). Analysis of d′ excluding 1 outlier in the control group with a high rate of false alarms (>3 SDs from the mean) showed a significant group difference (F3,47=4.29, P=.01) (Figure 2). Post hoc analysis showed a significantly higher d′ in the control group than in the ultra-high-risk group and the early schizophrenia group, and marginally higher than in the chronic group. Trend analysis using the JT test showed a significant trend (z = 2.9, P=.002). Analysis of B′′ to examine perseverative behavior or biases did not find significant group differences.
Figure 1.
Performance measured as percentage of correct responses to target stimuli (F3,48=3.4, P<.03). Bars represent mean values; error bars, standard error.
Figure 2.
Analysis of d′ showing a significant group difference (F3,47=4.29, P<.01). The value of d′ (|ZHit−ZFA|) is based on the hit rate and the false alarm (FA) rate (incorrect response to standard or novel). Bars represent mean values; error bars, standard error.
The cognitive subscale of the Positive and Negative Syndrome Scale,54,58 used to examine cognitive disorganization, disclosed marginally significant differences between the clinical groups (F2,32=3.3, P=.052). Trend analysis with the JT test demonstrated diminishing cognitive scores (ultra-high-risk>early>chronic) across the 3 clinical groups (z = 2.6, P=.005).
TARGET VS NOVEL DIFFERENTIAL ACTIVATION
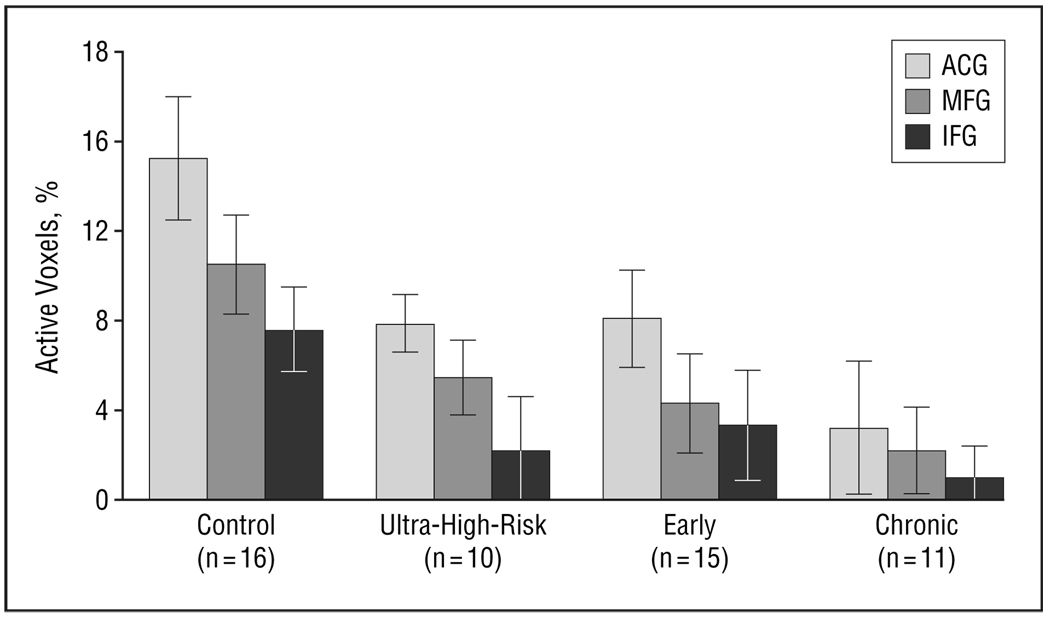
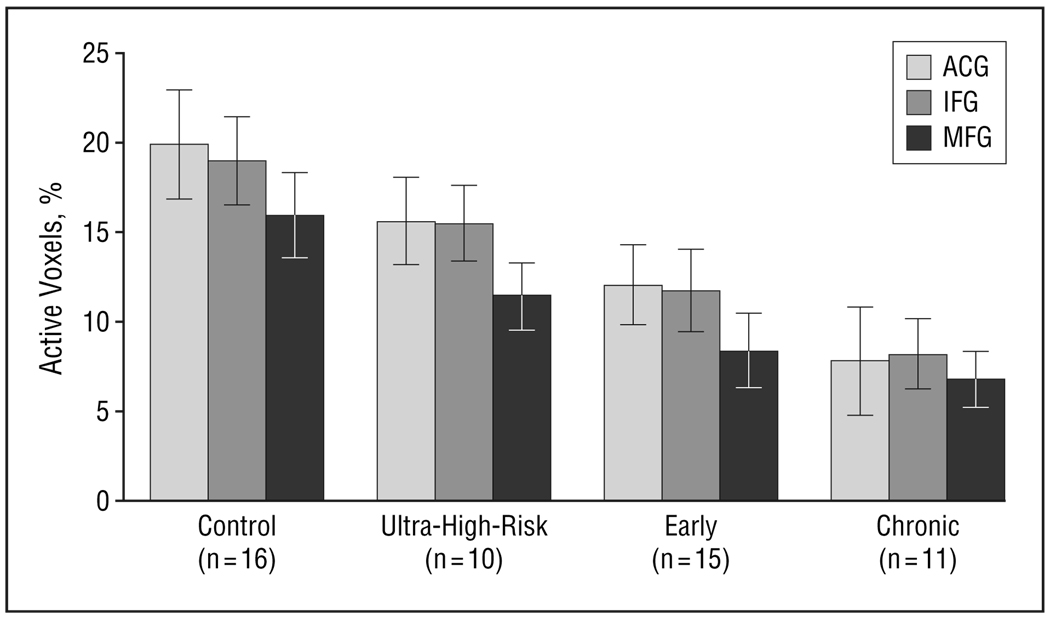
As expected, we found differential frontal (ACG, IFG, MFG) activation (PAV) to target vs novel stimuli and found a significant main effect of group (F3,48=4.0, P<.02) and of region (F2,98=12.2, P<.001), but no group×region interaction (Figure 3). Post hoc analysis showed greater activation in the controls than in the ultra-high-risk, early, and chronic groups and ACG greater than MFG and IFG activation. This finding suggests that prefrontal deficits associated with discrimination of task-relevant target stimuli from task-irrelevant novel stimuli are present in ultra-high-risk individuals experiencing prodromal symptoms of schizophrenia. Older controls showed greater differential activation than did the chronic group (F1,38=7.7, P=.02). Analysis using sex as a blocking factor did not show any effects for sex.
Figure 3.
Differential frontal (anterior cingulate gyrus [ACG], inferior frontal gyrus [IFG], middle frontal gyrus [MFG]) activation (percentage of active voxels) to target vs novel stimuli with a significant main effect of group (F3,48=4.0, P=.02) and region (F2,98=12.2, P<.001). Post hoc analysis showed greater activation in the controls than in ultra-high-risk, early, and chronic groups and ACG greater than MFG and IFG. Bars represent mean values; error bars, standard error.
ILLNESS EFFECTS ON FRONTOSTRIATAL FUNCTION
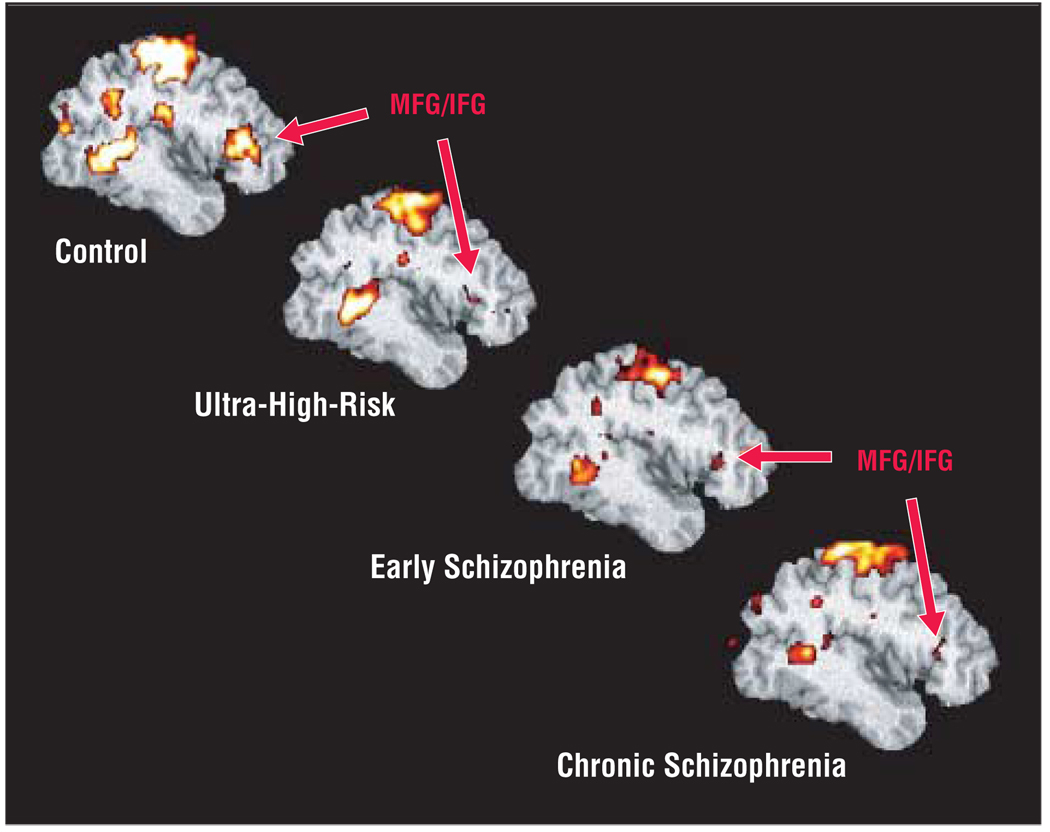
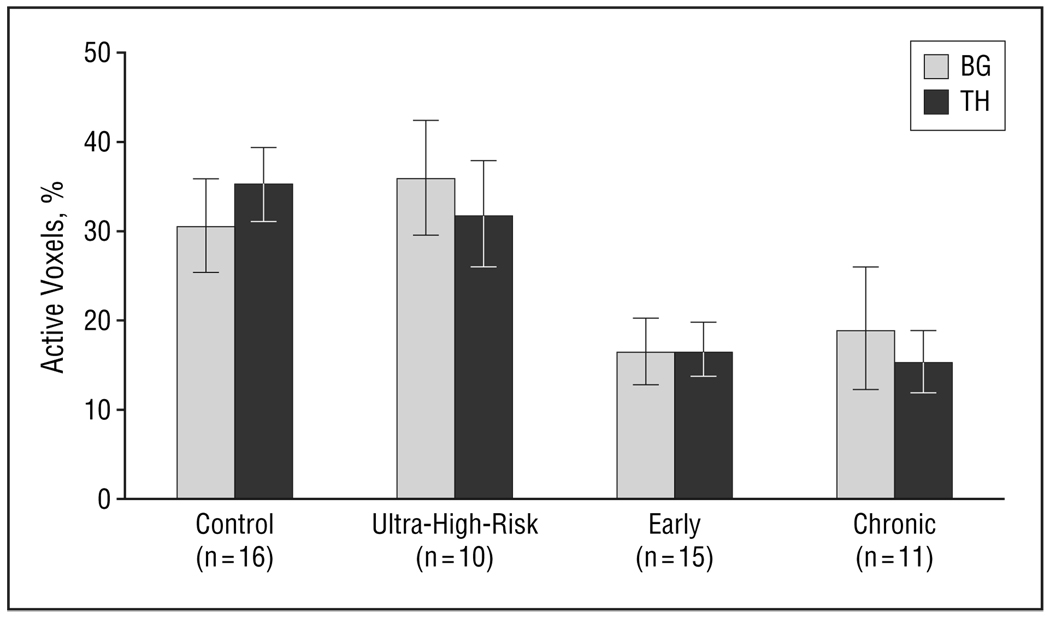
Figure 4 presents decline in target-related activation associated with progression of illness. Quantitative assessment showed significant differences in prefrontal targetrelated activation for group (F3,48=4.6, P=.01) and region (F1,48=12.8, P<.001), but no group×region interaction (Figure 5). Post hoc analysis showed greater extent of activation in controls than in the early and chronic groups, and in IFG than in MFG. Similarly, in the striatal region there was a significant difference for group (F3,48=5.6, P=.002), with greater activation in controls than in the early and chronic groups (Figure 6). These results were consistent with our hypothesis of diminished frontostriatal activation in the early and chronic groups, but did not find significantly reduced prefrontal activation in the ultra-high-risk group. In the medial frontal region, there was a significant difference for group (F3,48=3.5, P=.02) with greater activation in the control than the chronic group. We compared the chronic group directly with age-matched older controls and found that the chronic group still showed significantly lower target vs novel differential activation in the frontal region (F1,38=7.7, P=.02), lower target-associated medial frontal (F1,19=6.7, P=.02) and prefrontal (F1,19=7.9, P=.02) activation, and marginally lower striatal (F1,19=4.2, P=.06) activation. The JT trend analysis suggested a significant difference in extent of activation across the 4 groups (control>high-risk>early>chronic) in all the regions: ACG (z=3.52, P<.001), BG (z=2.16, P<.02), IFG (z=3.57, P<.001), MFG (z=3.32, P<.001), and TH (z=3.76, P<.001). Analysis using sex as a blocking factor did not show any effects for sex.
Figure 4.
Group-averaged, target-related prefrontal activation in control, ultra-high-risk, early, and chronic groups. ACG indicates anterior cingulate gyrus; IFG, inferior frontal gyrus; and MFG, middle frontal gyrus.
Figure 5.
Prefrontal target-related activation for group (F3,48=4.6, P<.01) and region (F1,48=12.8, P<.001), with greater activation in controls than early and chronic groups. There was medial frontal activation for group (F3,48=3.5, P=.02) with greater activation in the control than chronic group. Bars represent mean values; error bars, standard error. ACG indicates anterior cingulate gyrus; IFG, interior frontal gyrus; MFb, middle frontal gyrus.
Figure 6.
Striatal target-related activation for group (F3,48=5.6, P=.002) with greater activation in controls than early and chronic groups. Bars represent mean values; error bars, standard error. BG indicates basal ganglia; TH, thalamus.
EXPLORATORY EVALUATION OF LATERALITY EFFECTS
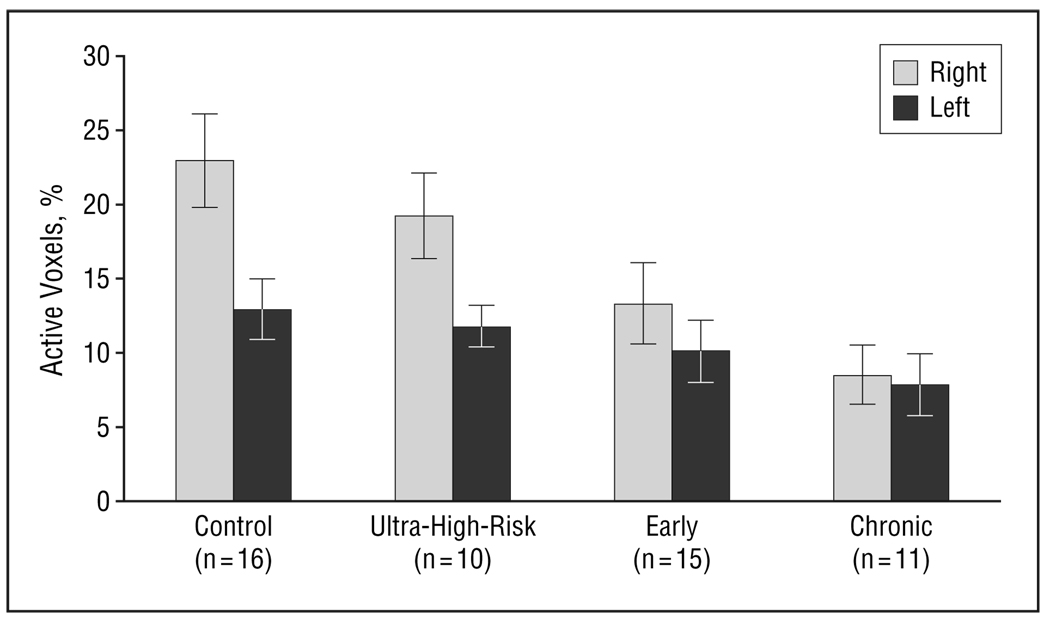
Lateralized structural and functional deficits, ranging from lateralized left hemisphere deficits59,60 to overall reduced laterality,61 have frequently been reported in schizophrenia. We performed exploratory analysis of laterality for each of the brain regions and found differences in target-related activation for group (F3,48=4.2, P=.01), side (F1,48=37.1, P<.001), and a group×side interaction (F3,49=6.5, P<.001), but only in the IFG (Figure 7). Post hoc analysis showed that controls had greater right- than left-sided activation as compared with early and chronic groups,59–62 but not greater than the ultra-high-risk group, reflecting a reduced laterality for both groups, consistent with previously reported laterality effects.
Figure 7.
Left and right inferior frontal gyrus target-related activation for group (F3,48=4.2, P=.01), side (F1,48=37.1, P<.001), and a group×side interaction (F3,49=6.5, P<.001). Bars represent mean values; error bars, standard error.
COMMENT
In summary, our fMRI findings show that the ability to differentially activate frontal brain regions (ACG, IFG, andMFG)to target vs novel stimuli was significantly lower in individuals experiencing prodromal symptoms of schizophrenia (ultra-high-risk group) compared with controls. The activation showed further decrements in groups that represent early and chronic stages of schizophrenia. This finding suggests regional impairments in circuits responsible for filtering task-relevant visual information from task-irrelevant but salient visual information. Our second major finding shows that frontal and striatal activation associated with target conditions is smaller in the early and chronic patient groups but not significantly lower in the ultra-high-risk group. However, trend analysis confirms a highly significant declining trend of activation in frontal and striatal regions across the 4 subject groups (control>ultra-high-risk>early>chronic) consistent with a neuroprogressive abnormality.35,63–67
BEHAVIORAL PERFORMANCE
Behavioral performance analyses indicated impaired target discrimination (d′) in ultra-high-risk and early groups relative to the control group. The trend analysis results confirmed a significant declining performance on discrimination measures in the absence of a significant difference between groups in overall performance bias, which successfully replicates behavioral performance studied in continuous performance tasks among ultra-high-risk individuals.17
Impaired attention has been related to personality development delays in subjects with a susceptibility to schizophrenia.68,69 Attention deficits dating back to childhood often lead to an inability to efficiently process information from the environment, especially subtle and highly complex interpersonal cues and communications. The long-term impact of chronic impairments in attention have also been associated with deficient social skills, leading to social isolation—a key index of negative symptomatology.17,68,70
fMRI MEASURES
The ability to differentially activate to target vs novel stimuli is diminished in individuals experiencing prodromal symptoms of schizophrenia. This fairly distributed frontal lobe abnormality is evident in the significant reduction of activation between the control group and the ultra-high-risk group, and persists in the early and chronic groups. In the absence of disease, the prefrontal regions (MFG and IFG) are critical in target and novelty detection9,71 and the medial frontal region (ACG) in decision making and action and conflict monitoring. 11,72 The inability to process and act on task-relevant sensory inputs while filtering out task-irrelevant sensory inputs may underlie impairments in executive function and attention that can be correlated with prefrontal abnormality and, as indicated by our findings, is present in individuals experiencing prodromal symptoms of schizophrenia. This finding, interpreted as assigning heightened salience to task-irrelevant stimuli, can be explained by means of perhaps the most widely adopted theory on the pathophysiology of schizophrenia: the dopamine hypothesis.73,74 Normally, the stimulus-linked release of dopamine mediates the acquisition and expression of relevant motivational saliences in response to the subjects’ environment. In schizophrenia, dysregulated dopamine transmission leads to a stimulus-independent release of dopamine. This neurochemical change overwhelms the normal process of context-driven salience attribution and leads to improper assignment of salience to external objects and to internal representations. Thus dopamine, which in healthy normal conditions is a mediator of contextually relevant salience, becomes a creator of inappropriate salience in schizophrenia. 75–77 The increased release of dopamine, mismatched with the context, leads to improper assignment of salience to external stimuli. Indeed, it is plausible that the proposed mechanism can explain our observed findings as well the clinical phenomenology observed in patients experiencing prodromal symptoms.75 Therefore, our findings are consistent with outcomes extrapolated from the dopamine hypothesis and its implications on mesocortical and mesolimbic dysregulation. The mechanisms for dopamine excess have been variously explained by theories of γ-aminobutyric acid–ergic interneuron dysfunction, glutamatergic dysregulation, and interrupted maturational pruning in adolescence.75,78–81
The pattern of results that we have obtained supports our hypothesis of diminished prefrontal function that progresses with chronicity of illness beginning with the onset of prodromal symptoms. As predicted, we found significantly greater activation in the control group than in both the early and the chronic groups in the prefrontal (MFG, IFG), medial frontal (ACG), and striatal (BG, TH) regions. Although the large differences between the control and ultra-high-risk groups did not meet conventional significance levels by means of parametric statistics, nonparametric trend analyses clearly demonstrated a significant stepwise decrement in prefrontal function across the clinical groups. It is also important to note that our high-risk subjects represent a heterogeneous sample of individuals who might develop schizophrenia or other psychiatric disorders, or might spontaneously remit. Therefore, it is likely that a group composed of strictly prodromal patients, ie, who have subsequently been documented to progress to schizophrenia, will disclose greater impairments on the outcome measures we investigated than our ultra-high-risk group did. An expanded sample size in the high-risk group or a longitudinal study of a confirmed prodromal sample would likely demonstrate a significant difference in activation.
Potential limitations of our study deserve consideration. While both the early and chronic groups were medicated, treatment duration was significantly longer for the chronic group, and only 2 patients in the ultra-high-risk group were receiving antipsychotic treatment. Furthermore, unlike controls, a subset of patients within each of the 3 clinical groups was taking antidepressant medication. While it is difficult to disentangle the possible effect of medication on task-related brain activation, a recent fMRI study of medication-naive first-episode patients performing a version of the AX–continuous performance task found diminished task-related activation in the dorsolateral prefrontal cortex,33 thus questioning the contention of medication-induced activation differences. Finally, confounding effects of a number of demographic variables such as age and sex in our study could partially be attributed to recruitment difficulties associated with the inherent nature of each population group, eg, prodromal patients being younger or females having typically later onset of psychosis.
Understanding the pathophysiology of the emerging and progressing clinical manifestations is critical to understanding the pathogenesis of schizophrenia and is likely to inform the development of preventive therapeutic strategies. Critical events of postnatal brain maturation of the glutamatergic systems take place in adolescence that coincide with onset of illness and a period of premorbid vulnerability. Therefore, evidence that early treatment can arrest neuronal damage has important therapeutic implications in ultra-high-risk patients. Although this study focuses on deficits in prefrontal and midbrain regions associated with executive function, a large body of literature has focused on temporal cortical dysfunction and associated deficits in the auditory modality in schizophrenia. 30 Recent studies have suggested that temporal cortical deficits in schizophrenia may not emerge until later stages of the disorder.82 Therefore, the pathophysiology of schizophrenia may affect various cortical circuits with a different chronology. Tracking the variable times of onset and rates of progression of spatially and temporally distributed neuropathology associated with schizophrenia will be best evaluated by longitudinal studies. Our data are limited by the cross-sectional experimental design. Longitudinal cohort studies that examine cortical and subcortical functions at the prodromal stage could lead to the discovery of discerning vulnerability markers.
Acknowledgments
We thank Margaret Rosemond, Jeremy Frye, Patricia Haak, Emily McClernon, MSW, LCSW, and Karen Graham, MD, for their help in patient recruitment; Joshua Bizzell, MS, and Chuck Michelich, PhD, for writing image analysis software; Kerry Anzenberger and Garrett Rosania for their help in data analysis; Robert Hamer, PhD, for consultation on statistical analysis; and MRI technologists Susan Music, Wandra Davis, Luther Poole, and Danny Thompson.
Funding/Support: This study was supported by grants from the National Institute of Mental Health (NIMH) Mental Health Clinical Research Center, Bethesda, Md; NIMH grant R01; NIMH grant MH58251; grant MH64065 to the University of North Carolina Schizophrenia Research Center (an NIMH Silvio O. Conte Center for the Neuroscience of Mental Disorders); the Foundation of Hope of Raleigh, NC; and a NARSAD Young Investigators Award National Alliance for Research on Schizophrenia and Depression, Great Neck, NY.
Footnotes
Financial Disclosure: Dr Perkins has received research grants from Eli Lilly and Company, Pfizer Inc, Bristol-Myers Squibb Company, Otsuka American Pharmaceuticals, Inc, and AstraZeneca International; is a consultant to Bristol-Myers Squibb Company; and is on the speakers’ bureau for Eli Lilly and Company, AstraZeneca International, and Bristol-Myers Squibb Company.
References
- 1.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 2.Bloom FE. Advancing a neurodevelopmental origin for schizophrenia. Arch Gen Psychiatry. 1993;50:224–227. doi: 10.1001/archpsyc.1993.01820150074008. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman JA, Perkins D, Belger A, Chakos M, Jarskog F, Boteva K, Gilmore J. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches [published correction appears in Biol Psychiatry. 2002;51:346] Biol Psychiatry. 2001;50:884–897. doi: 10.1016/s0006-3223(01)01303-8. [DOI] [PubMed] [Google Scholar]
- 4.Chapman LJ, Chapman JP, Kwapil TR, Eckblad M, Zinser MC. Putatively psychosisprone subjects 10 years later. J Abnorm Psychol. 1994;103:171–183. doi: 10.1037//0021-843x.103.2.171. [DOI] [PubMed] [Google Scholar]
- 5.Yung AR, Phillips LJ, McGorry PD, McFarlane CA, Francey S, Harrigan S, Patton GC, Jackson HJ. Prediction of psychosis: a step towards indicated prevention of schizophrenia. Br J Psychiatry Suppl. 1998;172:14–20. [PubMed] [Google Scholar]
- 6.Rosen JL, Woods SW, Miller TJ, McGlashan TH. Prospective observations of emerging psychosis. J Nerv Ment Dis. 2002;190:133–141. doi: 10.1097/00005053-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Cadenhead KS. Vulnerability markers in the schizophrenia spectrum: implications for phenomenology, genetics, and the identification of the schizophrenia prodrome. Psychiatr Clin North Am. 2002;25:837–853. doi: 10.1016/s0193-953x(02)00021-7. [DOI] [PubMed] [Google Scholar]
- 8.Funahashi S. Neuronal mechanisms of executive control by the prefrontal cortex. Neurosci Res. 2001;39:147–165. doi: 10.1016/s0168-0102(00)00224-8. [DOI] [PubMed] [Google Scholar]
- 9.Kirino E, Belger A, Goldman-Rakic P, McCarthy G. Prefrontal activation evoked by infrequent target and novel stimuli in a visual target detection task: an event-related functional magnetic resonance imaging study. J Neurosci. 2000;20:6612–6618. doi: 10.1523/JNEUROSCI.20-17-06612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens AA, Skudlarski P, Gatenby JC, Gore JC. Event-related fMRI of auditory and visual oddball tasks. Magn Reson Imaging. 2000;18:495–502. doi: 10.1016/s0730-725x(00)00128-4. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 12.Yoshiura T, Zhong J, Shibata DK, Kwok WE, Shrier DA, Numaguchi Y. Functional MRI study of auditory and visual oddball tasks. Neuroreport. 1999;10:1683–1688. doi: 10.1097/00001756-199906030-00011. [DOI] [PubMed] [Google Scholar]
- 13.Ardekani BA, Choi SJ, Hossein-Zadeh GA, Porjesz B, Tanabe JL, Lim KO, Bilder R, Helpern JA, Begleiter H. Functional magnetic resonance imaging of brain activity in the visual oddball task. Brain Res Cogn Brain Res. 2002;14:347–356. doi: 10.1016/s0926-6410(02)00137-4. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy G, Luby M, Gore J, Goldman-Rakic P. Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. J Neurophysiol. 1997;77:1630–1634. doi: 10.1152/jn.1997.77.3.1630. [DOI] [PubMed] [Google Scholar]
- 15.Huettel SA, McCarthy G. What is odd in the oddball task? prefrontal cortex is activated by dynamic changes in response strategy. Neuropsychologia. 2004;42:379–386. doi: 10.1016/j.neuropsychologia.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Huettel SA, Mack PB, McCarthy G. Perceiving patterns in random series: dynamic processing of sequence in prefrontal cortex. Nat Neurosci. 2002;5:485–490. doi: 10.1038/nn841. [DOI] [PubMed] [Google Scholar]
- 17.Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia [published correction appears in Schizophr Bull. 1994;20:248] Schizophr Bull. 1994;20:31–46. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- 18.Carter CS, Mintun M, Nichols T, Cohen JD. Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during singletrial Stroop task performance. Am J Psychiatry. 1997;154:1670–1675. doi: 10.1176/ajp.154.12.1670. [DOI] [PubMed] [Google Scholar]
- 19.Posner MI, Early TS, Reiman E, Pardo PJ, Dhawan M. Asymmetries in hemispheric control of attention in schizophrenia. Arch Gen Psychiatry. 1988;45:814–821. doi: 10.1001/archpsyc.1988.01800330038004. [DOI] [PubMed] [Google Scholar]
- 20.Neuchterlein KH, Dawson ME, Ventura J, Miklowitz D, Konishi G. Information-processing anomalies in the early course of schizophrenia and bipolar disorder. Schizophr Res. 1991;5:195–196. doi: 10.1016/0920-9964(91)90069-4. [DOI] [PubMed] [Google Scholar]
- 21.Nuechterlein KH, Dawson ME. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bull. 1984;10:160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- 22.Ford JM, Gray M, Whitfield S, Turken AU, Glover G, Faustman WO, Mathalon DH. Acquiring and inhibiting prepotent responses in schizophrenia. Arch Gen Psychiatry. 2004;61:119–129. doi: 10.1001/archpsyc.61.2.119. [DOI] [PubMed] [Google Scholar]
- 23.Carter CS, MacDonald AW, III, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry. 2001;158:1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- 24.Servan-Schreiber D, Cohen JD, Steingard S. Schizophrenic deficits in the processing of context: a test of a theoretical model. Arch Gen Psychiatry. 1996;53:1105–1112. doi: 10.1001/archpsyc.1996.01830120037008. [DOI] [PubMed] [Google Scholar]
- 25.Rubia K, Russell T, Bullmore ET, Soni W, Brammer MJ, Simmons A, Taylor E, Andrew C, Giampietro V, Sharma T. An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory function. Schizophr Res. 2001;52:47–55. doi: 10.1016/s0920-9964(00)00173-0. [DOI] [PubMed] [Google Scholar]
- 26.Potts GF, O’Donnell BF, Hirayasu Y, McCarley RW. Disruption of neural systems of visual attention in schizophrenia. Arch Gen Psychiatry. 2002;59:418–424. doi: 10.1001/archpsyc.59.5.418. [DOI] [PubMed] [Google Scholar]
- 27.Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155:1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- 28.Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry. 2001;158:1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- 29.Alain C, Hargrave R, Woods DL. Processing of auditory stimuli during visual attention in patients with schizophrenia. Biol Psychiatry. 1998;44:1151–1159. doi: 10.1016/s0006-3223(97)00478-2. [DOI] [PubMed] [Google Scholar]
- 30.Ford JM. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36:667–682. [PubMed] [Google Scholar]
- 31.Vianin P, Posada A, Hugues E, Franck N, Bovet P, Parnas J, Jeannerod M. Reduced P300 amplitude in a visual recognition task in patients with schizophrenia. Neuroimage. 2002;17:911–921. [PubMed] [Google Scholar]
- 32.Martin-Loeches M, Molina V, Munoz F, Hinojosa JA, Reig S, Desco M, Benito C, Sanz J, Gabiri A, Sarramea F, Santos A, Palomo T. P300 amplitude as a possible correlate of frontal degeneration in schizophrenia. Schizophr Res. 2001;49:121–128. doi: 10.1016/s0920-9964(00)00125-0. [DOI] [PubMed] [Google Scholar]
- 33.Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, III, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- 34.Szeszko PR, Bilder RM, Lencz T, Ashtari M, Goldman RS, Reiter G, Wu H, Lieberman JA. Reduced anterior cingulate gyrus volume correlates with executive dysfunction in men with first-episode schizophrenia. Schizophr Res. 2000;43:97–108. doi: 10.1016/s0920-9964(99)00155-3. [DOI] [PubMed] [Google Scholar]
- 35.DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R. Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res. 1997;74:129–140. doi: 10.1016/s0925-4927(97)00012-7. [DOI] [PubMed] [Google Scholar]
- 36.Gur R, Cowell P, Turetsky B, Gallacher F, Cannon T, Bilker W, Gur RC. A follow-up magnetic resonance imaging study of schizophrenia: relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry. 1998;55:145–152. doi: 10.1001/archpsyc.55.2.145. [DOI] [PubMed] [Google Scholar]
- 37.Lieberman J, Chakos M, Wu H, Alvir J, Hoffman E, Robinson D, Bilder R. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry. 2001;49:487–499. doi: 10.1016/s0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- 38.Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 39.Benes FM. What an archaeological dig can tell us about macro- and microcircuitry in brains of schizophrenia subjects. Schizophr Bull. 1997;23:503–507. doi: 10.1093/schbul/23.3.503. [DOI] [PubMed] [Google Scholar]
- 40.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 41.Lim KO, Hedehus M, Moseley M, de Crespigny A, Sullivan EV, Pfefferbaum A. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch Gen Psychiatry. 1999;56:367–374. doi: 10.1001/archpsyc.56.4.367. [DOI] [PubMed] [Google Scholar]
- 42.McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- 43.Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC. Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry. 1998;155:1711–1717. doi: 10.1176/ajp.155.12.1711. [DOI] [PubMed] [Google Scholar]
- 44.Ettinger U, Chitnis XA, Kumari V, Fannon DG, Sumich AL, O’Ceallaigh S, Doku VC, Sharma T. Magnetic resonance imaging of the thalamus in first-episode psychosis. Am J Psychiatry. 2001;158:116–118. doi: 10.1176/appi.ajp.158.1.116. [DOI] [PubMed] [Google Scholar]
- 45.Kumari V, Gray JA, Honey GD, Soni W, Bullmore ET, Williams SC, Ng VW, Vythelingum GN, Simmons A, Suckling J, Corr PJ, Sharma T. Procedural learning in schizophrenia: a functional magnetic resonance imaging investigation. Schizophr Res. 2002;57:97–107. doi: 10.1016/s0920-9964(01)00270-5. [DOI] [PubMed] [Google Scholar]
- 46.Menon V, Anagnoson RT, Glover GH, Pfefferbaum A. Functional magnetic resonance imaging evidence for disrupted basal ganglia function in schizophrenia. Am J Psychiatry. 2001;158:646–649. doi: 10.1176/appi.ajp.158.4.646. [DOI] [PubMed] [Google Scholar]
- 47.Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS. Thalamic volumes in patients with first-episode schizophrenia. Am J Psychiatry. 2001;158:618–624. doi: 10.1176/appi.ajp.158.4.618. [DOI] [PubMed] [Google Scholar]
- 48.Deicken RF, Eliaz Y, Chosiad L, Feiwell R, Rogers L. Magnetic resonance imaging of the thalamus in male patients with schizophrenia. Schizophr Res. 2002;58:135–144. doi: 10.1016/s0920-9964(01)00330-9. [DOI] [PubMed] [Google Scholar]
- 49.Heckers S. Neuropathology of schizophrenia: cortex, thalamus, basal ganglia, and neurotransmitter-specific projection systems. Schizophr Bull. 1997;23:403–421. doi: 10.1093/schbul/23.3.403. [DOI] [PubMed] [Google Scholar]
- 50.Andreasen NC, O’Leary DS, Flaum M, Nopoulos P, Watkins GL, Boles Ponto LL, Hichwa RD. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet. 1997;349:1730–1734. doi: 10.1016/s0140-6736(96)08258-x. [DOI] [PubMed] [Google Scholar]
- 51.Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 52.Buchsbaum MS, Nenadic I, Hazlett EA, Spiegel-Cohen J, Fleischman MB, Akhavan A, Silverman JM, Siever LJ. Differential metabolic rates in prefrontal and temporal Brodmann areas in schizophrenia and schizotypal personality disorder. Schizophr Res. 2002;54:141–150. doi: 10.1016/s0920-9964(01)00361-9. [DOI] [PubMed] [Google Scholar]
- 53.Squires-Wheeler E, Friedman D, Skodol AE, Erlenmeyer-Kimling L. A longitudinal study relating P3 amplitude to schizophrenia spectrum disorders and to global personality functioning. Biol Psychiatry. 1993;33:774–785. doi: 10.1016/0006-3223(93)90018-9. [DOI] [PubMed] [Google Scholar]
- 54.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 55.Perkins DO, Leserman J, Jarskog LF, Graham K, Kazmer J, Lieberman JA. Characterizing and dating the onset of symptoms in psychotic illness: the Symptom Onset in Schizophrenia (SOS) inventory. Schizophr Res. 2000;44:1–10. doi: 10.1016/s0920-9964(99)00161-9. [DOI] [PubMed] [Google Scholar]
- 56.Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Hoffman R, Davidson L. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70:273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- 57.Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, Woods SW. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- 58.Bell MD, Lysaker PH, Beam-Goulet JL, Milstein RM, Lindenmayer JP. Five-component model of schizophrenia: assessing the factorial invariance of the Positive and Negative Syndrome Scale. Psychiatry Res. 1994;52:295–303. doi: 10.1016/0165-1781(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 59.Hirayasu Y, Tanaka S, Shenton ME, Salisbury DF, DeSantis MA, Levitt JJ, Wible C, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Prefrontal gray matter volume reduction in first episode schizophrenia. Cereb Cortex. 2001;11:374–381. doi: 10.1093/cercor/11.4.374. [DOI] [PubMed] [Google Scholar]
- 60.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Highley JR, Esiri MM, McDonald B, Cortina-Borja M, Cooper SJ, Herron BM, Crow TJ. Anomalies of cerebral asymmetry in schizophrenia interact with gender and age of onset: a post-mortem study. Schizophr Res. 1998;34:13–25. doi: 10.1016/s0920-9964(98)00077-2. [DOI] [PubMed] [Google Scholar]
- 62.Wible CG, Anderson J, Shenton ME, Kricun A, Hirayasu Y, Tanaka S, Levitt JJ, O’Donnell BF, Kikinis R, Jolesz FA, McCarley RW. Prefrontal cortex, negative symptoms, and schizophrenia: an MRI study. Psychiatry Res. 2001;108:65–78. doi: 10.1016/s0925-4927(01)00109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lieberman JA, Sheitman BB, Kinon BJ. Neurochemical sensitization in the patho-physiology of schizophrenia: deficits and dysfunction in neuronal regulation and plasticity. Neuropsychopharmacology. 1997;17:205–229. doi: 10.1016/S0893-133X(97)00045-6. [DOI] [PubMed] [Google Scholar]
- 64.Lieberman JA. Prediction of outcome in first-episode schizophrenia. J Clin Psychiatry. 1993;54 suppl:13–17. [PubMed] [Google Scholar]
- 65.McGlashan TH, Fenton WS. Subtype progression and pathophysiologic deterioration in early schizophrenia. Schizophr Bull. 1993;19:71–84. doi: 10.1093/schbul/19.1.71. [DOI] [PubMed] [Google Scholar]
- 66.Nair TR, Christensen JD, Kingsbury SJ, Kumar NG, Terry WM, Garver DL. Progression of cerebroventricular enlargement and the subtyping of schizophrenia. Psychiatry Res. 1997;74:141–150. doi: 10.1016/s0925-4927(97)00013-9. [DOI] [PubMed] [Google Scholar]
- 67.Knoll JL, IV, Garver DL, Ramberg JE, Kingsbury SJ, Croissant D, McDermott B. Heterogeneity of the psychoses: is there a neurodegenerative psychosis? Schizophr Bull. 1998;24:365–379. doi: 10.1093/oxfordjournals.schbul.a033332. [DOI] [PubMed] [Google Scholar]
- 68.Penn DL, Mueser KT, Spaulding W, Hope DA, Reed D. Information processing and social competence in chronic schizophrenia. Schizophr Bull. 1995;21:269–281. doi: 10.1093/schbul/21.2.269. [DOI] [PubMed] [Google Scholar]
- 69.Cornblatt BA, Lenzenweger MF, Dworkin RH, Erlenmeyer-Kimling L. Childhood attentional dysfunctions predict social deficits in unaffected adults at risk for schizophrenia. Br J Psychiatry Suppl. 1992 October;:59–64. [PubMed] [Google Scholar]
- 70.Freedman LR, Rock D, Roberts SA, Cornblatt BA, Erlenmeyer-Kimling L. The New York High-Risk Project: attention, anhedonia and social outcome. Schizophr Res. 1998;30:1–9. doi: 10.1016/s0920-9964(97)00132-1. [DOI] [PubMed] [Google Scholar]
- 71.Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci U S A. 2002;99:11447–11451. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 73.Hietala J, Syvalahti E, Vuorio K, Rakkolainen V, Bergman J, Haaparanta M, Solin O, Kuoppamaki M, Kirvela O, Ruotsalainen U. Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet. 1995;346:1130–1131. doi: 10.1016/s0140-6736(95)91801-9. [DOI] [PubMed] [Google Scholar]
- 74.Reith J, Benkelfat C, Sherwin A, Yasuhara Y, Kuwabara H, Andermann F, Bachneff S, Cumming P, Diksic M, Dyve SE. Elevated dopa decarboxylase activity in living brain of patients with psychosis. Proc Natl Acad Sci U S A. 1994;91:11651–11654. doi: 10.1073/pnas.91.24.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 76.Cohen JD, Servan-Schreiber D. Context, cortex, and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol Rev. 1992;99:45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- 77.Servan-Schreiber D, Printz H, Cohen JD. A network model of catecholamine effects: gain, signal-to-noise ratio, and behavior. Science. 1990;249:892–895. doi: 10.1126/science.2392679. [DOI] [PubMed] [Google Scholar]
- 78.Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry. 1996;3:241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- 79.Keshavan MS. Development, disease and degeneration in schizophrenia: a unitary pathophysiological model. J Psychiatr Res. 1999;33:513–521. doi: 10.1016/s0022-3956(99)00033-3. [DOI] [PubMed] [Google Scholar]
- 80.Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158:1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- 81.Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- 82.Salisbury DF, Shenton ME, Griggs CB, Bonner-Jackson A, McCarley RW. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch Gen Psychiatry. 2002;59:686–694. doi: 10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]